Abstract
Topoisomerase V is a type I topoisomerase without structural or sequence similarities to other topoisomerases. Although it belongs to the type I subfamily of topoisomerases, it is unrelated to either type IA or IB enzymes. We used real-time single-molecule micromechanical experiments to show that topoisomerase V relaxes DNA via events that release multiple DNA turns, employing a constrained swiveling mechanism similar to that for type IB enzymes. Relaxation is powered by the torque in the supercoiled DNA and is constrained by friction between the protein and the DNA. Although all type IB enzymes share a common structure and mechanism and type IA and type II enzymes show marked structural and functional similarities, topoisomerase V represents a different type of topoisomerase that relaxes DNA in a similar overall manner as type IB molecules but by using a completely different structural and mechanistic framework.
Keywords: magnetic tweezers, single molecule, archaeon, DNA topology, type IC
Topoisomerase V (topo V) of the archaeon Methanopyrus kandleri is a 110-kDa enzyme belonging to the type I family of topoisomerases (1). Type I topoisomerases previously have been subdivided into two subtypes, IA and IB (2), based on whether they form a transient 5′ or 3′ covalent bond with the broken DNA strand (reviewed in ref. 3). Topo V possesses biochemical properties associated with type IB topoisomerases, namely, cleavage of a single DNA strand, formation of a covalent intermediate with the 3′ end of the broken strand, and the ability to relax both positive and negative supercoils in a magnesium- and ATP-independent manner (1). Despite these commonalities, topo V shows no sequence similarity to other type IB enzymes (4). Furthermore, the structure of topo V bears no resemblance to any known topoisomerase but instead reveals a unique fold (5). Although the active site of topo V includes a constellation of amino acids similar to that in type IB enzymes, their spatial arrangement is different. All these data suggest that, despite overall similarities, topo V and type IB enzymes use different catalytic mechanisms for DNA cleavage and religation. Furthermore, the mechanism of DNA relaxation used by topo V is unknown.
Two mechanisms of DNA relaxation have been discussed for type I topoisomerases: an enzyme-bridged strand passage mechanism and a swiveling or “controlled rotation” mechanism. Type IA topoisomerases have a characteristic toroidal shape (6) and use an enzyme-bridged strand passage mechanism where both ends of the broken DNA strand are bound to the enzyme, preventing DNA swiveling. The intact DNA strand is passed through the transient break in the other strand before the topoisomerase religates the broken strand. This mechanism changes the DNA linking number strictly in increments of one (ΔLk = ±1) (7–9). Type IB enzymes use a radically different mechanism involving swiveling, or rotation of one DNA strand around the other, to release supercoils. The protein embraces the DNA and cleaves one strand, allowing the free cleaved strand to rotate around the other until religation occurs (10). This controlled rotation mechanism changes the linking number by more than one during each cleavage/religation cycle. The average linking number change per cycle varies with torque and type of enzyme (11), but a hallmark of the swiveling mechanism is that the number of turns released per cycle is exponentially distributed.
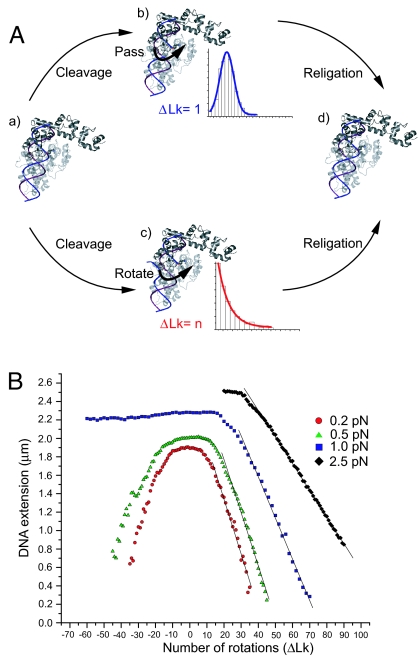
The structure of a 61-kDa fragment of topo V (5) revealed it to lack the hole required by the enzyme-bridged strand passage model. Instead, topo V possesses a large groove that could accommodate DNA more in agreement with the general features of type IB than type IA enzymes. Nevertheless, it is unclear whether topo V can completely surround DNA to constrain swiveling or whether it passes one strand through the other after cleavage (Fig. 1A). Given the unique structure and biochemistry of topo V, it is important to understand its mechanism in greater detail. Insights into the mechanistic pathway of topo V also may help understand better its role in vivo and establish whether there are alternative mechanisms of DNA relaxation not yet described.
Fig. 1.
Relaxation mechanisms and single DNA molecule calibration. (A) Possible mechanisms of DNA relaxation by topo V. Two possible mechanisms for DNA relaxation have been proposed for type I topoisomerases. Upon DNA binding (a), the relaxation cycle by topo V may proceed either by a strand passage mechanism (b) or a swiveling mechanism (c). The expected step-size distribution is shown for each case. The coordinates of topo-61 (PDB ID code 2CSB) were used to model possible interactions of topo V with DNA (5). (B) DNA calibration. The extension of a 9.7-kb DNA was monitored at different stretching forces. At a low force of 0.2 pN (red circles), the extension of DNA decreases irrespective of the sign of supercoiling because of the formation of plectonemic supercoils. At 0.5 pN (green triangles), DNA extension still decreases irrespective of the direction of rotation of the magnet, although plectonemes coexist with denatured DNA for negative supercoils. At high forces (1 pN, blue squares; 2.5 pN, black diamonds), DNA extension decreases only with the introduction of positive supercoils. The DNA denatures for negative supercoils, and there is no change in DNA extension with introduction of negative rotations. Events only were considered for analysis only when they fell in the linear range of the DNA calibration curve.
In this study, we probed the mechanism of DNA relaxation used by topo V by conducting real-time single-molecule experiments with magnetic tweezers with an N-terminal 78-kDa fragment of topo V (topo-78). The experiments reveal that topo V relaxes both positively and negatively supercoiled DNA via multiple-turn events. Experiments at different stretching forces show the relationship between the rotational relaxation rate (angular relaxation velocity) and the mean number of steps relaxed per cycle. We found the relationship between the torque in the DNA and the angular relaxation velocity. Experiments at different viscosities suggest that conformational changes in the DNA and enzyme may play an important role in the reaction cycle. We also have quantified the microscopic friction associated with a topoisomerase; we have found the linear rotational friction and also observed the onset of a nonlinear friction effect at large rotation rate.
Comparison of the results obtained for topo V and type IB enzymes reveals that despite drastic structural differences, there are overall commonalities in their mechanisms, although with significant and subtle differences. The sequence, biochemical, structural, and micromechanical data strongly suggest that topo V represents a topoisomerase unique to archaea, with a unique structure and catalytic mechanism of cleavage/religation, but with DNA relaxation mechanics similar to those of the type IB enzymes. Our observations indicate that topo V represents a discrete class of topoisomerases, type IC, with distinct structural and mechanistic characteristics (5, 12).
Results
Topo V is unusual in that it has topoisomerase and apurinic/apyrimidinic site-processing activity in the same molecule. Topoisomerase activity is present in the ≈30-kDa fragment at the N-terminal part of the enzyme, whereas the rest of the protein is formed by 24 tandem helix–hairpin–helix (HhH) repeats, some of which are involved in DNA repair (4, 13). Topo V is a hyperthermophilic enzyme with a temperature optimum of 108°C for its topoisomerase activity (1). The enzymatic activity decreases with decreasing temperature, exhibiting slow relaxation activity near 37°C and almost no activity at room temperature (data not shown).
To monitor single-molecule events in real time, we took advantage of this property and conducted experiments at 40°C. In addition, most topo V relaxation experiments were carried out in buffer with 40% glycerol added. The viscosity of this buffer solution was measured to be three times larger than the buffer without glycerol. The presence of glycerol slowed down the rotation rate and allowed us to resolve relaxation events more clearly. Addition of glycerol does not abolish relaxation in bulk experiments (data not shown); no attempt was made to quantitate the effect of glycerol on relaxation rate in the bulk experiments. A smaller number of experiments were carried out by using buffer with no glycerol added (Materials and Methods).
For low forces (≤0.5 pN), the behavior of the DNA is insensitive to the sign of supercoiling, and addition of either positive or negative plectonemic supercoils results in similar reductions of DNA extension (Fig. 1B). Under low force, addition of topo-78 to positively or negatively supercoiled DNA results in a stepwise increase in DNA extension, where each elongation step signifies removal of DNA supercoils (Fig. 2A). We did not observe a clear difference in DNA relaxation that depended on the sign of supercoiling (data not shown). We do expect differences for relaxation of supercoils of different signs [supporting information (SI)], but extensive and highly accurate measurements would be required to establish the magnitude of this asymmetry.
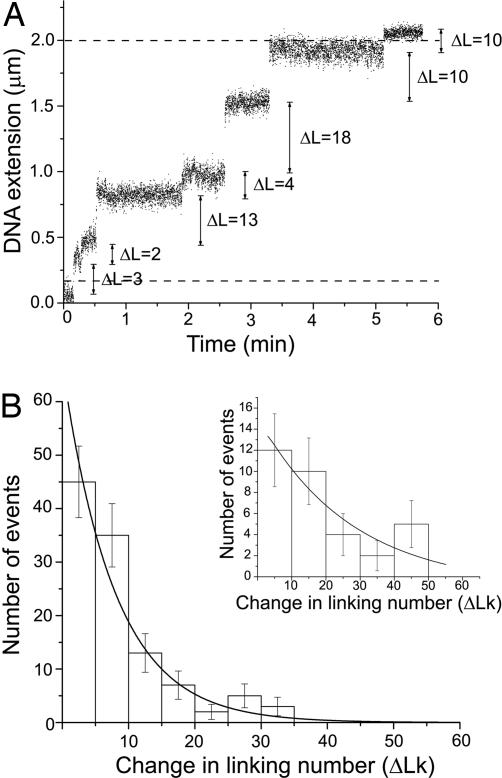
Fig. 2.
Monitoring of real-time DNA relaxation events and step-size distributions. (A) Real-time relaxation events on positively supercoiled DNA are shown at 1.5 pN in the presence of glycerol. An increase in DNA extension upon addition of topo-78 was observed. Each step corresponds to a relaxation event representing one cleavage/religation cycle. The change in linking number is of different size for different events. Hence, topo V relaxes DNA in steps of n supercoils. The dashed lines mark the extension limits used to select events for further analysis in this particular experiment. (B) A step-size distribution of topo-78 acting on a single DNA molecule is shown for events monitored at a force of 0.5 pN in presence of glycerol when 45 plectonemic supercoils were incorporated. A similar shape distribution was observed at all forces studied. The mean step size for each distribution was computed numerically by using the method of Koster et al. (14), yielding a mean ΔLk of 12.3 ± 1.8. (Inset) Step-size distribution for topo-78 measured in absence of glycerol when 45 plectonemic supercoils were applied at a force of 0.5 pN. The line was fit assuming an exponential decay. Error bars represent the square root of the number of events.
Relaxation reactions could be carried out many times; after full relaxation, the magnet was rotated again to reintroduce supercoils, and the DNA subsequently was relaxed by the topoisomerase. At higher forces (≥1 pN), however, DNA extension decreased only when positive supercoils were applied; introduction of negative supercoils led to DNA denaturation with no concomitant change in DNA extension (Fig. 1B). Because of this finding, experiments at or above 1 pN force were carried out with positively supercoiled DNA.
One of our major objectives was determining whether topo-78 relaxes DNA by changing linking number in single or multiple steps. The number of steps per event represents the change in linking number per cleavage/religation cycle, and hence its distribution gives information on the mechanism of DNA relaxation by topo V. Linking number changes (ΔLk) were calculated from changes in DNA length for each relaxation event. Fig. 2A shows that the linking number changes in steps of different size for each cleavage/religation cycle and follows an exponential distribution corresponding to ΔLk jumps in excess of one (Fig. 2B).
To estimate the mean number of supercoils removed, we used a maximum-likelihood approach to fit the step-size distribution to NE = Ae−s/〈ΔLk〉, where NE is the number of events, s is the step size, and 〈ΔLk〉 is the mean change in linking number per event (14). This approach takes into account the fact that the length of the DNA in the experimental setup is finite, and hence there is a constraint on the possible number of steps taken, particularly for events when the DNA is almost fully relaxed (14). Fig. 2B shows a typical histogram for the step-size distribution at a force of 0.5 pN. The mean change in linking number is 12.3 ± 1.8 for topo-78 in 40% glycerol buffer. Hence, topo V is similar to type IB enzymes in that it relaxes DNA with a mean change in linking number greater than one and with an exponential distribution of step size.
Relaxation events also were measured for topo-78 in buffer with no glycerol added; the shape of the step-size distribution was similar (Fig. 2B Inset). Too few events were recorded in the absence of glycerol to obtain a precise estimate of the mean step size, but we did observe that 〈ΔLk〉 is increased by more than a factor of two in the lower-viscosity glycerol-free experiments. Measurements done at several stretching forces both with and without glycerol indicated that in all cases 〈ΔLk〉 was not unity or any other fixed value.
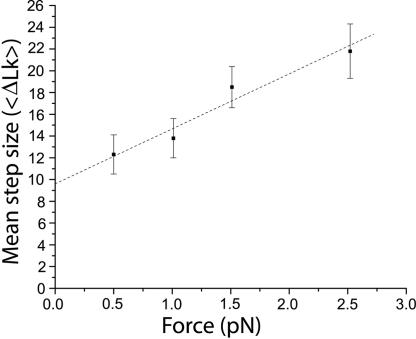
The mean number of supercoils released during each relaxation cycle by topo-78 depends on the initial stretching force on the DNA. Experiments at 0.5, 1.0, 1.5, and 2.5 pN were performed to obtain the mean step size at these stretching forces. In addition, a few data points were collected at 0.25 pN, enough to obtain estimates of the velocity but not enough to obtain a reliable mean step size. Although the shape of the distribution is similar for all of the forces tested and follows an exponential function (SI), the mean step size increases with applied force (Fig. 3), reaching ≈20 turns at 2.5 pN. Fitting the data to a model that describes the step-size distribution in terms of the rates of cleavage and religation (SI) allows us to estimate the mean step size at zero angular velocity to be ≈12 turns in the presence of 40% glycerol.
Fig. 3.
Dependence of mean step size on force. The mean step size (〈ΔLk〉) was plotted as a function of applied force and shows an increase in the mean step size with increasing force. The dashed line serves as a visual guide only. The error bars represent the standard error [standard deviation/(number of events)1/2].
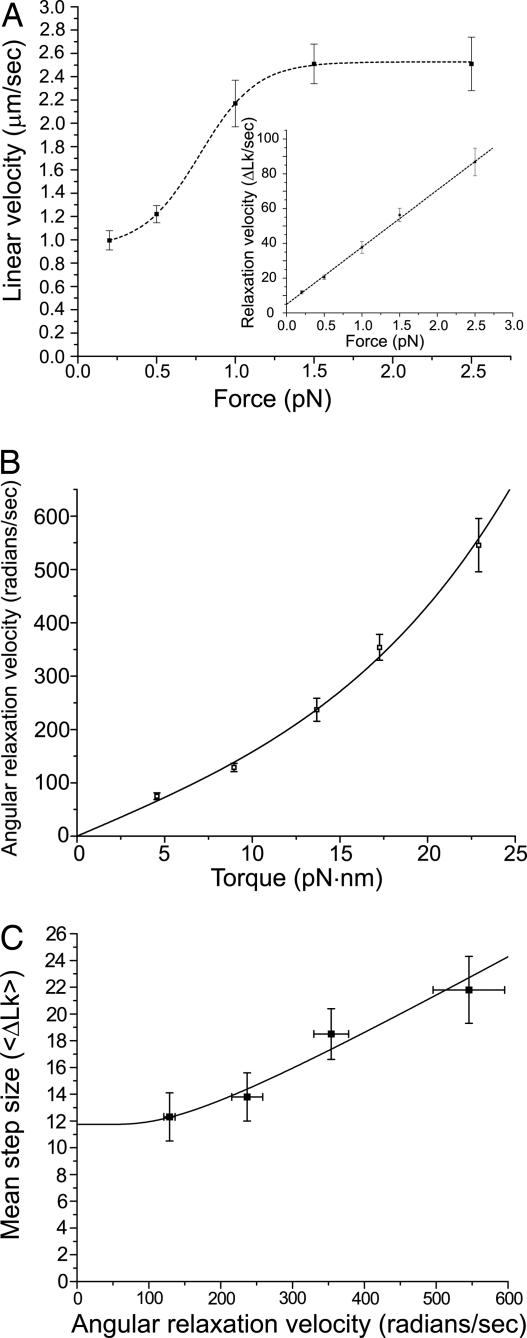
We also measured the “linear velocity” of each event, the rate at which the DNA extends as supercoils are released (11). For topo-78, this was found to depend highly nonlinearly on the applied force. The mean linear velocity of DNA relaxation was 1.2 ± 0.1 μm·sec−1 at 0.5 pN in 40% glycerol. With increase in force, a higher velocity of 2.2 ± 0.2 μm·sec−1 (at 1.0 pN) was observed; then the velocity appears to saturate, increasing to only 2.5 ± 0.2 μm·sec−1, at 2.5 pN (Fig. 4A). This apparent saturation simply is caused by the dependence of extension with linking number, which varies strongly with force (Fig. 1B): at higher forces, larger changes in DNA linking number are required to generate a given change in extension. The rate of change of the linking number, hereafter referred to as the “relaxation velocity” (i.e., the number of turns relaxed per second), increased with applied force (Fig. 4A Inset).
Fig. 4.
Linear and angular velocity dependencies on force and torque. (A) Linear velocity (μm/sec) has a nonlinear dependence on force, indicating that bead translational drag forces do not limit the rate of relaxation. The dashed line serves as a visual guide and does not correspond to a fit. (Inset) Plot of the angular relaxation velocity versus force whose dependence is linear over the range of forces studied. The linear regression fit has a correlation coefficient of 0.991. (B) Angular relaxation velocity ω (rad/sec) initially increases linearly with torque, indicating that rotational friction is the limiting source of friction in the relaxation over the range of forces studied (SI). The line corresponds to a fit of the data to the expression relating angular velocity to torque (SI), with positive and negative angular barrier widths set equal (θ+ = θ−), kBT = 4.1 pN·nm, and ηℓ3 = 10−9 pN·nm·sec. From the fit (reduced χ2 value = 1.4), the energy barrier (EB) is equal to 80.1 ± 0.7 pN·nm or 11.5 ± 0.1 kcal/mol. (C) The mean step size increases with increasing angular relaxation velocity. The line corresponds to a fit of the data to an equation describing the probability per turn that religation does not occur (P1), noting that 〈ΔLk〉 = −1/(1 − P1) (see SI). Using this model, we obtain values of kδ = 361.4 ± 132.4 sec−1 and k′δ = 33.8 ± 6.7 sec−1, respectively, where k and k′ are the cleavage and religation rates and δ is the angular range where cleavage and religation occur. The fit indicates that 〈ΔLk〉 = 11.7 ± 1.0 at zero angular velocity. In all cases, the error bars represent the standard error.
Velocity measurements also were made in buffer with no glycerol. The mean linear velocity of topo-78 events with no glycerol was found to be 3.0 ± 0.4 μm·sec−1 at 0.5 pN and 7.4 ± 1.5 μm/sec at 2.5 pN. These values correspond to relaxation velocities of 51.2 ± 6.1 Lk sec−1 at 0.5 pN and 254.8 ± 53.2 Lk sec−1 at 2.5 pN, compared with 20.5 ± 1.2 Lk sec−1 at 0.5 pN and 86.8 ± 7.9 Lk sec−1 at 2.5 pN in the presence of 40% glycerol. Hence, the 40% glycerol causes a reduction in the relaxation velocity by topo-78 by ≈2.5-fold, nearly equal to the change in viscosity introduced by the glycerol.
Relaxation of DNA occurs in the absence of an external energy source and is driven solely by the torque in the supercoiled DNA. We estimated the torque in the DNA by using Monte Carlo simulations of supercoiled DNA under tension (15) (SI). Plotting angular relaxation velocity, ω (radians of rotation per second), versus torque reveals a nonlinear relation between them (Fig. 4B). At low angular relaxation velocities, there appears to be a linear relation, but at higher torques, the linearity disappears. The lack of a linear approach to zero by the linear velocity (Fig. 4A) indicates that bead translational motion is not the primary source of friction in the experiments (SI). On the other hand, the linear approach to zero rotation rate at zero torque (Fig. 4B) indicates that the primary source of dissipation is rotational.
The rotational friction follows from the rate at which torque increases with angular velocity in Fig. 4B. Near ω = 0, this rotational friction is ζ = 0.071 ± 0.002 pN·nm·sec. We note that the equivalent friction associated with rotation of the entire 9.7-kb-long DNA molecule around its axis in glycerol is ≈100 times smaller (SI), indicating that the source of the rotational friction is not the fluid viscosity imparting drag to rotation of all or part of the DNA molecule but instead is caused by some other source, most likely the enzyme–DNA interaction. The same analysis applied to our glycerol-free experiments, where the rotational velocities were ≈2.5 times higher, indicates that the rotational friction in our experiments is approximately proportional to the solution viscosity. Finally, we note that if we use the torque formula (2kBTAf)1/2 (16) instead of our Monte Carlo results, we are led to a significantly different value of ζ = 0.131 ± 0.002 pN·nm·sec, an overestimate of the friction by ≈2.
Vaccinia virus topo IB has been characterized by single-molecule experiments similar to the ones described in ref. 11. We carried out experiments with the vaccinia IB enzyme to compare with topo V. We found vaccinia topo IB to be active in the presence of 40% glycerol (data not shown). Because of the short length of DNA (9.7 kb) used in our experiments, the maximum number of supercoils introduced at 1.5 pN was limited to 80. In the absence of glycerol, vaccinia topo IB has a mean step size of ≈80 at this force (11). In the presence of glycerol, the DNA was relaxed in one event in most instances, and we could not ascertain the effect of glycerol on the step-size distribution. Nevertheless, these experiments confirmed that the mean step size for vaccinia virus topo IB is much larger than the one observed for topo V at the same applied force and under the same conditions.
Another type IB topoisomerase with strong similarities to the viral enzymes is the bacterial type IB enzyme from Deinococcus radiodurans (17, 18). A step-size distribution for D. radiodurans topo IB was monitored at a force of 1 pN in the absence of glycerol, giving a mean step size of 54.4 ± 33.8 turns. At this force, vaccinia topo IB has been reported to have a mean step size of ≈50 supercoils (11), nearly the same as for D. radiodurans topo IB. The velocity of D. radiodurans topo IB (no glycerol) was found to be 7.9 μm·sec−1 at 1 pN force, a value comparable to that observed for vaccinia virus topo IB (6.7 μm·sec−1 at 0.2 pN) (11). The remarkably consistent topo IB results provide a solid baseline with which to compare topo V.
Discussion
Our micromechanical experiments establish that topo V relaxes DNA in multiturn steps and uses a swiveling mechanism for DNA relaxation. The step-size distribution reveals the mean change in linking number per cleavage/religation cycle. For type IA topoisomerases, there only is one turn removed per cycle, whereas for vaccinia topo IB, the mean step size is 19 turns per cycle in the absence of any applied force (11). By contrast, topo V releases ≈12 turns per cycle in the low-force limit in 40% glycerol, in accord with the value of ≈15 estimated from bulk experiments on topo V (1).
The mechanism of DNA relaxation by type IB topoisomerases has been shown to fit into a model in which friction and torque govern the relaxation activity (11). The major source of friction is thought to be the interaction between the protein and the DNA, whereas the torque is provided by the torsional stress in the supercoiled DNA. A hallmark of this model is the mean step-size dependence with force, which translates into a dependence of the mean step size with angular relaxation velocity. Fig. 4C shows that for topo V the mean step size increases with increasing angular relaxation velocity. This relationship can be understood easily by assuming that both ends of the broken strand have to be physically close for religation to occur. As the angular relaxation velocity increases, the rotating strand moves past the other one faster and is in optimal position for religation for a shorter time interval. This leads to a lower probability of religation per step, and consequentially longer mean steps, for larger angular speeds. Given this hypothesis, and our observation that increased viscosity leads to reduced relaxation velocities, the average step size should decrease as viscosity is increased. We carried out step-size measurements for topo V in buffers with different amounts of glycerol, with the result that the mean step size is smaller in 40% than in the 0% glycerol.
The dependence of the rotational velocity, and therefore the rotational friction, on viscosity indicates that relatively large changes (on the order of a few nanometers) and also dynamic conformational changes of either the DNA or the enzyme, which are sensitive to fluid viscosity, are the source of the friction. This is consistent with the model of Koster et al. (11), which posits thermal fluctuations of the protein–DNA complex over an energy barrier as the origin of the friction, because the rate of thermal fluctuations in such a model is inversely proportional to the fluid viscosity. Furthermore, as ω increases, we have observed the rotational friction to drop (slope increase for large ω in Fig. 4B), as expected if the main source of the friction is caused by thermal fluctuation over a free-energy barrier to rotation (11). We remark that the leading nonlinearity should be a term with quadratic dependence of angular velocity on torque, leading to differing frictions for relaxations in the two senses of rotation, i.e., differing magnitude of angular velocities for relaxation of positive and negative supercoils with equal magnitude torques (SI).
The mean step size for topo IB has been observed to be highly sensitive to the force applied to the DNA; as forces were varied from 1 pN to 3 pN, relaxation events varied in size from 20 to over 150 turns, roughly an eight-fold increase (11). In contrast to this, the topo V step size increases only by about a factor of two when the applied force is changed from 0.5 to 2.5 pN. This finding suggests that there is less dependence of the step size on angular relaxation velocity in the case of topo V (Fig. 4C). The smaller steps and the much lower level of sensitivity of step size to angular relaxation velocity for topo V relative to topo IB make clear that the modes of DNA relaxation of these two classes of enzymes have overall similarities, but the detailed mechanisms are distinct. Furthermore, topo-78 exhibits a slower linear velocity than does vaccinia topo IB. The linear velocity of topo-78 is 3.0 μm·sec−1 (0.5 pN) in glycerol-free buffer and it is likely to be slower at lower forces; the relaxation velocity for vaccinia topo IB is 6.7 ± 0.2 μm·sec−1 (0.2 pN) (11). For comparison, a nicking enzyme represents the fastest release of supercoils after cleavage of a DNA strand and has a velocity of 10.5 ± 0.2 μm·sec−1 (0.2 pN) (11). The slower linear velocity of topo V relative to topo IB indicates that there is even more rotary friction for topo V than for topo IB. A larger friction reduces the angular velocity, which reduces mean step size for a given nonzero torque or force (11), helping to explain the smaller step sizes observed for topo V relative to topo IB. However, we note that the nonzero mean step size in the limit of zero angular velocity (Fig. 4C) depends only on the competing ligation and recleavage rates during relaxation (ref. 11 and SI).
Although both topo V and topo IB appear to use a swiveling mechanism for DNA relaxation, the differences in mean step size, relaxation velocity, and the dependence of the mean step size with torque suggest that although the overall modes of DNA relaxation used by topo V and topo IB are the same, i.e., swiveling, the detailed mechanisms are distinct. The type IB model postulates that the enzyme introduces friction and hinders the free rotation of DNA and consequently slows down the velocity of the reaction. The source of friction is the interaction between protein and DNA and ensures that the steps are finite and that religation occurs. In support of this model, it has been observed that the linear velocity of vaccinia topo IB increases from 6.7 to 8.9 μm·sec−1 upon mutation of an amino acid involved in interactions with DNA (11, 19). Additionally, the structure of both a eukaryotic and a viral type IB enzyme in complex with DNA (20, 21) show that the DNA is completely encircled by the protein. There is no structure of a complex of topo V with DNA, but a model of the topoisomerase domain in complex with DNA (5) suggests extensive interaction of the protein with one face of the DNA, which could constrain free rotation of the strands. Although the binding groove in topo V appears to be longer than the equivalent region in type IB enzymes and may be able to exert similar friction without encircling the DNA, it is not clear that the type IB model will apply to topo V.
Finally, we also note that the generally slower rotation permitted by topo V may reflect differences between the physical conditions in our experiments and those found in the thermophilic bacterium where topo V occurs. Possibly the 40°C temperature used in the experiments, being much lower than the usual temperature of 108°C where topo V acts, leads to slow activity. Alternately, the absence of some of the C-terminal HhH repeats of topo V in topo-78 may affect the rotation rate. Experiments to study effects of temperatures above 40°C and inclusion of more of the HhH repeats will need to be the object of future studies.
In conclusion, despite differences in sequence, structure, and catalytic mechanism of DNA cleavage and religation, there is schematic conservation of the relaxation mechanism of type IB topoisomerases and topo V (Table 1). Both types of enzymes use a swiveling mechanism to relax DNA, but it appears that in the case of topo V, the swiveling is significantly more constrained, leading to a smaller mean step size and slower relaxation rates. These two occurrences of the same overall mechanism suggest convergent evolution of very similar functions of these two subtypes of type I topoisomerases. This observation is even more striking when viewed in the context of all other topoisomerases. Before the structure of topo V was known, there appeared to be two general motifs used by all topoisomerases; type IA and type II enzymes show biochemical similarities in the mechanism of DNA cleavage/religation, structural similarities in some regions (22), and overall mechanistic similarities in the strand passage method used. However, all type IB enzymes share a common fold and use the same cleavage/religation and DNA relaxation mechanism. Topo V does not fit into either of these two general groups. Structurally and biochemically, topo V illustrates a completely different solution to the same DNA relaxation problem but with mechanistic features reminiscent of type IB topoisomerases. Topo V therefore represents a unique type of topoisomerase, termed type IC, that independently acquires the same overall manner of relaxing DNA as type IB enzymes, while using a completely different structural and biochemical framework characterized by a more constrained mode of DNA swiveling.
Table 1.
Comparison of type I topoisomerases
| Properties | Type |
||
|---|---|---|---|
| IA | IB | IC | |
| Phylogenetic distribution | Archaea, Prokarya, and Eukarya (3) | Prokarya, Eukarya, and some eukaryotic viruses (3) | Archaea* (3) |
| Cleavage polarity | 5′ (25) | 3′ (3) | 3′ (1) |
| Substrate DNA | Negatively supercoiled DNA† (25) | Positively and negatively supercoiled DNA (3) | Positively and negatively supercoiled DNA (1) |
| Mg2+ dependence | Yes (25) | No (3) | No (1) |
| Change in linking number | Strictly in steps of +1 (9) | In steps of n (〈ΔLk〉 ≈19 at zero force) (11) | In steps of n (〈ΔLk〉 ≈12 at zero angular velocity in 40% glycerol) |
| Force dependence on linking number change | No (9, 26) | Yes (11) | Yes |
| Relaxation velocity | 0.0023‡ μm/sec (26) | 4.1§–6.7¶ μm/sec (11) | 3.0 μm/sec‖ |
| Structural properties | Toroidal shape; binds ssDNA along a groove and accepts passing strand in central hole (6, 27); structural and mechanistic similarities to type II enzymes (22) | Concave shape; surrounds DNA in a clamp-like manner; structural similarities to tyrosine recombinases suggest a common ancestor (21, 28) | Unique fold that includes a large positively charged groove containing a helix–turn–helix motif; some of the active site residues are found in the helix–turn–helix motif (5) |
| Mechanism | Enzyme-bridged strand passage (7–9) | Swiveling or ″controlled rotation″ (10, 11) | Constrained swiveling |
Materials and Methods
Micromanipulation of DNA.
All single-molecule micromanipulation experiments were performed by using the magnetic tweezers setup described (23, 24). Flow-cell preparation, force measurements, and calibration were performed as described (23, 24). Briefly, a 9.7-kb DNA fragment with multiple digoxygenins at one end was tethered to an anti-digoxygenin functionalized cover glass. A paramagnetic bead was attached to the other end of the DNA fragment through multiple biotin—streptavidin interactions (23, 24). The glass slide in the flow cell was passivated with BSA to minimize nonspecific interactions.
Magnetic Tweezers Calibration.
To ascertain whether the bead was attached to a single, intact DNA molecule, the bead in the flow cell was stretched with increasing force and the response of the DNA with respect to force plotted (Fig. 1B). DNA supercoils were introduced by rotating the magnet on the tweezers. The extension of a single DNA molecule attached to the bead decreased as supercoils were introduced, and its length was related directly to the number of introduced supercoils. Nicked molecules did not change their length upon supercoiling and were discarded.
Protein Purification.
An N-terminal fragment of M. kandleri topo V (topo-78, residues 1–685) had been cloned earlier (13), and purification was carried out as described elsewhere (2, 5). D. radiodurans topo IB and vaccinia topo IB enzymes were used as controls for comparisons with topo-78. D. radiodurans topo IB was purified as described (17, 18), and vaccinia virus topo IB was obtained from Epicentre Biotechnologies, Madison, WI.
Buffers and Relaxation Experiments.
Topo-78 relaxation experiments were performed at 40°C by using a small heater attached to the microscope lens. A thermometer monitored the temperature of the flow cell at all times. The experiments were performed in a buffer containing 50 mM Tris·HCl (pH 7.0) and 50 mM NaCl. Vaccinia virus topo IB reactions were performed in 50 mM Tris·HCl (pH 8.0), 50 mM NaCl, and 2 mM MgCl2 at 37°C. Most relaxation events for both topo-78 and topo IB were too fast to be monitored by the experimental setup and did not permit unambiguous calculation of the velocity. Hence, 40% glycerol was added to the buffer. Viscosity measurements were performed for the topo IB and topo V buffers at 37°C and 40°C, respectively, both in presence and absence of glycerol, by using a Physica Modular Compact Rheometer (Anton Paar, Graz, Austria) with a double-gap cuvette fixture. Glycerol causes the viscosity to increase by 3.1-fold at 40°C, resulting in an increase in drag force and slowing down of the rotation rate. Glycerol was excluded from a small number of experiments to confirm that it does not have a deleterious effect on enzymatic activity and that the functional form of the step-size distribution remained unchanged. D. radiodurans topo IB relaxations were performed in 40 mM sodium phosphate buffer (pH 8.0) and 200 mM potassium glutamate at 37°C. The number of supercoils introduced was 20–30 at 0.2 pN, 45 at 0.5 pN, 60 at 1.0 pN, 80 at 1.5 pN, and 110–115 at 2.5 pN. The concentrations of the proteins used were 1 nM, 0.1–10 nM, and 5–100 nM for topo-78, vaccinia virus topo IB, and D. radiodurans topo IB, respectively.
Data Analysis and Step-Size Estimation.
Data analysis to obtain the mean step size was performed by using the maximum likelihood method of Koster et al. (14) and solving the equation numerically. This approach ensures that we do not bias our results by the constraints imposed on the maximum step size because of the experimental setup, which may lead to overrepresentation of the smaller substeps. In addition, all steps that resulted in total relaxation of the molecule were ignored because the experimental constraints limited the maximum possible size of this final step (14). All other data fits were done by using Origin 7 (OriginLab, Northampton, MA).
Supplementary Material
Acknowledgments
We thank H. Bai for help with the single-molecule experiments, A. Schoch and W. Burghardt for help with the viscosity measurements, A. Patel (Northwestern University) for the gift of topo IB proteins, and members of the J.F.M. and A.M. laboratories for their help and advice. This research was supported by National Institutes of Health Grant GM51350 (to A.M.) and by National Science Foundation Grants PHY-0445565 and DMR-0715099 (to J.F.M.).
Abbreviations
- topo V
topoisomerase V
- topo-78
N-terminal 78-kDa fragment of topo V
- HhH
helix–hairpin–helix.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0701989104/DC1.
References
- 1.Slesarev AI, Stetter KO, Lake JA, Gellert M, Krah R, Kozyavkin SA. Nature. 1993;364:735–737. doi: 10.1038/364735a0. [DOI] [PubMed] [Google Scholar]
- 2.Slesarev AI, Lake JA, Stetter KO, Gellert M, Kozyavkin SA. J Biol Chem. 1994;269:3295–3303. [PubMed] [Google Scholar]
- 3.Champoux JJ. Annu Rev Biochem. 2001;70:369–413. doi: 10.1146/annurev.biochem.70.1.369. [DOI] [PubMed] [Google Scholar]
- 4.Belova GI, Prasad R, Kozyavkin SA, Lake JA, Wilson SH, Slesarev AI. Proc Natl Acad Sci USA. 2001;98:6015–6020. doi: 10.1073/pnas.111040498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Taneja B, Patel A, Slesarev A, Mondragon A. EMBO J. 2006;25:398–408. doi: 10.1038/sj.emboj.7600922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lima CD, Wang JC, Mondragon A. Nature. 1994;367:138–146. doi: 10.1038/367138a0. [DOI] [PubMed] [Google Scholar]
- 7.Brown PO, Cozzarelli NR. Proc Natl Acad Sci USA. 1981;78:843–847. doi: 10.1073/pnas.78.2.843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li Z, Mondragon A, DiGate RJ. Mol Cell. 2001;7:301–307. doi: 10.1016/s1097-2765(01)00178-2. [DOI] [PubMed] [Google Scholar]
- 9.Dekker NH, Rybenkov VV, Duguet M, Crisona NJ, Cozzarelli NR, Bensimon D, Croquette V. Proc Natl Acad Sci USA. 2002;99:12126–12131. doi: 10.1073/pnas.132378799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stewart L, Redinbo MR, Qiu X, Hol WG, Champoux JJ. Science. 1998;279:1534–1541. doi: 10.1126/science.279.5356.1534. [DOI] [PubMed] [Google Scholar]
- 11.Koster DA, Croquette V, Dekker C, Shuman S, Dekker NH. Nature. 2005;434:671–674. doi: 10.1038/nature03395. [DOI] [PubMed] [Google Scholar]
- 12.Forterre P. Trends Biotechnol. 2006;24:245–247. doi: 10.1016/j.tibtech.2006.04.006. [DOI] [PubMed] [Google Scholar]
- 13.Belova GI, Prasad R, Nazimov IV, Wilson SH, Slesarev AI. J Biol Chem. 2002;277:4959–4965. doi: 10.1074/jbc.M110131200. [DOI] [PubMed] [Google Scholar]
- 14.Koster DA, Wiggins CH, Dekker NH. Proc Natl Acad Sci USA. 2006;103:1750–1755. doi: 10.1073/pnas.0510509103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vologodskii AV, Marko JF. Biophys J. 1997;73:123–132. doi: 10.1016/S0006-3495(97)78053-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Strick T, Allemand J, Croquette V, Bensimon D. Prog Biophys Mol Biol. 2000;74:115–140. doi: 10.1016/s0079-6107(00)00018-3. [DOI] [PubMed] [Google Scholar]
- 17.Krogh BO, Shuman S. Proc Natl Acad Sci USA. 2002;99:1853–1858. doi: 10.1073/pnas.032613199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Patel A, Shuman S, Mondragon A. J Biol Chem. 2006;281:6030–6037. doi: 10.1074/jbc.M512332200. [DOI] [PubMed] [Google Scholar]
- 19.Sekiguchi J, Shuman S. EMBO J. 1996;15:3448–3457. [PMC free article] [PubMed] [Google Scholar]
- 20.Perry K, Hwang Y, Bushman FD, Van Duyne GD. Mol Cell. 2006;23:343–354. doi: 10.1016/j.molcel.2006.06.015. [DOI] [PubMed] [Google Scholar]
- 21.Redinbo MR, Stewart L, Kuhn P, Champoux JJ, Hol WG. Science. 1998;279:1504–1513. doi: 10.1126/science.279.5356.1504. [DOI] [PubMed] [Google Scholar]
- 22.Corbett KD, Berger JM. Annu Rev Biophys Biomol Struct. 2004;33:95–118. doi: 10.1146/annurev.biophys.33.110502.140357. [DOI] [PubMed] [Google Scholar]
- 23.Yan J, Skoko D, Marko JF. Phys Rev E Stat Nonlin Soft Matter Phys. 2004;70 doi: 10.1103/PhysRevE.70.011905. 011905. [DOI] [PubMed] [Google Scholar]
- 24.Skoko D, Wong B, Johnson RC, Marko JF. Biochemistry. 2004;43:13867–13874. doi: 10.1021/bi048428o. [DOI] [PubMed] [Google Scholar]
- 25.Wang JC. Annu Rev Biochem. 1996;65:635–692. doi: 10.1146/annurev.bi.65.070196.003223. [DOI] [PubMed] [Google Scholar]
- 26.Dekker NH, Viard T, de La Tour CB, Duguet M, Bensimon D, Croquette V. J Mol Biol. 2003;329:271–282. doi: 10.1016/s0022-2836(03)00320-6. [DOI] [PubMed] [Google Scholar]
- 27.Changela A, DiGate RJ, Mondragon A. Nature. 2001;411:1077–1081. doi: 10.1038/35082615. [DOI] [PubMed] [Google Scholar]
- 28.Cheng C, Kussie P, Pavletich N, Shuman S. Cell. 1998;92:841–850. doi: 10.1016/s0092-8674(00)81411-7. [DOI] [PubMed] [Google Scholar]
- 29.Slesarev AI, Zaitzev DA, Kopylov VM, Stetter KO, Kozyavkin SA. J Biol Chem. 1991;266:12321–12328. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.