Abstract
Glycerol nucleic acid (GNA) is an interesting alternative base-pairing system based on an acyclic, glycerol-phosphate backbone repeat unit. The question of whether DNA polymerases can catalyze efficient template-dependent synthesis using GNA as the template is of particular interest because GNA is unable to form a stable duplex with DNA. In the present study, we screened a variety of DNA polymerases for GNA-dependent DNA synthesis. We find that Bst DNA polymerase can catalyze full-length DNA synthesis on a dodecamer GNA template. The efficiency of DNA synthesis is increased by replacing adenine with diaminopurine in both the GNA template and the DNA monomers and by the presence of manganese ions. We suggest that the BstDNA polymerase maintains a short, transient region of base-pairing between the DNA product strand and the GNA template, but that stable duplex formation between product and template strands is not required for template-dependent polymerization.
Keywords: information transfer, polymerase
Nucleic acid analogs with altered backbones or bases are of significant interest in the search for biopolymers with novel chemical and biological properties, and many such analogs have been designed and synthesized (1–3). Evaluation of the hybridization and nuclease-resistance properties of these synthetic nucleic acids has led to several nucleic acid analogues with potential biological applications (4–6). However, much less is known about the potential for information transfer between synthetic nucleic acid systems and the modern DNA/RNA system via template-dependent polymerization, a crucial aspect of the chemical etiology of nucleic acids and directed evolution of functional biopolymers based on a synthetic nucleic acid system.
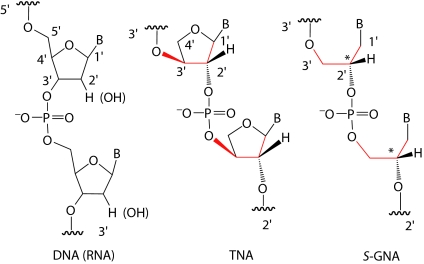
Recently, our group and others have studied the enzymatic synthesis of (3′ → 2′) α-l-threose nucleic acid (TNA; Fig. 1) (7–13). TNA was discovered by Eschenmoser and coworkers (7, 8) during a systematic evaluation of the base-pairing properties of nucleic acids containing alternative sugar-phosphate backbones. TNA was found to be capable of forming stable, antiparallel duplexes with RNA, DNA, and itself, a surprising property for a nucleic acid analog with a shorter backbone repeat unit than DNA or RNA (8, 14, 15). This remarkable intersystem duplex formation has been considered to be the basis of possible information transfer between TNA and DNA/RNA. Later studies by our group revealed that α-l-threose nucleoside 3′-triphosphates were substrates for efficient template-dependent enzymatic polymerization by Therminator DNA polymerase, a mutated archaeal family B DNA polymerase (10, 11, 16). Therminator DNA polymerase was also shown to be an efficient and accurate TNA-dependent DNA polymerase (9). The information transfer between TNA and DNA catalyzed by polymerases provides support for a possible role of TNA as a progenitor of DNA/RNA.
Fig. 1.
Structures of DNA/RNA (Left), TNA (Center), and S-GNA (Right). The similarity of backbone between TNA and S-GNA is highlighted in red.
The search for nucleic acid analogs with even simpler backbones led to studies of the glycerol nucleic acids (GNAs), which have a three-carbon, acyclic backbone (17–19) (Fig. 1). The S isomer of GNA can form stable antiparallel duplexes with itself and RNA, but not with DNA of the sequence 3′-taa aat tta tat tat taa-2′ (lowercase type denotes the GNA sequence) (18). These properties suggested that direct sequence information exchange between GNA and DNA might not be possible. In the present study, we screened a panel of polymerases for their ability to catalyze DNA synthesis on GNA templates. Surprisingly, even without stable duplex formation between GNA and DNA, as demonstrated by thermal denaturation studies using optical hyperchromicity and CD spectroscopy, Bst DNA polymerase is able to catalyze DNA synthesis on a GNA template with good fidelity. These results demonstrate template-dependent synthesis in the absence of a stable product–template duplex.
Results
Synthesis of GNA Oligoucleotides.
S-GNA oligonucleotides were prepared by solid-phase synthesis as described by Zhang et al. (19). In addition, for studies of duplex stability and primer extension reactions involving GNA, a diaminopurine (D) GNA monomer, which is a stronger base-pairing partner with T than A, was incorporated into GNA via solid-phase synthesis [supporting information (SI) Scheme 1]. The detailed synthetic procedures and characterization of synthetic intermediates and final products are described in SI Text. All GNA and GNA-DNA chimeric oligonucleotides were purified by denaturing gel electrophoresis and characterized by MALDI-TOF MS (SI Table 3).
In the present study, we found that GNA undergoes base-catalyzed decomposition, which is likely to involve nucleophilic attack on internal phosphodiester linkages by the 3′- or 2′-terminal hydroxyl groups (SI Fig. 6A). To quantitatively evaluate the stability of GNA, a ssDNA modified with a GNA trinucleotide at its 3′ end (5′-TAA TAC GAC TCA CTA TAG GG t a t-2′; lowercase type is the GNA sequence) was 32P-labeled at the 5′ end, and its degradation at different pH or Mg2+ concentrations was monitored by denaturing gel electrophoresis. GNA is stable at pH 9.0 at 20°C (SI Fig. 6B). At pH 13, the half-life of GNA was measured to be 141 h (SI Fig. 6C, red trace). Magnesium ion was found to accelerate the degradation of GNA (SI Fig. 6C, blue trace) by >4-fold at pH 13. To increase the stability of GNA oligonucleotides during the base deprotection step of the solid-phase synthesis, an extra deoxyadenosine was used to cap the free hydroxyl termini in most of the GNA sequences in this study (Table 1 and SI Table 3).
Table 1.
Sequences of GNA and DNA oligonucleotides for thermal denaturing and CD studies
| Sequence | Tm, °C | |
|---|---|---|
| 1 | 5′- TDT DGT CGT CDG -3′ | n.o. |
| 2′-A dtd tcd gcd gtc A-3′ | ||
| 2 | 5′- TDT DGT CGT CDG -3′ | 58.4 |
| 3′- DTD TCD GCD GTC -5′ | ||
| 3 | 3′-A tdt dgt cgt cdg A-2′ | ∼90 |
| 2′-A dtd tcd gcd gtc A-3′ | ||
| 4 | 5′- TDT DGT CGT CDG -3′ | |
| 5 | 2′-A dtd tcd gcd gtc A-3′ | |
| 6 | 5′- TAT AGT CGT CAG -3′ | n.o. |
| 2′-A ata tca gca gtc A-3′ | ||
| 7 | 5′- TAT AGT CGT CAG -3′ | 44.5 |
| 3′- ATD TCA GCA GTC -5′ | ||
| 8 | 3′-A tat agt cgt cag A-2′ | 67.9 |
| 2′-A ata tca gca gtc A-3′ | ||
| 9 | 5′- TAT AGT CGT CAG -3′ | |
| 10 | 2′-A ata tca gca gtc A-3′ | |
| 12 | 5′-TAA TAC GAC TCA CTA TAG GG TDT DGT CGT CDG T-3′ | <55 |
| 3′-ATT ATG CTG AGT GAT ATC CC dtd tcd gcd gtc A-3′ | ||
| 13 | 5′-TAA TAC GAC TCA CTA TAG GG -3′ | |
| 3′-ATT ATG CTG AGT GAT ATC CC dtd tcd gcd gtc A-3′ | ||
| 14 | 5′-TAA TAC GAC TCA CTA TAG GG -3′ | 59.0 |
| 3′-ATT ATG CTG AGT GAT ATC CC -5′ |
Tm values for stable duplexes are also listed. Uppercase type, DNA; lowercase type, GNA; D or d, diaminopurine; n.o., not observed.
Thermal Denaturation and CD Studies on GNA/DNA Heteroduplexes.
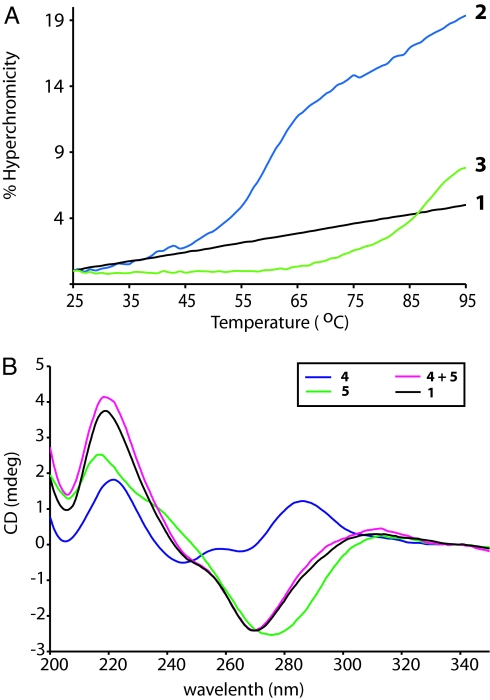
In a previous study by Zhang et al. (18), an AT-rich GNA strand (3′-taa aat tta tat tat taa-2′) was shown to be unable to form a stable, antiparallel duplex with a complementary DNA strand by thermal denaturation studies. Because all AT sequences form intrinsically weak duplexes, we asked whether sequences containing G and C, and in which A was replaced by diaminopurine (D), might allow the formation of stable GNA/DNA duplexes. GNA dodecamers with all four bases (G, T, A/D, and C) were synthesized (Table 1), and their ability to form homo- and hetero-duplexes with GNA or DNA under physiological conditions (100 mM NaCl, 10 mM sodium phosphate, pH 7.0) was studied by thermal denaturation using optical hyperchromicity and CD spectroscopy. Fig. 2A shows the thermal denaturation profile (black trace) of a diaminopurine-containing DNA/GNA construct (1 in Table 1), which reveals no cooperative transition or distinct melting temperature (Tm). The same sequence shows clear evidence of DNA/DNA (2; Fig. 2A, blue trace) and GNA/GNA (3; Fig. 2A, green trace) duplex formation (see Table 1 for sequences). In addition, the CD spectrum of 1 (Fig. 2B, black trace) is nearly identical to the linear combination (Fig. 2B, magenta trace) of its single-stranded components (Fig. 2B, blue and green traces for 4 and 5, Table 1) with no new features that can be attributed to possible duplex formation. Similar observations were obtained for constructs with adenine-containing GNA or DNA (6–10; Table 1) in the same sequence context (SI Fig. 7). These results suggest that this GNA dodecamer cannot form a stable antiparallel duplex with DNA, consistent with the previous study (18).
Fig. 2.
Thermal denaturation and CD studies of duplex DNA, GNA, and a 1:1 mixture of complementary GNA and DNA oligonucleotides. (A) Thermal denaturing curves of duplex DNA (2), GNA (3), and DNA/GNA hybrid (1). (B) CD spectra of ssDNA (4), GNA (5), and their hybrid (1). The magenta trace is the calculated linear combination of 4 and 5. Sequences of 1–5 are listed in Table 1.
Duplex formation between dodecamer GNA and DNA sequences was also investigated in the context of an adjacent 20-nt dsDNA clamp (12; Table 1). This structure represents the full-length product of the enzymatic synthesis of DNA by using the partial duplex 13 as the primer template. The thermal melting profile of 12 reveals a broad transition with a Tm < 55°C (SI Fig. 8A, magenta trace), compared with the sharp melting curve of the duplex DNA moiety alone (14; SI Fig. 8A, blue trace; Tm of 59°C). This result argues against stable duplex formation between GNA and DNA in 12, which would lead to an increased Tm. The initial increase in hyperchromicity may be caused by unstacking of the single-stranded GNA and DNA moieties in 12. In addition, the CD spectrum of 12 (SI Fig. 8B, black trace) is quite similar to the linear combination of the CD signals from 14 and single-stranded 4 and 5, which also suggests that the GNA moiety does not form a stable duplex even adjacent to 20 bp of duplex DNA, as in 12.
DNA Synthesis on a GNA Template.
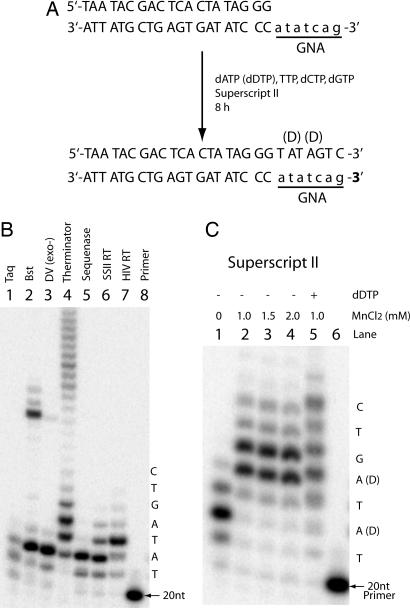
Although GNA appears not to form a stable duplex with DNA, preliminary studies revealed GNA-dependent DNA synthesis catalyzed by a variety of enzymes on a 7-mer GNA template (2′-atatcag-3′) using a 20-mer DNA primer template lead sequence (Fig. 3A). Fig. 3B shows the primer extension activity of these enzymes under their optimal conditions. For most polymerases, the primer extension process stalled after incorporation of two or three deoxynucleotides. Bst and Therminator polymerases (Fig. 3B, lanes 2 and 4) also produced longer products than expected, most likely because of template switching (see Discussion). SuperScript II and HIV-1 reverse transcriptase (Fig. 3B, lanes 6 and 7) extended the DNA primer by 4 and 5 nucleotides respectively, without overextended side products. Therefore, SuperScript II was used to further test the enzymatic extension activity under different conditions.
Fig. 3.
DNA synthesis on a GNA template. (A) Sequences of the primer–template complex and the final product. GNA is shown in lowercase type. (B) Denaturing gel analysis of DNA synthesis on the GNA template by different enzymes. (C) Effects of Mn2+ and dDTP on the polymerase activity of SuperScript II. Lanes 1–4, primer extension in the presence of 0–2.0 mM MnCl2 using dATP. Lane 5, primer extension with dDTP (1.0 mM MnCl2). Lane 6, primer only.
Attempts to optimize the conditions for SuperScript II reverse transcriptase in the presence of different metal ions revealed that the manganese (II) ion improved the GNA-dependent DNA synthesis (Fig. 3C). Compared with the primer extension reaction without MnCl2 (Fig. 3C, lane 1), a significant amount of full-length product (≈5%) was observed in the presence of 1 mM MnCl2 without overextension. Higher concentrations of MnCl2 did not further improve the efficiency of SuperScript II (Fig. 3C, lanes 3 and 4). However, substitution of 2′-deoxyadenosine-5′-triphosphate (dATP) with 2′-deoxydiaminopurine-5′-triphosphate (dDTP) resulted in two to three times more full-length products (Fig. 3C, lane 5), suggesting that a stronger base-pairing interaction between the GNA template and the incoming nucleotide triphosphate leads to higher efficiency of DNA synthesis on a GNA template.
DNA Synthesis on a Diaminopurine-Containing GNA Template.
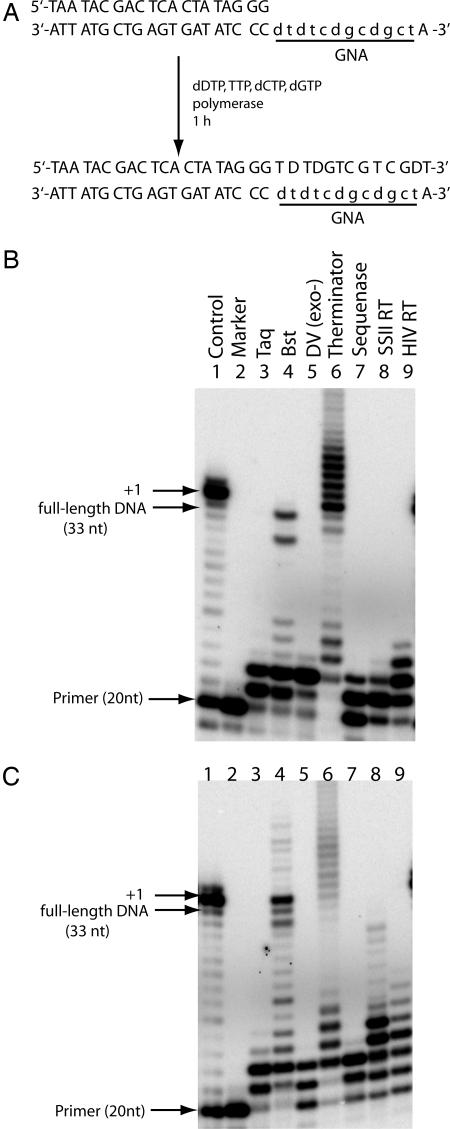
Based on the initial enzyme screen results with MnCl2 and dDTP, a dodecamer GNA template containing diaminopurine was constructed, using the same 20-nt DNA primer sequence (Fig. 4A). The dodecamer GNA template (2′-dtd tcd gcd gct-3′) is the same sequence as used in the thermal denaturation and CD studies (5; Table 1), which has been shown not to form a stable duplex with the complementary DNA sequence (4). The polymerases listed in Fig. 3B were rescreened for their ability to catalyze DNA synthesis on this template by using dDTP-supplemented triphosphates. In the absence of MnCl2, most enzymes could incorporate only 2–3 nt in 1 h (Fig. 4B). Bst polymerase (Fig. 4B, lane 4) was the most efficient polymerase under this condition, producing a significant amount of full-length product (6%) without overextension (Fig. 4B, lane 6). Similar to SuperScript II, MnCl2 increased the efficiency of several polymerases, including Bst, Sequenase, and HIV-1 reverse transcriptase (Fig. 4C, lanes 4 and 7–9). In the case of Bst polymerase, the fraction of full-length product increases from 6% to 22% in the presence of MnCl2 (compare lane 4s in Fig. 4 B and C).
Fig. 4.
Polymerase screen on a GNA template containing diaminopurine. (A) Sequences of the primer template and the product. The GNA sequence is in lowercase type. (B and C) Primer extension without (B) and with 1 mM MnCl2 (C).
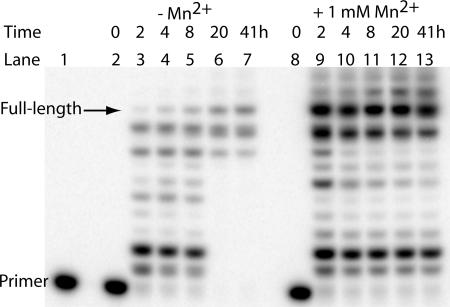
The time course of DNA synthesis on the GNA template was studied for Bst polymerase (Fig. 5). In the absence of Mn2+, the full-length product constitutes only a small fraction during the first 8-h incubation (7.6% in Fig. 5, lane 5). Longer incubation (up to 41 h) caused some aggregation of the extended products and prevented them from entering the gel, making it difficult to quantify the fraction of the full-length product. However, an increasing amount of the full-length product is observed (Fig. 5, lanes 6 and 7). In the presence of 1 mM MnCl2, the fraction of the full-length product increases from 25% at 2 h to 34% at 20 h (Fig. 5, lanes 9–12).
Fig. 5.
Time-dependent synthesis of DNA on a GNA template catalyzed by Bst polymerase. The sequences of the primer and the template are the same as in Fig. 4. Lane 1, primer only. Lanes 2–7, no MnCl2. Lanes 8–13, with 1 mM MnCl2. The time points are indicated above each lane.
Fidelity of DNA Synthesis on a GNA Template by Bst Polymerase.
Because Bst polymerase was found to be the most efficient GNA-dependent DNA polymerase, its fidelity was studied in the presence and absence of MnCl2 on the same template as shown in Fig. 4A. The full-length DNA product generated by Bst polymerase after 1-h incubation was purified by denaturing gel electrophoresis and PCR-amplified by Taq DNA polymerase before being cloned and sequenced (12). The final error rates were corrected by subtracting the error rate resulting from PCR amplification (12) and compared with the error rate on a DNA template (Table 2). The results show that without MnCl2 the error rate of DNA synthesis on the GNA template (0.025) is about four times the error rate on a DNA template (0.006). Compared with the DNA template, the GNA template caused a significant increase in A to G, C to G, and T to G mutations. Furthermore, whereas MnCl2 increased the overall efficiency of Bst polymerase on the GNA template, the error rate was also increased from 0.025 to 0.055 (Table 1), possibly because of the MnCl2-facilitated continuation of synthesis that had stalled because of misincorporation.
Table 2.
Fidelity of DNA synthesis by Bst polymerase on DNA and GNA templates with or without 1.0 mM MnCl2
| Measure | DNA | DNA + Mn | GNA | GNA + Mn |
|---|---|---|---|---|
| Nucleotides | 552 | 396 | 432 | 216 |
| Transitions | 0 | 3 | 3 | 4 |
| Transversions | 9 | 5 | 9 | 8 |
| Deletions | 0 | 3 | 3 | 2 |
| Insertions | 0 | 0 | 0 | 0 |
| Raw error rate | 0.016 | 0.028 | 0.035 | 0.065 |
| Corrected error rate | 0.006 | 0.018 | 0.025 | 0.055 |
The raw error rate equals (transition + transversion + deletions + insertions mutations)/total nucleotides sequenced. The corrected error rate is calculated by subtracting the error rate of Taq (∼0.01 from 33 rounds of PCR) from the raw error rate.
The fidelity analysis also revealed a class of sequences that could be explained by a partial primer extension on the GNA template followed by resumed synthesis using a free primer as a second template. The template switching was observed only for the GNA template. This observation might explain the presence of overextended products in the earlier primer extension studies (Fig. 3B, lanes 2 and 4; Fig. 4 B and C, lane 6). No template switching was observed for the DNA template, which suggests that the template switching might be caused by the absence of stable base-pairing of newly synthesized DNA to its GNA template. Template switching has also been observed during TNA-dependent DNA synthesis (9).
Discussion
It is generally assumed that stable duplex formation between the template and the growing product strand is required to allow enzymatic DNA polymerization to proceed. In contrast, we have found that Bst polymerase, a Family A DNA polymerase produced by thermophilic Bacillus stearothermophilus (20, 21), is able to carry out template-dependent DNA synthesis on a GNA template, which does not form a stable duplex with DNA. The catalytic efficiency of Bst polymerase on a GNA template increases in the presence of Mn2+ ions, which is known to relax the substrate specificity of many DNA polymerases, possibly by allowing enhanced binding of the β- and γ-phosphates of the dNTPs and/or by strengthening weak contacts between polymerases and their DNA substrates (22, 23). Substitution of adenine with diaminopurine in both template and monomer triphosphates further increases DNA synthesis on GNA templates, emphasizing the requirement for a strong base-pairing interaction between the incoming nucleoside triphosphate and the template for efficient synthesis by polymerases.
A series of structures of a related Bst DNA polymerase (BF) and its complexes with substrates have been solved (24–27) and provide insight into the possible interactions between Bst DNA polymerase and a GNA template. BF is produced by a different strain of Bacillus stearothermophilus and shares 86.1% sequence identity (with conserved catalytic and substrate-binding site) with Bst polymerases used in the present study (27). The catalytically active crystal of BF polymerase has provided a detailed molecular picture of primer/template-binding and conformational change during the course of dNTP incorporation (25, 26). The structure of BF in a complex with a primer/template shows that the single-stranded template forms a nearly 90° turn relative to the duplex region and binds to the O and O1 helices in the finger region. Therefore, the template is not preorganized for simultaneous base-pairing and primer-stacking interactions with the incoming nucleotide. A conserved tyrosine (Y714 of BF) of the O helix orchestrates the conformational change of the nucleotide on the template (n) from a preinsertion site [not stacked against the (n − 1) base pair, the open state] to an insertion site [stacked against the (n − 1) base, the closed state], which is capable of base-pairing with the incoming dNTP (25). The transition between the open and the closed state for each catalytic cycle involves a large conformational change of the O helix and the translocation of the template by 1 nt. Our results suggest that the single-stranded GNA can bind to Bst polymerase in a similar fashion as DNA. The conformational change between the open and closed state only requires the formation of 5 bp upstream from the 3′ terminus of the primer (25). Therefore, stable duplex formation over a longer region does not appear to be necessary for catalysis. In addition, BF polymerase actively binds to the duplex region of the primer/template by partially unwinding the duplex (up to 4 bp) and forming H-bonding interactions with N3 of purines and O2 of pyrimidines in the minor groove (26). Therefore, a B-form-like conformation in the primer/template is not required for the binding of the template strand to polymerases. Our results suggest that Bst polymerase could stabilize a short region of heteroduplex formation between the newly synthesized DNA product and the GNA template in the active site to maintain a catalytically active, “closed” conformation. This transient and local enzyme-stabilized base-pairing region would then translocate along the template as DNA synthesis continues.
The structural simplicity and the facile synthesis of GNA make it an attractive system for studying the evolution of functional biopolymers by in vitro selection (19, 28). As a step toward exploring potential biological functions of GNA, the present study has demonstrated that sequence information can be faithfully copied from GNA to DNA by using DNA polymerases. Future studies should search for polymerases capable of synthesizing long stretches of GNA by using S-glycerol nucleoside 3′-triphosphates as precursors. In addition, the structural resemblance of GNA to DNA/RNA suggests that GNA could be an ancestor or progenitor of the contemporary nucleic acids. GNA monomers can be assembled by condensing a nucleobase with a C3 unit (e.g., glycerol, glyceraldehyde, or glycidol). Previous studies have shown that glycidol can be produced from glycerol carbonate in a reaction catalyzed by zeolites (29), which suggests that GNA monomers might be formed under prebiotic conditions. Because highly activated GNA monomers such as phosphorimidazolides cyclize rapidly, template-directed primer extension by cyclic monomers or triphosphates deserves further study. Because duplex formation of GNA with itself and with RNA is stereospecific, GNA is also an interesting system in which to study the rise of homochirality during the origins of life. Future studies on the nonenzymatic polymerization of GNA will help shed light on its role as a possible genetic information carrier before the proposed “RNA World.”
Materials and Methods
Synthesis of DNA and GNA Oligonucleotides.
DNA primers and templates were synthesized on a 0.2-μmol scale by the Massachusetts General Hospital DNA Core. Synthesis of diaminopurine-containing GNA oligonucleotides is described in SI Text. All oligonucleotides were purified by denaturing PAGE and characterized by MALDI-TOF MS (see below).
MALDI-TOF MS.
To characterize purified single-stranded DNA or GNA oligonucleotides, a sample of ≈200 pmol oligonucleotide was absorbed on a C18 Zip Tip. Samples were eluted with 1.5 μl of a matrix solution containing a 2:1 mixture of 52.5 mg/ml 3-hydroxypicolinic acid in 50% acetontrile and 0.1 M ammonium citrate in water. Eluents were directly spotted onto a stainless steel MALDI-TOF plate and analyzed in positive mode on a Voyager MALDI-TOF mass spectrometer (Applied Biosystems, Foster City, CA) with an average of 200 scans. Results of MALDI-TOF MS analysis of all synthetic GNAs are summarized in SI Table 3.
Stability Studies on GNA.
A 20-mer ssDNA oligonucleotide with a GNA trinucleotide on the 3′ end (5′-TAA TAC GAC TCA CTA TAG GG t a t-2′; lowercase type denotes the GNA sequence) was 5′ 32P-labeled and incubated under different pH and salt conditions at 20°C. The final mixture of 50 μl contained 1 μM labeled oligonucleotide, 100 mM NaCl in a desired buffer system (10 mM sodium phosphate, pH 7.0, 10 mM Hepes-Mes, pH 9.0, or 100 mM NaOH, pH 13.0). An aliquot of 5 μl was removed from the mixture at 0, 2, 5, 9, 28, and 51 h and was analyzed by denaturing gel electrophoresis (20% polyacrylamide). The reactions were repeated with 20 mM MgCl2.
Tm Determination by Thermal Denaturation.
Thermal denaturation experiments were performed on a Cary 1E spectrophotometer (Varian, Palo Alto, CA) equipped with a programmable temperature control. The hyperchromicity was monitored at 260 nm (1 nm width) with a heating rate of 1°C/min. The sample (200 μl) contained 1 or 3 μM of each strand in 100 mM NaCl and 10 mM sodium phosphate, pH 7.0. The Tm values were calculated from the first derivatives of the melting curves in duplicate.
CD Spectroscopy.
CD spectroscopic studies were carried out on a CD spectrometer (model 202; Aviv, Lakewood, NJ) at 25°C. The sample (300 μl) contained 3 or 10 μM of each strand in 100 mM NaCl, 10 mM sodium phosphate, pH 7.0, path length 1.0 mm. The samples were scanned from 200 to 350 nm with a 1-nm increment. The signal was recorded from the average of 10 measurements for each wavelength with a time constant of 1 s. A sample containing only buffer was used as the control for all measurements.
Polymerase Screen.
The DNA primer (5′-TAA TAC GAC TCA CTATAG GG-3′) was 5′ 32P-labeled and annealed to a DNA/GNA chimeric template (3′-ATT ATG CTG AGT GAT ATC CC a t a t c a g-5′, or 3′-ATT ATG CTG AGT GAT ATC CC d t d t c d g c d g t c A-5′; lowercase type denotes the GNA templates). Primer extension reactions were performed in a mixture (10 μl) of 50 nM primer/template, 100 μM of each dNTP containing either dATP or dDTP (Trilink Biotech, San Diego, CA), and 0.5 μl of each enzyme in buffers supplied by the manufacturers [Taq, 2.5 units; Roche, Indianapolis, IN); Bst, 4 units; DeepVent (exo−), 1 unit; NEB, Beverly, MA; Therminator, 1 unit; NEB; Sequenase, 6.5 units; USB, Cleveland, OH; SuperScript II, 100 units; Invitrogen, Carlsbad, CA; HIV-1 RT, 13 units; Worthington Biochemical, Lakewood, NJ]. The reaction mixtures were incubated at 55°C for thermophilic enzymes or 37°C for mesophilic enzymes for 1 or 8 h. Reactions were analyzed by denaturing gel electrophoresis (20% polyacrylamide).
Fidelity of DNA Synthesis on GNA Templates.
The DNA primer (5′-TAA TAC GAC TCA CTATAG GG-3′) was 5′ 32P-labeled and annealed to a DNA or a DNA/GNA chimeric template (3′-ATT ATG CTG AGT GAT ATC CC DTD TCD GCD GTC A-5′; underline denotes either DNA or GNA sequences). The primer extension reaction was performed in a mixture (10 μl) of 500 nM primer/template, 100 μM of each dNTPs containing dDTP, and 48 units of Bst DNA Polymerase (590 nM) with or without 1.0 mM MnCl2. The reaction mixtures were incubated at 55°C for 60 min and quenched by adding 8 M urea and 100 mM EDTA. Full-length products were purified by 20% denaturing PAGE. The products were then extended with 4–6 deoxycytidine residues by terminal deoxynucleotidyl transferase (Promega, Madison, WI) as described (9) and PCR-amplified for 33 rounds. The amplified products were then cloned into TOPO vectors (Invitrogen) before sequencing (Massachusetts General Hospital Molecular Biology Core Facility).
Supplementary Material
Acknowledgments
We thank Xin Cai for help with synthesis of some GNA phosphoramidites. J.C. was supported by a Fellowship from the Harvard Origins Center. This work was supported by National Science Foundation Grant CHE 0434507 (to J.W.S.). J.W.S. is an Investigator of the Howard Hughes Medical Institute, and C.H.T. is a Research Associate of the Howard Hughes Medical Institute.
Abbreviations
- GNA
glycerol nucleic acid
- TNA
(3′ → 2′) α-l-threose nucleic acid
- Tm
melting temperature
- dDTP
2′-deoxydiaminopurine-5′-triphosphate.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0704211104/DC1.
References
- 1.Uhlman E, Peyman A. Chem Rev. 1990;90:543–584. [Google Scholar]
- 2.Eschenmoser A. Orig Life Evol Biosph. 2004;34:277–306. doi: 10.1023/b:orig.0000016450.59665.f4. [DOI] [PubMed] [Google Scholar]
- 3.Krueger AT, Lu H, Lee AH, Kool ET. Acc Chem Res. 2007;40:141–150. doi: 10.1021/ar068200o. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lundin KE, Good L, Stromberg R, Graslund A, Smith CI. Adv Genet. 2006;56:1–51. doi: 10.1016/S0065-2660(06)56001-8. [DOI] [PubMed] [Google Scholar]
- 5.Petersen M, Wengel J. Trends Biotechnol. 2003;21:74–81. doi: 10.1016/S0167-7799(02)00038-0. [DOI] [PubMed] [Google Scholar]
- 6.Gryaznov SM. Biochim Biophys Acta. 1999;1489:131–140. doi: 10.1016/s0167-4781(99)00151-7. [DOI] [PubMed] [Google Scholar]
- 7.Eschenmoser A. Science. 1999;284:2118–2124. doi: 10.1126/science.284.5423.2118. [DOI] [PubMed] [Google Scholar]
- 8.Schoning K, Scholz P, Guntha S, Wu X, Krishnamurthy R, Eschenmoser A. Science. 2000;290:1347–1351. doi: 10.1126/science.290.5495.1347. [DOI] [PubMed] [Google Scholar]
- 9.Chaput JC, Ichida JK, Szostak JW. J Am Chem Soc. 2003;125:856–857. doi: 10.1021/ja028589k. [DOI] [PubMed] [Google Scholar]
- 10.Chaput JC, Szostak JW. J Am Chem Soc. 2003;125:9274–9275. doi: 10.1021/ja035917n. [DOI] [PubMed] [Google Scholar]
- 11.Horhota A, Zou K, Ichida JK, Yu B, McLaughlin LW, Szostak JW, Chaput JC. J Am Chem Soc. 2005;127:7427–7434. doi: 10.1021/ja0428255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ichida JK, Horhota A, Zou K, McLaughlin LW, Szostak JW. Nucleic Acids Res. 2005;33:5219–5225. doi: 10.1093/nar/gki840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ichida JK, Zou K, Horhota A, Yu B, McLaughlin LW, Szostak JW. J Am Chem Soc. 2005;127:2802–2803. doi: 10.1021/ja045364w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vandendriessche F, Augustyns K, Van Aerschot A, Busson R, Hoogmartens J, Herdewijn P. Tetrahedron. 1993;49:7223–7238. [Google Scholar]
- 15.Nielsen P, Kirpekar F, Wengel J. Nucleic Acids Res. 1994;22:703–710. doi: 10.1093/nar/22.5.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gardner AF, Jack WE. Nucleic Acids Res. 1999;27:2545–2553. doi: 10.1093/nar/27.12.2545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang L, Meggers E. J Am Chem Soc. 2005;127:74–75. doi: 10.1021/ja043904j. [DOI] [PubMed] [Google Scholar]
- 18.Zhang L, Peritz A, Meggers E. J Am Chem Soc. 2005;127:4174–4175. doi: 10.1021/ja042564z. [DOI] [PubMed] [Google Scholar]
- 19.Zhang L, Peritz AE, Carrikk PJ, Meggers E. Synthesis. 2005;4:645–653. [Google Scholar]
- 20.Lu YY, Ye SY, Hong GF. BioTechniques. 1991;11:464–466. [PubMed] [Google Scholar]
- 21.Mead DA, McClary JA, Luckey JA, Kostichka AJ, Witney FR, Smith LM. BioTechniques. 1991;11:76–87. [PubMed] [Google Scholar]
- 22.Cadwell RC, Joyce GF. PCR Methods Appl. 1992;2:28–33. doi: 10.1101/gr.2.1.28. [DOI] [PubMed] [Google Scholar]
- 23.Tabor S, Richardson CC. Proc Natl Acad Sci USA. 1989;86:4076–4080. doi: 10.1073/pnas.86.11.4076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Johnson SJ, Beese LS. Cell. 2004;116:803–816. doi: 10.1016/s0092-8674(04)00252-1. [DOI] [PubMed] [Google Scholar]
- 25.Johnson SJ, Taylor JS, Beese LS. Proc Natl Acad Sci USA. 2003;100:3895–3900. doi: 10.1073/pnas.0630532100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kiefer JR, Mao C, Braman JC, Beese LS. Nature. 1998;391:304–307. doi: 10.1038/34693. [DOI] [PubMed] [Google Scholar]
- 27.Kiefer JR, Mao C, Hansen CJ, Basehore SL, Hogrefe HH, Braman JC, Beese LS. Structure (London) 1997;5:95–108. doi: 10.1016/s0969-2126(97)00169-x. [DOI] [PubMed] [Google Scholar]
- 28.Wilson DS, Szostak JW. Annu Rev Biochem. 1999;68:611–647. doi: 10.1146/annurev.biochem.68.1.611. [DOI] [PubMed] [Google Scholar]
- 29.Yoo JW, Mouloungui Z. Studies Surf Sci Cat. 2001;135:3806–3813. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.