Abstract
Two trinuclear copper [CuICuICuI(L)]1+ complexes have been prepared with the multidentate ligands (L) 3,3′-(1,4-diazepane-1,4-diyl)bis(1-((2-(dimethylamino)ethyl)(methyl)amino)propan-2-ol) (7-Me) and (3,3′-(1,4-diazepane-1,4-diyl)bis(1-((2-(diethylamino) ethyl)(ethyl) amino)propan-2-ol) (7-Et) as models for the active site of the particulate methane monooxygenase (pMMO). The ligands were designed to form the proper spatial and electronic geometry to harness a “singlet oxene,” according to the mechanism previously suggested by our laboratory. Consistent with the design strategy, both [CuICuICuI(L)]1+ reacted with dioxygen to form a putative bis(μ3-oxo)CuIICuIICuIII species, capable of facile O-atom insertion across the central C C bond of benzil and 2,3-butanedione at ambient temperature and pressure. These complexes also catalyze facile O-atom transfer to the C
C bond of benzil and 2,3-butanedione at ambient temperature and pressure. These complexes also catalyze facile O-atom transfer to the C H bond of CH3CN to form glycolonitrile. These results, together with our recent biochemical studies on pMMO, provide support for our hypothesis that the hydroxylation site of pMMO contains a trinuclear copper cluster that mediates C
H bond of CH3CN to form glycolonitrile. These results, together with our recent biochemical studies on pMMO, provide support for our hypothesis that the hydroxylation site of pMMO contains a trinuclear copper cluster that mediates C H bond activation by a singlet oxene mechanism.
H bond activation by a singlet oxene mechanism.
Keywords: density functional theory, methane monooxygenase, membrane-bound or particulate methane monooxygenase, soluble methane monooxygenase, mass spectroscopy
There presently is considerable interest in the development of efficient catalysts for the facile conversion of methane to methanol (1). Industrially, this is a difficult process. However, two methane monooxygenases (MMO) are known to mediate this process in methanotrophic bacteria: a membrane-bound MMO called particulate MMO (pMMO) and a water-soluble form referred to as soluble MMO (sMMO) (2). pMMO is a multicopper protein (3), and sMMO is a nonheme diiron protein (4, 5). Both systems exploit metal clusters to catalyze this difficult chemistry.
The pMMO is found in all methanotrophs; in contrast, the sMMO has only been isolated from certain strains of methanotrophic bacteria. As an MMO, the oxidation of the C H bond often is described by the chemical equation
H bond often is described by the chemical equation
Several possible mechanisms for the catalytic function of MMO have been considered. One involves a radical mechanism, wherein an activated “oxygen” species abstracts a hydrogen atom from the hydrocarbon substrate, followed by radical-rebound chemistry of the alkyl radical with the “hot” hydroxyl radical to form product. This mechanism has been implicated for the nonheme diiron cluster at the active site of sMMO (6–8). The other mechanism suggested by our laboratory invokes oxenoid or “singlet oxene” insertion across the C H bond (3). Evidence for a direct insertion mechanism has been provided by the turnover chemistry mediated by pMMO (9–12). Direct insertion of a singlet oxene across a C
H bond (3). Evidence for a direct insertion mechanism has been provided by the turnover chemistry mediated by pMMO (9–12). Direct insertion of a singlet oxene across a C H bond should result in facile bond closure of the C
H bond should result in facile bond closure of the C O bond after formation of the O
O bond after formation of the O H bond, and the process should proceed with full retention of configuration at the carbon center oxidized. Indeed, the hydroxylation of small straight-chain alkanes (C1–C5) mediated by pMMO occurs with total retention of configuration (11). In contrast, the hydroxylation of hydrocarbons mediated by sMMO has been reported to proceed with only partial retention of configuration (6, 8).
H bond, and the process should proceed with full retention of configuration at the carbon center oxidized. Indeed, the hydroxylation of small straight-chain alkanes (C1–C5) mediated by pMMO occurs with total retention of configuration (11). In contrast, the hydroxylation of hydrocarbons mediated by sMMO has been reported to proceed with only partial retention of configuration (6, 8).
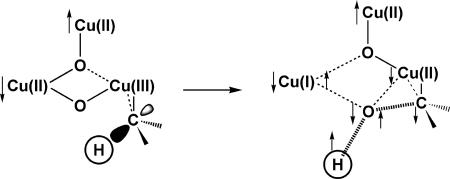
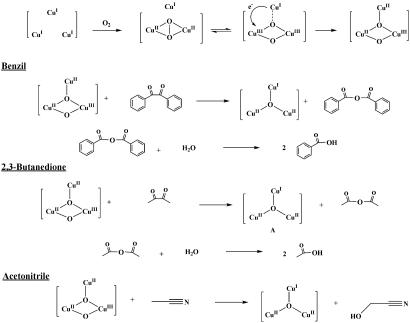
In our proposed singlet oxene mechanism, we have conjectured that the “active” species that promotes O-atom insertion in pMMO is a mixed-valence [CuIICuIICuIII(μ-O)2]3+ intermediate formed after reaction of a reduced trinuclear [CuICuICuI]3+ cluster with dioxygen (3). The main features of this mechanism are illustrated in Fig. 1. Although only two of the three reducing equivalents are required for hydroxylation chemistry, we have proposed that the third reducing equivalent in the hydroxylation cluster is required for efficient O-atom transfer based on the concerted singlet oxene insertion across the C H bond (3, 13). Analysis of this process by density functional theory (DFT) has ensured that the oxo-transfer chemistry proceeds on a “singlet” reaction potential surface without spin crossover (13). This analysis also indicates that the putative trinuclear copper cluster offers a significantly more facile pathway for alkane hydroxylation compared with the traditional dinuclear copper cluster.
H bond (3, 13). Analysis of this process by density functional theory (DFT) has ensured that the oxo-transfer chemistry proceeds on a “singlet” reaction potential surface without spin crossover (13). This analysis also indicates that the putative trinuclear copper cluster offers a significantly more facile pathway for alkane hydroxylation compared with the traditional dinuclear copper cluster.
Fig. 1.
Details of the adiabatic singlet oxene transfer from a dioxygen-activated trinuclear copper cluster to methane to form the transition state. ↑ and ↓ denote “up” and “down” directions of the unpaired electron spins. [Reproduced with permission from ref. 3 (Copyright 2004, American Chemical Society).]
Compelling evidence for the existence of a tricopper cluster has been recently reported for pMMO from Methylococcus capsulatus. (Bath) based on redox potentiometry and EPR spectroscopy (14). By fine-tuning the redox potential with redox mediators, an EPR signal characteristic of a trinuclear copper cluster could be observed.
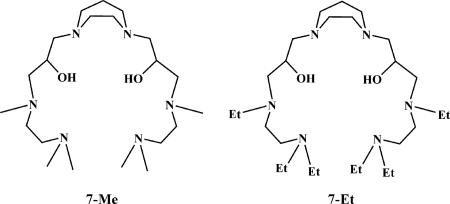
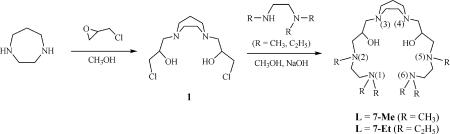
In this study we have extended our efforts on pMMO to a model compound that might provide fundamental test of the ideas derived from the enzyme studies. Building on the findings of our recent DFT calculations (13), we have designed and synthesized several trinuclear copper complexes based on the ligand (denoted as L) 3,3′-(1,4-diazepane-1,4-diyl)bis(1-((2-(dimethylamino)ethyl)(methyl)amino)propan-2-ol) (7-Me) or 3,3′-(1,4-diazepane-1,4-diyl)bis(1-((2-(diethylamino)ethyl)(ethyl)amino)propan-2-ol (7-Et) for the model studies (Scheme 1). These two ligands contain six neutral amines and two hydroxyl groups that are capable of trapping three copper ions simultaneously. We also report the oxygenation of these [CuICuICuI(L)]1+ complexes along with the subsequent oxo-transfer chemistry and characterize these reactions by electrospray ionization (ESI)-MS, GC-MS, x-ray crystal structure, EPR, and IR spectroscopy.
Scheme 1.
Ligands 7-Me and 7-Et used in this study.
Results
The [CuICuICuI(L)]1+ complexes were inherently unstable in acetonitrile. In most organic solvents, such as CH2Cl2, methanol, acetone, and tetrahydrofuran, they underwent disproportionation, resulting in the instantaneous color change of the solution from pale yellow to blue and the formation of a red copper powder precipitate under an argon atmosphere. It is well known that the CH3CN forms stable Cu(I) complexes, using its π* orbital to stabilize the Cu(I) ion. However, even in this solvent, the [CuICuICuI(L)]1+ complexes were stable for only several hours at room temperature. Nevertheless, the reaction product of the [CuICuICuI(L)]1+ complexes with dioxygen could be characterized by crystallography and by optical and mass spectroscopy.
Dioxygen Reactivity of [CuICuICuI(L)]1+(X) (L = 7-Et, 7-Me; X = BF4−, ClO4−).
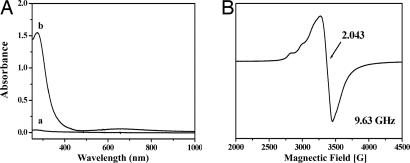
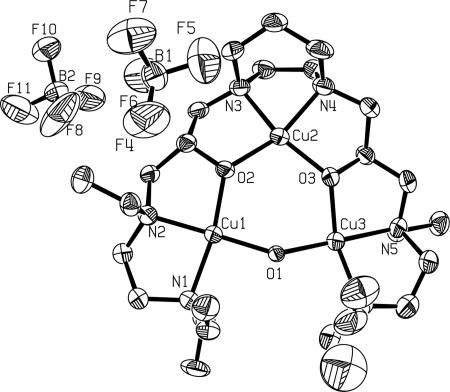
Bubbling dioxygen into a solution of [CuICuICuI(L)]1+(X) (L = 7-Et, 7-Me; X = BF4−, ClO4−) in CH3CN under ambient conditions of pressure and temperature induced an instantaneous color change from pale yellow to deep blue. The corresponding UV-visible spectra of the final products were similar for all four [CuICuICuI(L)]1+ complexes. The absorption at 272 nm could be assigned to ligand-to-CuII ligand-to-metal charge transfer (LMCT) absorptions, and the band at 650 nm to the Cu(II) d-d transitions, as expected for the deep blue [CuIICuIICuII(L)(O)]2+ species (Fig. 2A). The 4 K EPR spectrum of one of the tricopper clusters after dioxygen treatment is shown in Fig. 2B. The almost isotropic signal centered at g ∼ 2.043 is consistent with a ferromagnetically exchanged S = 3/2 species with a small zero-field interaction, as expected for the [CuIICuIICuII(7-Et)(O)]2+(BF4−)2 species, which is in accord with the x-ray crystal structure [Fig. 3, details described in the supporting information (SI) Text and SI Tables 1–3].
Fig. 2.
Spectroscopic characterization of complexes. (A) Optical absorption spectrum [CuICuICuI(L)]1+(X) (L = 7-Et, 7-Me; X = BF4−, ClO4−) before (line a) and after (line b) oxygenation. (B) 4 K EPR spectrum of the [CuIICuIICuII(7-Et)(O)]2+(BF4−)2 in the solvent of CH3CN.
Fig. 3.
ORTEP (30) representation of the [CuIICuIICuII(7-Et)(O)]2+(BF4−)2 showing the atom labeling scheme together with the 30% probability ellipsoids (H-atoms omitted for clarity). Selected bond distances (in angstroms) and angles (in degrees) are as follows: Cu1-O1, 1.934 (4); Cu1-O2, 1.934 (5); Cu2-O2, 1.927 (5); Cu2-O3, 1.926 (5); Cu3-O1, 1.925 (5); Cu3-O3, 1.926 (5); O1-Cu1-O2, 96.64 (19); O3-Cu2-O2, 108.3 (2); and O1-Cu3-O3, 92.72. Further details are published as SI Text.
MS Analysis of the Oxygenation Products.
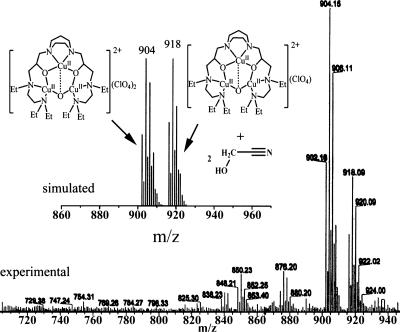
ESI-MS analyses were performed to identify the oxygenated species obtained from reactions of [CuICuICuI(L)]1+(X) (L = 7-Et, 7-Me; X = ClO4−, BF4−) with dioxygen. The ESI-MS spectrum of oxygenated [CuICuICuI(7-Et)]1+(ClO4−) revealed two primary positive ion clusters with multiple peaks. One cluster appeared in the range of m/z ≈902–910 atomic mass units (amu) with a maximum peak at m/z 904 amu; the other occurred in the range of m/z ≈916–924 amu with a maximum peak at m/z 918 amu (Fig. 4). The multiple peaks associated with the two signals were consistent with a complex with three copper ions based on the diagnostic statistical combination of the 63Cu (69%) and the 65Cu (31%) isotopes associated with three copper atoms in natural abundance, together with one or two chlorine atoms based on the natural abundances of 35Cl (76%) and 37Cl (24%). The signal at m/z 904 amu was assigned to {[CuIICuIICuII(7-Et)(O)]2+(ClO4−)2 + H}+ by the mass calculator (Fig. 4). The other signal at m/z 918 amu hypothetically could be assigned to {[CuIICuIICuII(7-Et)(O)]2+(ClO4−) + 2(CH2OHCN)}+ by an excellent coincidence in simulation. We preliminarily propose that this signal consists of the fragment of [CuIICuIICuII(7-Et)(O)]2+ with only one ClO4− but also coordinated to two oxidized acetonitrile molecules formed by oxo-transfer from two trinuclear copper-dioxygen adducts. Support of this tentative assignment is provided by the identification of glycolonitrile by IR spectroscopy and further ESI-MS experiments with [CuICuICuI(L)]1+(X) (L = 7-Et and X = BF4−) where the counteranion ClO4− has been replaced by BF4− (for details, see SI Fig. 7). Identical conclusions were derived when 7-Me was used as the ligand instead of 7-Et (see SI Fig. 8).
Fig. 4.
ESI mass spectrum of the oxygenated [CuICuICuI(7-Et)]1+(ClO4−), together with their simulated mass spectra. These spectra are assigned to the species [CuIICuIICuII(7-Et)(O)]2+(ClO4−)2 and {[CuIICuIICuII(7-Et)(O)]2+(ClO4−) + 2(CH2OHCN)}+, respectively.
The formation of CH2OHCN was corroborated by IR spectroscopy. The deep blue adduct was precipitated from the CH3CN solution by adding diethyl ether. IR spectrum of the adduct in KBr pellet revealed a broad band centered at 2,021 cm−1, which we have assigned to the C N stretch (vCN) of glycolonitrile coordinated to one of the Cu(II) ions in [CuIICuIICuII(L)(O)]2+(X) (L = 7-Me; X = ClO4−). This frequency was shifted by ≈76 cm−1 to lower frequency from that of glycolonitrile in CH3CN [CH2OHCN, vCN = 2,097 cm−1 (br) in CH3CN], which in turn was significantly lower than the vCN of ≈2,250 cm−1 in CH3CN itself. The shift of vCN in the deep blue adduct to lower frequencies relative to CH3CN would be consistent with the electron withdrawing effect of replacing one of the C
N stretch (vCN) of glycolonitrile coordinated to one of the Cu(II) ions in [CuIICuIICuII(L)(O)]2+(X) (L = 7-Me; X = ClO4−). This frequency was shifted by ≈76 cm−1 to lower frequency from that of glycolonitrile in CH3CN [CH2OHCN, vCN = 2,097 cm−1 (br) in CH3CN], which in turn was significantly lower than the vCN of ≈2,250 cm−1 in CH3CN itself. The shift of vCN in the deep blue adduct to lower frequencies relative to CH3CN would be consistent with the electron withdrawing effect of replacing one of the C H in the methyl group by an OH substituent, and the additional shift of vCN in the adduct should reflect an additional electron withdrawing effect of the bound Cu(II) ions in [CuIICuIICuII(L)(O)]2+(X) (L = 7-Me; X = ClO4−). Although direct oxo-insertion across a C
H in the methyl group by an OH substituent, and the additional shift of vCN in the adduct should reflect an additional electron withdrawing effect of the bound Cu(II) ions in [CuIICuIICuII(L)(O)]2+(X) (L = 7-Me; X = ClO4−). Although direct oxo-insertion across a C N might have led to the formation of methyl isocyanate, this scenario was not supported by IR spectroscopy. In methyl isocyanate, there is a broad band centered at ≈2,280 cm−1 in the IR that is diagnostic of
N might have led to the formation of methyl isocyanate, this scenario was not supported by IR spectroscopy. In methyl isocyanate, there is a broad band centered at ≈2,280 cm−1 in the IR that is diagnostic of  C
C N
N O stretch, but the region from 2,000 to 2,100 cm−1 is perfectly clean. Although it is evident that the active oxidant is capable of hydroxylation of acetonitrile, we have obtained no evidence for ligand dealkylation.
O stretch, but the region from 2,000 to 2,100 cm−1 is perfectly clean. Although it is evident that the active oxidant is capable of hydroxylation of acetonitrile, we have obtained no evidence for ligand dealkylation.
Thus, oxygenation of [CuICuICuI(L)]1+(X) (L = 7-Me or 7-Et; X = ClO4− or BF4−) complexes resulted in the formation of at least two species according to MS analysis: one detected as {[CuIICuIICuII(L)(O)]2+(X)2 + H}+ and the other as {[CuIICuIICuII(L)(O)]2+(X) + 2(CH2OHCN)+. The former species contained two coordinated ligands (ClO4− or BF4−), and the latter contained one coordinated ligand and two oxidized CH3CN. Subsequent experiments with 18O2 in lieu of 16O2 resulted in the shift in the mass peaks of the {[CuIICuIICuII(L)(O)]2+(X)2 + H}+ by +2 amu. Thus, during the activation of the ligands 7-Me or 7-Et by dioxygen, one oxygen atom from the exogenous O2 was trapped in the oxidized trinuclear copper complexes.
O-Atom Insertion into C C Bonds Mediated by the Putative [CuIICuIICuIII(L)(μ-O)2]3+.
C Bonds Mediated by the Putative [CuIICuIICuIII(L)(μ-O)2]3+.
In the pMMO enzyme system, electrons are supplied to the fully oxidized C-cluster ([CuIICuIICuII(μ-O)]4+) from NADH, or a membrane-bound ubiquinol, to initiate the hydroxylation event (3). To simulate this process in the present model studies, we have deployed either benzoin/triethylamine or acetoin/triethylamine as the two-electron donor in a single-turnover experiment (15).
Benzoin as Reductant.
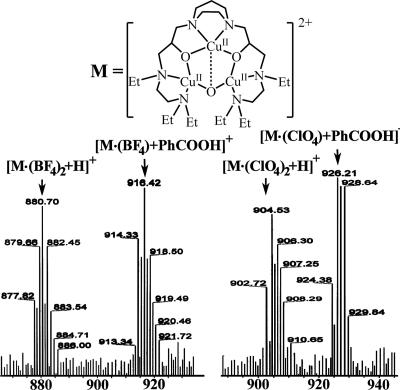
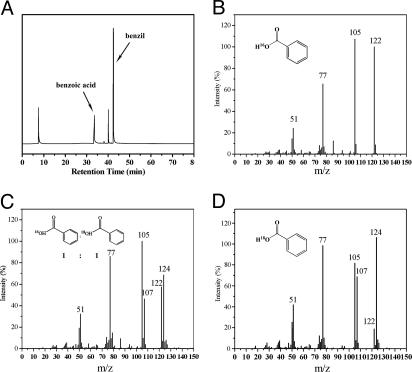
Three equivalents of benzoin (6 reducing equivalents) were added to a CH3CN solution of the [CuIICuIICuII(L)(O)]2+(X)2 (X = ClO4− or BF4−) complex (2 equivalents) at room temperature, and the mixture was stirred for 2 h under argon. During the reduction to form [CuICuICuI(L)]1+(ClO4− or BF4−), the solution color changed from deep blue to pale yellow. The complex was EPR silent, in accordance with a fully reduced tricopper complex. The benzoin was oxidized to benzil. After stirring the mixture under an atmosphere of O2 at room temperature, the color of the solution changed from pale yellow to green within 30 s. This green product was very stable and persisted even for several days at room temperature. The primary oxygenated products obtained were characterized by nanospray ESI-MS. Aside from the expected {[CuIICuIICuII(7-Et)(O)]2+(ClO4− or BF4−)2 + H}+, evidence was obtained for benzoic acid [PhC16O16OH] (Mr = 122) and the benzoic adduct {[CuIICuIICuII(7-Et)(O)]2+(ClO4− or BF4−) + PhCOOH}+ (Fig. 5) (for the full spectrum, see SI Figs. 9 and 10). In the GC-MS analysis, benzoic acid appeared at the retention time (tR) of 33.48 min with the following characteristic ions: m/z 122, M+; m/z 105, M with loss of hydroxyl group; and m/z 77, M with loss of carboxylic acid accompanied by a fragment at m/z 51 corresponding with loss of acetylene (Fig. 6 a and b). Because the green solution yielded the same mass spectrum even a week after it was generated, we have assigned the green product to the CuIICuIICuII benzoic acid adduct. In contrast, in the absence of the benzoic acid product, the solution will turn to deep blue after reoxygenation.
Fig. 5.
Nanospray ESI mass spectrum of the benzoic adduct {[CuIICuIICuII(L)(O)]2+(ClO4− or BF4−) + PhCOOH}+.
Fig. 6.
Reoxidation of reduced tricopper complex by isotopes of molecular oxygen. (A) Representative GC-MS chromatogram in an experiment in which the tricopper complex, [CuIICuIICuII(L)(O)]2+(BF4−)2, was reduced to [CuICuICuI(L)]1+(BF4−) by benzoin and then reoxidized by O2. The benzoic acid molecule appears in the retention time (tR) of 33.48 min. (B) The mass spectrum obtained when the experiment started with [CuIICuIICuII(L)(16O)]2+(BF4−)2 and the reoxidation was carried out in the presence of 16O2. (C) The same experiment as in b except that 18O2 was used instead of 16O2 in the reoxidation. (D) The mass spectrum obtained when [CuICuICuI(L)]1+(BF4−) was prepared by reduction of the [CuIICuIICuII(L)(18O)]2+(BF4−)2 and the reoxidation was undertaken with 18O2.
Acetoin as Reductant.
When acetoin was adopted as the reductant, following the same experimental procedure as with benzoin, the color of the solution of the complex changed from deep blue to pale yellow as well. As expected, the acetoin was oxidized to 2,3-butanedione, and the tricopper complex was reduced to [CuICuICuI(L)]1+(ClO4− or BF4−). Upon reoxygenation (within 30 s), the color of the solution changed from pale yellow to green. Nanospray ESI-MS analysis revealed {[CuIICuIICuII(7-Et)(O)]2+(ClO4− or BF4−)2 + H}+, acetic acid [CH3C16O16OH] (Mr = 60), and the acetic acid adduct {[CuIICuIICuII(7-Et)(O)]2+(ClO4− or BF4−) + CH3COOH}+. The latter was similar to the benzoic adduct {[CuIICuIICuII(7-Et)(O)]2+(ClO4− or BF4−) + PhCOOH}+ obtained when benzoin was used as the reductant (see SI Fig. 11). The reaction with acetoin as reductant was totally the same as that with benzoin as the reductant.
Experiments with 18O2.
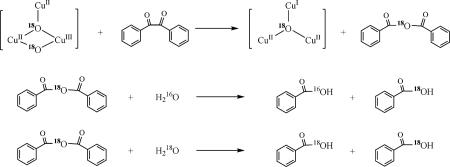
To verify that benzoic acid was indeed produced during the process of reduction of the CuIICuIICuII cluster by benzoin followed by reoxygenation, the reaction was repeated with 18O2 (96%) instead of normal dioxygen (Scheme 2). Two experiments were performed: (i) the starting CuIICuIICuII complex was first prepared with a bridging 16O-oxo, namely, [CuIICuIICuII(7-Et)(16O)]2+(BF4−)2 and (ii) the [CuIICuIICuII(7-Et)(18O)]2+(BF4−)2 complex, where the bridging 16O-oxo already had been replaced with an 18O-oxo to start with. In the case of [CuIICuIICuII(7-Et)(16O)]2+(BF4−)2, GC-MS analysis of the product mixture revealed the production of PhC16O18OH (Mr = 124) as well as PhC16O16OH (Mr = 122), with a product ratio close to one (Fig. 6c). Interestingly, only one of the two oxygen atoms of the exogenous 18O2 molecule was incorporated into the benzoic acid product. The remaining 18O was found in the product [CuIICuIICuII(7-Et)(18O)](X)2 complexes, according to nanospray ESI-MS analysis (see SI Fig. 12). By contrast, in the second experiment, where we started with [CuIICuIICuII(7-Et)(18O)]2+(BF4−)2, only PhC16O18OH (Mr = 124) was observed in the mass spectrum of the products at the same retention times as was obtained in the experiment with [CuIICuIICuII(7-Et)(16O)]2+(BF4−)2 (Fig. 6d). These observations strongly suggested that H2O was produced during the reduction of the CuIICuIICuII cluster, H216O in the case of [CuIICuIICuII(7-Et)(16O)]2+(BF4−)2, and H218O in the case of [CuIICuIICuII(7-Et)(18O)]2+(BF4−)2, and this H2O is involved in the disproportionation of the benzyl after the oxo-transfer chemistry and hydrolysis of the anhydride (see below).
Scheme 2.
O-atom transfer to benzil mediated by 18O2-activated tricopper cluster.
Competition of Substrates for “Oxo-Transfer” Chemistry.
To provide further insight into the role of the tricopper cluster in mediating oxo-transfer chemistry, we have compared the chemoselectivity of oxidation of benzil and 2,3-butanedione. As demonstrated earlier, both benzil and 2,3-butanedione products can be further oxidized by the dioxygen-activated [CuICuICuI(7-Et or 7-Me)]1+(X) complex. One interesting question is whether these products were dissociated before oxygenation and turnover. By treating the trinuclear copper cluster with one compound (e.g., acetoin) and performing the oxygenation in the presence of the already oxidized product (e.g., benzil), one could provide support for the direct involvement of the oxygenated tricopper species after dissociation of the oxidized reducing agent.
We previously proposed that the pMMO mediates methane oxidation by a tricopper cluster using a cluster-activated singlet oxene and have designed here the 7-Et and 7-Me ligands to promote the formation of a tricopper cluster to mediate this chemistry. If, as suggested by this design, the oxo-transfer by the putative bis(μ3-oxo)CuIICuIICuIII species is mediated by singlet oxene addition across the C C bond of the diketone to form an anhydride, the yield of oxidized substrates should be correlated with the central C
C bond of the diketone to form an anhydride, the yield of oxidized substrates should be correlated with the central C C bond energy of the diketone. Diketones with a lower central C
C bond energy of the diketone. Diketones with a lower central C C bond energy would be expected to be preferably attacked, and the corresponding cleavage products formed from hydrolysis of the anhydride would be produced in higher yields. To evaluate this possibility, we conducted substrate competition experiments between 2,3-butanedione and benzil. We used acetoin as the reductant to fully reduce [CuIICuIICuII(7-Et)(O)]2+(X)2 to [CuICuICuI(7-Et)]1+(X) and followed up by adding the same number of equivalents of exogenous benzil as the acetoin.
C bond energy would be expected to be preferably attacked, and the corresponding cleavage products formed from hydrolysis of the anhydride would be produced in higher yields. To evaluate this possibility, we conducted substrate competition experiments between 2,3-butanedione and benzil. We used acetoin as the reductant to fully reduce [CuIICuIICuII(7-Et)(O)]2+(X)2 to [CuICuICuI(7-Et)]1+(X) and followed up by adding the same number of equivalents of exogenous benzil as the acetoin.
As noted earlier, the acetoin is oxidized to 2,3-butanedione upon full reduction of the tricopper complexes. The bond energy of the central C C bond of 2,3-butanedione is estimated to 60 kcal/mol (16), only somewhat higher than the corresponding C
C bond of 2,3-butanedione is estimated to 60 kcal/mol (16), only somewhat higher than the corresponding C C bond energy in benzil, 51 kcal/mol (17). Accordingly, we expected a mixture of products, benzoic adduct {[CuIICuIICuII(7-Et)(O)]2+(ClO4− or BF4−) + PhCOOH}+ and the acetic adduct {[CuIICuIICuII(7-Et)(O)]2+(ClO4− or BF4−) + CH3COOH}+. Indeed, this outcome was confirmed by nanospray ESI-MS (see SI Fig. 13). The observed product ratio was ≈1.
C bond energy in benzil, 51 kcal/mol (17). Accordingly, we expected a mixture of products, benzoic adduct {[CuIICuIICuII(7-Et)(O)]2+(ClO4− or BF4−) + PhCOOH}+ and the acetic adduct {[CuIICuIICuII(7-Et)(O)]2+(ClO4− or BF4−) + CH3COOH}+. Indeed, this outcome was confirmed by nanospray ESI-MS (see SI Fig. 13). The observed product ratio was ≈1.
In contrast, when benzil was used as the external substrate in the same oxygenation, but [CuICuICuI(7-Et)]1+(ClO4− or BF4−) was obtained by mixing [Cu(CH3CN)4]1+(ClO4− or BF4−) and [7-Et]2−[Na2]2+ in CH3CN, the major product turned out to be benzoic acid, as characterized by GC-MS. There was no evidence for the product CH2OHCN noted earlier in the ESI mass spectrum. As the C H bond energy in CH3CN is 93–95 kcal/mol (18), CH3CN is hardly competitive with benzil as a substrate.
H bond energy in CH3CN is 93–95 kcal/mol (18), CH3CN is hardly competitive with benzil as a substrate.
The series of experiments highlighted in this section illustrate the chemoselectivity of the oxidation mediated by the putative [CuIICuIICuIII(L)(O2)]1+(X) complex.
Details of the crystal structure of [CuIICuIICuII(7-Et)(O)](BF4−)2(0.5 PF6−) are described in SI Text and SI Fig. 14.
Discussion
In this study, we have designed and prepared two ligands, each of which is capable of trapping three Cu(I) ions to form a pale yellow [CuICuICuI(L)]1+ complex that could be activated by dioxygen to form an intermediate capable of O-atom transfer chemistry. We showed that this putative [CuIICuIICuIII(L)(O2)]1+ species could mediate facile O-atom insertion across the C H bond in CH3CN to form glycolonitrile, and between the central C
H bond in CH3CN to form glycolonitrile, and between the central C C bond in benzil and 2,3-butanedione to yield benzoic acid and acetic acid, respectively (Scheme 3). The chemistry observed here is facile, in sharp contrast to the extremely slow O-atom transfer mediated by well characterized bis(μ-oxo)CuIIICuIII species or the structurally related (μ-η2:η2-peroxo)CuIICuII and (hydroperoxo) CuIICuII species, obtained when the corresponding dinuclear CuICuI complexes are reoxidized by dioxygen under the same conditions (19–27). Moreover, in addition to being relatively inert, these oxidized dicopper clusters typically support intramolecular ligand oxidation rather than mediate O-atom transfer to exogenous substrates. Together, the results of the present study provide strong support for the earlier suggestion of Chan and coworkers (3) that pMMO utilizes a tricopper cluster with an oxo-bridged [CuIICuIICuIII] intermediate capable of harnessing a singlet oxene for facile insertion across C
C bond in benzil and 2,3-butanedione to yield benzoic acid and acetic acid, respectively (Scheme 3). The chemistry observed here is facile, in sharp contrast to the extremely slow O-atom transfer mediated by well characterized bis(μ-oxo)CuIIICuIII species or the structurally related (μ-η2:η2-peroxo)CuIICuII and (hydroperoxo) CuIICuII species, obtained when the corresponding dinuclear CuICuI complexes are reoxidized by dioxygen under the same conditions (19–27). Moreover, in addition to being relatively inert, these oxidized dicopper clusters typically support intramolecular ligand oxidation rather than mediate O-atom transfer to exogenous substrates. Together, the results of the present study provide strong support for the earlier suggestion of Chan and coworkers (3) that pMMO utilizes a tricopper cluster with an oxo-bridged [CuIICuIICuIII] intermediate capable of harnessing a singlet oxene for facile insertion across C C or C
C or C H bonds of an exogenous substrates.
H bonds of an exogenous substrates.
Scheme 3.
Oxidation chemistry of benzil, 2,3-butanedione, and acetonitrile.
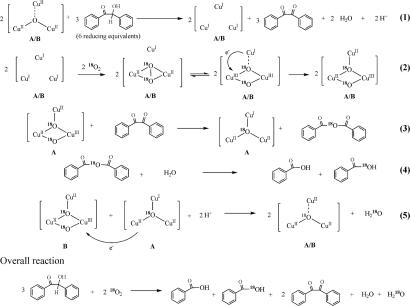
Using the lessons learned from our biochemical studies of pMMO, we have studied the oxo-transfer chemistry under conditions in which the oxidized tricopper cluster [CuIICuIICuII(7-Et or 7-Me)(O)]2+(X)2 initially is reduced by an exogenous reductant, benzoin or acetoin, and then reacted with dioxygen. The benzoin was oxidized to benzil, and acetoin to 2,3-butanedione, both of which in turn underwent further oxidation by O-atom insertion across the central C C bond from the putative [CuIICuIICuIII(L)(O2)]1+ intermediate formed by oxygenation of the reduced [CuICuICuI(L)]1+ complex, to give the corresponding anhydride, followed by hydrolysis to yield two molecules of benzoic acid or acetic acid, respectively. A mechanistic rationale for the reaction, which is consistent with the 16O2 and 18O2 data, is given in Scheme 4.
C bond from the putative [CuIICuIICuIII(L)(O2)]1+ intermediate formed by oxygenation of the reduced [CuICuICuI(L)]1+ complex, to give the corresponding anhydride, followed by hydrolysis to yield two molecules of benzoic acid or acetic acid, respectively. A mechanistic rationale for the reaction, which is consistent with the 16O2 and 18O2 data, is given in Scheme 4.
Scheme 4.
The proposed mechanism of O-atom transfer mediated by dioxygen-activated tricopper cluster.
The proposed mechanism described in Scheme 4 predicts a benzoic acid/benzil product ratio of 1, in excellent agreement with experiments performed under single-turnover conditions, as determined by using calibrated external benzil and benzoic acid as standards. In addition, according to Scheme 4, in a single-turnover experiment in which [CuICuICuI(7-Et)]1+(BF4−) was reduced from [CuIICuIICuII(7-Et)(16O)]2+(BF4−)2 in step 1, but exogenous 18O2 was used in step 2, only one 18O atom will be incorporated into the C C bond of benzil. Subsequent hydrolysis of the anhydride by the H216O produced in step 1 yielded a 1:1 product mixture of PhC16O18OH (Mr = 124) and PhC16O16OH (Mr = 122), in perfect conformity with the GC-MS data (Fig. 6c). On the other hand, when the same reduction was initiated with [CuIICuIICuII(7-Et)(18O)]2+(BF4−)2 in step 1, and 18O2 was used in the reoxygenation in step 2, only the production of PhC16O18OH (Mr = 124) was observed (Fig. 6d). As before, only one 18O atom was incorporated into the C
C bond of benzil. Subsequent hydrolysis of the anhydride by the H216O produced in step 1 yielded a 1:1 product mixture of PhC16O18OH (Mr = 124) and PhC16O16OH (Mr = 122), in perfect conformity with the GC-MS data (Fig. 6c). On the other hand, when the same reduction was initiated with [CuIICuIICuII(7-Et)(18O)]2+(BF4−)2 in step 1, and 18O2 was used in the reoxygenation in step 2, only the production of PhC16O18OH (Mr = 124) was observed (Fig. 6d). As before, only one 18O atom was incorporated into the C C bond of benzil, but the anhydride is hydrolyzed by one of two H218O molecules formed from the bridging 18O-oxo in the starting [CuIICuIICuII] complex in step 1. These 16O2/18O2 data also are in accord with the mechanistic scenario highlighted in Scheme 4. Together, these data provide strong support for the idea that the putative bis(μ3-oxo)CuIICuIICuIII complex harnesses a singlet oxene.
C bond of benzil, but the anhydride is hydrolyzed by one of two H218O molecules formed from the bridging 18O-oxo in the starting [CuIICuIICuII] complex in step 1. These 16O2/18O2 data also are in accord with the mechanistic scenario highlighted in Scheme 4. Together, these data provide strong support for the idea that the putative bis(μ3-oxo)CuIICuIICuIII complex harnesses a singlet oxene.
Finally, it is interesting that we have obtained no evidence for the degradation of the tricopper cluster even with many turnovers. Perhaps this is not surprising, given that the putative bis(μ3-oxo)[CuIICuIICuIII] species merely harnesses a singlet oxene, and the O-atom transfer only occurs when a substrate becomes bound, and then only when a suitable bond is in the proper stereochemical juxtaposition to form a transition state that allows the oxo-transfer to take place. In other words, the chemistry is inner sphere. It is a concerted direct O-atom insertion, unlike the hydrogen-abstraction radical rebound chemistry in the radical mechanism of oxo-transfer. The chemo- or regio-selectivity that we have observed for benzil, 2,3-butanedione, and acetonitrile only could be accounted for by this type of inner sphere chemistry. All these substrates somehow must be anchored to coordination sites of the bis(μ3-oxo)CuIICuIICuIII catalyst, and presumably the copper centers offer potential binding sites for these substrates.
Conclusions
We have reported here the synthesis of a series of multidentate ligands capable of forming a trinuclear copper cluster with facile oxo-transfer activity. We have demonstrated that the oxygenation of [CuICuICuI(L)]1+(X) (L = 7-Et, 7-Me; X = ClO4− and BF4−) yields a [CuIICuIICuII(L)(O)]2+(X)2 complex with a structure similar to that predicted and implicated for the fully oxidized hydroxylation site of pMMO. In the presence of suitable substrates, we show that one oxygen atom is trapped in the fully oxidized CuIICuIICuII complex, whereas the other oxygen is involved in oxo-transfer to the exogenous substrate. Oxo insertion into the C H of CH3C
H of CH3C N, and oxo insertion of the central C
N, and oxo insertion of the central C C bond of benzil and 2,3-butanedione were highlighted in this study. This chemistry is consistent with the formation of a putative bis(μ3-oxo)[CuIICuIICuIII] species, which we recently have argued to be capable of harnessing a singlet oxene for oxo-transfer to a suitable substrate under appropriate conditions. Specifically, we have proposed that the conversion of methane to methanol mediated by the tricopper cluster in pMMO occurs via such a singlet oxene mechanism (3). Our model tricopper cluster is unique in that it is capable of mediating facile oxo-transfer across a C
C bond of benzil and 2,3-butanedione were highlighted in this study. This chemistry is consistent with the formation of a putative bis(μ3-oxo)[CuIICuIICuIII] species, which we recently have argued to be capable of harnessing a singlet oxene for oxo-transfer to a suitable substrate under appropriate conditions. Specifically, we have proposed that the conversion of methane to methanol mediated by the tricopper cluster in pMMO occurs via such a singlet oxene mechanism (3). Our model tricopper cluster is unique in that it is capable of mediating facile oxo-transfer across a C C or a C
C or a C H bond. Thus, this copper cluster should provide a structural and functional model for the hydroxylation of alkanes as reported for pMMO. Indeed, we have obtained preliminary evidence for the hydroxylation of C
H bond. Thus, this copper cluster should provide a structural and functional model for the hydroxylation of alkanes as reported for pMMO. Indeed, we have obtained preliminary evidence for the hydroxylation of C H bonds when hexane was introduced as a substrate. As expected, the yield was very low, because association of the apolar hydrocarbon substrate with the polar tricopper cluster was too weak to facilitate frequent oxo-transfer events. Nevertheless, it is clear that the same chemistry is operative with our model system here as in the case of pMMO.
H bonds when hexane was introduced as a substrate. As expected, the yield was very low, because association of the apolar hydrocarbon substrate with the polar tricopper cluster was too weak to facilitate frequent oxo-transfer events. Nevertheless, it is clear that the same chemistry is operative with our model system here as in the case of pMMO.
Materials and Methods
Preparation of Ligands.
The ligands 7-Me and 7-Et are variants of a polyamine alcohol described previously (28). In the present ligand design, all of the amino groups are capped with methyl or ethyl groups, including the secondary amino groups at N(2) and N(5) as well as the terminal amino groups at N(1) and N(6). In addition, the hydroxyl group axially disposed with respect to the seven-membered chelate ring at the apex in the earlier ligands is not included here to create an open coordination site for each of the three copper ions in the metal complexes to be prepared as part of this study. 7-Me and 7-Et were prepared according to Scheme 5.
Scheme 5.
Synthesis of ligands 7-Me and 7-Et.
Details of the synthesis and spectroscopic characterization of the ligands are described in SI Text.
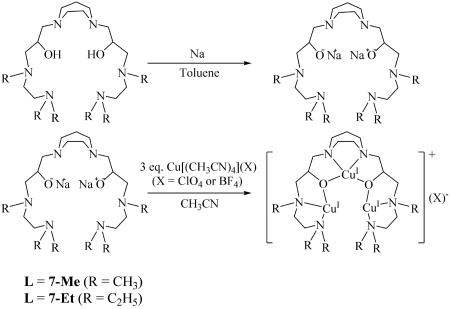
Preparation of CuICuICuI Complexes.
The [CuICuICuI(L)]1+ complexes were extremely sensitive to air, thus it was necessary to handle the experimental procedures under a purified argon atmosphere. The salts CuI[(CH3CN)4]X (X = BF4− and ClO4−) were prepared as described in ref. 29. The sodium salts of 7-Me or 7-Et could be obtained by allowing the free ligand to react with two equivalents of sodium in toluene for 48 h under argon followed by removal of the toluene under high-vacuum conditions. The [CuICuICuI(L)]1+ complexes (Scheme 6) readily were prepared by treating 3 equivalents of Cu[(CH3CN)4](X) (X = ClO4− or BF4−) in anhydrous CH3CN solution with one equivalent of [7-Me]2−[Na2]2+ or [7-Et]2−[Na2]2+ and used directly.
Scheme 6.
Preparation of [CuICuICuI(L)]1+.
Supplementary Material
Acknowledgments
We thank Dr. Mei-Chun Tseng of the Institute of Chemistry of Academia Sinica for kind assistance in nanospray ESI-MS, Yi-Hung Liu of the Instrumentation Center of National Taiwan University in Taipei for solving the x-ray structure, and Dr. Michael K. Chan of the Department of Biochemistry and Chemistry at Ohio State University (Columbus, OH) for numerous discussions on this scientific project as well as valuable comments on this manuscript. This work was supported by Academia Sinica and by grants from the National Science Council (NSC95-2113-M-001-046-MY2) in Taiwan.
Abbreviations
- MMO
methane monooxygenase
- pMMO
particulate MMO
- sMMO
soluble MMO
- DFT
density functional theory
- 7-Me
3,3′-(1,4-diazepane-1,4-diyl)bis(1-((2-(dimethylamino)ethyl)(methyl)amino)propan-2-ol)
- 7-Et
3,3′-(1,4-diazepane-1,4-diyl)bis(1-((2-(diethylamino)ethyl)(ethyl)amino)propan-2-ol
- ESI
electrospray ionization
- amu
atomic mass unit.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0707119104/DC1.
References
- 1.Periana RA, Taube DJ, Gamble S, Taube H, Satoh T, Fujii H. Science. 1998;280:560–564. doi: 10.1126/science.280.5363.560. [DOI] [PubMed] [Google Scholar]
- 2.Hanson RS, Hanson TE. Microbiol Rev. 1996;60:439–471. doi: 10.1128/mr.60.2.439-471.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chan SI, Chen KHC, Yu SSF, Chen CL, Kuo SSJ. Biochemistry. 2004;43:4421–4430. doi: 10.1021/bi0497603. [DOI] [PubMed] [Google Scholar]
- 4.Feig AL, Lippard SJ. Chem Rev. 1994;94:759–805. [Google Scholar]
- 5.Lipscomb JD. Annu Rev Microbiol. 1994;48:371–399. doi: 10.1146/annurev.mi.48.100194.002103. [DOI] [PubMed] [Google Scholar]
- 6.Valentine AM, Wilkinson B, Liu KE, KomarPanicucci S, Priestley ND, Williams PG, Morimoto H, Floss HG, Lippard SJ. J Am Chem Soc. 1997;119:1818–1827. [Google Scholar]
- 7.Valentine AM, LeTadic-Biadatti MH, Toy PH, Newcomb M, Lippard SJ. J Biol Chem. 1999;274:10771–10776. doi: 10.1074/jbc.274.16.10771. [DOI] [PubMed] [Google Scholar]
- 8.Nesheim JC, Lipscomb JD. Biochemistry. 1996;35:10240–10247. doi: 10.1021/bi960596w. [DOI] [PubMed] [Google Scholar]
- 9.Wilkinson B, Zhu M, Priestley ND, Nguyen HHT, Morimoto H, Williams PG, Chan SI, Floss HG. J Am Chem Soc. 1996;118:921–922. [Google Scholar]
- 10.Elliott SJ, Zhu M, Tso L, Nguyen HHT, Yip JHK, Chan SI. J Am Chem Soc. 1997;119:9949–9955. [Google Scholar]
- 11.Yu SSF, Wu LY, Chen KHC, Luo WI, Huang DS, Chan SI. J Biol Chem. 2003;278:40658–40669. doi: 10.1074/jbc.M301018200. [DOI] [PubMed] [Google Scholar]
- 12.Huang DS, Wu SH, Wang YS, Yu SSF, Chan SI. ChemBioChem. 2002;3:760–765. doi: 10.1002/1439-7633(20020802)3:8<760::AID-CBIC760>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 13.Chen PPY, Chan SI. J Inorg Biochem. 2006;100:801–809. doi: 10.1016/j.jinorgbio.2005.12.014. [DOI] [PubMed] [Google Scholar]
- 14.Chan SI, Wang VCC, Lai JCH, Yu SSF, Chen PPY, Chen KHC, Chen CL, Chan MK. Angew Chem Int Ed. 2007;46:1992–1994. doi: 10.1002/anie.200604647. [DOI] [PubMed] [Google Scholar]
- 15.Itoh S, Kondo T, Komatsu M, Ohshiro Y, Li CM, Kanehisa N, Kai Y, Fukuzumi S. J Am Chem Soc. 1995;117:4714–4715. [Google Scholar]
- 16.Szwarc M. Proc R Soc London Ser A Math Phys Sci. 1951;207:5–13. [Google Scholar]
- 17.Carson AS, Fine DH, Gray P, Laye PG. J Chem Soc B Phys Org. 1971;1971:1611–1615. [Google Scholar]
- 18.Penn JH, Owens WH. J Am Chem Soc. 1993;115:82–86. [Google Scholar]
- 19.Gelling OJ, Meetsma A, Feringa BL. Inor Chem. 1990;29:2816–2822. [Google Scholar]
- 20.Cruse RW, Kaderli S, Karlin KD, Zuberbuhler AD. J Am Chem Soc. 1988;110:6882–6883. [Google Scholar]
- 21.Holland PL, Rodgers KR, Tolman WB. Angew Chem Int Ed. 1999;38:1139–1142. doi: 10.1002/(SICI)1521-3773(19990419)38:8<1139::AID-ANIE1139>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 22.Mahapatra S, Halfen JA, Tolman WB. J Am Chem Soc. 1996;118:11575–11586. [Google Scholar]
- 23.Halfen JA, Young VG, Tolman WB. J Am Chem Soc. 1996;118:10920–10921. [Google Scholar]
- 24.Itoh S, Nakao H, Berreau LM, Kondo T, Komatsu M, Fukuzumi S. J Am Chem Soc. 1998;120:2890–2899. [Google Scholar]
- 25.Arii H, Saito Y, Nagatomo S, Kitagawa T, Funahashi Y, Jitsukawa K, Masuda H. Chem Lett. 2003;32:156–157. [Google Scholar]
- 26.Cole AP, Mahadevan V, Mirica LM, Ottenwaelder X, Stack TDP. Inorg Chem. 2005;44:7345–7364. doi: 10.1021/ic050331i. [DOI] [PubMed] [Google Scholar]
- 27.Shearer J, Zhang CX, Zakharov LN, Rheingold AL, Karlin KD. J Am Chem Soc. 2005;127:5469–5483. doi: 10.1021/ja045191a. [DOI] [PubMed] [Google Scholar]
- 28.Bernhardt PV, Sharpe PC. J Chem Soc Dalton Trans. 1998;1988:1087–1088. [Google Scholar]
- 29.Knapp S, Trope AF, Theodore MS, Hirata N, Barchi JJ. J Org Chem. 1984;49:608–614. [Google Scholar]
- 30.Burnett MN, Johnson CK. ORTEP-III: Oak Ridge Thermal Ellipsoid Plot Program for Crystal Structure Illustrations. Oak Ridge, TN: Oak Ridge National Laboratory; 1996. Report ORNL-6895. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.