Abstract
We previously reported that ovalbumin (OVA) and IL-18 nasally administered act on memory type T helper (Th)1 cells to induce airway hyperresponsiveness (AHR) and inflammation, which is characterized by peribronchial infiltration with neutrophils and eosinophils. Here, we report this administration also induces lung fibrosis in an IL-13-dependent manner. Th1 cells secrete several cytokines, including IFN-γ and bronchogenic cytokine IL-13, when stimulated with antigen (Ag) and IL-18. However, IL-13 blockade failed to attenuate AHR, although this treatment inhibited eosinophilic infiltration. To understand the mechanism by which Th1 cells induce AHR after Ag plus IL-18 challenge, we established “passive” and “active” Th1 mice by transferring OVA-specific Th1 cells into naïve BALB/c mice or by immunizing naïve BALB/c mice with OVA/complete Freund's adjuvant, respectively. Administration of Ag and IL-18 induced both types of Th1 mice to develop AHR, airway inflammation, and lung fibrosis. Furthermore, this treatment induced deposition of periostin, a novel component of lung fibrosis. Neutralization of IL-13 or IFN-γ during Ag plus IL-18 challenges inhibited the combination of eosinophilic infiltration, lung fibrosis, and periostin deposition or the combination of neutrophilic infiltration and AHR, respectively. We also found that coadministration of OVA and LPS into Th1 mice induced AHR and airway inflammation via endogenous IL-18. Thus, IL-18 becomes a key target molecule for the development of a therapeutic regimen for the treatment of Th1-cell-induced bronchial asthma.
Keywords: bronchial asthma, LPS, periostin, hydroxy proline, airway inflammation
Bronchial asthma is a complex syndrome characterized by airway hyperresponsiveness (AHR) and reversible airflow obstruction associated with airway inflammation and remodeling and occasional high serum level of IgE (1–7). Histologically, there are infiltrates of eosinophils, degranulated mast cells, subbasement membrane thickening, hyperplasia and hypertrophy of bronchial smooth muscle, and hyperplasia of airway goblet cells (1, 2). Th2 cells have been recognized as inducing bronchial asthma by production of Th2 cytokines (1–12). Particularly, IL-13 is suggested to play a critical role in induction of AHR, eosinophilic infiltration, goblet cell metaplasia, and lung fibrosis (9–13). In contrast, Th1 cells previously had been regarded to inhibit bronchial asthma by virtue of IFN-γ (14–16). However, several studies have disclosed the disability of Th1 cell to suppress Th2 cell-induced AHR (17–21). Moreover, a combination of Th1 and Th2 cells or their products augment each activity to induce airway inflammation and AHR (17, 18, 21). Thus, bronchial asthma is a complicated disease induced by the functions of Th1 and Th2 cells.
Recently, we have demonstrated that antigen (Ag) plus IL-18 acts on adoptively transferred memory type Th1 cells to induce airway inflammation and AHR in a naïve host mouse (22). These Th1 cells express ovalbumin (OVA)-specific T cell antigen receptor and IL-18 receptor (22, 23). They produce IFN-γ in response to OVA and increase further IFN-γ production in response to additional IL-18 stimulation (22, 23). Most surprisingly, they simultaneously produce Th2 cytokines (e.g., IL-9 and IL-13), granulocyte–macrophage colony-stimulating factor and chemokines (e.g., RANTES and macrophage inflammatory protein 1α) when stimulated with OVA and IL-18 (22). Human Th1 cells also produce Th1 and Th2 cytokines and IL-8 in response to anti-CD3 plus IL-18 (24). Recently, we demonstrated Th1 cells induce intrinsic atopic dermatitis by production of Th1 and Th2 cytokines and chemokines (25). Thus, IL-18 has added its new function to its growing functional list (26–29). Based on this unique function of Ag- plus IL-18-stimulated Th1 cells, we proposed to designate them as “super Th1 cells” (25). It is important to determine the mechanism by which super Th1 cells induce bronchial asthma by production of both Th1 and Th2 cytokines. However, as we reported previously (22), IL-13 blockade fails to attenuate Ag- plus IL-18-induced AHR, although this treatment markedly diminishes eosinophilic infiltration. These results prompted us to examine the role of Th1 cytokine in induction of AHR and airway inflammation.
In our previous report, we established “passive Th1 mice” by transferring OVA-specific memory Th1 cells (1 × 107 cells per mouse) into naïve BALB/c mice (22). Here, we prepared “active Th1 mice” by immunizing BALB/c mice with OVA in complete Freund's adjuvant (CFA) 2 weeks before experimentation. Both types of Th1 mice develop AHR, airway inflammation, and lung fibrosis after challenge with OVA and IL-18. Furthermore, they express periostin, a novel component of lung fibrosis under the control of IL-4 and IL-13 signals (30). Administration of anti-IFN-γ Ab almost completely inhibited AHR and neutrophilic infiltration, whereas IL-13 neutralization inhibited lung fibrosis and eosinophilic infiltration without affecting AHR. Thus, Th1 cells become very harmful super Th1 cells when stimulated with OVA and IL-18 by production of IFN-γ and IL-13 in the lungs. Of interest, OVA/CFA-immunized mice develop AHR and airway inflammation upon challenge with OVA and LPS via endogenous IL-18. Importantly, administration of anti-IL-18 almost completely inhibited this OVA/LPS-induced AHR, thereby rationalizing the development of reagents that down-regulate IL-18 for the treatment of Th1-cell-induced bronchial asthma.
Results
OVA and IL-18 Induce AHR in Th1 Cell-Bearing Mice in an IFN-γ-Dependent Manner.
We previously reported that mice receiving memory Th1 cells develop AHR and airway inflammation after intranasal administration of OVA and IL-18 (22). Th1 cells produce IFN-γ, IL-9, IL-13, granulocyte–macrophage colony-stimulating factor, and chemokines in response to Ag, IL-2, and IL-18 in vitro (22, 25). Among the cytokines produced, IL-13 is most bronchogenic and participates in AHR (7–13). However, neutralization of IL-13 in the lungs fails to inhibit Th1-cell-induced AHR (22). Thus, we sought to determine the causative factor critically involved in this Th1-cell-induced AHR.
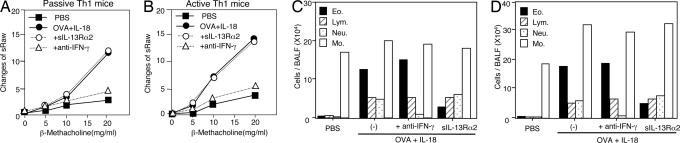
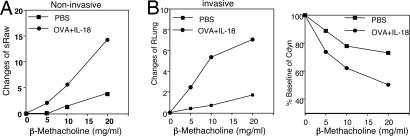
We first examined the relevant role of IFN-γ in induction of bronchial asthma. For this purpose, we constructed a convenient Th1-cell-induced bronchial asthma model. We immunized BALB/c mice with OVA in CFA (OVA/CFA) to actively induce OVA-specific Th1 cells in vivo. Indeed, this treatment strongly induced OVA-specific Th1 cells in vivo [supporting information (SI) Fig. 8A]. We designated these OVA/CFA-primed mice as active Th1 mice and tested their development of bronchial asthma after their nasal exposure to OVA and IL-18. We compared their responses to those of naïve mice receiving adoptively transferred OVA-specific Th1 cells (passive Th1 mice). As shown in Fig. 1 A and B, both types of Th1 mice developed AHR. Consistent with our previous report (22), each stimulus alone did not induce AHR (SI Fig. 8B). Furthermore, both noninvasive and invasive measurements of AHR provided basically identical results, excluding a contribution from the upper airway component to the induction of AHR (Fig. 2). Invasive measurement also indicated that OVA- plus IL-18-challenged Th1 mice reduced lung compliance. Bronchoalveolar lavage fluid (BALF) examination revealed that administration of OVA and IL-18 induced increases in the numbers of eosinophils, lymphocytes, and neutrophils in Th1-cell-bearing mice (Fig. 1 C and D) but not in normal control mice (SI Fig. 8C) (22). Therefore, both active and passive Th1 mice showed similar responses to OVA plus IL-18 in terms of AHR and airway inflammation.
Fig. 1.
Anti-IFN-γ Ab treatment protected against Ag- plus IL-18-induced AHR and accumulation of neutrophils in Th1 mice. Passive (A and C) and active (B and D) Th1 mice were exposed to daily intranasally administered PBS (50 μl) or OVA (100 μg per 50 μl of PBS) plus IL-18 (0.5 μg per 50 μl of PBS) for 3 days. A total of 20 μg of sIL-13Rα2-Fc chimera (sIL13Rα2) was used for IL-13 blockade in vivo. For the blockade of IFN-γ in vivo, 50 μg of anti-IFN-γ Ab was intranasally coadministered with OVA and IL-18 for 3 days. (A and B) At 24 h after the final exposure to OVA plus IL-18, AHR in response to increased concentrations of inhaled β-methacholine and inflammatory cell composition of BALF was examined. (C and D) Cell differential percentages were determined by light microscopic evaluation of cytospin preparation. Representative results of five to seven animals per group are shown.
Fig. 2.
Noninvasive or invasive measurement of Ag- plus IL-18-induced AHR. Active Th1 mice were exposed daily to intranasal administration of PBS (50 μl) or OVA (100 μg per 50 μl of PBS) plus IL-18 (0.5 μg per 50 μl of PBS) for 3 days. At 24 h after the final exposure to OVA plus IL-18, AHR in response to increased concentrations of inhaled β-methacholine was determined by noninvasive [specific airway resistance (sRaw)] (A) and invasive [pulmonary resistance (RLung) and Cdyn] (B) measurement. Baseline values for pulmonary resistance (cmH2O/ml·sec−1) and Cdyn (ml/cmH2O) were 4.58 for PBS, 4.65 for OVA plus IL-18, 0.0119 for PBS, and 0.0133 for OVA plus IL-18. Representative results of five to seven animals per group are shown.
To evaluate the effects of IFN-γ and IL-13 on AHR and airway inflammation, we treated Th1 mice with an Ab against IFN-γ or sIL-13Rα2-Fc chimera (sIL-13Rα2). As shown in Fig. 1 A and B, neutralization of IFN-γ almost completely inhibited Ag- plus IL-18-induced AHR, whereas neutralization of IL-13 failed to do so, suggesting a contribution from IFN-γ but not from IL-13 to the induction of AHR. Nevertheless, this IL-13 neutralization inhibited eosinophilic infiltration, excluding a contribution from eosinophilic infiltration to the induction of AHR in Th1-cell-induced bronchial asthma. To understand the mechanism whereby only IFN-γ neutralization inhibited AHR in both types of Th1 mice, we compared the cellular components in BALFs from Th1 mice either receiving these treatments or not. As shown in Fig. 1 C and D, neutralization of IFN-γ or IL-13 selectively diminished neutrophilic or eosinophilic infiltration, respectively, suggesting their differential regulation by IFN-γ and IL-13.
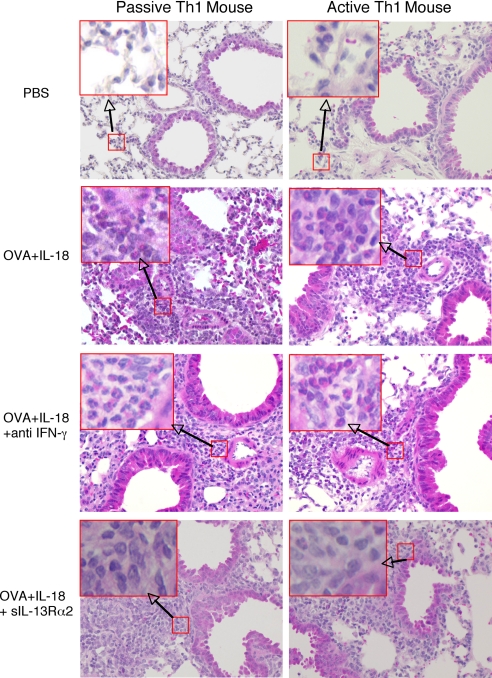
Histological analysis revealed that both types of Th1 mice expressed peribronchial and perivenular infiltrations composed mainly of eosinophils and neutrophils after OVA plus IL-18 treatment (Fig. 3). The degree of eosinophilic infiltrations in the lungs of the Th1-cell-bearing mice is comparable to that of Th2-cell-bearing mice after exposure to OVA (22). Consistent with the result of Fig. 1 C and D, neutralization of IFN-γ and IL-13 appeared to reduce more selectively neutrophilic and eosinophilic infiltrations in the lungs, respectively (Fig. 3). These results strongly indicated that Th1 cells induce AHR and eosinophilic infiltration in response to Ag and IL-18 by production of IFN-γ and IL-13, respectively.
Fig. 3.
Histological examination of the lung tissues from Th1 mice exposed to OVA and IL-18. Both passive and active Th1 mice were exposed daily to intranasally administered PBS or OVA plus IL-18 with anti-IFN-γ or sIL-13Rα2 as described in the legend of Fig. 1. At 24 h after final exposure, lungs from each group of mice were prepared for histological examination by perfusing the animal via the right ventricle with 10 ml of PBS; the lungs were then fixed in formalin, cut into 3-μm sections, and stained with H&E as described in Material and Methods. [Original magnification, ×40 (Insets, ×200).]
IL-4Rα−/− Mice Normally Develop Bronchial Asthma After OVA Plus IL-18 Challenge.
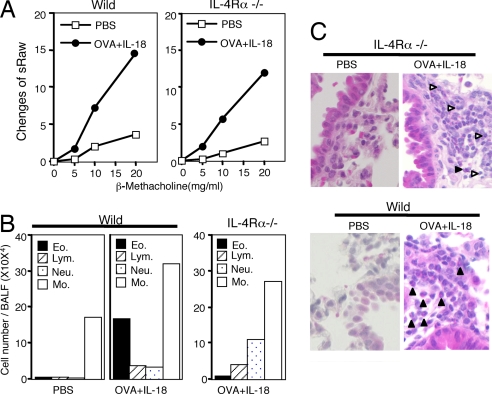
It is important to exclude entirely the contribution from IL-13 to AHR because residual IL-13 might collaborate with IFN-γ for the induction of AHR. For this purpose, we primed BALB/c background IL-4Rα−/− mice with OVA/CFA and subsequently challenged them with OVA and IL-18. As expected, IL-4Rα−/− mice normally developed AHR (Fig. 4A), which formally excluded a contribution from IL-13 to the induction of AHR in Th1-cell-bearing mice.
Fig. 4.
IL-4Rα−/− mice often developed Th1-cell-induced AHR. BALB/c WT or BALB/c background IL-4Rα−/− mice, which were immunized with OVA in CFA 2 weeks previously, were exposed to daily intranasal administration of PBS or OVA plus IL-18 for 3 days. At 24 h after final exposure, the mice were analyzed for β-methacholine-induced AHR (A) and BALF (B) and their histological changes (C), as described for in the legends of Figs. 1 and 2. White arrowhead, neutrophil; black arrowhead, eosinophil. Representative results obtained from five to seven animals per group are shown. (Magnification, ×200.)
We simultaneously examined the cell components in the BALFs (Fig. 4B). We also performed histological evaluation of the lungs (Fig. 4C). As expected from the BALFs, results of sIL-13Rα2-treated mice (Fig. 1 C and D), OVA/CFA-primed, and OVA/IL-18-challenged IL-4Rα−/− mice markedly reduced the numbers of eosinophils and, conversely, increased the number of neutrophils in their BALFs (Fig. 4B). Histological examination revealed that OVA and IL-18 induced peribronchial infiltration in all cases of WT mice, as well as IL-4Rα−/− mice (Fig. 4C). However, compared with WT mice, IL-4Rα−/− mice markedly increased the cell infiltrates composed mainly of neutrophils (Fig. 4C), substantiating further the differential induction of neutrophils and eosinophil by IFN-γ and IL-13, respectively. Thus, IL-13 is not required for the induction of AHR in OVA- plus IL-18-administered Th1 mice.
Coadministration of Ag and LPS Induces AHR in Th1-Immunized Mice.
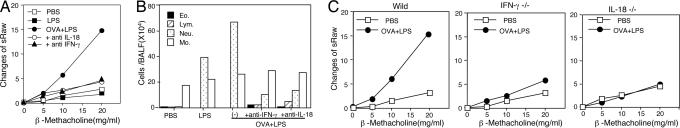
Patients with bronchial asthma often develop AHR and airway inflammation after viral or bacterial infection (2, 31–33). It has been reported that infected animals often display an increase in serum levels of IL-18 after viral or bacterial infection (27). Thus, we assumed that pathogen or pathogen-associated molecular pattern might induce AHR by the induction of IL-18. To examine this possibility, we treated Th1 mice with intranasal administration of OVA and LPS instead of OVA and IL-18. We found that mice receiving such treatment developed AHR and severe airway inflammation (Fig. 5 A and B).
Fig. 5.
Neutralization of IFN-γ or IL-18 abolished OVA plus LPS-induced AHR and neutrophilic infiltration in active Th1 mice. BALB/c WT mice or BALB/c background IFN-γ−/− or IL-18−/− mice immunized with OVA/CFA 2 weeks previously were challenged intranasally with 5 μg of LPS and 100 μg of OVA. To block endogenous IL-18 or IFN-γ, Ab against IL-18 or IFN-γ mixed with OVA and LPS was coadministered daily. At 24 h after final administration, β-methacholine-induced AHR (A and C) and cell population in BALF (B) were analyzed as described in the legend of Fig. 1. Representative results of five to seven animals per group are shown. The results shown in A Left and C Left were obtained from the same experiment.
We next tried to determine whether OVA and LPS induced AHR by the action of endogenous IL-18 that, in turn, induces IFN-γ production from Th1 cells. Thus, we examined the capacity of anti-IL-18 or anti-IFN-γ Ab treatment to inhibit this OVA plus LPS-induced AHR (Fig. 5). Each Ab treatment markedly diminished AHR (Fig. 5A). Furthermore, IFN-γ−/− or IL-18−/− mice were resistant to the sequential treatment with OVA/CFA priming and OVA/LPS challenge (Fig. 5C), substantiating further the importance of IL-18-dependent IFN-γ production for AHR. However, this OVA plus LPS challenge only increased the number of neutrophils in BALFs (Fig. 5B). Thus, OVA plus LPS partially replaced the action of OVA and IL-18. However, anti-IFN-γ Ab treatment modestly increased the number of eosinophils and lymphocytes but markedly reduced the number of neutrophils (Fig. 5B). These results strongly indicated that OVA plus LPS induced Th1 cells to produce IFN-γ via endogenous IL-18, resulting in induction of neutrophilic infiltration and inhibition of eosinophilic infiltration.
Ag- and IL-18-Induced Lung Fibrosis Depends on Endogenous IL-13.
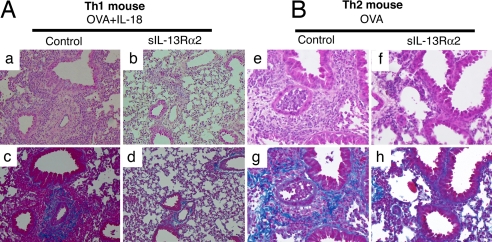
IL-13 is indispensable for eosinophilic inflammation (7, 9–13, 34). Furthermore, IL-13 has the potential to induce lung fibrosis by activating macrophages, bronchoepithelial cells, and eosinophils to produce fibrogenic cytokine TGF-β (35, 36). Therefore, we tested the pathological effect of IL-13 derived from Ag- plus IL-18-stimulated Th1 cells on lung fibrosis. We compared the degree of lung fibrosis in the lungs of Th1 and Th2 mice after challenge with OVA plus IL-18 or OVA alone, respectively. Both Th1 and Th2 mice similarly developed lung fibrosis (Fig. 6). Development of fibrosis was proven to depend on the function of endogenous IL-13 because blockade of endogenous IL-13 inhibited lung fibrosis (Fig. 6 Ad and Bh). Thus, OVA- plus IL-18-stimulated Th1 cells or OVA-stimulated Th2 cells induced lung fibrosis by production of IL-13.
Fig. 6.
IL-13-dependent lung fibrosis in Ag- plus IL-18-administered Th1 mice or Ag-administered Th2 mice. Active Th1 mice or Th2 were induced by immunization with OVA/CFA (A) or OVA/alum (B), respectively. Immunized mice were exposed daily to intranasally administered OVA or OVA plus IL-18 with or without sIL-13Rα2 as described in the legend of Fig. 1. At 24 h after final exposure, lungs from each group of mice were prepared, stained with H&E (a, b, e, and f) or Azan–Mallory (c, d, g, and h), and used for histological examination. (Original magnification, ×40.)
To examine further the extent of fibrosis, we measured the lung hydroxyproline content (Table 1). Th1 mice challenged with OVA plus IL-18 significantly increased hydroxyproline content in their lungs, whereas Th1 mice challenged identically but under IL-13 neutralization conditions did not, indicating that IL-18 induces lung fibrosis by acting on Th1 cells to produce IL-13. Furthermore, anti-IFN-γ Ab treatment did not affect lung hydroxyproline content in OVA- plus IL-18-challenged Th1 mice. These results allowed us to conclude that IL-18 induces lung fibrosis by the induction of endogenous IL-13.
Table 1.
Lung hydroxyproline level in active Th1 mice challenged with OVA plus IL-18
| Active Th1 mouse | n | Hydroxyproline, μg per lung |
|---|---|---|
| PBS | 4 | 110.17 ± 6.4 |
| OVA + IL-18 | 4 | 149.9 ± 12.3* |
| OVA + IL-18 + sIL-13Rα2 | 4 | 118.48 ± 3.5 |
| OVA + IL-18 + anti-IFNγ | 4 | 158.83 ± 8.3† |
Active Th1 mice were exposed daily to intranasal administration of PBS (50 μl), OVA (100 μg per 50 μl of PBS), or OVA (100 μg per 50 μl of PBS) plus IL-18 (0.5 μg per 50 μl of PBS) for 3 days. sIL-13Rα2 or anti-IFN-γ antibody was used for IL-13 or IFN-γ blocking, respectively, in vivo. At 24 h after the final exposure, lung total hydroxyproline levels were measured.
*, P < 0.01 (vs. PBS treatment or OVA + IL-18 + sIL-13Rα2 treatment).
†Not significant (vs. OVA + IL-18 treatment).
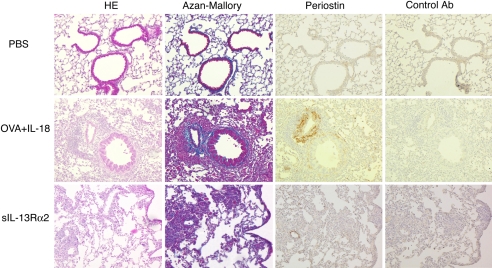
We finally examined the expression of periostin, a novel component of lung fibrosis developing at the early stage of bronchial asthma and colocalizing with the extracellular matrix protein involved in lung fibrosis (30). Induction of periostin is shown to depend on IL-4 and IL-13 but not TGF-β signaling (30). OVA/CFA-primed mice expressed this molecule in response to OVA plus IL-18 challenge (Fig. 7). IL-13 blockade inhibited this expression, suggesting that Th1 cells induce lung fibrosis and periostin deposition by production of IL-13 (Fig. 7). In conclusion, after being stimulated with Ag and IL-18, Th1 cells became very pathological super Th1 cells, which induce AHR and lung fibrosis by production of IFN-γ and IL-13, respectively, in this mouse model of bronchial asthma.
Fig. 7.
Immunohistochemical staining of periostin in the lungs of Th1 mice after exposure to OVA and IL-18. Active Th1 mice were exposed daily to intranasally administered OVA plus IL-18 or OVA plus IL-18 plus sIL-13Rα2 as described in the legend of Fig. 1. At 24 h after final exposure, lungs from each group of mice were fixed and stained with H&E or Azan–Mallory. Immunohistochemical staining for periostin was as described in Material and Methods. (Original magnification, ×40.)
Discussion
We have established passive and active Th1 mice by transferring OVA-specific Th1 cells into or OVA/CFA immunization of naïve BALB/c mice, respectively. Both types of Th1 mice developed AHR and airway inflammation associated with lung fibrosis after intranasal challenge with OVA and IL-18. BALF analysis and histological evaluation revealed that they increased the number of eosinophils, neutrophils, and lymphocytes in their lungs (Figs. 1 and 3). Infiltrations of eosinophils and neutrophils in the lungs are regulated differentially by IL-13 and IFN-γ (Figs. 1 and 3). Results from IL-4Rα−/− mice excluded the potential contribution from IL-13 to Th1-cell-induced AHR (Fig. 4). Results from anti-IFN-γ-treated mice revealed that IFN-γ is a true causative factor responsible for inducing AHR (Fig. 1). OVA plus LPS replaced partly the effect of OVA and IL-18 by the induction of endogenous IL-18 (Fig. 5). We also showed that, like Th2 mice challenged with OVA, Ag- plus IL-18-challenged Th1 mice develop lung fibrosis associated with periostin deposition and increased lung hydroxyproline content, which are induced by the action IL-13 (Table 1 and Figs. 6 and 7). Thus, Th1 cells become very pathological super Th1 cells when stimulated with Ag and IL-18 by the production of IFN-γ and IL-13, which in combination induce AHR, peribronchial inflammation, and lung fibrosis in this mouse model of bronchial asthma.
It is well defined that the immunological situation toward Th1 cell development protects Th2 cell development and vice versa (14–16). Thus, the concept of Th1/Th2 development has been believed to be a dichotomy. However, our previous studies have clearly demonstrated that IL-18 disrupts the biologically reciprocal actions of Th1 and Th2 cells in mice (22, 24, 25). We showed that OVA-specific monoclonal Th1 cells, which we developed in vitro, have the potential to produce Th1 cytokine (IFN-γ) and Th2 cytokine (IL-9, IL-13) as well as chemokines (e.g., RANTES and macrophage inflammatory protein 1α) in response to Ag, IL-2, and IL-18 in vitro (22, 25). Thus, Ag- and IL-18-stimulated Th1 cells produce a similar spectrum of cytokines and chemokines that are produced by activated mast cells. In this study, we first examined whether OVA-specific Th1 cells, which we newly developed in vivo by immunization of mice with OVA/CFA, can produce IFN-γ, IL-9, IL-13, granulocyte–macrophage colony-stimulating factor, and chemokines in response to Ag, IL-2 and IL-18 in vitro. Furthermore, we confirmed that they had such potential. Next, we examined whether OVA/CFA-immunized active Th1 mice developed AHR and airway inflammation after administration of OVA and IL-18. Consistent with our previous report obtained from passive Th1 mice (22), active Th1 mice exposed to OVA alone did not develop AHR (SI Fig. 8B). However, when nasally exposed to OVA and IL-18, both passive and active Th1 mice equally developed AHR and severe airway inflammation (Figs. 1–3). Histological examination revealed that additional IL-18 administration induced massive cell infiltrates composed of eosinophils, lymphocytes, and neutrophils (Fig. 3).
In our previous report, we tried to understand the mechanism by which Th1 cells induced AHR and airway inflammation when they were stimulated with Ag and IL-18 in vivo. We initially envisaged that IL-13 from Ag- plus IL-18-stimulated Th1 cells was a causative factor because IL-13 has been shown to induce AHR in Th2-cell-bearing mice (9–13). However, neutralization of IL-13 did not affect Th1-cell-induced AHR, although this treatment strongly diminished eosinophilic infiltration in the lungs (22) (Figs. 1 and 3). We could not reduce completely BALF levels of eosinophils to that in PBS-treated mice even if we performed daily administration of sIL-13Rα for neutralization of IL-13 (Fig. 1 C and D). Furthermore, the effects of this treatment on lung tissue eosinophils or neutrophils were less effective than those on BALF levels of these cells (Figs. 1 and 3). IL-13 neutralization in BALF might be more efficient than that in lung tissue.
In this study, we formally could exclude the contribution from IL-13 to AHR by showing the capacity of IL-4Rα−/− mice to normally develop AHR after OVA/CFA priming and subsequent OVA/IL-18 challenge (Fig. 4). Because Ag- plus IL-18-stimulated Th1 cells produced IFN-γ, IL-9, and IL-13 but not IL-5, we could assume that eosinophilic infiltration was induced by the action of IL-13. Of interest, compared with WT BALB/c mice, IL-4Rα−/− mice augmented neutrophilic infiltration in their lungs, suggesting that IL-13 down-regulates neutrophilic infiltration but up-regulates eosinophilic infiltration. We recently demonstrated that IL-13 recruits eosinophils in the lungs by the induction of eotaxin from lung epithelial cells (34).
We tried to determine the causative factor. Here, we could demonstrate that IFN-γ from Ag- plus IL-18-stimulatd Th1 cells was responsible for inducing AHR and airway inflammation (Figs. 1 and 3). Neutralization of IFN-γ inhibited AHR and neutrophilic accumulation in BALF and lung tissues. However, at present, we do not know how IFN-γ induces recruitment of neutrophils in the lungs. It is quite reasonable to speculate that IFN-γ induces production of some chemokines, which have the capacity to recruit neutrophils in the lungs.
Bronchial asthma is often induced by viral or bacterial infection (2, 31–33). We tested whether pathogen-associated molecular pattern (LPS) can induce IL-18 production from alveolar macrophage and/or lung epithelial cells. The lung epithelial cell line TGMBE02-3 cells (37) express Toll-like receptor 4 and produce IL-18 upon LPS stimulation in vitro (SI Fig. 9). Furthermore, OVA/CFA-immunized and OVA/LPS-challenged mice increased their serum levels of IL-18 (SI Fig. 10). IL-18 is also involved in human bronchial asthma. Serum levels of IL-18 are elevated in patients with bronchial asthma (SI Fig. 10), and a significant correlation between IL-18 serum levels and the disease severity of bronchial asthma has been reported (38).
In this study, we could demonstrate the unique capacity of OVA-specific Th1 cells to induce AHR after intranasal administration of OVA and LPS. Neutralization of IL-18 or IFN-γ attenuated AHR and decreased the number of neutrophils in BALF. Furthermore, OVA/CFA-immunized IL-18−/− or IFN-γ−/− mice failed to develop AHR upon challenge with OVA and LPS (Fig. 5). These results clearly indicated that endogenous IL-18 played a critical role in induction of this mouse model of bronchial asthma by activation of Th1 cells to produce IFN-γ.
We have shown that IL-13 induces lung fibrosis associated with periostin deposition (Figs. 6 and 7), which binds to extracellular matrix proteins to form a reticular structure (30). Indeed, neutralization of IL-13 inhibited lung fibrosis (Fig. 6), lung hydroxyproline content (Table 1), and periostin deposition (Fig. 7) without inhibiting AHR (Fig. 1). Thus, Ag- plus IL-18-stimulated Th1 cells exhibit two pathological effects on the development of bronchial asthma: one is IFN-γ-induced AHR, and the other is IL-13-induced lung fibrosis. Here, we also demonstrated that LPS induces Th1-cell-induced asthma by the induction of IL-18 production. It is well known that Th2 cell-induced asthma can be controlled by the treatment with anti-Th2 cytokines or anti-IgE (2, 39). However, there are no appropriate treatments for infection- or Th1-cell-induced bronchial asthma, in which super Th1 cells play very pathological roles. Our present results clearly indicated that we could regulate AHR and lung fibrosis by down-regulation of IL-18. Thus, IL-18 becomes rational target for the treatment of Th1-cell-induced bronchial asthma. Because difficult asthma or refractory asthma is often induced by bacterial or viral infection, anti-IL-18 therapy might be applicable for its treatment.
Materials and Methods
Animals and Reagents.
Specific pathogen-free female BALB/c mice and IL-4Rα−/− mice (BALB/c background) were purchased from The Jackson Laboratory (Bar Harbor, ME). BALB/c background IFN-γ−/− mice were kindly provided by Y. Iwakura (University of Tokyo, Tokyo, Japan). BALB/c background IL-1−/− mice were established in our laboratory (40) and used for experimentation. Mice transgenic for αβ T cell antigen receptor recognizing OVA323–339 (DO11.10; BALB/c background) (41) were generously provided by D. Loh (Washington University, St. Louis, MO). All experiments were performed on the line heterozygous for the transgene. All mice were bred under specific pathogen-free conditions at the animal facilities of Hyogo College of Medicine (Nishinomiya, Japan) and were used at 6 to 10 weeks of age. Recombinant mouse IL-12 and IL-18 were purchased from R & D Systems (Minneapolis, MN) and MBL (Nagoya, Japan), respectively. LPS from Salmonella minnesota Re-595 was purchased from Sigma–Aldrich (St Louis, MO). Anti-CD3 (2C11) and anti-IL-4 (11B11) were prepared by Harlan Bioproducts for Science (Indianapolis, IN). Purified anti-mouse IL-18 rabbit serum was prepared in our laboratory.
Generation of Th1 Cells in Vivo.
We induced Th1 cells by immunization of mice with OVA (50 μg) in CFA. Endotoxin contaminated in OVA was removed by END-X 15 (Seikagaku America, Cape Cod, MA). After removal, endotoxin level was <0.5 pg/ml in 1 mg/ml OVA. We used the resultant endotoxin-depleted OVA in all of our experiments.
In Vivo Intranasal Antigen Challenge.
The preparation of passive Th1 mice has been described previously (22, 42). Briefly, 1 × 107 Th1 cells, which we developed in vitro (22, 42), were injected i.v. into normal BALB/c mice (10–15 mice per group). At 4 weeks, or even later, after cell transfer, transplanted Th1 cells expressed the Ag-specific resting Th1 memory phenotype in host mice. Active Th1 mice were prepared by immunization of mice with OVA/CFA. Two weeks after OVA/CFA immunization or 4 weeks after Th1 cell transfer, both types of Th1 mice were exposed to daily intranasal administration of 50 μl of PBS or 50 μl of PBS containing OVA (100 μg) or OVA (100 μg) plus IL-18 (0.5 μg) for 3 days. In some experiments, Th1 mice were exposed to intranasal administration of LPS (5 μg) and OVA (100 μg). Mice were analyzed for their AHR and airway inflammation at 24 h after the final exposure. sIL-13Rα2-Fc chimera (20 μg; R & D Systems) was administered daily intranasally for blocking of IL-13 in vivo as described previously (22). For the blockade of IFN-γ or IL-18 in vivo, 50 μg of anti-IFN-γ or 60 μg of anti-IL-18, as well as its corresponding control Ab (50 μg of rat IgG or 60 μg of rabbit IgG), was administered daily intranasally at the time of OVA challenge.
Noninvasive or Invasive Measurement of AHR.
Noninvasive measurement of specific airway resistance has been described previously (22, 34). Briefly, we measured AHR to β-methacholine inhalation in mice by using Pulmos-I hardware and software (MIPS, Osaka, Japan). We placed a mouse in a chamber and first exposed it to aerosols of saline (baseline) and then to increased concentrations of β-methacholine (5, 10, 15, and 20 mg/ml). After each 2-min exposure, we measured enhanced pause, a dimensionless index that reflects changes in amplitude of pressure waveform and expiratory time (43), for 2 min. Invasive measurement of AHR was assessed as an increase in pulmonary resistance and a decrease in dynamic compliance (Cdyn) in response to aerosolized β-methacholine. Briefly, mechanical ventilation was achieved by using a MiniVent Model 845 ventilator (HSE, March–Hugstetten, Germany). Saline and increased concentrations of β-methacholine were aerosolized as noninvasive measurement. Pulmonary resistance and Cdyn were measured by Pulmos-II (MIPS) hardware and software (MIPS) (44).
Bronchoalveolar Lavage.
Bronchoalveolar lavage was performed with three aliquots of 0.5 ml of PBS per mouse. Total cell counts were performed. Cytospin preparations of BALF were stained with Dif-Quik (Baxter Healthcare, Miami, FL), and differentials were performed based on morphology and staining characteristics.
Histology.
Lungs were prepared for histological examination by perfusion of the animal via the right ventricle with 10 ml of PBS and then were fixed in 10% buffered formalin, cut into 3-mm sections, and stained with H&E for H&E stain or azocarmine G and aniline blue orange G for Azan–Mallory stain. Immunohistochemical staining for periostin has been described previously (30). Briefly, lung tissues were fixed with 10% formalin and incubated with primary Ab or normal IgG (control) overnight. The antigens were detected by the ENVISION+/HRP (DAB) system (Dako Cytomation, Glostrup, Denmark).
Hydroxyproline.
Total hydroxyproline content was measured by HPLC (SRL, Tokyo, Japan).
Supplementary Material
Acknowledgments
We thank Dr. H. Tsutsui of Hyogo College of Medicine for critical reading of the manuscript; Dr. K. Yasuda, Dr. H. Tanaka, and Dr. M. Kuroda of Hyogo College of Medicine for enthusiastic discussion; and Ms. S. Yumikura-Futatsugi and Ms. M. Uemura for excellent technical assistance. This study was supported by a Grant-in-Aid for Scientific Research on Priority Areas and Hitech Research Center from the Ministry of Education, Culture, Sports, Science, and Technology of Japan.
Abbreviations
- Ag
antigen
- AHR
airway hyperresponsiveness
- BALF
bronchoalveolar lavage fluid
- Cdyn
dynamic compliance
- CFA
complete Freund's adjuvant
- OVA
ovalbumin
- Th
T helper.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0706378104/DC1.
References
- 1.Bochner BS, Undem BJ, Lichtenstein LM. Annu Rev Immunol. 1994;12:295–335. doi: 10.1146/annurev.iy.12.040194.001455. [DOI] [PubMed] [Google Scholar]
- 2.Busse WW, Lemanske RJ. N Engl J Med. 2001;344:350–362. doi: 10.1056/NEJM200102013440507. [DOI] [PubMed] [Google Scholar]
- 3.Cohn L, Elias JA, Chupp GL. Annu Rev Immunol. 2004;22:789–815. doi: 10.1146/annurev.immunol.22.012703.104716. [DOI] [PubMed] [Google Scholar]
- 4.Davies DE, Wicks J, Powell RM, Puddicombe SM, Holgate ST. J Allergy Clin Immunol. 2003;111:215–225. doi: 10.1067/mai.2003.128. [DOI] [PubMed] [Google Scholar]
- 5.Elias JA, Lee CG, Zheng T, Ma B, Homer RJ, Zhu Z. J Clin Invest. 2003;111:291–297. doi: 10.1172/JCI17748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Umetsu DT, McIntire JJ, Akbari O, Macaubas C, DeKruyff RH. Nat Immunol. 2002;3:715–720. doi: 10.1038/ni0802-715. [DOI] [PubMed] [Google Scholar]
- 7.Wills-Karp M. Annu Rev Immunol. 1999;17:255–281. doi: 10.1146/annurev.immunol.17.1.255. [DOI] [PubMed] [Google Scholar]
- 8.Nakamura Y, Ghaffar O, Olivenstein R, Taha RA, Soussi-Gounni A, Zhang DH, Ray A, Hamid Q. J Allergy Clin Immunol. 1999;103:215–222. doi: 10.1016/s0091-6749(99)70493-8. [DOI] [PubMed] [Google Scholar]
- 9.Kuperman DA, Huang X, Koth LL, Chang GH, Dolganov GM, Zhu Z, Elias JA, Sheppard D, Erle DJ. Nat Med. 2002;8:885–889. doi: 10.1038/nm734. [DOI] [PubMed] [Google Scholar]
- 10.Wills-Karp M, Luyimbazi J, Xu X, Schofield B, Neben TY, Karp CL, Donaldson DD. Science. 1998;282:2258–2261. doi: 10.1126/science.282.5397.2258. [DOI] [PubMed] [Google Scholar]
- 11.Grunig G, Warnock M, Wakil A, Venkayya R, Brombacher F, Rennick D, Sheppard D, Mohrs M, Donaldson D, Locksley R, et al. Science. 1998;282:2261–2263. doi: 10.1126/science.282.5397.2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhu Z, Homer R, Wang Z, Chen Q, Geba G, Wang J, Zhang Y, Elias J. J Clin Invest. 1999;103:779–788. doi: 10.1172/JCI5909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wynn T. Annu Rev Immunol. 2003;21:425–456. doi: 10.1146/annurev.immunol.21.120601.141142. [DOI] [PubMed] [Google Scholar]
- 14.Cohn L, Homer RJ, Niu N, Bottomly K. J Exp Med. 1999;190:1309–1318. doi: 10.1084/jem.190.9.1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huang TJ, MacAry PA, Eynott P, Moussavi A, Daniel KC, Askenase PW, Kemeny DM, Chung KF. J Immunol. 2001;166:207–217. doi: 10.4049/jimmunol.166.1.207. [DOI] [PubMed] [Google Scholar]
- 16.Iwamoto I, Nakajima H, Endo H, Yoshida S. J Exp Med. 1993;177:573–576. doi: 10.1084/jem.177.2.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ford JG, Rennick D, Donaldson DD, Venkayya R, McArthur C, Hansell E, Kurup VP, Warnock M, Grunig G. J Immunol. 2001;167:1769–1777. doi: 10.4049/jimmunol.167.3.1769. [DOI] [PubMed] [Google Scholar]
- 18.Hansen G, Berry G, DeKruyff RH, Umetsu DT. J Clin Invest. 1999;103:175–183. doi: 10.1172/JCI5155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li L, Xia Y, Nguyen A, Feng L, Lo D. J Immunol. 1998;161:3128–3135. [PubMed] [Google Scholar]
- 20.Randolph DA, Carruthers CJ, Szabo SJ, Murphy KM, Chaplin DD. J Immunol. 1999;162:2375–2383. [PubMed] [Google Scholar]
- 21.Randolph DA, Stephens R, Carruthers CJ, Chaplin DD. J Clin Invest. 1999;104:1021–1029. doi: 10.1172/JCI7631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sugimoto T, Ishikawa Y, Yoshimoto T, Hayashi N, Fujimoto J, Nakanishi K. J Exp Med. 2004;199:535–545. doi: 10.1084/jem.20031368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yoshimoto T, Takeda K, Tanaka T, Ohkusu K, Kashiwamura S, Okamura H, Akira S, Nakanishi K. J Immunol. 1998;161:3400–3407. [PubMed] [Google Scholar]
- 24.Hata H, Yoshimoto T, Hayashi N, Hada T, Nakanishi K. Int Immunol. 2004;16:1733–1739. doi: 10.1093/intimm/dxh174. [DOI] [PubMed] [Google Scholar]
- 25.Terada M, Tsutsui H, Imai Y, Yasuda K, Mizutani H, Yamanishi K, Kubo M, Matsui K, Sano H, Nakanishi K. Proc Natl Acad Sci USA. 2006;103:8816–8821. doi: 10.1073/pnas.0602900103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Okamura H, Tsutsi H, Komatsu T, Yutsudo M, Hakura A, Tanimoto T, Torigoe K, Okura T, Nukada Y, Hattori K, et al. Nature. 1995;378:88–91. doi: 10.1038/378088a0. [DOI] [PubMed] [Google Scholar]
- 27.Nakanishi K, Yoshimoto T, Tsutsui H, Okamura H. Annu Rev Immunol. 2001;19:423–474. doi: 10.1146/annurev.immunol.19.1.423. [DOI] [PubMed] [Google Scholar]
- 28.Tsutsui H, Yoshimoto T, Hayashi N, Mizutani H, Nakanishi K. Immunol Rev. 2004;202:115–138. doi: 10.1111/j.0105-2896.2004.00205.x. [DOI] [PubMed] [Google Scholar]
- 29.Dinarello C, Novick D, Puren A, Fantuzzi G, Shapiro L, Muhl H, Yoon D, Reznikov L, Kim S, Rubinstein M. J Leukoc Biol. 1998;63:658–664. [PubMed] [Google Scholar]
- 30.Takayama G, Arima K, Kanaji T, Toda S, Tanaka H, Shoji S, McKenzie A, Nagai H, Hotokebuchi T, Izuhara K. J Allergy Clin Immunol. 2006;118:98–104. doi: 10.1016/j.jaci.2006.02.046. [DOI] [PubMed] [Google Scholar]
- 31.Cook DN, Pisetsky DS, Schwartz DA. Nat Immunol. 2004;5:975–979. doi: 10.1038/ni1116. [DOI] [PubMed] [Google Scholar]
- 32.Eisenbarth SC, Piggott DA, Huleatt JW, Visintin I, Herrick CA, Bottomly K. J Exp Med. 2002;196:1645–1651. doi: 10.1084/jem.20021340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gern JE, Busse WW. Nat Rev Immunol. 2002;2:132–138. doi: 10.1038/nri725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ishikawa Y, Yoshimoto T, Nakanishi K. Int Immunol. 2006;18:847–855. doi: 10.1093/intimm/dxl021. [DOI] [PubMed] [Google Scholar]
- 35.Lee C, Homer R, Zhu Z, Lanone S, Wang X, Koteliansky V, Shipley J, Gotwals P, Noble P, Chen Q, et al. J Exp Med. 2001;194:809–821. doi: 10.1084/jem.194.6.809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Booth B, Adler K, Bonner J, Tournier F, Martin L. Am J Respir Cell Mol Biol. 2001;25:739–743. doi: 10.1165/ajrcmb.25.6.4659. [DOI] [PubMed] [Google Scholar]
- 37.Sugiyama N, Tabuchi Y, Numata F, Uchida Y, Horiuchi T, Ishibashi K, Ono S, Obinata M, Furusawa M. Cell Struct Funct. 1998;23:119–127. doi: 10.1247/csf.23.119. [DOI] [PubMed] [Google Scholar]
- 38.Tanaka H, Miyazaki N, Oashi K, Teramoto S, Shiratori M, Hashimoto M, Ohmichi M, Abe S. J Allergy Clin Immunol. 2001;107:331–336. doi: 10.1067/mai.2001.112275. [DOI] [PubMed] [Google Scholar]
- 39.Holgate S, Djukanovic R, Casale T, Bousquet J. Clin Exp Allergy. 2005;35:408–416. doi: 10.1111/j.1365-2222.2005.02191.x. [DOI] [PubMed] [Google Scholar]
- 40.Takeda K, Tsutsui H, Yoshimoto T, Adachi O, Yoshida N, Kishimoto T, Okamura H, Nakanishi K, Akira S. Immunity. 1998;8:383–390. doi: 10.1016/s1074-7613(00)80543-9. [DOI] [PubMed] [Google Scholar]
- 41.Murphy KM, Heimberger AB, Loh DY. Science. 1990;250:1720–1723. doi: 10.1126/science.2125367. [DOI] [PubMed] [Google Scholar]
- 42.Hayashi N, Liu D, Min B, Ben SS, Paul WE. Proc Natl Acad Sci USA. 2002;99:6187–6191. doi: 10.1073/pnas.092129899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hamelmann E, Schwarze J, Takeda K, Oshiba A, Larsen GL, Irvin CG, Gelfand EW. Am J Respir Crit Care Med. 1997;156(3 Pt 1):766–775. doi: 10.1164/ajrccm.156.3.9606031. [DOI] [PubMed] [Google Scholar]
- 44.Giles RE, Finkel MP, Mazurowski J. Arch Int Pharmacodyn Ther. 1971;194:213–222. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.