Abstract
‘Naked’ nucleic acid vaccines are potentially useful candidates for the treatment of patients with cancer1-3, but their clinical efficacy has yet to be demonstrated. We sought to enhance the immunogenicity of a nucleic acid vaccine by making it ‘self-replicating’. We accomplished this by using a gene encoding an RNA replicase polyprotein derived from the Semliki forest virus, in combination with a model antigen. A single intramuscular injection of a self-replicating RNA immunogen elicited antigen-specific antibody and CD8+ T-cell responses at doses as low as 0.1 μg. Pre-immunization with a self-replicating RNA vector protected mice from tumor challenge, and therapeutic immunization prolonged the survival of mice with established tumors. The self-replicating RNA vectors did not mediate the production of substantially more model antigen than a conventional DNA vaccine did in vitro. However, the enhanced efficacy in vivo correlated with a caspase-dependent apoptotic death in transfected cells. This death facilitated the uptake of apoptotic cells by dendritic cells, providing a potential mechanism for enhanced immunogenicity. Naked, non-infectious, self-replicating RNA may be an excellent candidate for the development of new cancer vaccines.
To enhance the efficacy of nucleic acid vaccines, we explored the use of ‘self-replicating’ RNA as a cancer vaccine. These RNA vaccines replicate through the activity of a replicase derived from the alphavirus Semliki forest virus (SFV). The RNA immunogens were produced by in vitro transcription and capping using a variety of DNA templates (Fig. 1a). The RNA replicase encoded by Rep-LacZ and Rep-empty mediate the production of an RNA replicase polyprotein that cleaves itself (ultimately into four subunits) to form the mature replicase complex. This complex drives the replication of viral genomic as well as subgenomic RNA that encodes the structural proteins of the viral coat4. We chose β-galactosidase (β-gal) as the model antigen for these initial studies because of the ease and specificity of the X-gal assay and because of previous experimental work with β-gal as a model tumor antigen5.
Fig. 1.
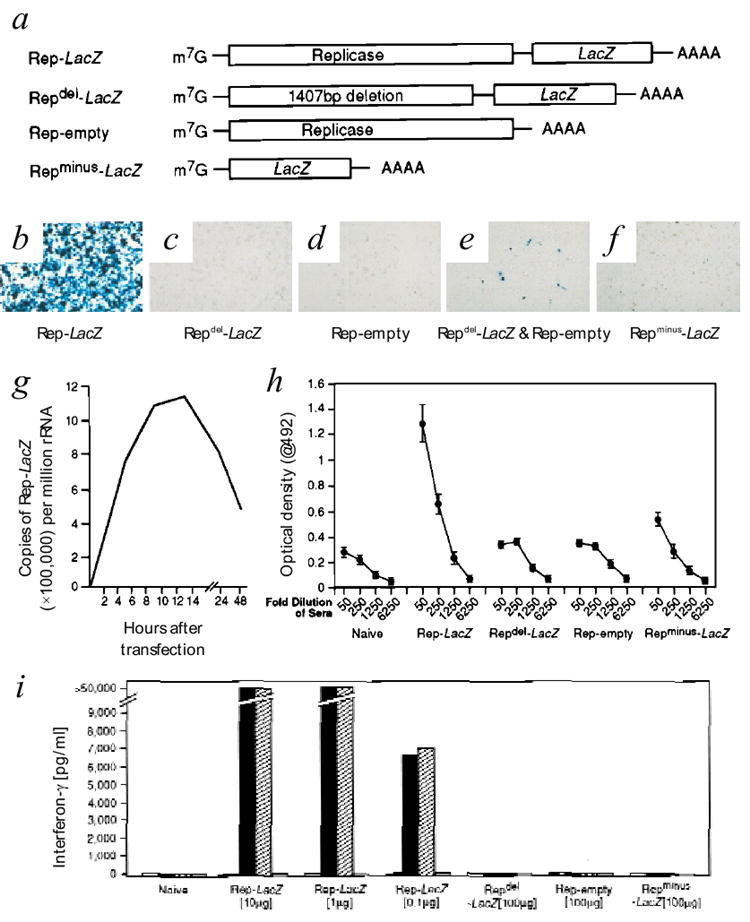
Structure, function and immunogenicity of self-replicating RNA vaccines. a, RNA constructs used for immunization. m7G, ‘cap’ at the 5′ end of the mRNA. b–f, RNA constructs transcribed and capped in vitro were transfected into BHK21 cells; 24 h later, cells were tested for β-gal expression using the X-gal assay. Data are representative of three independent experiments with similar results. g, Quantitative analysis of RNA replication using TaqMan real-time RT–PCR. Total cellular RNA was isolated at 6, 10, 14, 24 and 48 h after transfection with the Rep-LacZ RNA. Using vector-specific primers, the copy number of the mRNA Rep-LacZ was then quantified relative to 18s ribosomal RNA. This experiment was done three independent times with similar results. h, Induction of antigen-specific antibody responses in mice by self-replicating RNA vaccines. Sera obtained from mice 21 d after immunization with RNA were tested by ELISA for the presence of IgG antibodies against the recombinant β-gal protein. i, Induction of CD8+ T-cell responses in mice by self-replicating RNA vaccines. Splenocytes obtained from mice 21 d after immunization with RNA were re-stimulated in vitro in the presence of β-gal876-884, then cultures were assayed for β-gal-specific CD8+ T-cell recognition by measuring IFN-γ release after exposure to CT26 alone (□) or pulsed with the synthetic peptides derived from β-gal876-884 (▨) or P815A35-43 (
 ) or transduced with a retrovirus encoding LacZ (■).
) or transduced with a retrovirus encoding LacZ (■).
To evaluate the function of the RNA immunogens, we tested for the production of β-gal. By X-gal staining, the Rep-LacZ RNA construct produced a large amount of β-gal (Fig. 1b), whereas BHK21 cells transfected with Repdel-LacZ RNA or Rep-empty RNA did not produce measurable levels of β-gal (Fig. 1c and d). To further test the function of the Repdel-LacZ and Rep-empty, we co-transfected both RNAs into BHK21 cells. β-gal expression was intensely expressed by approximately 1% of the cells, demonstrating that the Repdel-LacZ construct encoded functional β-gal (but not replicase) and Rep-empty encoded functional replicase complex (but not β-gal)(Fig. 1e). Finally, transfection with Repminus-LacZ RNA yielded a faint blue stain, probably due to the activity of the transfected positive-stranded RNA itself (Fig. 1f).
To quantify the actual replication, as evidenced by the intense blue stain of cells transfected with Rep-LacZ, we performed real-time, quantitative RT–PCR using the TaqMan system (Fig. 1g). There was substantial amplification of the Rep-LacZ RNA. Indeed, 10 hours after transfection, the amount of RNA measured in transfected BHK21 cells was equal to that of the 18s rRNA (Fig. 1g, relative expression values).
To assess whether an antigen-specific antibody response could be induced, we tested sera from mice immunized with RNA vaccines by ELISA for β-gal-specific antibodies. A single injection of the RNA Rep-LacZ was sufficient to elicit a β-gal-specific antibody response, whereas mice immunized with the control RNAs Repdel-LacZ, Rep-empty or Repminus-LacZ did not develop β-gal-specific antibody responses above the threshold of detection by our assay (Fig 1h).
To test for the elicitation of β-gal-specific CD8+ T-cell responses, we immunized mice as in Fig. 1h. Mice immunized with a single dose of RNA Rep-LacZ developed CD8+ T cells that specifically recognized target cells even when as little as 0.1 μg of Rep-LacZ RNA was used for immunization (Fig. 1i). To our knowledge, such immunogenicity after a single intramuscular injection has not been reported using conventional ‘naked’ DNA (or RNA) immunization. A single immunization of mice with as much as 100 μg of control RNA was not effective at eliciting β-gal-specific, CD8+ T-cell responses.
To determine if the immune response we measured correlates with anti-tumor activity, we assessed the efficacy of self-replicating RNA in preventing antigen-expressing tumor in vivo. Mice were challenged with tumor 21 days after immunization. As little as 1 μg of Rep-LacZ RNA consistently mediated complete tumor protection. In contrast, little or no protection was produced in mice immunized with Repminus-LacZ, Repdel-LacZ or Rep-empty RNA, even when doses of 100 μg were used (Fig. 2a). In an active immunotherapy model in which tumor was given 2 days before immunization, mice treated with Rep-LacZ RNA survived an average of 10–20 days longer than those treated with Repdel-LacZ, which had no measurable therapeutic effect (Fig. 2b). In addition, the number of pulmonary metastases in a parallel experiment was significantly lower in mice immunized with Rep-LacZ RNA than in mice immunized with Repdel-LacZ (Fig. 2c).
Fig. 2.
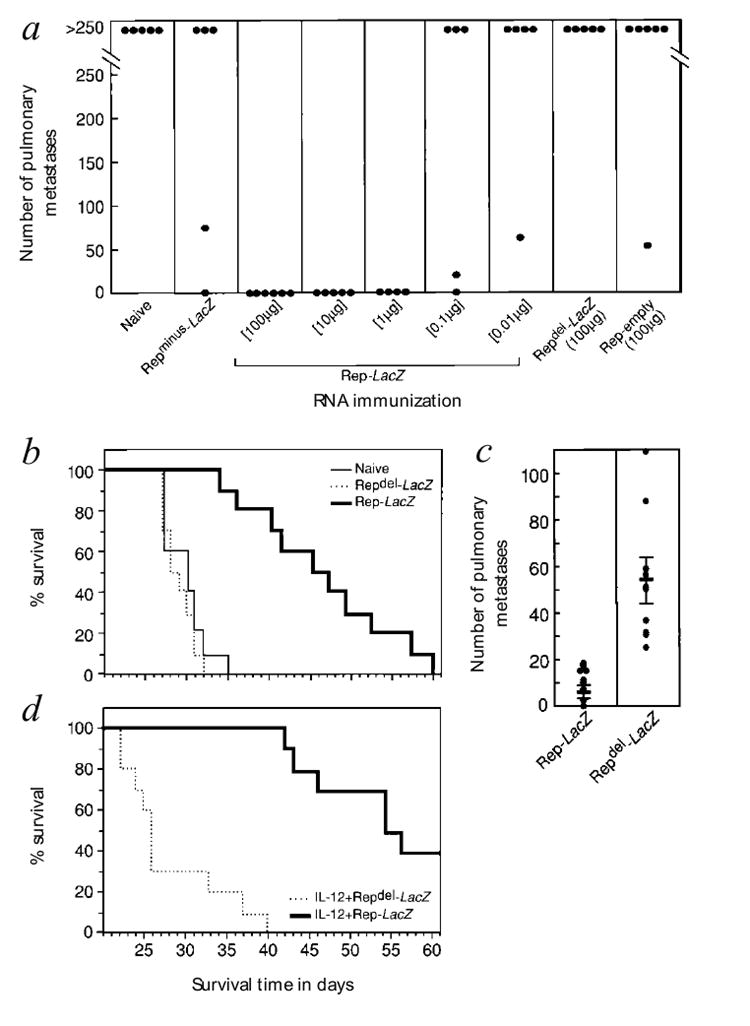
Anti-tumor effects mediated by self-replicating RNA vaccines. a, Self-replicating RNA immunization protects mice from intravenous tumor challenge. Mice immunized with RNA vaccines were injected 21 d later with CT26.CL25 cells, then pulmonary metastases were counted 14 d after tumor challenge. Identically immunized mice were not protected from challenge with the β-gal-negative, parental CT26 (not shown). P < 0.001, Rep-LacZ (100 μg or 10 μg) compared with Repminus-LacZ, Repdel-LacZ or Rep-empty; P < 0.002, Rep-LacZ (1 μg) compared with Repminus-LacZ, Repdel-LacZ or Rep-empty, Kruskal-Wallis test. This experiment was repeated three times with similar results. b, Self-replicating RNA vaccine treats established CT26.CL25 tumor. Mice with established (2-day) CT26.CL25 tumors were immunized with RNA and assessed for survival. P < 0.001, Rep-LacZ compared with naive or Rep-LacZ compared with Repdel-LacZ, Mantel-Haenszel test. c, A separate group of mice from the experiment in b were evaluated 12 d after RNA vaccination for pulmonary metastases. P < 0.001, Rep-LacZ compared with Repdel-LacZ, Kruskal-Wallis test. d, The effect of adjuvant IL-12 on the survival of mice treated with self-replicating RNA. Mice with established (2-day) CT26.CL25 tumors were immunized with RNA, then given IL-12 and assessed for survival. P < 0.0001, Rep-LacZ +IL-12 compared with Repdel-LacZ +IL-12; P < 0.0022, Rep-LacZ +IL-12 compared with Rep-LacZ, Mantel-Haenszel test. Experiments in b, c and d were repeated with similar results.
Studies of the efficacy of cancer vaccines delivered alone6,7 or with cytokines, especially IL-12 (refs. 2 and 8) have demonstrated that cytokines such as IL-12 can considerably enhance the efficacy of nucleic acid vaccines2. Indeed, the combination of Rep-LacZ immunization and IL-12 significantly prolonged the survival of treated mice, with 40% surviving more than 90 days (Fig. 2d).
The rationale for expressing antigen under the control of an alphavirus replicon was to enhance antigen expression by enabling RNA self-replication. To test the relative levels of the model antigen in transfected BHK21 cells, we evaluated β-gal protein production by ELISA, correcting for transfection efficiency. The self-replicating Rep-LacZ RNA induced the production of 211 ng of β-gal protein per μg of total cellular protein, compared with 90 ng of β-gal per μg produced by cells transfected with a control CMV promoter-based DNA plasmid. Thus, the self-replicating RNA produced only slightly more than 200% the amount of antigen produced by a conventional DNA immunogen. Although intramuscular injection of 1 μg of the conventional CMV promoter-based DNA plasmid elicited a minimal immune response (data not shown), an equivalent amount of self-replicating RNA was very immunogenic. Therefore, it seemed unlikely that the substantial enhancement of immunogenicity of the self-replicating vector was merely due to enhanced antigen production.
The cytopathic effect of infection with alphaviruses or transfection with a SFV-based DNA construct has been described9. To assess the effects of self-replicating RNA on the survival of transfected cells, we constructed a vector expressing enhanced green fluorescence protein (EGFP) under the control of SFV-replicase (Rep-EGFP). After being transfected with Rep-EGFP RNA, cells rounded up, shrunk and stopped dividing within 24 hours. Nearly all of the cells died by 96 hours (Fig. 3a). In contrast, BHK21 cells transfected with a conventional DNA plasmid encoding EGFP (pEGFP-C1) remained nearly as adherent and proliferative as non-transfected control cells (Fig. 3a). RNA encoding EGFP but no replicase had no effects on the cell growth (not shown).
Fig. 3.
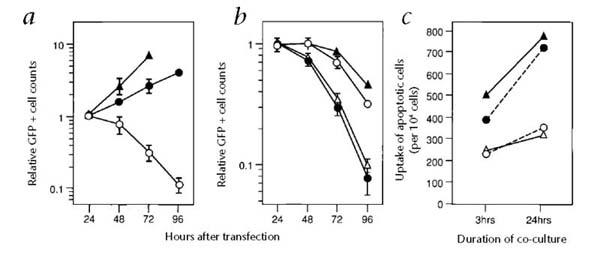
Induction of apoptotic death of host a cells by self-replicating RNA and its effect on the uptake by dendritic cells. a, Self-replicating RNA induces host cell death. RNA transcribed in vitro from plasmid pRep-EGFP (○) was transfected into BHK21 cells; a control plasmid containing the EGFP gene under the control of the CMV promoter (●) was also transfected into BHK21 cells. GFP-positive cells were sorted by FACS, then counted every 24 h to assess their survival. Filled triangles, GFP-negative cells. Relative GFP + cell counts b, Prevention of apoptosis by caspase inhibitors. BHK21 cells were transfected with Rep-EGFP RNA in the absence (●) or presence of peptide caspase inhibitors z-VAD (○), BAF (▲) or z-FA (△; control). The total number of green cells was assessed every 24 h. c, Uptake of RNA-induced apoptotic cells by dendritic cells. Unmanipulated (○) or Rep-EGFP RNA-transfected (▲) BHK21 cells were labeled with PKH26-GL, then fed to immature den-dritic cells. Identically prepared groups of cells were evaluated in the presence of z-VAD (△) or z-FA (●; control). Cells were evaluated at 3 and 24 h using FACS analysis for PKH (red) and I-Ab (green). Double-positive cells were counted as a fraction of total FACS events. This experiment was repeated with similar results.
Cells transfected with Rep-EGFP RNA remained intensely bright during the onset of the cytopathic effect, indicating the loss of EGFP-positive cells was not merely due to dilution. At 48 hours, these cells had nuclear fragmentation characteristic of apoptosis after staining with 4’, 6-diamidino-2-phenylindole (data not shown).
To more rigorously explore if the cell death associated with the function of the replicon was in fact apoptotic death, we used caspase inhibitors. Caspases are an ICE/Ced3 family of cysteine endoproteases known to mediate signals that direct many of the proteolytic events that occur during apoptosis10. We used peptide analogs capable of specifically inhibiting caspase activity, including z-VAD and its truncated analog BAF (refs. 11,12). As a control, we used a chemically related compound called z-FA. Caspase inhibitors substantially delayed cell death (Fig. 3b). However, all transfected cells eventually died, even in the presence of caspase inhibitors, indicating that the blockade of caspase activity was ‘leaky’, that caspase-independent death pathways were engaged or that host metabolism was disrupted by the activity of the RNA replicase. Nonetheless, much of the loss of cells after transfection with the self-replicating RNA was due to caspase-dependent apoptotic cell death.
Replicase-based RNA vaccines thus seemed to be much more immunogenic than non-replicase-containing RNA vaccines. Moreover, they immunized mice at doses far lower than those previously reported for conventional DNA vaccines. Nonetheless, the levels of antigen that were produced did not reflect this difference in efficacy. Other work with alphavirus replicase-based immunizations has demonstrated a similar discordance between immunogenicity and antigen production9,13. One clue to a solution of this enigma was the observed apoptotic cell death.
The immunologic implications of some forms of apoptotic death have been explored by demonstration of enhanced uptake of apoptotic cells by dendritic cells in vitro14. We sought to determine whether the induction of apoptosis by the activity of the RNA replicase was associated with increased uptake of dead and dying cells by ‘professional’ antigen-presenting cells. We generated a population of bone marrow-derived dendritic cells as described15. We used ‘immature’ dendritic cells, as they retain their phagocytic activity. BHK21 cells were labeled with PKH26-GL, a non-toxic, red fluorescent marker that is incorporated into the cell membrane. The baseline uptake of labeled BHK21 cells was considerably increased after transfection with the Rep-LacZ RNA (Fig 3c). However, this increased uptake was completely abrogated by the addition of z-VAD, but not the control z-FA.
Given the observations described here, one of the mechanisms underlying the enhanced immunogenicity of self-replicating RNA vaccines may be the enhanced uptake of antigen by dendritic cells and other ‘professional’ antigen-presenting cells of cells that die apoptotically as a result of the replicase activity. Production of many immunostimulatory signals may also be elicited by the presence of requisite double-stranded (ds) RNA intermediates that are formed as a result of replicase activity. For example, in the presence of type I interferons, dsRNA is a potent activator of 2’, 5′-oligoadenylate (2-5A) synthetase and the serine/threonine protein kinase. The 2-5A synthetase produces 2’, 5′-linked oligoadenylates, which activate RNaseL. RNaseL degrades both viral and cellular RNA, predisposing the cell to apoptosis. Activation of the serine/threonine protein kinase-RNA activated pathway mediated by dsRNA (ref. 16) shuts off protein synthesis, arrests the cell cycle, predisposes cells to die by apoptosis and increases the expression of proteins that are involved in antigen processing and presentation, including major histocompatibility complex, transporter associated with antigen processing and large multifunctional proteosome component molecules. Moreover, dsRNA has direct effects on dendritic cells, increasing their expression of major histocompatibility complex molecules and inducing their rapid maturation17.
Transfection of cells with a replicase-based RNA vaccine may mimic the activity of alphaviruses (such as a Sindbis virus), which are known to trigger a series of host defense responses. The virus-like RNA replication elevates RNA copy number and antigen production. Simultaneously, dsRNA intermediates are formed that induce host cell death and also result in the production of other molecular ‘danger signals’. These factors could account for the greatly enhanced immunogenicity of self-replicating, RNA-based immunogens.
These data demonstrate a powerful new tool that may be useful in the therapeutic immunization of patients with cancer and infectious diseases. ‘Naked’, non-infectious, self-replicating vaccines are potentially useful for many reasons. There seems to be little, if any, humoral immune response directed against ‘naked’ nucleic acid, making multiple immunizations possible. This is particularly important because the efficacy of vaccines based on recombinant viruses like vaccinia and adenovirus in murine models18,19, are often compromised in human patients because of pre-existing neutralizing antibodies because of prior immunization or environmental exposure20.
The self-replicating RNA constructs described here are also safe. ‘Naked’, infectious RNA induces protective immunity against flavivirus infection with less than 1 ng RNA administered by gene gun21. However, safety issues remain a concern because infectious RNA could result in reversion mutants22. Alphavirus replicon-based RNA vaccines, in contrast, are non-infectious, and reversion mutants cannot occur because the constructs do not contain any structural proteins. Because self-replicating RNA does not go through a DNA intermediate, there would be little chance for genomic integration, potentially allowing for immunization with antigens such as oncogene products. Finally, self-replicating RNA uniformly induced caspase-dependent apoptotic death in transfected cells, which represents an additional measure of safety. ‘Naked’, non-infectious, self-replicating RNA could serve as a powerful tool in anticancer immunization.
Methods
Tumor cell lines, peptides and animals
CT26.WT, a clone of the N-nitroso-N-methylurethane-induced BALB/c (H-2d) undifferentiated colon carcinoma, and CT26.CL25, which expresses the model antigen β-gal as a result of its transduction with a retrovirus containing LacZ, have been described5. Both cell lines were maintained in complete medium, and CT26.CL25 was grown in the presence of 400 μg/ml G418 (Life Technologies) as described5. The synthetic peptide TPHPARIGL, representing a naturally processed H-2 Ld-restricted peptide consisting of amino-acid residues 876–884 of β-gal, and the peptide LPYLGWLVF, representing residues 35–43 of the P815A protein, were synthesized and made more than 99% pure by Peptide Technologies (Washington, DC). Female BALB/c mice, 6–10 weeks old, were obtained from the Animal Production Colonies, Frederick Cancer Research Facility, NIH (Frederick, Maryland).
Construction of plasmids encoding self replicating RNA
The plasmid SFV3-LacZ contains a SP6 promoter, a 7-kb fragment encoding the SFV RNA replicase, and a subgenomic promoter that is bound by the RNA replicase to synthesize large quantity of subgenomic RNA. The LacZ gene was located in the 5′ region of the subgenomic promoter. The plasmid pRep-LacZ was constructed by inserting an SphI fragment containing the CMV immediate early promoter/enhancer into the SphI site of SFV3-LacZ (Life Technologies). The CMV promoter/enhancer fragment was inserted so that the resulting plasmid could potentially be used for DNA immunization studies similar to those reported9,13. An additional SpeI site was inserted 5′ of the SP6 promoter together with the CMV promoter/enhancer fragment. Digestion of the plasmid with SpeI generates a DNA fragment that contains only the SFV RNA replicase and β-gal coding sequences without sequences encoding ampicillin-resistant β-lactamase, which is used as the template for in vitro transcription of Rep-LacZ RNA. The primers used for generating a PCR fragment containing the CMV promoter/enhancer from pCR3.1 plasmid (Invitrogen, Carlsbad, California) were: 5′ primer, 5′–ACATGCATGCACTAGTGCGCGCGTTGACATTGATTA–3′ (SpeI site (underlined) added for subsequent linearization) and 3′ primer, 5′–ACATG-CATGCATGTGAGAGCTCTGCTTATATAGACC–3′. pRepdel-LacZ was created by cleavage of the Rep-LacZ plasmid with XhoI and BglII (creating a 1407-bp deletion) which was then end-filled and self-ligated. pRep-EGFP was constructed by replacing the LacZ fragment in pRep-LacZ with EGFP fragment from the plasmid pEGFP-C1pEGFP-C1 (Clontech, Palo Alto, California).
In vitro transcription
SpeI was used to linearize DNA templates for the synthesis of Rep-LacZ, Repdel-LacZ and the Rep-empty RNA. The SFV1 plasmid (Life Technologies) was used to synthesize Rep-empty RNA, as it contains sequences encoding the replicase but not β–gal. The RNA Repminus-LacZ was in vitro transcribed using pCMV-Sport-β–gal (Life Technologies) as a template. RNA vaccines were transcribed in vitro and capped using SP6 RNA polymerase and capping analog from Life Technologies. The functional utility of the transcripts was assessed by transfection of the RNA into BHK21 cells using lipofactamine (Life Technologies). β-gal expression was analyzed by X-gal staining and by ELISA (Boehringer).
Real-time quantitative RT–PCR
Quantitative analysis of RNA replication in BHK21cells used the ABI prism 7700 Sequence Detection System (Perkin-Elmer, Foster City, California) as described23. Thermal cycler parameters include 2 min at 50 °C, 10 min at 95 °C, and 40 cycles comprising denaturation at 95 °C for 15 s and annealing/extension at 60 °C for 1 min. Samples were normalized by dividing the copies of the RNA of interest by copies of the reference RNA derived from the 18s ribosomal RNA using primers and probes purchased from Perkin-Elmer (TaqMan Ribosomal RNA Control Reagents; Perkin-Elmer, Foster City, California). The primers and the probe for Rep-EGFP were: 5′ primer, 5′–GTCCGCCCTGAGCAAAGA–3′; 3′ primer, 5′–TCACGAACTCCAGCAGGACC–3′; TaqMan probe, 5′–CC-CAACGAGAAGCGCGATCACA–3′.
Cell death analysis
The self-replicating RNA was transcribed in vitro from the pRep-EGFP plasmid, then transfected into BHK21 cells. As a control, the plasmid pEGFP-C1 (Clontech, Palo Alto, California) was also transfected into BHK21 cells. The GFP-positive (green) and -negative cells were sorted and plated into 96-well plates (100–200 cells per well; 5–10 wells per group) then counted every 24 h. Caspase inhibition experiments were done by plating BHK21 cells transfected with 2 μg/well at 1,000 cells/well in 96-well plates (5 wells per group). Where indicated, 20 μM of the following caspase inhibitors were added: Z-Val-Ala-Asp-CH2F (z-VAD), BOC-Asp-CH2F (BAF) or Z-Phe-Ala-CH2F (z-FA; control) (Enzyme Systems, Dublin, California). The total number of fluorescent cells was assessed every 24 h, and the cells were followed up to 96 h. In uptake experiments, BHK21 cells were trans-fected with Rep-LacZ RNA, then labeled 24 h later with PKH26-GL (Sigma). Labeled cells (5 ×105) were co-cultured with 5 ×105 bone marrow-derived immature dendritic cells prepared by culture of bone marrow cells for 4 d in the presence of 20 ng/ml murine granulocyte–monocyte colony-stimulating factor and 40 ng/ml IL-4 (both from Peprotech, Rocky Hill, New Jersey) as described15. Cells were then stained with FITC-labeled anti-I-Ab mAb (PharMingen, La Jolla, California) and analyzed by fluorescence-activated cell sorting (FACS).
Assessment of responses in mice to self-replicating RNA vaccines
BALB/c mice were injected intramuscularly with 100 μg RNA. Then, 21 d after the immunization, sera were tested by ELISA for the presence of IgG antibodies against the recombinant β-gal protein as described2. Splenocytes obtained 21 d after immunization were re-stimulated in vitro for 6 d in the presence of 1 μg/ml of a Ld-restricted peptide, β-gal876-884. Cultures were then assayed for β-gal-specific CD8+ T cell recognition by measuring IFN-γ release after exposure to the following targets: CT26 alone or pulsed with the synthetic peptides derived from β-gal876-884 or P815A35-43 or transduced with a retrovirus encoding LacZ (CT26.CL25) as described24.
Intravenous tumor experiments
BALB/c mice were immunized intramuscularly with RNA vaccines, then 21 d after immunization, mice were injected intravenously with 5 × 105 CT26.CL25 cells. Then 12 d later, pulmonary metastases were counted by experimenters ‘blinded’ to sample identity, as described 24. For experiments with established CT26.CL25 tumor, BALB/c mice were injected intravenously with 1 × 105 CT26.CL25 cells, and tumor was allowed to establish itself for 2 d. Mice were then therapeutically immunized with 100 μg RNA, then assessed for survival. Alternatively, 0.1 μg IL-12 (Genetics Institute, Cambridge, Masssachusetts) was administered intravenously once daily for 3 d starting 12 h after the therapeutic immunization and survival was assessed.
Acknowledgments
The authors thank S.A. Rosenberg for reading the manuscript and for discussions, A. Atwood for help with vector construction, T. Dubensky (Chiron) for advice, M. Blalock for graphics, P. Spiess for help with animal experiments, and S. Wolf (Genetics Institute) for his gift of rmIL-12.
References
- 1.Liu MA, Fu TM, Donnelly JJ, Caulfield MJ, Ulmer JB. DNA vaccines. Mechanisms for generation of immune responses. Adv Exp Med Biol. 1998;452:187–191. [PubMed] [Google Scholar]
- 2.Irvine KR, Rao JB, Rosenberg SA, Restifo NP. Cytokine enhancement of DNA immunization leads to effective treatment of established pulmonary metastases. J Immunol. 1996;156:238–245. [PMC free article] [PubMed] [Google Scholar]
- 3.Gurunathan S, et al. CD40 ligand/trimer DNA enhances both humoral and cellular immune responses and induces protective immunity to infectious and tumor challenge. J Immunol. 1998;161:4563–4571. [PMC free article] [PubMed] [Google Scholar]
- 4.Atkins GJ, Sheahan BJ, Liljestrom P. Manipulation of the Semliki Forest virus genome and its potential for vaccine construction. Mol Biotechnol. 1996;5:33–38. doi: 10.1007/BF02762410. [DOI] [PubMed] [Google Scholar]
- 5.Wang M, et al. Active immunotherapy of cancer with a nonreplicating recombinant fowlpox virus encoding a model tumor-associated antigen. J Immunol. 1995;154:4685–4692. [PMC free article] [PubMed] [Google Scholar]
- 6.Rosenberg SA, et al. Immunologic and therapeutic evaluation of a synthetic peptide vaccine for the treatment of patients with metastatic melanoma. Nature Med. 1998;4:321–327. doi: 10.1038/nm0398-321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Overwijk WW, et al. Vaccination with a recombinant vaccinia virus encoding a “self” antigen induces autoimmune vitiligo and tumor cell destruction in mice: requirement for CD4(+) T lymphocytes. Proc Natl Acad Sci USA. 1999;96:2982–2987. doi: 10.1073/pnas.96.6.2982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rao JB, et al. IL-12 is an effective adjuvant to recombinant vaccinia virus-based tumor vaccines: enhancement by simultaneous B7-1 expression. J Immunol. 1996;156:3357–3365. [PMC free article] [PubMed] [Google Scholar]
- 9.Berglund P, Smerdou C, Fleeton MN, Tubulekas I, Liljestrom P. Enhancing immune responses using suicidal DNA vaccines. Nature Biotechnol. 1998;16:562–565. doi: 10.1038/nbt0698-562. [DOI] [PubMed] [Google Scholar]
- 10.Miller DK. The role of the Caspase family of cysteine proteases in apoptosis. Semin Immunol. 1997;9:35–49. doi: 10.1006/smim.1996.0058. [DOI] [PubMed] [Google Scholar]
- 11.Chappell DB, Zaks TZ, Rosenberg SA, Restifo NP. Human melanoma cells do not express Fas (Apo-1/CD95) ligand. Cancer Res. 1999;59:59–62. [PMC free article] [PubMed] [Google Scholar]
- 12.Zaks TZ, Chappell DB, Rosenberg SA, Restifo NP. Fas-mediated suicide of tumor-reactive T cells following activation by specific tumor: selective rescue by caspase inhibition. J Immunol. 1999;162:3273–3279. [PMC free article] [PubMed] [Google Scholar]
- 13.Hariharan MJ, et al. DNA immunization against herpes simplex virus: enhanced efficacy using a Sindbis virus-based vector. J Virol. 1998;72:950–958. doi: 10.1128/jvi.72.2.950-958.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Albert ML, Sauter B, Bhardwaj N. Dendritic cells acquire antigen from apoptotic cells and induce class I- restricted CTLs. Nature. 1998;392:86–89. doi: 10.1038/32183. [DOI] [PubMed] [Google Scholar]
- 15.Specht JM, et al. Dendritic cells retrovirally transduced with a model antigen gene are therapeutically effective against established pulmonary metastases. J Exp Med. 1997;186:1213–1221. doi: 10.1084/jem.186.8.1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Der SD, Yang YL, Weissmann C, Williams BR. A double-stranded RNA-activated protein kinase-dependent pathway mediating stress-induced apoptosis. Proc Natl Acad Sci USA. 1997;94:3279–3283. doi: 10.1073/pnas.94.7.3279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cella M, Salio M, Sakakibara Y, Langen H, Julkunen I, Lanzavecchia A. Maturation, Activation, and Protection of Dendritic Cells Induced by Double-stranded RNA. J Exp Med. 1999;189:821–829. doi: 10.1084/jem.189.5.821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Restifo NP. The new vaccines: building viruses that elicit antitumor immunity. Curr Opin Immunol. 1996;8:658–663. doi: 10.1016/s0952-7915(96)80082-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pardoll DM. Cancer vaccines. Nature Med. 1998;4:525–531. doi: 10.1038/nm0598supp-525. [DOI] [PubMed] [Google Scholar]
- 20.Rosenberg SA, et al. Immunizing patients with metastatic melanoma using recombinant adenoviruses encoding MART-1 or gp100 melanoma antigens. J Natl Cancer Inst. 1998;90:1894–1900. doi: 10.1093/jnci/90.24.1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mandl CW, Aberle JH, Aberle SW, Holzmann H, Allison SL, Heinz FX. In vitro-synthesized infectious RNA as an attenuated live vaccine in a flavivirus model. Nature Med. 1998;4(12):1438–1440. doi: 10.1038/4031. [DOI] [PubMed] [Google Scholar]
- 22.Dubensky TW, Polo JM, Liu MA. Live virus vaccines: Something old, something new, something borrowed…. Nature Med. 1998;4:1357–1358. doi: 10.1038/3939. [DOI] [PubMed] [Google Scholar]
- 23.Fink L, et al. Real-time quantitative RT-PCR after laser-assisted cell picking. Nature Med. 1998;4:1329–1333. doi: 10.1038/3327. [DOI] [PubMed] [Google Scholar]


