Abstract
Soil-transmitted helminth (STH) infections are among the most prevalent of chronic human infections worldwide. Based on the demonstrable impact on child development, there is a global commitment to finance and implement control strategies with a focus on school-based chemotherapy programmes. The major obstacle to the implementation of cost-effective control is the lack of accurate descriptions of the geographical distribution of infection. In recent years considerable progress has been made in the use of geographical information systems (GIS) and remote sensing (RS) to better understand helminth ecology and epidemiology, and to develop low cost ways to identify target populations for treatment. This chapter explores how this information has been used practically to guide large-scale control programmes. The use of satellite-derived environmental data has yielded new insights into the ecology of infection at a geographical scale that has proven impossible to address using more traditional approaches, and has in turn allowed spatial distributions of infection prevalence to be predicted robustly by statistical approaches. GIS/RS have increasingly been used in the context of large-scale helminth control programmes, including not only STH infections but also those focusing on schistosomiasis, filariasis and onchocerciasis. The experience indicates that GIS/RS provides a cost-effective approach to designing and monitoring programs at realistic scale. Importantly, the use of this approach has begun to transition from being a specialist approach of international vertical programs to become a routine tool in developing public sector control programs. GIS/RS is used here to describe the global distribution of STH infections and to estimate the number of infections in school age children in sub-Saharan Africa (89.9 million) and the annual cost of providing a single anthelmintic treatment using a school-based approach (US$5.0-7.6 million). These are the first estimates at a continental scale to explicitly include the fine spatial distribution of infection prevalence and population, and suggest that traditional methods have overestimated the situation. The results suggest that continent-wide control of parasites is, from a financial perspective, an attainable goal.
1. Introduction
There are four main nematode species of human soil-transmitted helminth (STH) infections, also known as geohelminths: Ascaris lumbricoides (roundworm), Trichuris trichiura (whipworm), Ancylostoma duodenale and Necator americanus (hookworms). These infections are most prevalent in tropical and sub-tropical regions of the developing world where adequate water and sanitation are lacking, with recent estimates suggesting that A. lumbricoides infects 1,221 million people, T. trichiura 795 million, and hookworms 740 million (de Silva et al., 2003). The greatest numbers of STH infections occur in sub-Saharan Africa, East Asia, China, India and South America.
Chronic and intense STH infections can contribute to malnutrition and iron-deficiency anaemia, and also can adversely affect physical and mental growth in childhood (Drake et al., 2000; Stephenson et al., 2000; Hotez et al., 2004). In recognition of the global health importance of STH infections, there is a renewed global commitment to finance and implement control strategies to reduce the disease burden of STH and other helminths, including schistosomiasis (Fenwick et al., 2003), filariasis and onchocerciasis (Molyneux et al., 2003). The development of effective helminth control is possible because of the availability of proven, cost-effective and logistically feasible intervention strategies. In the case of STH infections, regular periodic chemotherapy, using benzimidazole anthleminthics, of school-aged children delivered through the school system is the main intervention strategy (Aswashi et al., 2004; Hotez et al., 2005; Bundy et al., 2005). Understanding where at-risk populations live is fundamental for appropriate resource allocation and cost-effective control. In particular, it allows for reliable estimation of the overall drug needs of programmes and efficient geographical targeting of control efforts (Brooker & Michael, 2000). The precise global distribution of STH infection and how many people are infected and at risk of morbidity however remains poorly defined. This limits how national governments and international organizations define and target resources to combat the disease burden due to STH infection.
A previous review in this series highlighted the potential use of Geographical Information Systems (GIS) and remote sensing (RS) to better understand helminth distributions and their ecological correlates, but also to serve as geographic decision-making tools for identifying areas of particular risk as well as for the design, implementation and monitoring of control programmes (Brooker & Michael, 2000). As an increasing number of large-scale control efforts are underway, it is timely to assess how the potential of GIS and RS to guide control has been realized in practice. This article begins by describing the scientific basis of how environmental factors affect the biology and transmission dynamics of STH infection. We then show how satellite data can be used to establish and predict species-specific distributions, and how these tools can help shed additional light on the ecology and epidemiology of infection. Next, we describe how these tools have been effectively used within the context of large-scale control programmes. Finally, we adopt a data-driven approach to map the contemporary global distributions of STH infection, and relate these to global human population distribution data to derive regional and national estimates of population at risk by parasite species. Although focusing on STH infections, examples will also be presented for other helminthiases, including schistosomiasis, filariasis and onchocerciasis.
2. Transmission dynamics and the environment
To understand and ultimately predict the global distribution of STH infections it is essential to appreciate their biology, ecology and transmission dynamics. The life cycles of STH infection follow a general pattern. The adult parasite stages inhabit some part of the host intestine (A. lumbricoides and hookworm in the small intestine; T. trichiura in the colon), reproduce sexually and produce eggs, which are passed in human faeces and deposited in the external environment. Adult worms survive for several years and produce large numbers of eggs after 4–6 weeks (Table 1). Eggs can remain viable in the soil for several months (A. lumbricoides and T. trichiura) and larvae several weeks (hookworms), dependent on prevailing environmental conditions. A. duodenale larvae can undergo hypobiosis (arrested development at a specific point in the nematode life cycle) in the human body under certain environmental conditions for several months. Infection occurs through accidental ingestion of eggs (A. lumbricoides and T. trichiura) or penetration of the skin (by hookworm larvae).
Table 1.
Population parameters, development rates and life expectancies of parasites and free-living STH infective stages
| A. lumbricoides | T. trichiura | Hookworm | |
|---|---|---|---|
| Infective stage | Ova | Ova | Larvae |
| Egg production (eggs/female worm/day)† |
10,000-200,000 | 2,000-20,000 | 3,000-20,000 |
| Life expectancy of free-living infective stages† |
28-84 days | 10-30 days | 3-10 days |
| Adult life span‡ | 1-2 years | 1-2 years | 3-4 years |
| Pre-patency (adult development to sexual maturity) ‡ |
50-80 days | 50-84 days | 28-50 days |
| Larvae development time to infective stage§ |
8-37 days | 20-100 days | 2-14 days |
| Max. temp. of viable development§ |
35-39 °C | 37-39 °C | 40 °C |
| Basic reproductive number‡ | 1-5 | 4-6 | 2-3 |
Data taken from Anderson (1982); Bundy & Cooper (1989); Crompton (2001)
Data taken from Anderson & May (1991)
Data on A. lumbricoides (Seamster (1950); T. suis (Beer, 1976); hookworm (Nwosu, 1978; Smith & Schad, 1989).
As is common for infectious diseases, the transmission of STH infections can be summarized by the basic reproductive number (R0). This is defined as the average number of female offspring produced by one adult female parasite that attain reproductive maturity, in the absence of density dependent constraints (Anderson & May, 1991). R0 values of between 1 and 6 are estimated, with rates intrinsically highest for T. trichiura and lowest for hookworm. In practice, epidemiological studies fail to differentiate between the main hookworm species, A. duodenale and N. americanus, which will have different epidemiological and ecological characteristics.
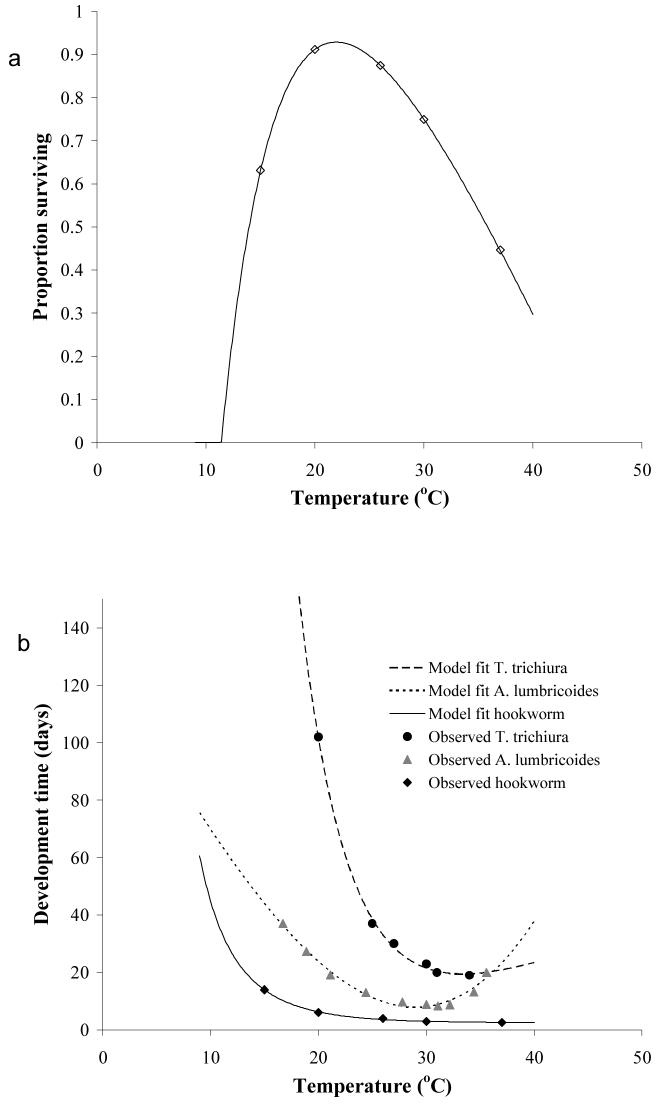
Increases in R0 give rise to increases in infection prevalence (percentage of individuals infected) and infection intensity (number of worms per human host). The dynamic processes involved in STH transmission, such as free-living infective stage development and survival, depend on the prevailing environmental conditions (Pavlovsky, 1966; Anderson, 1982). For example, as indicated in Figure 1, free-living infective stages present in the environment develop and die at temperature-dependent rates. Maximum survival rates of hookworm larvae, as indicated by proportion of larvae surviving, occur at 20-30 °C (Figure 1a). Experimental studies suggest that maximum development rates of free-living infective stages occur at temperatures between 28 and 32 °C, with development of A. lumbricoides and T. trichiura arresting below 5 and above 38 °C (Beer, 1976; Seamster, 1950), and development of hookworm larvae ceasing at 40 °C (Udonsi & Atata, 1987; Smith & Schad, 1989) (Figure 1b). It is suggested that A. lumbricoides eggs are more resistant to extreme temperatures than T. trichiura eggs (Bundy & Cooper, 1989).
Figure 1.
Relationship between temperature (a) parasite survival and (b) development duration. Points indicate experimental data (Seamster, 1950; Beer, 1973; Nwosu, 1978; Udonsi & Atata, 1987) and lines are fits derived from fractional polynomials analyses. Regression details: parasite survival y = 0.884 + −22.88x−0.5 + −7.73ln(x); A. lumbricoides duration y = 8.601 + −63.718x− −2 + 2.526x−3; T. trichiura duration y = 26.079 + 41209.68x−−2 + −1715.02x—2; hookworm duration y = 3.701 + 40.88x−−2 + −46.18x−−2
Soil moisture and relative atmospheric humidity are also known to influence the development and survival of ova and larvae: higher humidity is associated with faster development of ova; and at low humidity (<50%) the ova of A. lumbricoides and T. trichiura do not embryonate (Otto, 1929; Spindler, 1929). Field studies show that the abundance of hookworm larvae is related to atmospheric humidity (Nwosu and Anya, 1980; Udonsi et al., 1980).
These differing rates of development and survival will influence parasite establishment in the human host and hence the infection levels. Thus, a climate-induced increase in the rate of establishment, while holding parasite mortality constant, causes the parasite equilibrium to rise (Bundy & Medley, 1992). Although seasonal dynamics in transmission may occur, such fluctuations may be of little significance to the overall parasite equilibrium within communities. This is because the life-span of adult worms is typically much longer (1-10 years) than the periods in the year during which Ro is less than unity, and Ro will on average will be greater than one, maintaining overall endemicity (Anderson, 1982). For all these reasons, spatial variability in long-term synoptic environmental factors will have a greater influence on transmission success and patterns of STH infection than seasonal variability in a location.
3. Ecological correlates
Given the importance of environmental factors on transmission processes, it is unsurprising that statistical relationships between environmental factors and spatial patterns of infection can be observed. Several studies, some dating back to the early twentieth century, report ecological associations between STH distributions and temperature, rainfall and altitude (reviewed in Brooker & Michael, 2000). As outlined by Hay et al. (this volume), the ability to investigate ecological correlates of infection has been enhanced by satellite imagery, which can provide data from which information about temperature, humidity and vegetation conditions can be derived, and by the use of GIS to overlay multiple layers of data. These tools have been used successfully to describe the environmental factors associated with patterns of STH infection in selected geographical locations, and helped identify the relative importance of different environmental factors in determining geographic distributions (Appleton & Gouws, 1996; Appleton et al., 1999; Brooker et al., 2002a, 2000c, 2003, 2004b; Saathoff et al., 2005).
Here we extend these analyses and investigate the spatial ecology of STH infection across sub-Saharan Africa (SSA), where surprisingly little is still known about the distributions of STH infection and their underlying environmental determinants. Although intensity of STH infection is a key determinant of transmission dynamics and morbidity (Anderson & May, 1991), prevalence of infection, based on microscopic examination of STH eggs in stool samples, remains the most widely used indicator of infection status and the need for control. We derive estimates of STH prevalence from dedicated surveys, conducted among schoolchildren after 1985 and geo-referenced using global positioning systems. Detailed data are available for Cameroon, Chad, Eritrea, Guinea, South Africa, Tanzania, Uganda, Zambia, and show that prevalence of A. lumbricoides and T. trichiura is greatest in equatorial, central and west Africa and southeast South Africa, whereas hookworm has a wider distribution across the continent (Figure 2). By relating these distributions to satellite-derived environmental data, we can investigate their large-scale ecological correlates.
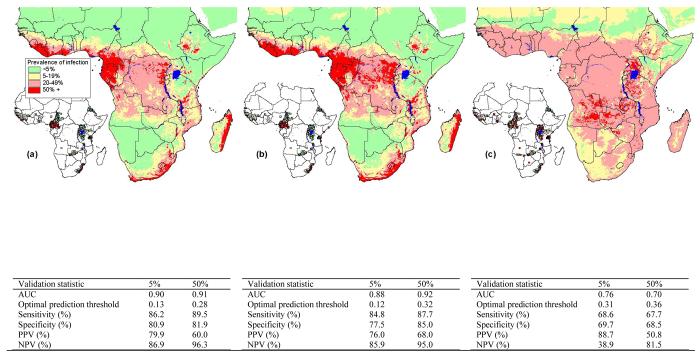
Figure 2.
Predicted prevalence of (a) A. lumbricoides, (b) T. trichiura and (c) hookworm, based on relationships between observed prevalence of infection among school-aged children (insert) and AVHRR satellite data (see Hay et al., this volume for details) and elevation obtained from an interpolated digital elevation model from the Global Land Information System (GLIS) of the United States Geological Survey (http://edcwww.cr.usgs.gov/landdaac/gtopo30/). Prevalence data are available for 1172 sites across sub-Saharan Africa including 84,412 children. All surveys were conducted using similar diagnostic techniques (direct smear, typically using the Kato-Katz method) and based on random samples of children in areas where no control measures have previously been undertaken. Due to non-linear relationships between observed prevalence and predictor variables, the predictors were categorised before being entered into the models. The coefficients from these models were then applied to the categories of the predictor variables to generate a predicted prevalence of infection. Model coefficients for A. lumbricoides included LST 29 - 32°C: −0.3 (95% CI −0.3, −0.2), LST 32 - 37.5°C: −1.7 (95% CI −1.8, −1.6), LST 37.5 - 45°C: −3.8 (95% CI −3.9, −3.7), LST >45°C: −5.1 (95% CI −5.5, −4.7), NDVI −7.8 − −6: 0.3 (95% CI −0.1, 0.6), NDVI −6 − −5: −0.02 (95% CI −0.4, 0.3), NDVI >−5: 0.4 (95% CI 0.1, 0.8), elevation 500 - 1000 m: −0.05 (95% CI −0.1, 0.01), elevation 500 - 1000m: −1.0 (95% CI −1.0, −0.9) and elevation 1000 - 1500m: −1.5 (95% CI −1.6, −1.4) and explained 28.2% of the variance of the data (R2=0.282); for T. trichiura they included LST 29 - 32°C: −0.6 (95% CI −0.7, −0.5), LST 32 - 37.5°C: −2.2 (95% CI −2.3, −2.1), LST 37.5 - 45°C: −4.2 (95% CI −4.4, −4.1), LST >45°C: −6.0 (95% CI −6.3, −5.7), NDVI −7.8 − −6: −1.5 (95% CI −1.7, −1.4), NDVI −6 − −5: −1.6 (95% CI −1.8, −1.4), NDVI >−5: −1.2 (95% CI −1.4, −1.0), elevation 500 - 1000 m: −0.1 (95% CI −0.2, −0.1), elevation 1000 - 1500m: −1.3 (95% CI −1.3, −1.2) and elevation >1500m: −2.4 (95% CI −2.6, −2.3) (R2=0.335) and for hookworm they included LST 29 - 32°C: 0.02 (95% CI −0.1, 0.1), LST 32 - 37.5°C: 0.8 (95% CI 0.7, 0.8), LST 37.5 - 45°C: 0.9 (95% CI 0.8, 1.0), LST >45°C: −0.1 (95% CI −0.2, 0.1), NDVI −7.8 − −6: 1.3 (95% CI 1.1, 1.4), NDVI −6 − −5: 1.9 (95% CI 1.8, 2.1), NDVI >−5: 2.4 (95% CI 2.2, 2.5), elevation 500 - 1000 m: −0.5 (95% CI −0.6, −0.5), elevation 1000 - 1500m: 0.4 (95% CI 0.3, 0.4) and elevation >1500m: −0.7 (95% CI −0.8, −0.6) (R2=0.071). Validation statistics including area under the curve (AUC), optimal prediction threshold and sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) at the optimal prediction threshold are presented for observed prevalence thresholds of 5% and 50%.
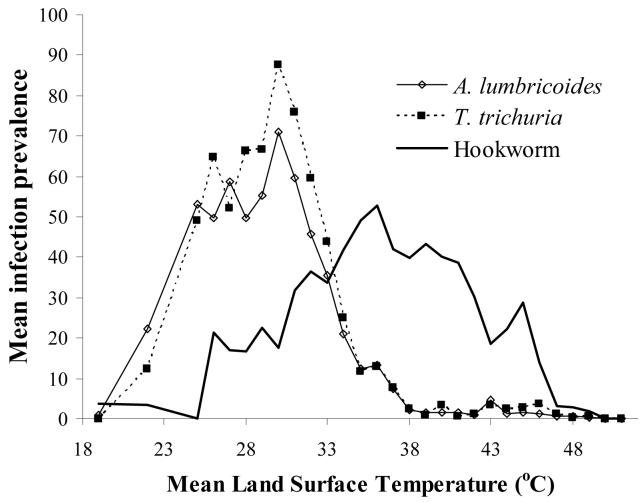
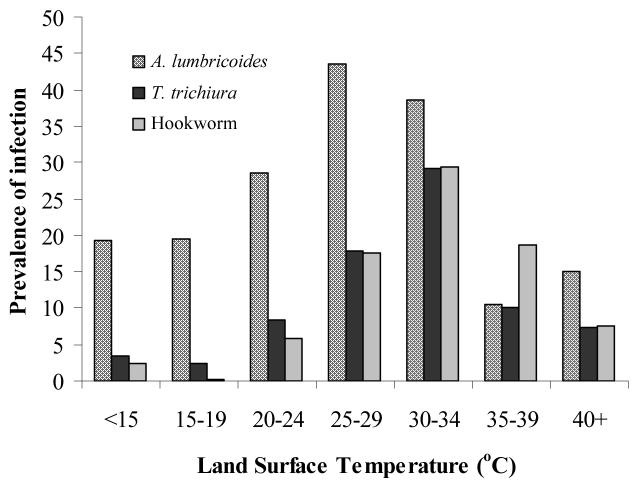
For each species, a clear relationship exists between prevalence of infection and remotely sensed Land Surface Temperature (LST) (Cracknell, 1997): prevalence of A. lumbricoides and T. trichiura is generally <5% in areas where LST exceeds 38-40 °C, whereas hookworm infection remains highly prevalent throughout the upper end of the thermal range (Figure 3). This is an intriguing observation since experimental studies suggest that each STH species has similar thermal thresholds (Table 1). The apparent ability of hookworm to survive hotter conditions may be explained in part by the ability of mobile larvae to migrate to more suitable thermal and moisture conditions. In particular, whereas hookworm larvae stages have some limited motility and can move downward into the soil, thereby avoiding desiccation, the ova of A. lumbricoides and T. trichiura are non-motile, and high surface temperatures will result in ova dying from desiccation (Beaver, 1953).
Figure 3.
Relationship between mean Land Surface Temperature, estimated from satellite data, and prevalence of STH infection. Estimates are derived for each survey location and locations with the same degree Celsius are averaged for presentation. (see legend of Figure 2 for details.)
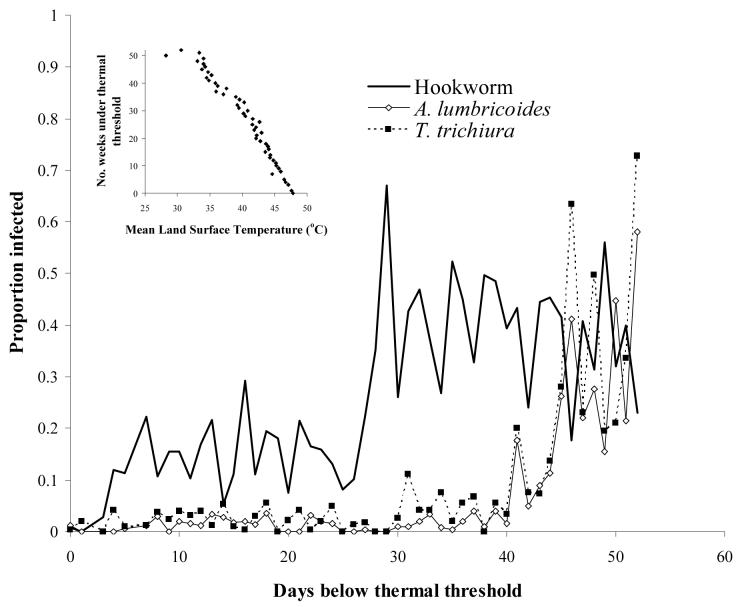
Some features of helminth life cycles, such as hypobiosis, equip the parasite to survive periods that are unsuitable for transmission. However, hypobiosis only occurs for A. duodenale in humans (Schad et al., 1973) and not for N. americanus, the hookworm species predominant in SSA (Kilima, 1990) and is therefore excluded here as a possible explanation for hookworm's apparent wider thermal tolerance. Other species-differences in life history traits may play an important role. It is suggested that the probability of surviving to infectivity and finding a new host is enhanced for hookworm by its shorter time for larval development to infective stage in the soil of hookworm larvae (3-10 days) compared to that of A. lumbricoides (28-84 days) and T. trichiura (10-30 days) (Table 1). This hypothesis is supported by an observed relationship between prevalence and the number of weeks available which are below the thermal threshold (Figure 4). The prevalence of A. lumbricoides and T. trichiura was generally low in locations where temperatures fall below the thermal threshold for less than 35 to 40 weeks, and increased with increasing number of weeks. Hookworms however required a much smaller (8 week) window of thermal suitability for transmission and so were able to persist even when the period available for development was of the order of ten weeks.
Figure 4.
Relationship between number of day which temperature <40 °C, which is suitable for survival of free-living STH infective stages, and proportion infected based on observed data for 601 locations from nationwide surveys in Cameroon (Ratard et al., 1991, 1992), Chad (Brooker et al. 2002a), and Uganda (Brooker et al., 2004b). Data were collected in cross-sectional school surveys using similar diagnostic technique (Kato-Katz method) and sampling designs (stratified, random), and encompass a broad range of infection rates. Insert: Relationship between mean LST and number of weeks temperature falls below 40 °C (y= −3.006x + 147.8, r=0.94, p<0.001). Estimates were derived for each survey location and locations with the same number of days under the thermal threshold were averaged for presentation.
Finally, a potentially very important factor is the longevity of the adult worm, since the location inside the host is essentially a refuge from the high external temperatures. Hookworms have a longer adult stage life expectancy (3-4 years) than either A. lumbricoides or T. trichiura (1-2 years). This implies that hookworms can find refuge from external temperatures for more than twice the length of time of the other species, effectively protected from extreme temperatures over a 3-4 year period for appropriate conditions for development. This greatly increases the chances of hookworm transmission stages being deposited and developing in suitable thermal conditions.
4. Predicting distributions
These analyses have enabled the first continent-wide predictions of infection patterns on the basis of satellite-derived environmental covariates as well as providing insight into the ecology of infection. Predictions of prevalence were based on binomial logistic regression analysis, where, for each location, the response variable contained the total number of positive responses and the total number examined, and the independent variables were satellite-derived mean Land Surface Temperature (LST) and Normalized Difference Vegetation Index (NDVI) for 1982-1998 (Hay et al., this volume) and elevation (http://edcwww.cr.usgs.gov/landdaac/gtopo30/). Models were then cross-validated using a jack-knife procedure (King et al. 2004) and predicted values were compared to observed values using Receiver Operating Characteristics (ROC) analysis (Brooker et al., 2002c). The models for A. lumbricoides and T. trichiura provided impressive descriptive accuracies of low transmission (prevalence <5%) and areas which would be the target of mass treatment programmes (prevalence >50%) (Figure 2). By contrast, the hookworm model has only moderate accuracy, a probable reflection of the apparent wider distribution of hookworm. The models indicate that prevalence of A. lumbricoides and T. trichiura is greatest in equatorial, central and west Africa, eastern Madagascar and southeast Africa, whereas hookworm is more widely distributed across the continent. A limitation of this analysis is the use of infection prevalence rather than infection intensity, which has greater relevance to transmission dynamics and morbidity, but which few studies quantify. There remains the need to investigate geographical heterogeneity in infection intensity.
Satellite data can therefore help define the large-scale distributions of STH infection, which are demonstrated to be influenced by heterogeneities in climate. At smaller spatial scales other factors, including variability in human behaviour, including personal hygiene, as well as differences in sanitation and socio-economic status, have to be considered. For example, in climatically unsuitable areas, microhabitats, influenced by local housing and sanitation, may provide suitable transmission foci, and vice-versa. Such microhabitats are commonly found in urban areas.
5. Urbanization
In common with many other parasitic infections, STH infections flourish in impoverished areas characterized by inadequate sanitation and overcrowding. It is commonly assumed that A. lumbricoides and T. trichiura are more prevalent in urban areas whereas hookworm is more often found in rural areas (Crompton & Savioli, 1993). However, comparable data STH infections in urban and rural settings are remarkably few and those that do exist indicate a more complicated picture. Studies which surveyed similar age groups and socio-economic areas indicate that the prevalence of A. lumbricoides and T. trichiura differ between urban and rural communities, but in no systematic manner (Table 2). By contrast, hookworm appears to be equally prevalent in both urban and rural settings (Table 2). The precise reasons for the urban-rural dichotomies for A. lumbricoides and T. trichiura are as yet unclear. Differences in prevalence of A. lumbricoides and T. trichiura in urban and rural areas may reflect differences in sanitation or population density; socio-economic differences will also play an important role. It is clear that further work is needed to resolve these issues.
Table 2.
Prevalence of soil-transmitted helminth infections among schoolchildren in urban and rural communities in developing countries. Included studies sought to restrict investigation to areas of similar socio-economic characteristics
| Setting | Sample size |
A. lumbricoides |
T. trichiura |
Hookworm |
Reference | |||
|---|---|---|---|---|---|---|---|---|
| Urban | Rural | Urban | Rural | Urban | Rural | |||
| Blantyre, Malawi |
553 children (3-14 years old) |
15.4 | 0.7 | - | - | 0.4 | 2.1 | Phiri et al. (2000) |
| Pemba, Tanzania |
256 children (3-14 years old) |
60.6 | 63.6 | 100 | 100 | 97.6 | 94.6 | Albonico et al. 1993 |
| Buea, Cameroon |
211 children (8-15 years old) |
33.9 | 56.4 | 32.3 | 59 | 0 | 5.1 | Ndenecho et al. (2002) |
| Rolandia, Brazil |
236 children (5-15 years old) |
6.1 | 1.3 | 0.7 | 0.1 | 4.3 | 4.4 | Giraldi et al. (2001) |
| Malaysia | 3073 children (<15 years old) |
51.7 | 21.2 | 65.3 | 29.1 | 5.7 | 5.9 | Kan et al. (1989) |
| Penang, Malaysia |
192 children (7-12 years old) |
37.4 | 33.4 | 100 | 92 | 18.7 | 19.7 | Rahman (1998) |
By 2007, it is predicted that more than half of the global human population will be urban citizens, most of them living in the rapidly growing cities of Africa, Asia and Latin America (United Nations, 2003). Urbanization often accompanies social and economic development, with better opportunities for education, adequate living standards and higher incomes. However, overcrowding and lack of adequate water and sanitation of urban slum communities may increase transmission of STH infections. Investigation of the impact of increased urbanisation on STH infections together with assessment of the effectiveness of urban helminth control measures in low-income settings is clearly warranted as increased urbanization may promote the transmission of STH infections, especially A. lumbricoides and T. trichiura.
6. Global control strategies
Recommended drugs for use in public health programmes to control STH infection are the benzimidazole anthelmintics (BZAs), albendazole or mebendazole; older drugs including pyrantel pamoate and levamisole are also occasionally used in some developing countries (WHO, 2002; Utzinger & Keiser, 2004). In areas where STH infections co-occur with schistosomiasis, BZAs are co-administered with praziquantel (PQZ), the major drug used for the treatment of schistosomiasis (Fenwick et al., 2003).
Current efforts to control STH infection, as well as schistosomiasis, focus on the school age population. It is estimated that between 25 and 35 percent of school-aged children are infected with one or more of the major species worms (Bundy, 1997; de Silva et al., 2003). The most intense worm infections and related illnesses occur at school age (Partnership for Child Development, 1998, 1999).
Infection can result in significant consequences for health and development, affecting growth, promoting anaemia and causing some overt clinical disease, much of which is rapidly reversed by treatment (Warren et al., 1993; Hotez et al., 2005). In addition to these impacts on health and physical development, infected schoolchildren perform poorly in tests of cognitive function; when treated, immediate educational and cognitive benefits are apparent only for children with heavy worm burdens or with concurrent nutritional deficits (Bundy et al., 2005). Treatment alone cannot reverse the cumulative effects of lifelong infection nor compensate for years of missed learning, but studies suggest that children are more ready to learn after treatment for worm infections and may be able to catch up if this learning potential is exploited effectively in the classroom. In Kenya treatment reduced absenteeism by one quarter, with the largest gains for the youngest children who suffered the most ill health (Miguel & Kremer, 2004).
For these reasons, school age children are the natural targets for treatment, and school based treatment delivery programmes offer major cost advantages because of the use of the existing school infrastructure and the fact that schoolchildren are accessible through schools. An important element of the approach is to minimize the need for clinical diagnosis, which is often more expensive than the treatment itself, and to focus on mass delivery of services. Evidence suggests that mass delivery of deworming is preferable on efficacy, economic and equity grounds to approaches that require diagnostic screening (Warren et al., 1993). School-based deworming also has major externalities for untreated children and the whole community by reducing disease transmission in the community as a whole (Bundy et al., 1990).
Recognizing the centrality of school age children to the response to helminth infection, in 2001, the 54th World Health Assembly of the WHO passed a resolution to provide regular deworming treatment to 75 percent of school-age children at risk (an estimated target population of 398 million) by 2010. School health and nutrition programs provide the vehicle for delivering regular but infrequent (every 6 months or more) anthelmintic treatment to school children. Operational research by Partnership for Child Development (PCD) has demonstrated how interventions can be implemented and evaluated at the country level, for example enabling mass deworming of schoolchildren (Bundy & Guyatt, 1996; PCD, 1998, 1999; Guyatt et al., 2000).
A major step forward in international coordination and cohesion was achieved when a framework to Focus Resources on Effective School Health (FRESH) was launched at the World Education Forum in Dakar in April 2000 (World Bank, 2000). Among the early partners in this effort were UNESCO, UNICEF, the World Food Programme (WFP), the WHO, and the World Bank, with the Education Development Centre, Education International, and the Partnership for Child Development. The FRESH framework provides a consensus approach of agreed good practice for the effective implementation of health and nutrition services within school health programmes. The framework proposes four core components that should be considered in designing an effective school health and nutrition programme and suggests that the program will be most equitable and cost-effective if all of these components are made available, together, in all schools. The four components also provide the appropriate mix of interventions for responding to helminth infection globally: (1) Policy: health- and nutrition-related school policies that promote the nutrition and health of staff and children (and promote the role of teachers in delivering anthelmintic treatment); (2) School environment: access to safe water and provision of effective sanitation facilities (which helps break the helminth transmission cycle); (3) Education: skills-based education, including life skills that addresses health and hygiene issues and promotion of positive behaviors (including promoting handwashing and other hygienic behaviours that protect against helminth infection); and (4) Services: simple, safe, and familiar health and nutrition services that can be delivered cost-effectively in schools (such as deworming).
The common focus has encouraged concerted action by the participating agencies and has increased significantly the number of countries implementing school health reforms. The simplicity of the approach, combined with the enhanced resources available from donor coordination, has helped ensure that these programs can go to scale. For example, annual external support from the World Bank for these actions approaches US$90 million, targeting some 100 million schoolchildren.
The FRESH framework does not prescribe the design of school based deworming programs, and in practice these are highly variable and country specific (Bundy et al., 2005). In low income countries a public sector model is commonly used, involving the Ministry of Health in supervising the activity, and the Ministry of Education in implementing the intervention through teachers. In middle income countries, including Indonesia and, historically, Japan and South Korea, a private sector model involving nongovernmental organizations delivering treatment that is paid for by the community has proven sustainable and effective.
Whatever the design, identifying which schools and communities require treatment is an essential part of the process, and a key role for GIS.
7. Control applications of GIS/RS
As recently as five years ago, applications of GIS and RS in helminthology had only been attempted for schistosomiasis and filariasis (reviewed in Brooker & Michael, 2000; Brooker, 2002). Since then studies have investigated spatial patterns of STH infection (Brooker et al., 2002c, 2003, 2004b; Saathoff et al., 2005), Loa loa (Thomson et al., 2004) and onchocerciasis (Carabin et al., 2003). These studies have focused on the use of RS data to identify ecological correlates of infection and develop statistical models of disease risk. While these applications are attractive research objectives, the challenge remains to apply these geographic tools in the context of large-scale control programmes. Here we examine how GIS and RS have contributed to the design and implementation of helminth control programmes.
Human onchocerciasis or river blindness results from infection with a parasitic filarial worm, Onchocerca volvulus, which is transmitted by female Simulium blackflies. Blackflies breed in areas close to fast-flowing and well oxygenated rivers and seldom travel more than 15km in search of a bloodmeal. This means that high-prevalence communities are located close to breeding sites. Rapid Epidemiological Mapping of Onchocerciasis (REMO), developed by TDR/WHO, has been a key geographic tool for the control of onchocerciasis (Ngoumou et al., 1994; Katabarwa et al., 1999). With REMO, it is possible to assess quickly and cheaply which communities are at high risk of onchocerciasis and where they are located. REMO uses geographical information, particularly the locations of river basins, to identify communities that are likely to be at high risk. A subsample of these communities is then rapidly assessed by screening individuals for onchocercal nodules. This enables communities to be classified into three categories: priority areas which require community-directed treatment with ivermectin; areas which do not require treatment; and possible endemic areas but which require further investigation. Results of REMO have been effectively incorporated into a GIS to visualize priority areas for mass distribution of ivermectin and estimate the number of individuals to be treated (Noma et al., 2002). This has helped the African Programme for Onchocerciasis Control (APOC) to prioritize allocation of resources according to need. The robustness of REMO following several rounds of interventions remains, however, to be fully investigated since there has been little validation of the approach since its initial development.
Severe adverse (and sometimes fatal) encephalopathic reactions following treatment with ivermectin have been reported in individuals co-infected with O. volvulus and Loa loa (loiasis), and as such, there is an operational necessity to identify areas with a high prevalence of L. loa (Addiss et al., 2003). Thomson et al. (2000, 2004) developed a spatial model that predicted the prevalence of L. loa microfilaraemia on the basis of satellite-derived environmental data in Cameroon, with applications for defining areas at-risk of post-ivermectin Loa-related severe adverse reactions. Like onchocerciasis, treatment strategies have been defined, according to levels of endemicity of onchocerciasis and loiasis. In areas where both diseases are highly endemic, detailed measures, such as training of medical staff, provision of medical supplies and heightened surveillance of treated individuals, are required.
A rapid mapping method has also been developed for lymphatic filariasis. This disease is caused by the filarial parasite Wuchereria bancrofti and is being targeted for eradication by the Global Alliance for the Elimination of Lymphatic Filariasis (www.filariasis.org). As with other control programmes, delimitation of endemic localities is an essential prerequisite for planning control elimination programmes, based on treatment with diethylcarbamazine (DEC) plus albendazole or ivermectin plus albendazole. To address this, a method for the Rapid Geographical Assessment of Bancroftian Filariasis (RAGFIL) has been developed by TDR/WHO. This is based on the use of a spatial sampling grid with either 25km or 50km between sampled communities, rapid prevalence assessments using immunochromatographic card tests (ICT) for the detection of circulating antigen from adult W. bancrofti filarial antigenaemia, and geostatistical methods for predicting the distribution of filariasis throughout the target area (WHO, 1998). Using this method, Gyapong et al. (2002) predicted prevalence in four countries in West Africa, enabling control planning to be initiated. Other analyses suggest, however, that endemic foci can persist within the interstices of the proposed grid and that smaller grids are required (Srividya et al., 2002; Alexander et al., 2003).
The Schistosomiasis Control Initiative (SCI) is currently supporting six countries in sub-Saharan Africa to implement national control programmes for schistosomiasis and STH infections, including Burkina Faso, Mali, Niger, Tanzania, Uganda and Zambia (www.schisto.org). In Uganda, where Schistosoma mansoni is widespread, GIS and RS have been employed to classify the country according to different treatment strategies. Regular chemotherapy with praziquantel and albendazole is being provided to schoolchildren and other high-risk groups (Kabatereine et al., 2005). Following WHO guidelines, the programme is classifying communities according to three strategies: (1) in communities with a high prevalence (>=50%) schoolchildren are treated every year and high risk groups, such as fishermen, are treated; (2) in communities with a moderate prevalence (>=20% and <50%) schoolchildren are treated once every two years; and (3) in communities with a low prevalence (<20%) chemotherapy are made available in health facilities for treatment of suspected cases. With the use of GIS coupled with satellite and climatic data, geographical analysis found that no transmission of S. mansoni typically occurs in areas of Uganda where total annual rainfall was <850 mm or altitude was >1400 m (Kabatereine et al., 2004). These areas were subsequently set aside without the need for further surveys (Brooker et al., 2004a) (Figure 5). It was also shown that prevalence consistently exceeded 50% in areas within 5km of Lakes Victoria and Albert, and thus in these areas warranted mass treatment without the need for further surveys. Prospective surveys have validated these predicted classifications (Kabatereine et al., unpublished results).
Figure 5.
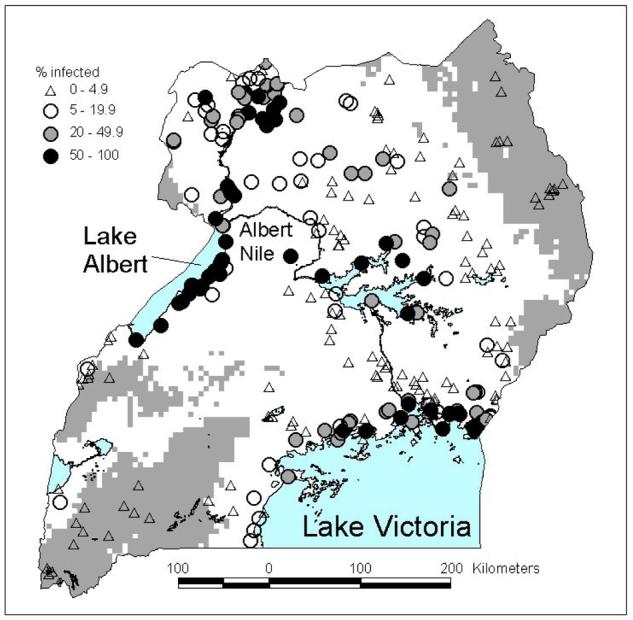
Distribution of S. mansoni in Uganda and classification of the country according to treatment category: (1) mass treatment without further surveys, <5km from Lake Victoria and Lake Albert (not shown); (2) non-treatment areas, altitude >1400 m or annual rainfall <850 mm (grey areas); (3) and areas requiring further investigation using LQAS (white areas). For further details see Kabatereine et al. (2004), Brooker et al. (2004a, 2005).
Outside these two ecological areas, where smaller rivers and water bodies are numerous, it is suggested that individual communities are surveyed using standard parasitological methods (Brooker et al., 2004a). Rapid mapping of communities is based on the Lot Quality Assurance Sampling (LQAS) method (Brooker et al., 2005). Developed in industry for quality control, LQAS makes it possible to use small sample sizes when conducting surveys among populations (lots), best used in situations where classification of a population is useful and where the emphasis is on decision making (e.g. whether or not to intervene in a particular community) rather than estimation of prevalence and intensity of infection. Field testing showed that LQAS sampling plan where only 15 children are sampled and a decision to intervene is made if seven children are found to be infected had excellent diagnostic performance, while economic analysis demonstrated that screening using LQAS was more cost-effective than mass treating all schools in a single sub-county (Brooker et al., 2005). Based on these findings, LQAS is employed in Uganda to quantify the fine-scale distribution of S. mansoni in areas of potential risk. Thus, GIS/RS coupled with field data can target schistosomiasis control from the national to the local level.
Predictive risk-mapping has also been employed to determine target areas for mass treatment in Tanzania (Clements et al., 2005). First, simulation studies were conducted to determine the number of individuals and schools that were required to give a desired level of precision in risk estimates. Parasitological surveys were then conducted in 143 schools in northwestern Tanzania. The proportions of children found to have S. haematobium and S. mansoni infections were determined for each school and these were used as the outcome variables in separate spatially-explicit binomial logistic regression models. The models were developed in a Bayesian framework and included environmental covariates derived from remote-sensing and a geostatistical component, using a powered exponential function to describe spatial correlation in the datasets. Models were externally-validated against an independently-collected dataset from one district within the study area and were used to make spatial predictions for S. haematobium and S. mansoni risk at prediction co-ordinates, defined by a grid of equally-spaced locations and predictions were interpolated to produce a continuous risk surface for the study area (Figures 6a-b).
Figure 6.
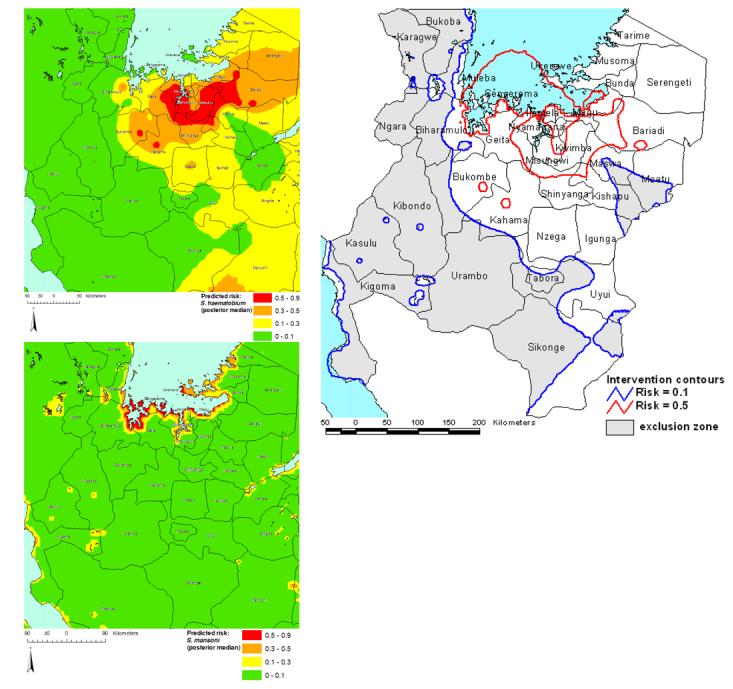
(a) Risk prediction surface for prevalence of S. haematobium infection in northwest Tanzania. Values presented are interpolated median posterior risk estimates from a Bayesian geostatistical binomial logistic regression model. Model parameters were: α (intercept) = 2.3 (95% Bayesian CI −0.7 - 5.9), κ (smoothing parameter) = 0.9 (95% Bayesian CI 0.6 - 1.2), ϕ (decay of spatial correlation) = 0.2 (95% Bayesian CI 0.1 - 0.5) and σ (overall variance) = 4.8 (95% Bayesian CI 2.7 - 7.6). (b) Risk prediction surface for prevalence of S. mansoni infection in northwest Tanzania. Values presented are interpolated median posterior risk estimates from the Bayesian geostatistical binomial logistic regression model. Model parameters were: α (intercept) = −12.3 (95% Bayesian CI −18.8 − −4.5), coefficient for distance to perennial water body, <0.04 decimal degrees = 4.1 (95% Bayesian CI 2.8 - 5.4), coefficient for distance to perennial water body, 0.04 – 0.1 decimal degrees = 2.3 (95% Bayesian CI 1.2 - 3.4), coefficient for distance to perennial water body, 0.1 - 0.4 decimal degrees = 1.1 (95% Bayesian CI 0.1 −2.0), coefficient for annual minimum temperature = 0.4 (95% Bayesian CI 0.0 - 0.8), κ (smoothing parameter) = 0.8 (95% Bayesian CI 0.5 - 1.3) = ϕ (decay of spatial correlation) = 2.8 (95% Bayesian CI 1.0 - 5.7) and σ (overall variance) = 1.2 (95% Bayesian CI 0.8 - 1.9). (c) Intervention contour map overlying districts of northwest Tanzania. Areas outside the 0.1 risk contour will be excluded from the mass treatment programme and praziquantel will be made available in health centres. Areas between the 0.1 and 0.5 risk contour will receive mass treatment, targeted at school-age children. Areas within the 0.5 risk contour are priority areas where mass treatment will be targeted at school-age children and other high-risk groups.
Subsequently, prediction surfaces for S. haematobium and S. mansoni were combined to make a single intervention map (as the treatment programme and its use of prazquantel, makes no distinction between the two schistosome species), consisting of contours that equated to a prevalence of S. haematobium or S. mansoni of 10% and 50% (Figure 6c). The lower contour (10%) delineated areas that would and would not be targeted by the mass treatment campaign and the upper contour (50%) delineated areas where mass treatment would only be conducted in school-age children and areas where both school-age children and other high-risk groups in the community would be targeted. Estimates of uncertainty in the spatial predictions help identify area where further data collection is required.
The experience of SCI in Tanzania and Uganda amply demonstrates the usefulness of GIS/RS as geographic decision-making tools for implementing helminth control at both national scales and local scales. Geographical distributions are continually updated as new epidemiological data are collected, and as intervention reduces the prevalence of infection. Analysis of the cost-effectiveness of the tools, which is germane to their long-term and sustainable use, is currently underway.
The above examples have shown how research and international programmes have led the way in developing the use of GIS/RS for directing control programs. An important emerging trend is that national governments are beginning to use this approach for designing and developing sustainable national programs. GIS/RS has been employed by national governments to plan and conduct nationwide rapid epidemiological assessments of STH and schistosomiasis in Chad (Brooker et al., 2002a) and Eritrea (Partnership for Child Development, 2003), and to design and implement national parasite control programs, in both cases as part of national development programs with World Bank assistance. In Chad, RS data was used to define seven ecological zones which were combined with population data in a GIS to define the sample protocol, whereby 20 schools, in different ecological zones were surveyed. This approach substantially reduced the cost of the sample survey, while preserving its utility and effectiveness. The analysis showed that the patterns of hookworm and S. haematobium had a close association with the RS/GIS defined ecological zones, and significant relationships with environmental variables; it was correctly predicted that A. lumbricoides and T. trichiura would not occur in the country. The results from the survey helped the government plan the country's school-based control programme, and resulted in significant cost savings for the program since it identified the need to target far fewer schools than had first been anticipated. A similar geographical approach was adopted in planning the school health programme in Eritrea (Partnership for Child Development, 2003). Again the sampling methodology proved substantially less expensive, and more practical, than traditional approaches developed without benefit of GIS/RS. The national survey revealed that infection was highly focal, and that deworming interventions could be precisely targeted, with significant savings in financial and technical resources.
8. Global distributions
The above examples show that, used appropriately, GIS/RS can provide a practical and low cost tool for designing and implementing sustainable helminth control programs. GIS has the potential to promote evidence-based priority setting and careful targeting of finite financial resources, resulting in savings in resources and enhancement of sustainability. For this to become a reality, a critical first step is defining the geographical distribution of infection globally.
As with all diseases, there is long history of attempts to define global distributions of STH infections, and provide estimates of numbers infected. A first seminal effort was provided by Stoll (1947), which has been frequently updated (Peters, 1978; Crompton & Tulley, 1987; Bundy & Cooper, 1989; Chan et al., 1994; Brooker et al., 2000; Bundy et al., 2004), with the most recent estimates provided by de Silva et al. (2003).
Since Stoll provided his estimates nearly 60 years ago, efforts to control STH infections and morbidity have varied across different regions of the world. The analysis by de Silva et al. (2003) showed that in certain regions there has been a reduction in STH infection prevalence. In the Americas, for example, there has been a precipitous decline in prevalence and in absolute numbers since the 1960s, a change largely attributable to national treatment programmes coincidental with social and economic development which have brought about improved access to clean water and proper sanitation (PAHO, 2000; Ehrenberg et al. 2003). Well-documented declines in prevalence exist for Brazil and Mexico, the two most populous countries in the region (Tay et al., 1976, 1995; Vinha, 1971; PAHO, 2000). Because of the success of control we do not consider the geographical distribution of infection in the region any further, although we recognise that there may be small foci of high prevalence, which require control activities.
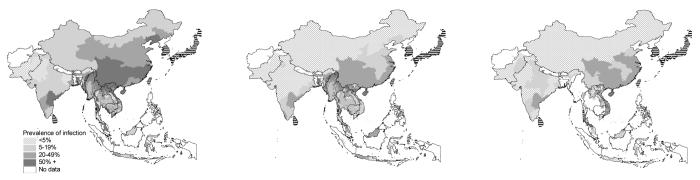
In Asia, several countries, notably Japan, South Korea and Taiwan, have achieved sustained and successful control of STH infections over the last forty years (WHO, 1996). More recently, a national control programme in Sri Lanka has reduced prevalence to less than five percent. Control programes have also been launched in several other Asian countries including China, Indonesia, Malaysia, Nepal, The Philippines and Thailand. Despite these control efforts, data available for south and southeast Asia suggest the need for effective control (Figure 7). STH infection remains prevalent in China and India, countries which account for a third of the world's population. The highest prevalence rates are observed for southern China and the northern regions of southeast Asia, and the lowest rates in northern China, northern India and Pakistan. In northern China, A. lumbricoides is more prevalent than either the prevalence of T. trichiura and hookworm. STH species are also widespread in the Pacific Islands (Hughes et al., 2004).
Figure 7.
Prevalence of STH infection by province in Asia. (a) A. lumbricoides, (b) T. trichiura and (c) hookworm. Horizontal hatched areas indicate areas where sustained control has resulted in prevalence levels of <5%; white areas indicate a lack of data. Data were derived by published surveys or reviews: Afghanistan (Albis Gabrielli, unpublished data), Bangladesh (Hall & Nahar, 1994; Mascie-Taylor et al., 1999), Bhutan (Allen et al., 2004), Cambodia (Sinuon et al., 2003; Urbani et al., 2001), China (Xu et al., 1995), India (de Silva et al., 2003), Indonesia (Margono, 2001), Lao PDR (Rim et al., 2003), Malaysia (Singh & Cox-Singh, 2001) Myanmar (Montresor et al., 2004), Pakistan (Government of Pakistan, 1988), Thailand (Anantaphruti et al 2000, 2002, 2004; Chongsuvivatwong et al 1994; Kasuya et al 1989; Nacher et al 2002; Waikagul et al 2002), Pacific Islands (Hughes et al., 2004); Vietnam (Anon 1995; van der Hoek et al. 2003). In Cambodia and Myanmar, where empirical data are lacking, prevalence of A. lumbricoides and T. trichiura is estimated from RS-based prediction models (Brooker et al., 2003).
We suggest that such distribution patterns reflect environmental suitability of STH transmission, especially thermal constraints. Here, this hypothesis is explored by investigating the relationship between prevalence of infection and satellite data for each administrative unit in the region. LST was expressed as the median value for each unit, and then STH infection prevalences were averaged for each five degrees Celsius (Figure 8). Median LST is estimated to be <20 °C in northern China and >40 °C in northern India and Pakistan, where STH infection prevalence is lowest. It appears that A. lumbricoides is more widespread in Asia and is able to survive colder temperatures than either T. trichiura or hookworm. It can thus be interpreted that these represent the lower thermal limits of STH transmission. Insufficient data were available to adequately explore the upper thermal limits of transmission in the region, as was possible for SSA (Figure 3). The current regional analysis for Asia supports previous studies which show that heterogeneities in STH infection prevalences are correlated with temperature and humidity in China (Xu et al., 1995; Lai & His, 1996) and southeast Asia (Brooker et al., 2003). The latter study used satellite data to predict prevalence in areas lacking detailed data (Brooker et al., 2003).
Figure 8.
The relationship between prevalence of STH infection in Asia and satellite-derived mean land surface temperature for 1982-1998, obtained from NOAA's AVHRR. Prevalence is expressed as median prevalence for each temperature category and the median temperature was calculated for each geographical region; it is recognized that this approach masks the heterogeneity in STH prevalence and temperature within regions, but epidemiological data are available are not available at finer spatial resolution.
While there have been declines in the prevalence of STH infection in the Americas and parts of Asia, estimated prevalence rates for sub-Saharan Africa (SSA) are equivalent to those first estimated by Norman Stoll more than 60 years ago (de Silva et al., 2003). Yet, because the region has weak disease surveillance systems, the prevalence and distribution of STH infection remains largely unknown. The helminthological data that do exist in the formal and ‘grey’ literature have been collated into a GIS database (Brooker et al., 2000). However, this continental database includes data for only 15% of districts in SSA, with most data on hookworm infection. Furthermore, for reasons outlined previously (Stoll, 1947; Brooker & Michael, 2000), the inherent variability in the data, because of differences in the timing, methodology and study population of studies, makes it difficult to use these data to reliably define the distribution of STH species. Instead, we have utilized the robust predictions of STH prevalence developed here (Figure 2). These predictions provide perhaps the most detailed description of STH infection in SSA to date.
9. Predicted numbers of infections
As indicated, school-based deworming represents the most cost-effective and feasible intervention strategy for STH infections and the greatest need for control exists in sub-Saharan Africa. For these reasons, we derive country-specific estimates of school-aged children infected in the region. Consistent with other analyses in this edition, population was estimated from the Gridded Population of the World version 3 (GPW3) global human population distribution for 2005 (Balk et al. this volume). Using a “West” model life table with a female life expectancy of 50 years and an annual rate of growth of 3% (Coale & Demeny, 1966), it was assumed that 27.2% of the total population was school-aged (5-14 years). These population data were spatially related to our species-specific risk models (Figure 2), which were re-sampled at one kilometre, the spatial resolution of GPW3. Since the models were developed using prevalence estimates among school-aged children it is assumed that models define prevalence for this age group. For each district, population totals and predicted infection prevalence were extracted and used to estimate numbers of school-aged children infected with each species. Since no clear patterns in urban-rural differences in species prevalence exist (Table 2) no adjustment was made for the effect of urbanization.
Infections A. lumbricoides, T. trichiura and hookworm are often found in the same communities and individuals and benzimidazole anthelmintics, such as albendazole and mebendazole, are broadly effective against each species. In order to estimate the cost of interventions against multiple STH infections, an estimate of the numbers of infections of any species, either single or multiple species infections, is required. To quantify the numbers of multiple species infections we use a validated probabilistic model to predict the prevalence of multiple-species infections for each district for which only overall prevalence data exist (Booth & Bundy, 1995), thereby allowing the number of children infected with any STH species to be estimated.
It is estimated that over 35.4 million African school-aged children are infected with A. lumbricoides, 40.1 million with T. trichiura and 41.1 million with hookworm. Since many children have multiple infections, it is estimated that 89.9 million are infected with any STH species. Forty four per cent of the infections are concentrated in just four countries: in descending order of magnitude these are Nigeria, the Democratic Republic of Congo, South Africa and Tanzania (Table 3). Previous estimates have suggested larger numbers of infections; the most recent estimate suggesting that 53 million school-aged children (5-15 years) are infected with A. lumbricoides, 50 million with T. trichiura and 47 million with hookworm (de Silva et al., 2003). Previous estimates at a regional or continental scale have all been based on the approach first established by Stoll in 1947: the prevalence data from the few studies available within a country are used to estimate a mean prevalence for the country as a whole, and then expressed as a product of the estimated number of school age children in the country. The estimates we present here are the first at this scale to explicitly include the fine spatial variation in distribution of both infection and population, and indicate that the earlier methodology tended to overestimate. It is also worth emphasizing that this level of precision has been achieved affordably only because of the use of GIS/RS: using traditional survey methods to obtain these data would be prohibitively expensive.
Table 3.
Estimated numbers of STH infection among school-aged children in sub-Saharan Africa by country, 2005
|
Country |
School-aged population (1000s) |
Estimated numbers of infections (1000s) |
Estimated numbers requiring mass treatment based 50% threshold (1000s) |
Total annual per treatment cost (US$ 1000s)† |
|||
|---|---|---|---|---|---|---|---|
| A. lumbricoides | T. trichiura | Hookworm | Any STH species | ||||
| Angola | 3,697 | 722 | 860 | 841 | 1,894 | 2,031 | 122-183 |
| Benin | 1,751 | 303 | 426 | 320 | 831 | 836 | 50-75 |
| Botswana | 426 | 10 | 11 | 97 | 113 | - | - |
| Burkina Faso | 3,250 | 35 | 42 | 609 | 671 | - | - |
| Burundi | 1,783 | 366 | 412 | 467 | 955 | 753 | 45-68 |
| Cameroon | 4,125 | 1,452 | 1,800 | 850 | 2,726 | 3,017 | 181-272 |
| Cape Verde | 112 | 8 | 15 | 35 | 50 | 22 | 1-2 |
| CAR* | 1,022 | 236 | 275 | 205 | 554 | 640 | 38-58 |
| Chad | 2,204 | 30 | 32 | 425 | 474 | - | - |
| Comoros | 147 | 85 | 98 | 36 | 131 | 147 | 9-13 |
| DRC* | 14,269 | 4,865 | 5,690 | 3,041 | 9,637 | 13,410 | 805-1,207 |
| Congo, Republic of | 847 | 286 | 374 | 161 | 574 | 828 | 50-75 |
| Coted' Ivoire | 4,436 | 1,657 | 2,154 | 837 | 3,187 | 3,967 | 238-357 |
| Djibouti | 171 | 1 | 1 | 33 | 34 | - | - |
| Equatorial Guinea | 126 | 83 | 98 | 30 | 118 | 126 | 8-11 |
| Eritrea | 1,029 | 13 | 17 | 260 | 281 | 4 | 0.3-0.4 |
| Ethiopia | 17,571 | 1,956 | 1,983 | 4,882 | 7,357 | 4,476 | 269-403 |
| Gabon | 335 | 184 | 231 | 63 | 295 | 331 | 20-30 |
| Gambia | 356 | 18 | 25 | 80 | 112 | 41 | 2-4 |
| Ghana | 5,371 | 1,574 | 2,053 | 1,043 | 3,282 | 3,551 | 213-320 |
| Guinea | 2,245 | 598 | 751 | 469 | 1,324 | 1,450 | 87-131 |
| Guinea Bissau | 333 | 55 | 79 | 67 | 161 | 105 | 6-9 |
| Kenya | 8,455 | 1,343 | 1,399 | 2,152 | 3,919 | 3,661 | 220-330 |
| Lesotho | 553 | 208 | 262 | 166 | 423 | 553 | 33-50 |
| Liberia | 813 | 474 | 586 | 149 | 734 | 813 | 49-73 |
| Madagascar | 4,451 | 1,820 | 2,101 | 982 | 3,144 | 3,312 | 199-298 |
| Malawi | 3,139 | 558 | 662 | 687 | 1,524 | 1,308 | 79-118 |
| Mali | 3,186 | 48 | 60 | 601 | 688 | - | - |
| Mauritania | 339 | 6 | 13 | 60 | 75 | - | - |
| Mauritius | 295 | 198 | 229 | 68 | 277 | 295 | 18-27 |
| Mozambique | 5,049 | 1,177 | 1,435 | 976 | 2,765 | 3,319 | 199-299 |
| Namibia | 477 | 8 | 11 | 112 | 126 | - | - |
| Niger | 3,060 | 16 | 23 | 581 | 613 | - | - |
| Nigeria | 32,009 | 5,552 | 7,666 | 6,685 | 15,183 | 14,286 | 857-1,286 |
| Rwanda | 2,122 | 561 | 635 | 573 | 1,271 | 1,326 | 80-119 |
| Réunion | 194 | 137 | 155 | 49 | 185 | 194 | 12-17 |
| Senegal | 2,596 | 502 | 598 | 472 | 1,022 | 560 | 34-50 |
| Seychelles | 22 | 8 | 9 | 13 | 19 | 22 | 1-2 |
| Sierra Leone | 1,234 | 562 | 713 | 256 | 1,003 | 1,234 | 74-111 |
| Somalia | 2,479 | 161 | 187 | 641 | 802 | 274 | 16-25 |
| South Africa | 11,886 | 4,067 | 4,981 | 2,694 | 7,773 | 7,770 | 466-699 |
| Sudan | 8,644 | 102 | 126 | 1,717 | 1,896 | - | - |
| Swaziland | 258 | 99 | 120 | 53 | 189 | 258 | 15-23 |
| São Tomé & Príncipe | 37 | 30 | 35 | 7 | 37 | 37 | 2-3 |
| Tanzania | 9,709 | 1,640 | 1,863 | 2,277 | 4,588 | 3,906 | 234-352 |
| Togo | 1,260 | 176 | 257 | 236 | 550 | 348 | 21-31 |
| Uganda | 6,595 | 1,567 | 1,676 | 1,586 | 3,628 | 3,498 | 210-315 |
| Zambia | 2,877 | 452 | 511 | 674 | 1,333 | 1,044 | 63-94 |
| Zimbabwe | 3,463 | 343 | 390 | 804 | 1,311 | 302 | 18-27 |
| Total | 180,808 | 36,350 | 44,130 | 40,123 | 89,841 | 84,056 | 5,043-7,565 |
CAR = Central African Republic; DRC = Democratic Republic of Congo
Range based on delivery costs of US$ 0.03 to 0.04 per child and drug costs US$ 0.03 to 0.05 per child
The estimates provide a basis for estimating the financial resources required to support school-based deworming in sub-Saharan Africa. It is assumed that mass treatment is only provided in districts where prevalence of any STH infection exceeds 50%, and in these districts treatment is provided to all school age children irrespective of infection status, according to WHO guidelines on mass treatment (WHO, 2002). Cost analyses estimate that the school based delivery costs of albendazole or mebendazole, anthelmintics that are effective against all the common STH species, are in the range US$ 0.03 to 0.04 per capita (Partnership for Development, 1999), while the drug costs are in the range US$ 0.03 to 0.05 per capita (Guyatt, 2003). Under these assumptions, using the school based approach to provide a single annual treatment to all school age children in all districts in Africa where mass treatment is justified would cost US$ 5.0 to 7.6 million.
These cost estimates are for maintaining a program and do not include the higher costs of start-up. Nevertheless they suggest that continent-wide control of parasites is, from a financial perspective, an attainable goal.
10. The future
GIS/RS is a powerful tool that has evolved from supporting sophisticated epidemiological research, through a role in directing public health interventions, to now showing a real potential for assisting the design of sustainable development programmes. The initial epidemiological research was critical to this evolution, because it has provided the evidence for the link between transmission dynamics and the environmental factors that can be detected by RS. Furthermore, as illustrated here, it can provide important new insights into patterns of transmission at a geographical scale that has proven impossible to address using more traditional approaches.
The present analyses have shown that, with the available evidence, GIS/RS can predict patterns of STH transmission, and can do so at a scale that is relevant to the design of national control programs. Most importantly, this approach is able to achieve this even for areas for which limited empirical data are available and at low cost relative to traditional survey technologies. This approach has the potential to facilitate the design of national control programmes, and the key question now is how best to realize this potential.
The experience of international vertical programmes aimed at controlling lymphatic filariasis, schistosomiasis and onchocerciasis provides some insights, but it is perhaps the examples from Chad, Eritrea, Tanzania and Uganda of national government efforts that more clearly point the way forward. GIS/RS has emerged as a tried and tested and increasingly acceptable technology that can provide national governments with a relatively low cost approach to surveying and programme design, and which can significantly reduce the cost of practical programmes through more precise geographical targeting and simplifying the processes of monitoring and evaluation. It is important to recognize that GIS/RS can reduce both the upstream (e.g. survey and design) and downstream (e.g. targeting, monitoring and evaluation) costs of programmes, while at the same time enhancing programme effectiveness.
As more use is made of GIS/RS in control, there is a need to assess their cost and cost-effectiveness in targeting control efforts. At present, most funding for their use comes from international agencies and northern researchers. Determining the long-term sustainability of the use of GIS/RS in disease control and how they influence allocation of resources is crucial. It is also important to assess the inherent uncertainties in spatial data (epidemiological and environmental) within a GIS and the propagation of uncertainty in predictions and estimations. Such research is underway for ecological modeling but similar research is clearly needed for epidemiological applications.
Notwithstanding these issues, the clear message is that this technology should be made available to policy makers and planners at the national level. In terms of helminth control an essential component is to enhance access to the predicted geographical patterns of transmission, such as those described here. As a contribution to improving access, all the maps presented in this text are made available at www.schoolsandhealth.org, and are available separately to individual countries in CD format by request from the same site. In this same spirit we welcome the release of the population and environmental data with this volume.
Acknowledgement
We thank for following individuals who have generously contributed data to this review: Henrietta Allen, Chris Appleton, Narcis Kabatereine, Nicholas Lwambo and Antonio Montresor. We are also grateful to Paul Coleman for his contribution to the STH ecological analysis and to Christine Budke, Maiza Campos Ponce, Bob Snow and Anthony Wilson for comments on this manuscript. SB is supported by a Wellcome Trust Advanced Training Fellowship (073656) and ACAC is funded by the Schistosomiasis Control Initiative, which receives support from the Bill and Melinda Gates Foundation.
References
- Addiss DG, Rheingans R, Twum-Danso NA, Richards FO. A framework for decision-making for mass distribution of Mectizan in areas endemic of Loa loa. Filaria Journal. 2003;2(Suppl. 1):S9. doi: 10.1186/1475-2883-2-S1-S9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albonico M, Chwaya HM, Montresor A, Stolfzfus RJ, Tielsch JM, Alawi KS, Savioli L. Parasitic infections in Pemba Island school children. East African Medical Journal. 1997;74:294–298. [PMC free article] [PubMed] [Google Scholar]
- Allen H, Sithey G, Padmasiri EA, Montresor A. Epidemiology of soil-transmitted helminths in the western region of Bhutan. Southeast Asian Journal of Tropical Medicine and Public Health. 2004;35:1–3. [PMC free article] [PubMed] [Google Scholar]
- Alexander ND, Moyeed RA, Hyun PJ, Dimber ZB, Bockarie MJ, Stander J, Grenfell BT, Kazura JW, Alpers MP. Spatial variation of Anopheles-transmitted Wuchereria bancrofti and Plasmodium falciparum infection densities in Papua New Guinea. Filaria Journal. 2003;2:14. doi: 10.1186/1475-2883-2-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anon . Report of the National Anemia and Nutrition Risk Factor Survey Vietnam 1995. Hanoi: UNICEF; 1995. [Google Scholar]
- Anantaphruti MT, Nuamtanong S, Muennoo C, Sanguankiat S, Pubampen S. Strongyloides stercoralis infection and chronological changes of other soil-transmitted helminthiases in an endemic area of southern Thailand. Southeast Asian Journal of Tropical Medicine and Public Health. 2000;31:378–382. [PubMed] [Google Scholar]
- Anantaphruti MT, Jongsuksuntigul P, Imsomboon T, Nagai N, Muennoo C, Saguankiat S, Pubampen S, Kojima S. School-based helminthiases control: I. A baseline study of soil-transmitted helminthiases in Nakhon Si Thammarat Province, Thailand. Southeast Asian Journal of Tropical Medicine and Public Health. 2002;33(Suppl 3):113–119. [PubMed] [Google Scholar]
- Anantaphruti MT, Waikagul J, Maipanich W, Nuamtanong S, Pubampen S. Soil-transmitted helminthiases and health behaviors among schoolchildren and community members in a west-central border area of Thailand. Southeast Asian Journal of Tropical Medicine and Public Health. 2004;35:260–266. [PubMed] [Google Scholar]
- Anderson RM. The population dynamics and control of hookworm and roundworm infection. In: Anderson RM, editor. Population Dynamics of Infectious Diseases: Theory and Applications. London: Chapman and Hall; 1982. pp. 67–109. [Google Scholar]
- Anderson RM, May RM. Population dynamics of human helminth infections: control by chemotherapy. Nature. 1982;297:557–563. doi: 10.1038/297557a0. [DOI] [PubMed] [Google Scholar]
- Anderson RM, May RM. Infectious Diseases of Humans: dynamics and control. Oxford: Oxford University Press; 1991. [Google Scholar]
- Appleton CC, Gouws E. The distribution of common intestinal nematodes along an altitudinal transect in Kwa-Zulu Natal, South Africa. Annals of Tropical Medicine and Parasitology. 1996;90:181–188. doi: 10.1080/00034983.1996.11813042. [DOI] [PubMed] [Google Scholar]
- Appleton CC, Maurihungirire M, Gouws E. The distribution of helminth infections along the coastal plain of Kwazulu-Natal province, South Africa. Annals of Tropical Medicine and Parasitology. 1999;93:859–868. doi: 10.1080/00034989957862. [DOI] [PubMed] [Google Scholar]
- Awasthi S, Bundy DAP, Savioli L. Helminthic infections. British Medical Journal. 2003;23:431–433. doi: 10.1136/bmj.327.7412.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaver PC. Persistence of Hookworm Larvae in Soil. American Journal of Tropical Medicine and Hygiene. 1953;2:102–108. doi: 10.4269/ajtmh.1953.2.102. [DOI] [PubMed] [Google Scholar]
- Beer RJ. The relationship between Trichuris trichiura (Linnaeus 1758) of man and Trichuris suis (Schrank 1788) of the pig. Research in Veterinary Science. 1976;20:47–54. [PubMed] [Google Scholar]
- Booth M, Bundy DAP. Estimating the number of multiple-species geohelminth infections in human communities. Parasitology. 1995;111:645–553. doi: 10.1017/s0031182000077131. [DOI] [PubMed] [Google Scholar]
- Brooker S, Rowlands M, Haller L, Savioli L, Bundy DAP. Towards an atlas of human helminth infection in sub-Saharan Africa: the use of geographical information systems (GIS) Parasitology Today. 2000;16:303–307. doi: 10.1016/s0169-4758(00)01687-2. [DOI] [PubMed] [Google Scholar]
- Brooker S. Schistosomes, snails and satellites. Acta Tropica. 2002;82:209–216. doi: 10.1016/s0001-706x(02)00012-8. [DOI] [PubMed] [Google Scholar]
- Brooker S, Beasley NMR, Ndinaromtan M, Madjiouroum EM, Baboguel M, Djenguinabe E, Hay SI, Bundy DAP. Use of remote sensing and a geographical information system in a national helminth control programme in Chad. Bulletin of the World Health Organization. 2002a;80:783–789. [PMC free article] [PubMed] [Google Scholar]
- Brooker S, Hay SI, Bundy DAP. Tools from ecology: useful for evaluating infection risk models? Trends in Parasitology. 2002b;18:70–74. doi: 10.1016/s1471-4922(01)02223-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooker S, Hay SI, Tchuem Tchuenté LA, Ratard R. Using NOAA-AVHRR data to model helminth distributions for planning disease control in Cameroon, West Africa. Photogrammetric Engineering and Remote Sensing. 2002c;68:175–179. [Google Scholar]
- Brooker S, Pratap S, Waikagul J, Suvanee S, Kojima S, Takeuchi T, Luong TV, Looareesuwan S. Mapping soil-transmitted helminth infections in Southeast Asia and implications for parasite control. Southeast Asian Journal of Tropical Medicine and Public Health. 2003;34:24–35. [PubMed] [Google Scholar]
- Brooker S, Kabatereine NB, Clements ACA, Stothard JR. Schistosomiasis control. Lancet. 2004a;363:658–659. doi: 10.1016/S0140-6736(04)15604-3. [DOI] [PubMed] [Google Scholar]
- Brooker S, Kabatereine NB, Tukahebwa EM, Kazibwe F. Spatial analysis of the distribution of intestinal nematode infections in Uganda. Epidemiology and Infection. 2004b;132:1065–1071. doi: 10.1017/s0950268804003024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooker S, Kabatereine NB, Myatt M, Stothard JR, Fenwick A. Rapid assessment of Schistosoma mansoni: the validity, applicability and cost-effectiveness of the Lot Quality Assurance Sampling method in Uganda. Tropical Medicine and International Health. 2005;10:647–658. doi: 10.1111/j.1365-3156.2005.01446.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooker S, Michael E. The potential of geographical information systems and remote sensing in the epidemiology and control of human helminth infections. Advances in Parasitology. 2000;47:245–288. doi: 10.1016/s0065-308x(00)47011-9. [DOI] [PubMed] [Google Scholar]
- Bundy DAP. This wormy world then and now. Parasitology Today. 1997;13:407–408. [Google Scholar]
- Bundy DAP, Chan MS, Medley GF, Jamison D, Savioli L. Chapter 9, Intestinal Nematode Infections. In: Murray CJL, Lopez AD, Mathers CD, editors. Global Epidemiology of Infectious Disease. WHO; 2004. pp. 243–300. (Global Burden of Disease Volume IV). http://whqlibdoc.who.int/publications/2004/9241592303.pdf. [Google Scholar]
- Bundy DAP, Cooper ES. Trichuris and trichuriasis in humans. Advances in Parasitology. 1989;28:107–173. doi: 10.1016/s0065-308x(08)60332-2. [DOI] [PubMed] [Google Scholar]
- Bundy DAP, Guyatt HL. Schools for health: focus on health, education and the school-aged child. Parasitology Today. 1996;12:1–16. doi: 10.1016/0169-4758(96)30011-2. [DOI] [PubMed] [Google Scholar]
- Bundy DAP, Medley GF. Immuno-epidemiology of human geohelminthiasis: ecological and immunological determinants of worm burden. Parasitology. 1992;104:S105–S119. doi: 10.1017/s0031182000075284. [DOI] [PubMed] [Google Scholar]
- Bundy D, Shaeffer S, Jukes M, Beegle K, Gillespie A, Drake L, Lee Seung-hee Frances, Hoffman A-M, Jones J, Mitchell A, Wright C, Barcelona D, Camara B, Golmar C, Savioli L, Takeuchi T, Sembene M. Chapter 61, School Based Health and Nutrition Programs. In: Jamison D, Claeson M, Breman J, Meacham A, editors. Disease Control Priorities for Developing Countries. Oxford: Oxford University Press; 2005. [Google Scholar]
- Bundy DAP, Wong MS, Lewis LL, Horton J. Control of geohelminths by delivery of targeted chemotherapy through schools. Transactions of the Royal Society of Tropical Medicine and Hygiene. 1990;84:115–120. doi: 10.1016/0035-9203(90)90399-y. [DOI] [PubMed] [Google Scholar]
- Carabin H, Escalona M, Marshall C, Vivas-Martinez S, Botto C, Joseph L, Basanez MG. Prediction of community prevalence of human onchocerciasis in the Amazonian onchocerciasis focus: Bayesian approach. Bulletin of the World Health Organization. 2003;81:482–490. [PMC free article] [PubMed] [Google Scholar]
- Chan MS, Medley GF, Jamison D, Bundy DAP. The evaluation of potential global morbidity attributable to intestinal nematode infections. Parasitology. 1994a;109:373–387. doi: 10.1017/s0031182000078410. [DOI] [PubMed] [Google Scholar]
- Chongsuvivatwong V, Pas-Ong S, Ngoathammatasna W, McNeil D, Vithsupakorn K, Bridhikitti V, Jongsuksuntigul P, Jeradit C. Evaluation of hookworm control program in southern Thailand. Southeast Asian Journal of Tropical Medicine and Public Health. 1994;25:745–751. [PubMed] [Google Scholar]
- Clements ACA, Lwambo NJS, Blair L, Nyandindi U, Kataano G, Fenwick A, Webster JP, Brooker S. Bayesian spatial analysis and risk-mapping: tools to enhance planning of a schistosomiasis control programme in Tanzania. American Journal of Tropical Medicine and Hygiene. 2005 doi: 10.1111/j.1365-3156.2006.01594.x. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coale AJ, Demeny P. Regional Model Life Tables and Stable Populations. Princeton: Princeton University Press; 1966. [Google Scholar]
- Cracknell AP. The Advanced Very High Resolution Radiometer. London: Taylor & Francis; 1997. Earth surfaces temperatures; pp. 181–231. [Google Scholar]
- Crompton DWT. Ascaris and ascariasis. Advances in Parasitology. 2001;48:285–375. doi: 10.1016/s0065-308x(01)48008-0. [DOI] [PubMed] [Google Scholar]
- Crompton DWT, Savioli L. Intestinal parasitic infections and urbanization. Bulletin of the World Health Organization. 1993;71:1–7. [PMC free article] [PubMed] [Google Scholar]
- Crompton DWT, Tulley JJ. How much Ascariasis is there in Africa? Parasitology Today. 1987;3:123–127. doi: 10.1016/0169-4758(87)90054-8. [DOI] [PubMed] [Google Scholar]
- de Silva NR, Brooker S, Hotez PJ, Montresor A, Engels D, Savioli L. Soil-transmitted helminth infections: updating the global picture. Trends in Parasitology. 2003;19:547–551. doi: 10.1016/j.pt.2003.10.002. [DOI] [PubMed] [Google Scholar]
- Drake LJ, Jukes MCH, Sternberg RJ, Bundy DAP. Geohelminth infections (Ascariasis, Trichuriasis and Hookworm): cognitive and developmental impacts. Seminars in Pediatric Infectious Diseases. 2000;11:245–251. [Google Scholar]
- Ehrenberg JP, de Mérida AM, Sentz J. An epidemiological overview of geohelminth and schistosomiasis in the Caribbean. Washington DC: PAHO; 2003. (PAHO/DPC/CD/P/242/03). [Google Scholar]
- Fenwick A, Savioli L, Engels D, Bergquist RN, Todd MH. Drugs for the control of parasitic diseases: current status and development in schistosomiasis. Trends in Parasitology. 2003;19:509–515. doi: 10.1016/j.pt.2003.09.005. [DOI] [PubMed] [Google Scholar]
- Giraldi N, Vidotto O, Navarro IT, Garcia JL. Enteroparasites prevalence among daycare and elementary school children of municipal schools, Rolandia, PR, Brazil. Revista da Sociedade Brasileira de Medicina Tropical. 2001;34:385–357. doi: 10.1590/s0037-86822001000400014. [DOI] [PubMed] [Google Scholar]
- Government of Pakistan . National nutrition survey 1985-87. Islamabad: National Institute of Health, Ministry of Health; 1988. [Google Scholar]
- Guyatt HL, Brooker S, Hall A, Kihamia CK, Bundy DAP. Evaluation of efficacy of school-based anthelmintic treatments against anaemia in children in the United Republic of Tanzania. Bulletin of the World Health Organization. 2001;79:695–703. [PMC free article] [PubMed] [Google Scholar]
- Guyatt HL. The cost of delivering and sustaining a control programme for schistosomiasis and soil-transmitted helminthiasis. Acta Tropica. 2003;86:267–274. doi: 10.1016/s0001-706x(03)00047-0. [DOI] [PubMed] [Google Scholar]
- Gyapong JO, Kyelem D, Kleinschmidt I, Agbo K, Ahouandogbo F, Gaba J, Owusu-Banahene G, Sanou S, Sodahlon YK, Biswas G, Kale OO, Molyneux DH, Roungou JB, Thomson MC, Remme J. The use of spatial analysis in mapping the distribution of bancroftian filariasis in four West African countries. Annals of Tropical Medicine and Parasitology. 2002;96:695–705. doi: 10.1179/000349802125001735. [DOI] [PubMed] [Google Scholar]
- Hotez PJ, Brooker S, Bethony JM, Bottazzi ME, Loukas A, Xiao S. Current concepts: Hookworm infection. New England Journal of Medicine. 2004;351:799–807. doi: 10.1056/NEJMra032492. [DOI] [PubMed] [Google Scholar]
- Hotez PJ, Bundy DAP, Beegle K, Brooker S,, de Silva N, Montresor A, Engels D, Drake L, Chitsulo L, Michaud C, Bethony JM, Oliveira R, Xiao SH, Fenwick A, Savioli L. Disease Control Priorities in Developing Countries. 2nd Edition. WHO, World Bank, NIH, Oxford University Press; 2005. Helminth Infections. in press. [Google Scholar]
- Hughes RG, Sharp DS, Hughes MC, Akau'ola S, Heinsbroek P, Velayudhan R, Schulz D, Palmer K, Cavalli-Sforza T, Galea G. Environmental influences on helminthiasis and nutritional status among Pacific schoolchildren. International Journal of Environmental Health Research. 2004;14:163–177. doi: 10.1080/0960312042000218589. [DOI] [PubMed] [Google Scholar]
- Kabatereine NB, Brooker S, Tukahebwa EM, Kazibwe F, Onapa A. Epidemiology and geography of Schistosoma mansoni in Uganda: implications for planning control. Tropical Medicine and International Health. 2004;9:372–380. doi: 10.1046/j.1365-3156.2003.01176.x. [DOI] [PubMed] [Google Scholar]
- Kabatereine NB, Tukahebwa EM, Kazibwe F, Namwangye H, Zaramba S, Brooker S, Stothard JR, Kamenka C, Whawell S, Webster JP, Fenwick A. Progress towards country-wide control of schistosomiasis and soil-transmitted helminthiasis in Uganda. Transactions of the Royal Society of Tropical Medicine and Hygiene. 2005 doi: 10.1016/j.trstmh.2005.03.015. in press. [DOI] [PubMed] [Google Scholar]
- Kan SP, Guyatt HL, Bundy DAP. Geohelminth infection of children from rural plantations and urban slums in Malaysia. Transactions of the Royal Society of Tropical Medicine and Hygiene. 1989;83:817–820. doi: 10.1016/0035-9203(89)90342-8. [DOI] [PubMed] [Google Scholar]
- Kasuya S, Khamboonruang C, Amano K, Murase T, Araki H, Kato Y, Kumada Y, Koyama A, Higuchi M, Nakamura J. Intestinal parasitic infections among schoolchildren in Chiang Mai, northern Thailand: an analysis of the present situation. Journal of Tropical Medicine and Hygiene. 1989;92:360–364. [PubMed] [Google Scholar]
- Katabarwa M, Onapa AW, Nakileza B. Rapid epidemiological mapping of onchocerciasis in areas of Uganda where Simulium neavei sl is the vector. East Africa Medical Journal. 1999;76:440–446. [PubMed] [Google Scholar]
- Kilma WL. Hookworm infection and disease in Africa and the Middle East. In: Schad GA, Warren KS, editors. Hookworm Disease. Current Status and New Directions. London: Taylor & Francis; 1990. pp. 17–32. [Google Scholar]
- King RJ, Campbell-Lendrum DH, Davies CR. Predicting geographic variation in cutaneous leishmaniasis, Colombia. Emerging Infectious Diseases. 2004;10:598–607. doi: 10.3201/eid1004.030241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai D, His BP. Soil-transmitted helminthiasis in China: a spatial statistical analysis. Southeast Asian Journal of Tropical Medicine and Public Health. 1996;27:754–759. [PubMed] [Google Scholar]
- Lengeler C, Utzinger J, Tanner M. Questionnaires for rapid screening of schistosomiasis in sub-Saharan Africa. Bulletin of the World Health Organization. 2002;80:235–242. [PMC free article] [PubMed] [Google Scholar]
- Margono SS. Large-scale control against intestinal helminthic infections in Japan, with special reference to the activities of Japan Association of Parasite Control. In: Hayashi S, editor. Collected Papers on the Control of Soil-transmitted Helminthiases. VII. Tokyo: Asian Parasite Control Organization; 2001. pp. 169–172. [Google Scholar]
- Mascie-Taylor CGN, Alam M, Montanari RM, Karim R, Ahmed T, Karim E, Akhtar S. A study of the cost effectiveness of selective health interventions for the control of intestinal parasites in rural Bangladesh. Journal of Parasitology. 1999;85:6–11. [PubMed] [Google Scholar]
- Miguel E, Kremer M. Worms: identifying impacts on education and health in the presence of treatment externalities. Econometrica. 2004;72:159–217. [Google Scholar]
- Molyneux DH, Bradley M, Hoerauf A, Kyelem D, Taylor MJ. Mass drug administration for lymphatic filariasis and onchocerciasis. Trends in Parasitology. 2003;19:516–522. doi: 10.1016/j.pt.2003.09.004. [DOI] [PubMed] [Google Scholar]
- Montresor A, Zin TT, Padmasiri E, Allen H, Savioli L. Soil-transmitted helminthiasis in Myanmar and approximate costs for countrywide control. Tropical Medicine and International Health. 2004;9:1012–1015. doi: 10.1111/j.1365-3156.2004.01297.x. [DOI] [PubMed] [Google Scholar]
- Nacher M, Singhasivanon P, Yimsamran S, Manibunyong W, Thanyavanich N, Wuthisen R, Looareesuwan S. Intestinal helminth infections are associated with increased incidence of Plasmodium falciparum malaria in Thailand. Southeast Asian Journal of Tropical Medicine and Public Health. 2002;88:55–58. doi: 10.1645/0022-3395(2002)088[0055:IHIAAW]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Ndenecho L, Ndamukong KJ, Matute MM. Soil transmitted nematodes in children in Buea Health District of Cameroon. East Africa Medical Journal. 2002;79:442–445. doi: 10.4314/eamj.v79i8.8832. [DOI] [PubMed] [Google Scholar]
- Ngoumou P, Walsh JF, Mace JM. A rapid mapping technique for the prevalence and distribution of onchocerciasis: a Cameroon case study. Annals of Tropical Medicine and Parasitology. 1994;88:463–474. doi: 10.1080/00034983.1994.11812893. [DOI] [PubMed] [Google Scholar]
- Noma M, Nwoke BEB, Nutall I, Tambala PA, Enyong P, Namsenmo A, Remme J, Amazigo UV, Kale OO, Seketeli A. Rapid epidemiology mapping of onchocerciasis (REMO): its application by the African Programme for Onchocerciasis Control (APOC) Annals of Tropical Medicine and Parasitology. 2002;96:S29–S39. doi: 10.1179/000349802125000637. [DOI] [PubMed] [Google Scholar]
- Nwosu ABC. Investigations Into the Free-Living Phase of the Cat Hookworm Life-cycle. Zeitschrift fur Parasitenkunde. 1978;56:243–249. doi: 10.1007/BF00931717. [DOI] [PubMed] [Google Scholar]
- Nwosu ABC, Anya AO. Seasonality in human hookworm infection in an endemic area of Nigeria, and its relationship to rainfall. Tropenmedizin Und Parasitologie. 1980;31:201–208. [PubMed] [Google Scholar]
- Otto GF. A study of the moisture requirements of the eggs of the horse, the dog, human and pig ascarids. American Journal of Hygiene. 1929;10:497–520. [Google Scholar]
- Pan-American Health Organization (PAHO) Meeting on the Control of Intestinal Helminthiasis in the Context of AIEPI. Washington DC: PAHO; 2000. (PAHO/HTC/AIEPI-21-E). [Google Scholar]
- Partnership for Child Development The health and nutritional status of school children in Africa: evidence from school-based health programmes in Ghana and Tanzania. Transactions of the Royal Society of Tropical Medicine and Hygiene. 1998;92:254–261. doi: 10.1016/s0035-9203(98)90999-3. [DOI] [PubMed] [Google Scholar]
- Partnership for Child Development The cost of large-scale school health programmes which deliver anthelmintics to children in Ghana and Tanzania. Acta Tropica. 1999;73:183–204. doi: 10.1016/s0001-706x(99)00028-5. [DOI] [PubMed] [Google Scholar]
- Partnership for Child Development . A situation analysis of the health of school children in Eritrea. London: Imperial College; 2003. [Google Scholar]
- Pavlovsky EP. Natural Nidality of Transmissible Diseases - with special reference to the landscape epidemiology of zooanthroponoses. Urbana: University of Illinois Press; 1966. [Google Scholar]
- Peters W. The relevance of parasitology to human welfare today. Symposia of the British Society for Parasitology. 1978;16:25–40. [Google Scholar]
- Phiri K, Whitty CJ, Graham SM, Ssembatya-Lule G. Urban/rural differences in prevalence and risk factors for intestinal helminth infection in southern Malawi. Annals of Tropical Medicine and Parasitology. 2000;94:381–387. doi: 10.1080/00034983.2000.11813553. [DOI] [PubMed] [Google Scholar]
- Rahman WA. Helminthic infections of urban and rural schoolchildren in Penang Island, Malaysia: implications for control. Asian Journal of Tropical Medicine and Public Health. 1998;29:596–598. [PubMed] [Google Scholar]
- Ratard RC, Kouemeni LE, Bessala MME, Ndamkou CN, Sama MT, Cline BL. Ascariasis and trichuriasis in Cameroon. Transactions of the Royal Society of Tropical Medicine and Hygiene. 1991;85:84–88. doi: 10.1016/0035-9203(91)90170-4. [DOI] [PubMed] [Google Scholar]
- Ratard RC, Kouemeni LE, Ekani Bessala MK, Ndamkou CN. Distribution of hookworm infection in Cameroon. Annals of Tropical Medicine and Parasitology. 1992;86:413–8. doi: 10.1080/00034983.1992.11812686. [DOI] [PubMed] [Google Scholar]
- Rim HJ, Chai JY, Min DY, Cho SY, Eom KS, Hong SJ, Sohn WM, Yong TS, Deodato G, Standgaard H, Phommasack B, Yun CH, Hoang EH. Prevalence of intestinal parasite infections on a national scale among primary schoolchildren in Laos. Parasitological Research. 2003;91:267–272. doi: 10.1007/s00436-003-0963-x. [DOI] [PubMed] [Google Scholar]
- Saathoff E, Olsen A, Sharp B, Kvalsvig JD, Appleton CC, Kleinschmidt I. Ecologic covariates of hookworm infection and reinfection in rural KwaZulu-Natal/South Africa: a geographic information system-based study. American Journal of Hygiene and Tropical Medicine. 2005;72:384–391. [PubMed] [Google Scholar]
- Schad GA, Chowdhury AB, Dean CG, Kochar V, Nawalinski TA, Thomas J, Tonascia JA. Arrested development in human hookworm infectios: an adaptation to seasonality unfavourable external environment. Science. 1973;180:502–504. doi: 10.1126/science.180.4085.502. [DOI] [PubMed] [Google Scholar]
- Seamster AP. Developmental studies concenring the eggs of Ascaris lumbricoides var. suum. The American Midland Naturalist. 1950;43:450–468. [Google Scholar]
- Singh B, Cox-Singh J. Parasites that cause problems in Malaysia: soil-transmitted helminths and malaria parasites. Trends in Parasitology. 2001;17:597–600. doi: 10.1016/s1471-4922(01)02148-1. [DOI] [PubMed] [Google Scholar]
- Sinuon M, Anantaphruti MT, Socheat D. Intestinal helminthic infections in schoolchildren in Cambodia. Southeast Asian Journal of Tropical Medicine and Public Health. 2003;34:254–258. [PubMed] [Google Scholar]
- Smith G, Schad GA. Ancylostoma duodenale and Necator americanus: effect of temperature on egg development and mortality. Parasitology. 1990;99:127–132. doi: 10.1017/s0031182000061102. [DOI] [PubMed] [Google Scholar]
- Spindler LA. The relation of moisture to the distribution of human trichuris and ascaris. American Journal of Hygiene. 1929;10:476–496. [Google Scholar]
- Srividya A, Michael E, Palaniyandi M, Pani SP, Das PK. A geostatistical analysis of the geographic distribution of lymphatic filariasis prevalence in southern India. American Journal of Tropical Medicine Hygiene. 2002;67:480–489. doi: 10.4269/ajtmh.2002.67.480. [DOI] [PubMed] [Google Scholar]
- Stephenson LS, Latham MC, Ottesen EA. Malnutrition and parasitic helminth infections. Parasitology. 2000;121(Suppl):S23–S38. doi: 10.1017/s0031182000006491. [DOI] [PubMed] [Google Scholar]
- Stoll NR. This Wormy World. Journal of Parasitology. 1947;33:1–18. [PubMed] [Google Scholar]
- Tay J, Salazar-Schettino PM, de Haro Arteaga I, Bucio Torres MI. Incidence of intestinal helminthiasis in Mexico. Revista de Investigacíon en Salud Pública. 1976;36:241–280. [PubMed] [Google Scholar]
- Tay J, Ruiz A, Sanchez Vega JT, Romero-Cabello R, Robert L, Becerril MA. Intestinal helminthiasis in the Mexican Republic. Boletín Chileno de Parasitología. 1995;50:10–16. [PubMed] [Google Scholar]
- Thomson MC, Obsomer V, Dunne M, Connor SJ, Molyneux DH. Satellite mapping of Loa loa prevalence in relation to ivermectin use in west and central Africa. Lancet. 2000;356:1077–1078. doi: 10.1016/s0140-6736(00)02733-1. [DOI] [PubMed] [Google Scholar]
- Thomson MC, Obsomer V, Kamgno J, Gardon J, Wanji S, Takougang I, Enyong P, Remme JH, Molyneux DH, Boussinesq M. Mapping the distribution of Loa loa in Cameroon in support of the African Programme for Onchocerciasis Control. Filaria Journal. 2004;3:7. doi: 10.1186/1475-2883-3-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Udonsi JK, Atata G. Necator americanus: temperature, pH, light, and larval development, longevity, and desiccation tolerance. Experimental Parasitology. 1987;63:136–142. doi: 10.1016/0014-4894(87)90154-8. [DOI] [PubMed] [Google Scholar]
- Udonsi JK, Nwosu ABC, Anya AO. Necator americanus: Population Structure, Distribution, and Fluctuations in Population Densities of Infective Larvae in Contaminated Farmlands. Zeitschrift fur Parasitenkunde. 1980;63:251–259. doi: 10.1007/BF00931987. [DOI] [PubMed] [Google Scholar]
- United Nations . World Urbanization Prospects: The 2003 Revision. Department of Economic and Social Affairs, Population Division. New York: United Nations; 2003. [Google Scholar]
- Urbani C, Odermatt P, Socheat D, Sinoun M, Hoyer S, Hatz C. Control of soil-transmitted helminth infections in schoolchildren in Cambodia,. Implications for an integrated approach to helminth control. In: Crompton DWT, Nesheim MC, Savioli L, editors. Controlling Disease Caused by Helminth Infections. Geneva: WHO; 2001. [Google Scholar]
- Utzinger J, Keiser J. Schistosomiasis and soil-transmitted helminthiasis: common drugs for treatment and control. Expert Opinion in Pharmacotherapy. 2004;5:263–286. doi: 10.1517/14656566.5.2.263. [DOI] [PubMed] [Google Scholar]
- van der Hoek W, De NV, Konradsen F, Cam PD, Hoa NT, Toan ND, Cong le D. Current status of soil-transmitted helminths in Vietnam. Southeast Asian Journal of Tropical Medicine and Public Health. 2003;34(Suppl 1):1–11. [PubMed] [Google Scholar]
- Vinha C. Occurrence, in Brazil, of helminthiasis transmitted by the soil. Croposcopic routine of the ex-Departmento Nacional de Endemias Rurais. Revista Brasileira de Malariologia e Doencas Tropicais. 1971;23:3–17. [PubMed] [Google Scholar]
- Waikagul J, Krudsood S, Radomyos P, Radomyos B, Chalemrut K, Jonsuksuntigul P, Kojima S, Looareesuwan S, Thaineau W. A cross-sectional study of intestinal parasitic infections among schoolchildren in Nan Province, Northern Thailand. Southeast Asian Journal of Tropical Medicine and Public Health. 2002;33:218–223. [PubMed] [Google Scholar]
- Warren KS, Bundy DAP, Anderson RM, Davis AR, Henderson DA, Jamison DT, Prescott N, Senft A. Helminth infections. In: Jamison DT, Mosley WH, Measham AR, Bobadilla JL, editors. Disease Control Priorities in Developing Countries. Oxford: Oxford University Press; 1993. pp. 131–160. [Google Scholar]
- WHO (World Health Organization) Report of the WHO Informal Consultation on the use of chemotherapy for the control of morbidity due to soil-transmitted nematodes in humans. Geneva: WHO; 1996. (WHO/ CTD/ SIP/ 96.2). [Google Scholar]
- WHO (World Health Organization) (TDR/TDF/COMDT/98.2).Research on Rapid Geographical Assessment of Bancroftian Filariasis. 1998 [Google Scholar]
- WHO (World Health Organization) Prevention and Control of Schistosomiasis and Soil-Transmitted Helminthiasis. Geneva: World Health Organization; 2002. (WHO Technical Series Report 912). [PubMed] [Google Scholar]
- World Bank . FRESH: Focusing Resources on Education and School Health. A FRESH start to improving the quality and equity of education. UNESCO, UNICEF, WHO and World Bank; 2000. http://www.schoolsandhealth.org/FRESH.htm. [Google Scholar]
- Xu L-Q, Yu S-H, Jiang Z-X, Yang J-L, Lai C-Q, Zhang XJ, Zheng C-Q. Soil-transmitted helminthiases: nationwide survey in China. Bulletin of the World Health Organization. 1995;73:507–513. [PMC free article] [PubMed] [Google Scholar]