Abstract
Oligomeric state makes important contributions to the signaling mechanisms of costimulatory molecules. In this study we address the biological relevance of the disulfide-linked dimeric structure of CD28. Fluorescence Resonance Energy Transfer (FRET) demonstrates that removal of the interdomain disulfide bond (C123) does not interfere with the formation of CD28 oligomers on the cell surface. Although the C123S mutant shows 40% lower binding affinity to the ligand B7-1, it is able to costimulate anti-CD3-induced IL-2 production but at a lower level (150%) compared to the wild-type (270%). Interestingly, binding to B7-2 was not affected. Thus, the covalently linked dimeric structure of CD28 represents an important mechanistic determinant for the optimal costimulatory activity in the immunological synapse.
Keywords: CD28, costimulatory, oligomerization, dimer, disulfide, FRET, B7-1
Introduction
Activation of T lymphocytes requires the engagement of their T cell receptor (TCR) by the MHC-peptide complex expressed on the surface of antigen presenting cells (APC), and a second signal provided by the CD28:B7 family of costimulatory molecules [1; 2]. The finely regulated integration of costimulatory signals is essential for the initiation, regulation and modulation of T cell responses. Upon binding to the B7-1 and B7-2 ligands expressed on APCs, the CD28 costimulatory receptor promotes T cell proliferation and differentiation by directing enhanced IL-2 production and initiating survival signals for T cells [3; 4]. B7-1 and B7-2 bind with higher affinity to CTLA-4, an inhibitory receptor expressed on activated T cells, which attenuates the T cell response [5]. CD28 and CTLA-4 belong to the immunoglobulin superfamily, and share a similar molecular architecture. They both exist as disulfide-linked homodimers due to an extracellular interchain disulfide formed by the equivalent of C123 in CD28, which resides in the linker region connecting the IgV and transmembrane domains. The extracellular cysteine at position 123 is highly conserved in CD28 [6–9] chicken CD28 being the only known exception [10]. In this study we address the biological relevance of the disulfide-linked dimeric structure of CD28 by examining the biochemical, biophysical, and functional properties of the C123S mutant, which is missing the interdomain disulfide bond.
Materials and methods
Constructs and mutagenesis
Mouse and human CD28 cDNAs were cloned in-frame into ECFP-N1 and EYFP-N1 (Clontech) expression vectors that had been mutated (A206K) to prevent self-dimerization [11]. The C123S mutants of human and mouse CD28 were generated by site-directed mutagenesis.
Mice
CD28-/- mice [12] on the C57BL/6 background were purchased from Jackson Laboratories and were maintained in pathogen free conditions. All animal work was performed according to the guidelines set by the AECOM Institutional Animal Care and Use Committee.
Western blotting
HEK293T cells transiently transfected with wild-type or mutant CD28 were lysed in the presence of 1% protease inhibitor mixture (Sigma) and 10 mM iodoacetamide, to prevent non-specific disulfide formation. Proteins were analyzed by Western blotting using goat anti-CD28 and HRP-coupled anti-goat antibodies (Santa Cruz Biotechnology).
Fusion proteins and binding assay
B7-1-Ig and B7-2-Ig were constructed as dimeric fusion proteins of the extracellular domain (residues 1-214) of human B7-1 or B7-2 and the Fc part of mouse IgG2a with a thrombin cleavage site and a His6-tag at the C-terminus of the Fc. The fusion proteins were purified from the cell culture supernatants of transfected HEK293T cells by Ni-NTA (Qiagen) affinity chromatography. CD28-transfected HEK293T cells were incubated with B7-1-Ig or B7-2-Ig (0.01–50µg/ml) and with Cy5-conjugated anti-mouse IgG F(ab)’ (Jackson Immunoresearch) and analyzed by flow cytometry.
Photobleaching FRET by Confocal Microscopy
Sample preparation and photobleaching FRET (pbFRET) studies were performed as previously described [11]. Fixed cells were examined with a Leica TCS SP II AOBS laser-scanning confocal microscope at the Analytical Imaging Facility at AECOM. Excitation wavelengths of 405 nm for CFP and 514 nm for YFP, and emission ranges of 416–492 nm for CFP and 525–600 nm for YFP were used. FRET efficiency (E) was calculated using the formula E(%) = [(Dpost - Dpre)/Dpost]×100, where Dpre is the fluorescence intensity of the donor CFP before, and Dpost after photobleaching the acceptor. 4–5 regions of interest were analyzed for each cell. Data were collected from 30–50 different cells per sample.
Retroviral infection of primary CD4+ T cells
CD4+ T cells isolated from the spleens and lymph nodes of CD28-/- mice using anti-CD4 Dynabeads (Invitrogen) were activated with plate-bound a-CD3ε (BD Biosciences) and 20U/ml IL-2 added 24 hours after activation. Retrovirus was made by transfecting Phoenix ecotropic packaging cells (kindly provided by GP Nolan’s laboratory, Stanford University) with CD28 cloned into RV-IRES-GFP retroviral vector [13]. Retroviruses were pre-adsorbed onto RetroNectin (Takara Bio Inc) coated plates[14], over which T cells were added and cultured for 2–3 days, then sorted.
In vitro T cell assay
CD28-reconstituted CD4+ T cells were activated with plate-bound anti-CD3ε in the presence of B7-1-expressing CHO cells, or untransfected CHO cells. 24 hours later IL-2 expression was determined by ELISA (BD Bioscience).
Statistical analysis
Student’s t test was used to analyze the difference between two groups. A p value of <0.01 was considered significant.
Results
C123S mutant CD28 is expressed on the cell surface and binds to its ligands
The oligomeric state of C123S and wild-type CD28 was confirmed by SDS-PAGE (Figure 1A). Removal of the disulfide bond associated with the C123S mutation does not affect cell surface expression detected by monoclonal antibody staining (Figure 1B). However, our binding data show that C123S CD28 binds 40% less well to B7-1 than wild-type CD28 (Figure 1C). Interestingly, there was no difference in binding to B7-2- Ig (Figure 1D).
Figure 1. C123S mutant CD28 is expressed on the cell surface but it binds to the ligand B7-1 with a lower affinity than the wild-type.
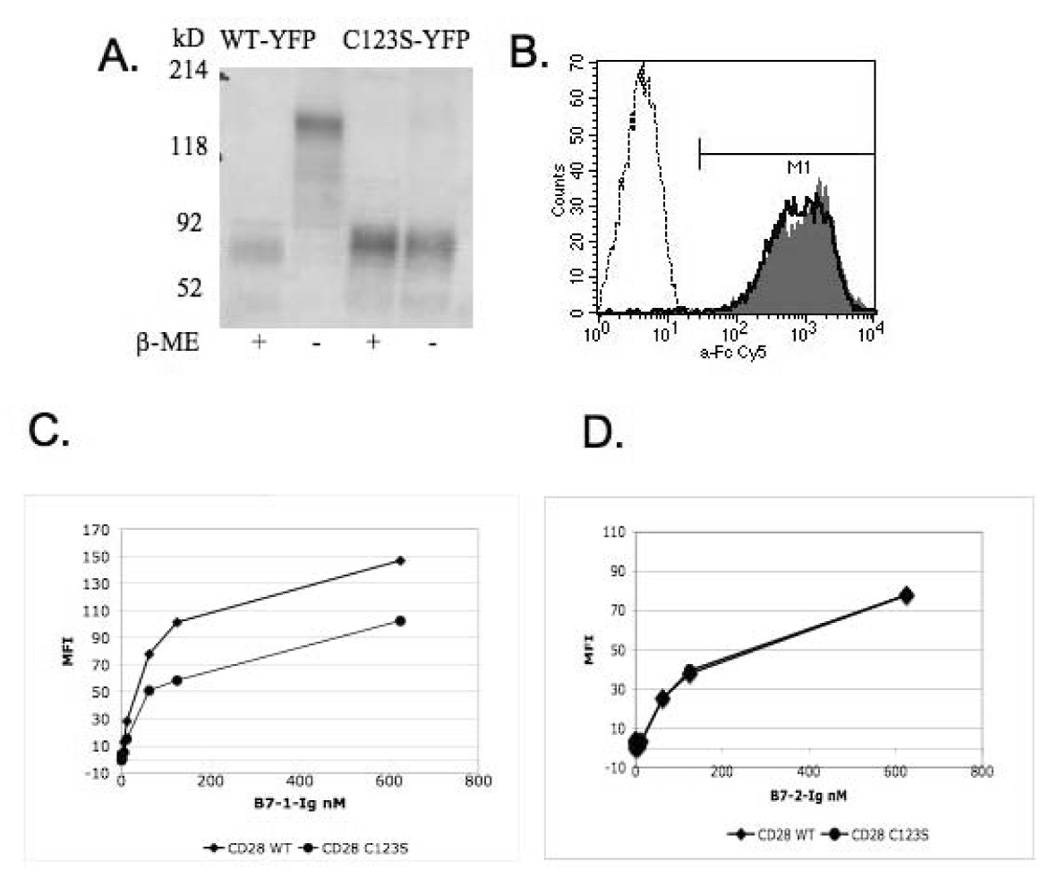
CD28 constructs were transiently expressed in HEK293T cells as YFP fusion proteins. A) On SDS-PAGE, C123S CD28 shows a single band under both reduced and non-reduced conditions equivalent with the monomeric form, as opposed to the wild-type CD28 that is a dimer under non-reduced conditions. B) Both C123S mutant and wild-type CD28 are expressed equally well on the cell surface. Cells transfected with wild-type (filled histogram) and C123S (solid line) CD28, as well as mock-transfected (dashed line) cells stained with an anti-mouse CD28 monoclonal antibody (37.51). C) Binding assay with B7-1-Ig. D) Binding assay with B7-2-Ig. Detection was performed using anti-Fc-Cy5 secondary staining. Normalized MFI values are plotted after substracting the background MFI of mock-transfected cells.
Lack of the interchain disulfide does not prevent non-covalent oligomerization of CD28 on the cell surface
The removal of the interchain disulfide may result in the expression of either purely monomeric form of CD28, or in the formation of non-covalent oligomers. To best address this question, we have used pbFRET, a combination of confocal microscope imaging and FRET that allows for the interaction of molecules to be studied on the intact cell surface. An increase in donor fluorescence after the acceptor is photobleached will indicate that donor and acceptor were in close proximity (1–10 nm) (Supplementary Figure A). Previous studies suggest that FRET data can be used to distinguish between a population of randomly distributed molecules (e.g. monomers) and specific molecular clusters ranging from dimers to complex higher order oligomers [11; 15; 16]. For a clustered distribution of molecules (i.e. oligomers), theoretical models [17] indicate that E is independent of acceptor densities, but increases with an increase of the acceptor-donor ratio. However, in case of a random distribution (monomer), E increases with increasing acceptor density but will not depend on the acceptor-donor ratio.
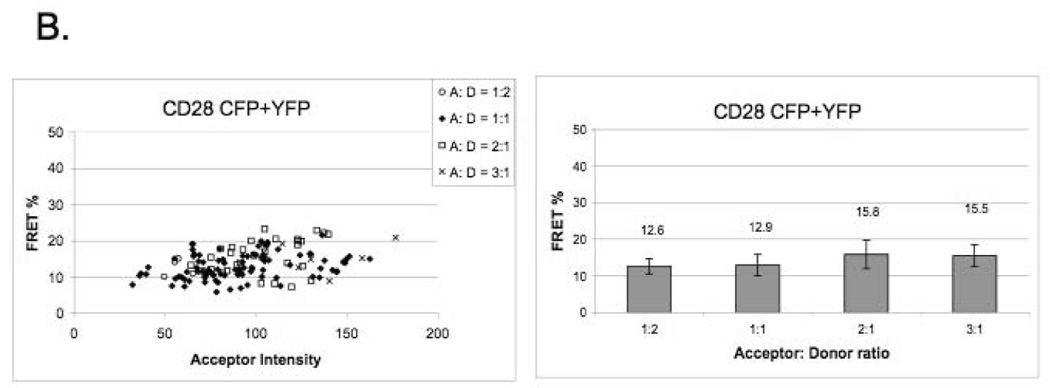
Our data indicate that the C123S mutant CD28 does not exist as monomer on the cell surface, but it rather exists as a dimer (or higher oligomer) (Figure 2A), since in correlation with the theoretical model, FRET efficiency is not dependent on acceptor density over wide ranges of YFP intensity (expressed in arbitrary units). Our FRET data are consistent with biochemical data that wild-type CD28 is a disulfide-linked dimer, since the E values do not depend on the intensity of the acceptor and there is a modest increase of E in function of the YFP-to-CFP ratio. (Figure 2B). Our FRET data were validated by using tandem B7-1-CFP-YFP expressing cells as positive control [11], and cells expressing non-interacting proteins as negative control (Supplementary Figure B).
Figure 2. CD28 exists as a dimer on the cell surface in the absence of the extracellular disulfide bond.
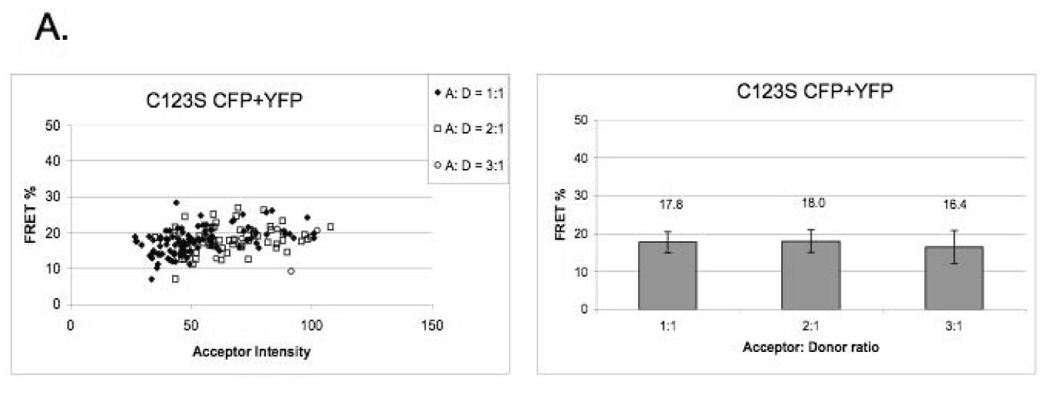
A) C123S mutant CD28 shows a clustered distribution on the cell surface. FRET efficiency (E) does not depend on acceptor density over a wide range of YFP intensity (expressed in arbitrary units).
B) FRET efficiency of covalently linked wild-type CD28 shows a clustered distribution. E values do not depend on increasing YFP intensity and there is a modest increase of E in function of the YFP-to-CFP ratio.
CD28 lacking the interchain disulfide is functional but it costimulates T cells less well than the wild-type
In order to test whether the interchain disulfide of CD28 is a prerequisite for signaling, we have performed in vitro T cell assays by comparing the costimulatory activity of the C123S mutant to that of the wild-type. CD28-/- CD4+ T cells were reconstituted by retroviral infection [13; 14] with either the wild-type or C123S CD28 molecule (expressed at the same level, data not shown) and were activated in vitro with anti-CD3 (0.1-1 µg/ml) in the presence or absence of B7-1. Figure 3A shows that T cells expressing either wild-type or mutant CD28 produce more IL-2 in the presence of B7-1 but there is no such difference when T cells are reconstituted with the retroviral vector (RV-IRES-GFP). However, by comparing the relative costimulatory activity of wild-type and C123S mutant CD28 (Figure 3B), it is apparent that C123S CD28 is less potent in inducing IL-2 production, this difference being more pronounced at low dose (0.1 µg/ml) of anti-CD3 activation (150% versus 270%).
Figure 3. Costimulatory activity of CD28 is lower in the absence of the interchain disulfide in an in vitro T cell assay.
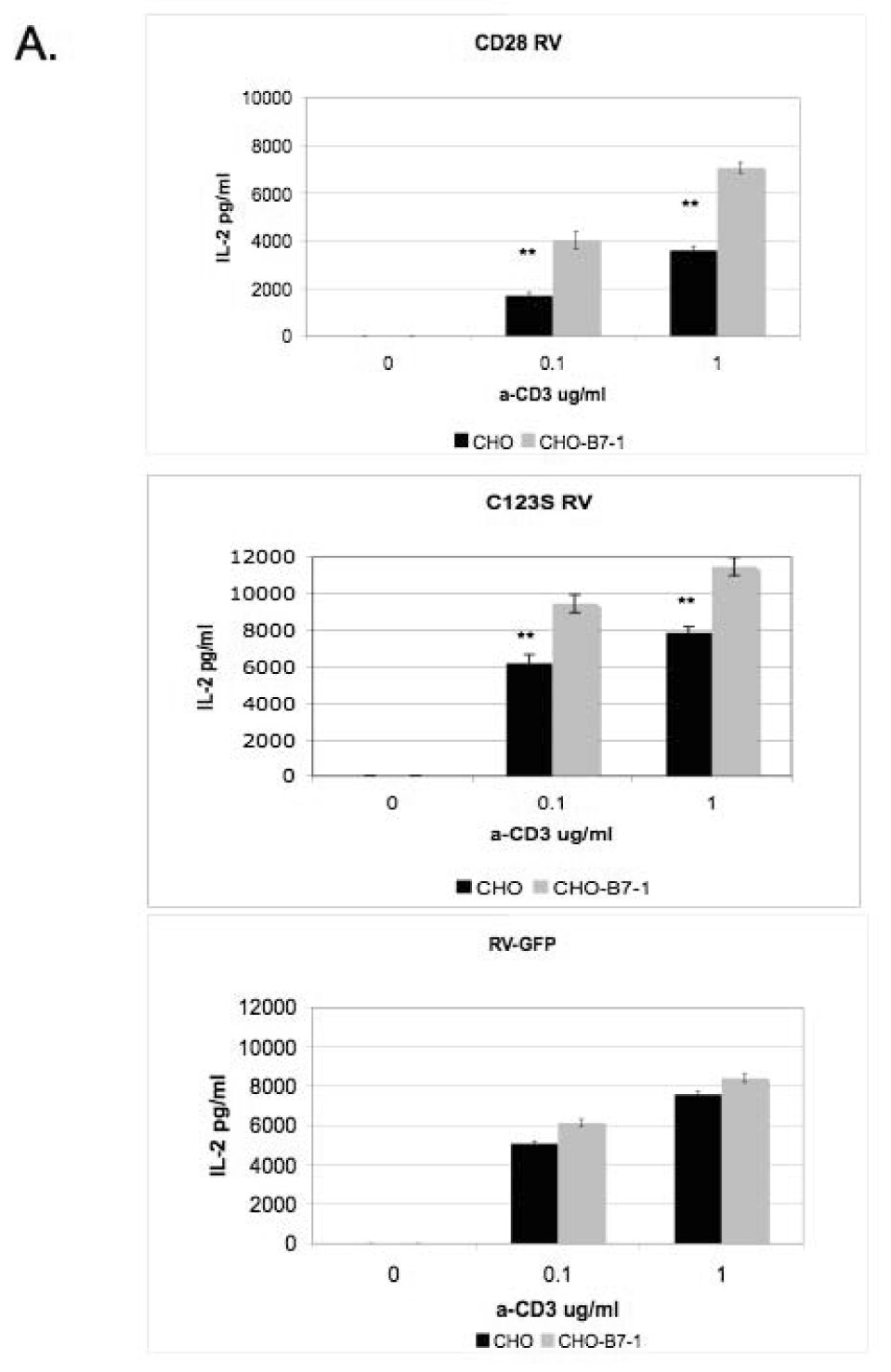
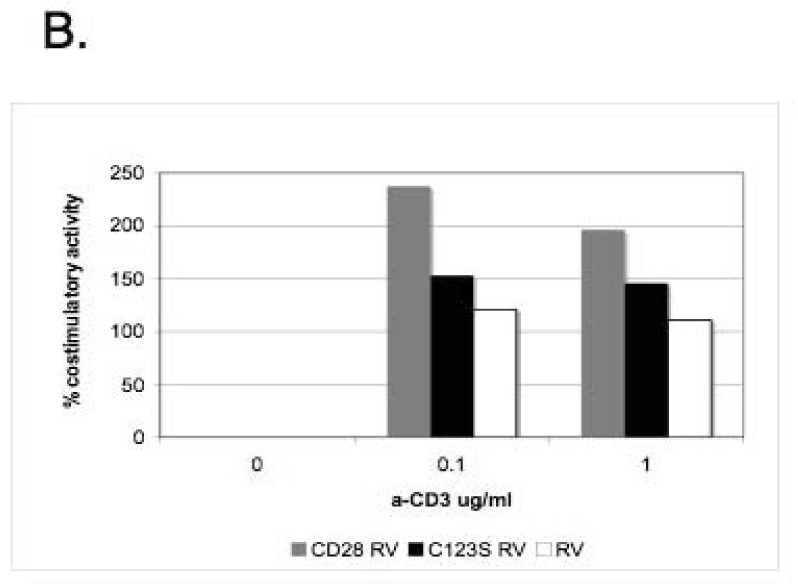
A) T cells expressing wild-type or C123S mutant CD28 produce more IL-2 upon stimulation in the presence of stably transfected CHO-B7- 1 cells. CD28-/- T cells were reconstituted with wild-type (top panel) or C123S (middle panel) CD28 or empty retroviral vector (RV-IRES-GFP, lower panel) and stimulated with anti-CD3, IL-2 was measured by ELISA. **, p < 0.01 C) Relative costimulatory activity is lower for C123S compared to wild-type CD28. Ratios of IL-2 from costimulated (anti-CD3+CHO-B7-1) and non-costimulated (anti-CD3+CHO) T cells are shown. The results shown are representative of three independent experiments.
Discussion
The oligomeric state of costimulatory molecules is an important structural feature that has direct implications for the mechanism of signaling in the immunological synapse. We studied the physiological significance of the covalent dimeric structure of CD28 by disrupting it using site-directed mutagenesis. Our data show that the C123S mutant CD28 is expressed on the cell surface as demonstrated by specific antibody staining and it is properly folded since it binds to B7-2-Ig (Figure 1D). However, it is less able to bind to B7-1-Ig than the disulfide linked wild-type molecule. Although the cysteine is not located in the immediate proximity of the ligand-binding groove, it may stabilize the homodimer in a way that is more optimal for ligand binding.
Our results show that the C123S mutant CD28 is not monomeric, but it forms higher oligomeric clusters since detailed analysis of the FRET efficiency values shows the characteristics of a clustered distribution. Thus, our data support the idea that CD28 has an intrinsic propensity to dimerize and the disulfide bond “traps” pre-existing dimers. In the absence of the disulfide bond (as in the case of C123S mutant), non-covalent interactions of the dimer interface [18] support the formation and stabilization of a non-covalent dimer on the cell surface. The importance of the dimeric structure is supported by observations that disrupting it by mutating the residues involved will reduce or abolish cell surface expression ([18] and unpublished data).
Moreover, our studies show for the first time that when C123S mutant CD28 is reconstituted into primary CD28-/- CD4+ T cells, it can efficiently induce costimulatory signals by enhancing IL-2 production in an in vitro T cell assay; however, its costimulatory activity is reduced compared to the wild-type (150% versus 270% compared to anti-CD3 alone). This decrease in the biological activity is most probably due to the observed 40% difference in the ligand binding (Figure 1C). However, given its membrane proximal location, this cysteine might also have a constraining effect to stabilize the homodimer and to bring the cytoplasmic tails into an arrangement that is optimal for recruitment of intracellular signaling molecules. Thus, the covalently linked dimeric structure of CD28 represents an important mechanistic determinant for the optimal costimulatory activity in the immunological synapse.
Acknowledgements
E.L-M was supported by a postdoctoral fellowship from Cancer Research Institute.
We thank Sumeena Bhatia for providing the stably transfected CHO-B7-1 cell line and the B7-1-CFP-YFP expression plasmid; we acknowledge Sanchari Bhattacharya’s contributions to this project. We thank the Analytical Imaging Facility at Albert Einstein College of Medicine and especially Michael Cammer; the Flow Cytometry Facility supported by the grant of the National Cancer Institute’s cancer center (P30CA013330) and especially William King; Fernando Macian and Olga Ujhelly for help with the retroviral infection; Teresa DiLorenzo, Fernando Macian and Kausik Chattopadhyay for critically reviewing the manuscript. This work was supported by National Institutes of Health Grant AI07289 (to S.G.N.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Supplementary Material
A) Pre- and postbleach images of cells expressing CD28-CFP and -YFP. CFP intensity is enhanced after photobleaching, shown by the color gradient FRET image (from blue to red). E = FRET efficiency, Dpre = intensity of the donor before photobleaching, Dpost = intensity of the donor after photobleaching. B) Validation of FRET: covalently linked B7-1-CFP-YFP tandem protein is used as positive control, in which case FRET efficiency does not depend on the acceptor density (expressed in arbitrary units). In case of a negative control such as non-interacting CD28 and PD-1 any increase in E values is due to the non-specific random clustering of the molecules due to high expression levels associated with transient transfection.
References
- 1.Greenwald RJ, Freeman GJ, Sharpe AH. The B7 family revisited. Annu Rev Immunol. 2005;23:515–548. doi: 10.1146/annurev.immunol.23.021704.115611. [DOI] [PubMed] [Google Scholar]
- 2.Sharpe AH, Freeman GJ. The B7-CD28 superfamily. Nat Rev Immunol. 2002;2:116–126. doi: 10.1038/nri727. [DOI] [PubMed] [Google Scholar]
- 3.Fraser JD, Irving BA, Crabtree GR, Weiss A. Regulation of interleukin-2 gene enhancer activity by the T cell accessory molecule CD28. Science. 1991;251:313–316. doi: 10.1126/science.1846244. [DOI] [PubMed] [Google Scholar]
- 4.Burr JS, Savage ND, Messah GE, Kimzey SL, Shaw AS, Arch RH, Green JM. Cutting edge: distinct motifs within CD28 regulate T cell proliferation and induction of Bcl-XL. J Immunol. 2001;166:5331–5335. doi: 10.4049/jimmunol.166.9.5331. [DOI] [PubMed] [Google Scholar]
- 5.Walunas TL, Lenschow DJ, Bakker CY, Linsley PS, Freeman GJ, Green JM, Thompson CB, Bluestone JA. CTLA-4 can function as a negative regulator of T cell activation. Immunity. 1994;1:405–413. doi: 10.1016/1074-7613(94)90071-x. [DOI] [PubMed] [Google Scholar]
- 6.Aruffo A, Seed B. Molecular cloning of a CD28 cDNA by a high-efficiency COS cell expression system. Proc Natl Acad Sci U S A. 1987;84:8573–8577. doi: 10.1073/pnas.84.23.8573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Greene JL, Leytze GM, Emswiler J, Peach R, Bajorath J, Cosand W, Linsley PS. Covalent dimerization of CD28/CTLA-4 and oligomerization of CD80/CD86 regulate T cell costimulatory interactions. J Biol Chem. 1996;271:26762–26771. doi: 10.1074/jbc.271.43.26762. [DOI] [PubMed] [Google Scholar]
- 8.Gross JA, St John T, Allison JP. The murine homologue of the T lymphocyte antigen CD28. Molecular cloning and cell surface expression. J Immunol. 1990;144:3201–3210. [PubMed] [Google Scholar]
- 9.Harper K, Balzano C, Rouvier E, Mattei MG, Luciani MF, Golstein P. CTLA-4 and CD28 activated lymphocyte molecules are closely related in both mouse and human as to sequence, message expression, gene structure, and chromosomal location. J Immunol. 1991;147:1037–1044. [PubMed] [Google Scholar]
- 10.Young JR, Davison TF, Tregaskes CA, Rennie MC, Vainio O. Monomeric homologue of mammalian CD28 is expressed on chicken T cells. J Immunol. 1994;152:3848–3851. [PubMed] [Google Scholar]
- 11.Bhatia S, Edidin M, Almo SC, Nathenson SG. Different cell surface oligomeric states of B7-1 and B7-2: implications for signaling. Proc Natl Acad Sci U S A. 2005;102:15569–15574. doi: 10.1073/pnas.0507257102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shahinian A, Pfeffer K, Lee KP, Kundig TM, Kishihara K, Wakeham A, Kawai K, Ohashi PS, Thompson CB, Mak TW. Differential T cell costimulatory requirements in CD28-deficient mice. Science. 1993;261:609–612. doi: 10.1126/science.7688139. [DOI] [PubMed] [Google Scholar]
- 13.Macian F, Garcia-Cozar F, Im SH, Horton HF, Byrne MC, Rao A. Transcriptional mechanisms underlying lymphocyte tolerance. Cell. 2002;109:719–731. doi: 10.1016/s0092-8674(02)00767-5. [DOI] [PubMed] [Google Scholar]
- 14.Hanenberg H, Xiao XL, Dilloo D, Hashino K, Kato I, Williams DA. Colocalization of retrovirus and target cells on specific fibronectin fragments increases genetic transduction of mammalian cells. Nat Med. 1996;2:876–882. doi: 10.1038/nm0896-876. [DOI] [PubMed] [Google Scholar]
- 15.Kenworthy AK, Edidin M. Distribution of a glycosylphosphatidylinositol-anchored protein at the apical surface of MDCK cells examined at a resolution of <100 A using imaging fluorescence resonance energy transfer. J Cell Biol. 1998;142:69–84. doi: 10.1083/jcb.142.1.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pentcheva T, Edidin M. Clustering of peptide-loaded MHC class I molecules for endoplasmic reticulum export imaged by fluorescence resonance energy transfer. J Immunol. 2001;166:6625–6632. doi: 10.4049/jimmunol.166.11.6625. [DOI] [PubMed] [Google Scholar]
- 17.Edidin M. John Wiley & Sons I, editor. Fluorescence Resonance Energy Transfer: Techniques for Measuring Molecular Conformation and Molecular Proximity. Current Protocols in Immunology. 2003 doi: 10.1002/0471142735.im1810s52. [DOI] [PubMed] [Google Scholar]
- 18.Evans EJ, Esnouf RM, Manso-Sancho R, Gilbert RJ, James JR, Yu C, Fennelly JA, Vowles C, Hanke T, Walse B, Hunig T, Sorensen P, Stuart DI, Davis SJ. Crystal structure of a soluble CD28-Fab complex. Nat Immunol. 2005;6:271–279. doi: 10.1038/ni1170. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
A) Pre- and postbleach images of cells expressing CD28-CFP and -YFP. CFP intensity is enhanced after photobleaching, shown by the color gradient FRET image (from blue to red). E = FRET efficiency, Dpre = intensity of the donor before photobleaching, Dpost = intensity of the donor after photobleaching. B) Validation of FRET: covalently linked B7-1-CFP-YFP tandem protein is used as positive control, in which case FRET efficiency does not depend on the acceptor density (expressed in arbitrary units). In case of a negative control such as non-interacting CD28 and PD-1 any increase in E values is due to the non-specific random clustering of the molecules due to high expression levels associated with transient transfection.


