Abstract
In response to inflammatory stimuli (e.g., endotoxin, proinflammatory cytokines) or oxidative stress, macrophages actively release a ubiquitous nuclear protein, high-mobility group box 1 (HMGB1), to sustain an inflammatory response to infection or injury. In this study, we demonstrated mild heat shock (e.g., 42.5°C, 1 h), or enhanced expression of heat shock protein (Hsp) 72 (by gene transfection) similarly rendered macrophages resistant to oxidative stress-induced HMGB1 cytoplasmic translocation and release. In response to oxidative stress, cytoplasmic Hsp72 translocated to the nucleus, where it interacted with nuclear proteins including HMGB1. Genetic deletion of the nuclear localization sequence (NLS) or the peptide binding domain (PBD) from Hsp72 abolished oxidative stress-induced nuclear translocation of Hsp72-ΔNLS (but not Hsp72-ΔPBD), and prevented oxidative stress-induced Hsp72-ΔPBD-HMGB1 interaction in the nucleus. Furthermore, impairment of Hsp72-ΔNLS nuclear translocation, or Hsp72-ΔPBD-HMGB1 interaction in the nucleus, abrogated Hsp72-mediated suppression of HMGB1 cytoplasmic translocation and release. Taken together, these experimental data support a novel role for nuclear Hsp72 as a negative regulator of oxidative stress-induced HMGB1 cytoplasmic translocation and release.
Oxidative stress is caused by excessive accumulation of reactive oxygen species (ROS)4 as a result of a defective antioxidant defense system of the cell, and has been implicated in a variety of pathophysiological conditions including inflammation and the aging process (1-3). An increase in intracellular ROS levels can lead to cell damages via lipid peroxidation, protein cross-linkage, and DNA breakage. ROS generation is also inducible by various proinflammatory cytokines such as IL-1 (4) and TNF-α (5), and occupies a pathogenic role in various inflammatory diseases (3).
High mobility group box 1 (HMGB1) is a major component of mammalian chromatin endowed with an architectural function. It binds within the minor groove of DNA and bends the double helix to facilitate the formation of multiprotein complexes (6). It facilitates numerous nuclear transactions, including transcription, replication, V(D)J recombination, and DNA transposition and interacts with p53, steroid receptors, NF-κB, homeobox-containing proteins, TATA-binding protein, and several viral proteins (6). Recently, HMGB1 has been established as an inflammatory mediator of lethal endotoxemia and sepsis (7-9). Although residing predominantly in the nucleus of quiescent macrophages, HMGB1 can be actively secreted in response to exogenous and endogenous inflammatory stimuli such as endotoxin, TNF-α, IL-1, and IFN-γ, and hydrogen peroxide (7, 10-13). In addition, HMGB1 can be passively released by necrotic cells (9, 14), and extracellular HMGB1 mediates a wide range of inflammatory responses. In vitro, extracellular HMGB1 can activate macrophages/monocytes (15) and dendritic cells (16-18) and promote cell proliferation, migration, and differentiation (19-24). In vivo, HMGB1 cause acute lung inflammation, and derangement of the epithelial-cell barrier function (25, 26).
Representing a universal response to diverse adverse stimuli, cells rapidly express stress-inducible heat shock proteins (Hsps) such as Hsp90, Hsp70, Hsp60, and Hsp27 (27). As major stress-inducible proteins, the Hsp70 family consists of ubiquitous Hsp73 and of Hsp72 inducible by heat shock, oxidative stress, and infection. Intracellular Hsp72 functions as a molecular chaperone to maintain cellular homeostasis (27-29), and nuclear Hsp72 confers a protective role against various environmental stress (30-33). In addition, Hsp72 can be released to the extracellular milieu and functions as a danger signal for the immune system (34).
We have recently demonstrated that oxidative stress (induced by hydrogen peroxide) induces active HMGB1 release in macrophage/monocyte cultures (13). However, the potential role of Hsp72 in the regulation of oxidative stress-induced HMGB1 release was previously unknown. In this study, we demonstrated that mild heat shock (e.g., 42.5°C, 1 h), or enhanced expression of Hsp72 (by gene transfection) similarly rendered RAW 264.7 cells resistant to hydrogen peroxide-induced HMGB1 cytoplasmic translocation and release. In response to oxidative stress, cytoplasmic Hsp72 translocated to the nucleus, where it interacted with nuclear proteins including HMGB1 and prevented oxidative stress (H2O2)-induced HMGB1 cytoplasmic translocation and release. The nuclear Hsp72-HMGB1 interaction may be a universal nuclear stress response to various adverse stimuli.
Materials and Methods
Cell culture and treatment
Murine macrophage-like RAW 264.7 cells and K562 human myeloid leukemia cells were obtained from the Shanghai Type Culture Collection and cultured in RPMI 1640 (Invitrogen Life Technologies) supplemented with 5–10% heat-inactivated FBS, 2 mM glutamine, and antibiotic-antimycotic mix in a humidified incubator with 5% CO2 and 95% air. Hydrogen peroxide was dissolved in PBS and further diluted in culture medium. Quercetin (Sigma-Aldrich) was dissolved in DMSO. Cells were pretreated with or without quercetin for the indicated time, before the addition of H2O2.
Heat shock treatment
Cells were sealed in screw-cap flasks containing an atmosphere of 5% CO2, 95% air. These flasks were then immersed completely in a water bath with a measured temperature of 42.5°C. After 1 h of immersion, cells were returned to a 37°C incubator and subsequently stimulated with H2O2 at indicated concentrations.
Cell viability assays
Cells were plated at a density of 104 cells/well on 96-well plates in 100 μl of RPMI 1640, and cell viability was evaluated by the conventional MTT reduction assay. Briefly, MTT (0.5%, 20 μl) was added to each microwell and incubated for 2 h at 37°C. The amount of MTT formazan product was determined by measuring optical density using a microplate reader (Bio-Rad) at a test wavelength of 570 nm and a reference wavelength of 630 nm. Alternatively, cell viability was measured by the lactate dehydrogenase (LDH) release assay, which was based on measurement of LDH activity in culture medium or cell lysate using commercial test kit (Nanjing Jiancheng Bioengineering Institute). The percentage release was calculated as (LDH in medium/total LDH activity) × 100.
Plasmid constructs and cell transfection
A full-length human Hsp72 gene from pH2.3 (ATCC 57495) was sub-cloned into the pcDNA3.1Myc/His− mammalian expression vector (Invitrogen Life Technologies). Mutants of Hsp72-ΔPBD (lacking a peptide binding domain (PBD); aa 383–542) and Hsp72-ΔNLS (lacking a nuclear localization sequence (NLS); aa 246–273) were generated from full-length Hsp72 by PCR followed by subcloning of the products into pcDNA3.1Myc/His− vector. Stable or transient transfection was done with Lipofectamine 2000 (Invitrogen Life Technologies) according to the manufacturer's instructions.
Concentration of the cell culture medium
After various treatments, cell-conditioned medium was harvested and filtered through Millex-GP (Millipore) to remove cell debris and macromolecular complexes. Samples were then concentrated with Amicon Ultra-4–10000 NMWL (Millipore) following the manufacturer's instructions.
Preparation of nuclear extracts
At the appropriate time after the treatment, cells were harvested and washed twice with cold PBS; nuclear extracts were prepared according to the method of Schreiber et al. (35). The protein content of the nuclear extracts was determined by a Bradford method.
Western blotting analysis
Proteins in the whole-cell lysate, subcellular fractions, or concentrated cell culture supernatants were resolved on 10% SDS-PAGE gel and transferred to a polyvinylidene fluoride membrane. After the membrane was blocked at room temperature for 6 h, the membrane was incubated for 2 h with various primary Abs specific for HMGB1 (BD Biosciences and Stressgen), Hsp72 and Hsp72/73 (Stressgen), proliferating cell nuclear Ag (BD Biosciences), GAPDH and β-actin (KangChen Biotechnology), β-tubulin (Sigma-Aldrich), and Myc tag (Upstate Biotechnology), respectively. After incubation with peroxidase-conjugated secondary Abs for 1 h at 25°C, the signals were visualized by diaminobenzidine detection (Boster Biotech) according to the manufacturer's instruction, and the bands of protein were scanned and quantitated with the Gel-pro Analyzer software (Media Cybernetics).
Immunocytochemical analysis
Cells were cultured on glass coverslips and fixed in 4% formaldehyde for 30 min at room temperature before detergent extraction with 0.1% Triton X-100 for 10 min at 4°C. Cover slips were saturated with PBS containing 2% BSA for 1 h at room temperature and processed for immunofluorescence with rabbit anti-HMGB1 polyclonal Ab (BD Biosciences) or mouse anti-Hsp72 mAb (Stressgen) followed by Cy3-conjugated sheep anti-rabbit Ig (Sigma-Aldrich) or FITC-conjugated sheep anti-mouse Ig (Boster Biotech), respectively. Nuclear morphology was analyzed with the fluorescent dye Hoechst 33258 (Sigma-Aldrich). Between all incubation steps, cells were washed three times for 3 min with PBS containing 0.2% BSA. Images were taken with a fluorescence microscope (ECLIPSE 80i; Nikon) equipped with a CFI Plan Achromat DL 40 × 0.65 Ph2 objective (Nikon). The observation was made at 25°C, and the image was recorded using a digital camera (DS Cooled Camera Head DS-5Mc; Nikon). Relative fluorescence intensity of HMGB1 in the nuclear and cytoplasmic regions of multiple representative cells (three to five different fields containing ∼50 cells) was assayed using the ImageProPlus software (Media Cybernetics).
Immunoprecipitation and coimmunoprecipitation analysis
Whole cell lysates or nuclear extracts were lysed at 4°C in ice-cold lysis buffer (50 mM Tris-HCl, pH 7.4, containing 150 mM NaCl, 1% Nonidet P-40, 0.5% sodium deoxycholate, 0.1% SDS, protease inhibitors mixture). Lysates were cleared by centrifugation at 12000 × g for 10 min and then incubated for 2 h or overnight at 4°C with 5 μg/ml of the appropriate Ab and protein A or G agarose-Sepharose beads (Amersham Biosciences). Immune complexes were washed extensively with PBS, and proteins were eluted by boiling in 2× SDS sample buffer. Proteins were assayed by Western blotting as above.
Statistical analysis
Significance of differences between groups was determined by a two-tailed Student t test or Fisher's least significant difference test, as indicated. p < 0.05 was considered significant.
Results
Mild HS pretreatment inhibits oxidative stress (H2O2)-induced HMGB1 release
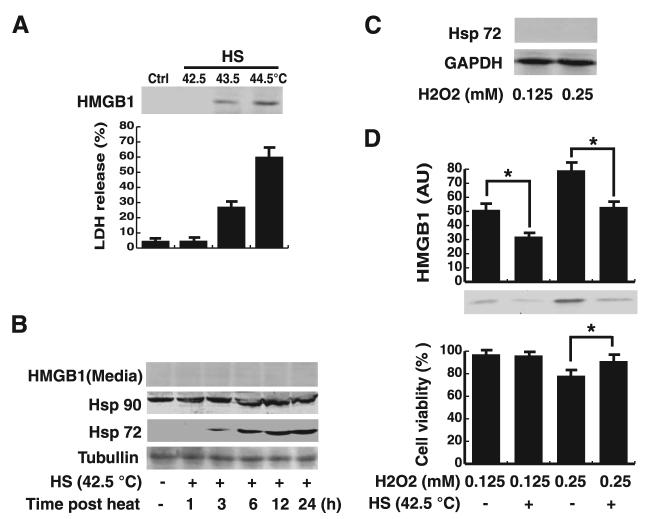
In response to a wide variety of stressful stimuli (such as heat shock), there is a marked increase in expression of Hsps, which function as molecular chaperones responsible for maintaining cellular homeostasis or cell survival. For instance, a mild heat shock (42.5°C for 1 h followed by recovery at 37°C for 12 h) was not cytotoxic to macrophage cultures (Fig. 1A) but induced the expression of a number of Hsps (e.g., Hsp72 and Hsp90; Fig. 1B). Hsp72 was not detectable in resting RAW 264.7 cells, but its expression levels were substantially increased following mild heat shock in a time-dependent manner (Fig. 1B). Consistent with previous report (36), Hsp90 was constitutively expressed in quiescent RAW 264.7 cells (Fig. 1B), and its cellular levels were further increased after mild heat shock (Fig. 1B). As expected, severe heat shock (e.g., 43.5–44.5°C) caused cell damage, and consequently led to passive release of LDH and HMGB1 from macrophage cultures (Fig. 1A).
FIGURE 1.
A mild heat shock (HS) pretreatment attenuates oxidative stress (H2O2)-induced HMGB1 release in macrophages cultures. A, Effects of mild or severe heat shock on HMGB1 release. RAW 264.7 cells were subjected to a brief heat shock (1 h) at 42.5°C (mild heat shock), 43.5°C or higher (severe heat shock), after which was returned to 37°C and incubated for additional 12 h. HMGB1 in the culture medium were detected by Western blotting analysis (top). In parallel, the cell viability was determined by LDH release assay (bottom). Ctrl, control cells. Blot is representative of three experiments with similar results. Values are mean ± SEM (n = 3) of three experiments in duplicate. B, Mild HS pretreatment induced Hsp expression. RAW 264.7 cells were subjected to heat shock (42.5°C, 1 h), and cellular levels of Hsp90 and Hsp72 were detected by Western blotting analysis at the indicated time points after heat shock. In parallel, levels of HMGB1 in the culture medium were determined by Western blotting analysis. Tubulin was used as a loading control. Values are representative of three independent experiments with similar results. C, Effects of oxidative stress on Hsp72 expression. RAW 264.7 cells were stimulated with H2O2 at nontoxic (0.125 mM), or low-toxic (0.25 mM) concentrations, and cellular Hsp72 were detected at 12 h poststimulation by Western blotting analysis. GAPDH was used as a loading control. Values are representative of three independent experiments with similar results. D, Mild heat shock pretreatment inhibited H2O2-induced HMGB1 release. After mild heat shock (42.5°C, 1 h), cells were allowed to recover for 12 h at 37°C and then stimulated for 12 h with H2O2 at nontoxic (0.125 mM) or low-toxic (0.25 mM) concentrations. Levels of HMGB1 in the culture medium were determined by Western blotting and expressed (in arbitrary units; AU) as mean ± SEM of three experiments in duplicate. In parallel, the cell viability was determined by MTT assay, and expressed as mean ± SEM (n = 4) of three experiments in duplicate. *, p < 0.05.
We recently demonstrated that a reactive oxygen species, H2O2, stimulates macrophages to actively secrete or passively release HMGB1 in a dose-dependent manner (13). In this study, we assessed the effects of mild heat shock on oxidative stress-induced HMGB1 release. By itself, hydrogen peroxide, at either nontoxic doses (0.125 mM) or low-toxic doses (0.25 mM), did not induce Hsp72 expression in macrophage cultures (Fig. 1C) but induced marked HMGB1 active secretion and/or passive release (Fig. 1D). Interestingly, a mild heat shock pretreatment (e.g., 42.5°C, 1 h) rendered macrophage cultures resistant to HMGB1 release induced by hydrogen peroxide at both nontoxic (0.125 mM), and low-toxic concentrations (0.25 mM; Fig. 1D). Consistently, mild heat shock pretreatment conferred cytoprotective effects against oxidative stress (0.25 mM H2O2)-induced cell death, implicating a possibility that mild heat shock not only attenuates active HMGB1 secretion but also inhibits passive HMGB1 release induced by H2O2 at low-toxic concentrations (Fig. 1D).
Overexpression of Hsp72 inhibits H2O2-induced HMGB1 release
To elucidate the mechanisms underlying heat shock-mediated suppression of HMGB1 release, we determined the effect of Hsp72 expression on oxidative stress-induced HMGB1 release. We stably transfected RAW 264.7 cells with Hsp72 expression pcDNA3.1-Hsp72 construct and verified Hsp72 expression by Western blot (Fig. 2A) or immunocytochemistry (Fig. 2B). Hsp72 was not detected in RAW 264.7 cells transfected with empty pcDNA3.1 plasmid (Fig. 2A) but was highly detectable in RAW 264.7 cells transfected with pcDNA3.1-Hsp72 construct (Fig. 2A). Immunocytochemistry analysis revealed strong Hsp72 staining in the cytoplasm of RAW 264.7 cells transfected with pcDNA3.1-Hsp72 expression construct, rather than the empty pcDNA3.1 plasmid (Fig. 2B), indicating that pcDNA3.1-Hsp72-transfected quiescent cells maintained a pool of Hsp72 in the cytoplasm.
FIGURE 2.
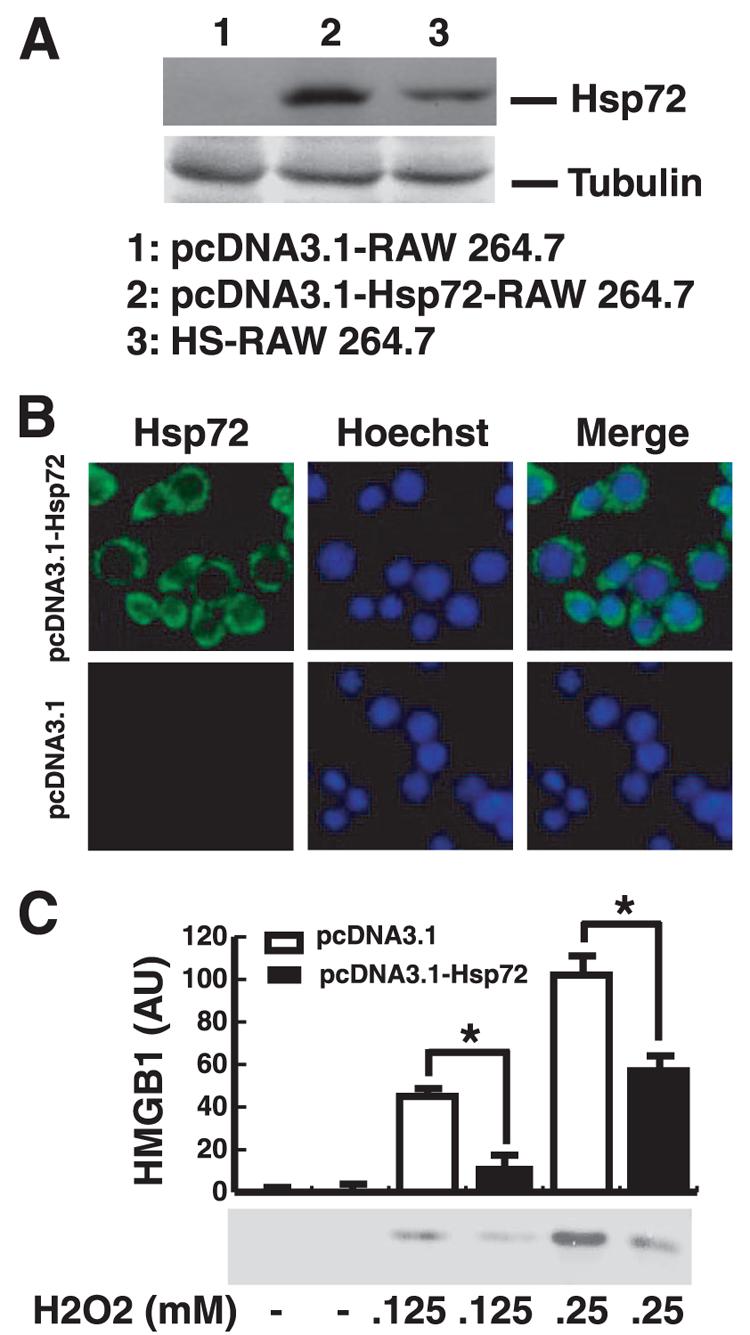
Overexpression of Hsp72 renders macrophages resistant to H2O2-induced HMGB1 release. A, Western blotting analysis of Hsp72 levels in RAW 264.7 cells transfected with empty plasmid (lane 1), Hsp72 expression construct (lane 2) or challenged with mild heat shock (HS, 42.5°C, 1 h). Tubulin was used as a loading control. B, Visualization of fluorescent Hsp72 protein in RAW 264.7 cells transfected with Hsp72 expression construct (top), or empty plasmid (bottom). Nuclei were visualized by Hoechst staining. C, Western blotting analysis of H2O2-induced HMGB1 release in RAW 264.7 cells transfected with empty plasmid, or Hsp72 expression construct. RAW 264.7 cells were stimulated with H2O2 (0.125 and 0.25 mM) for 12 h, and HMGB1 levels in the culture medium were determined, and expressed as mean ± SEM of three experiments in duplicate. *, p < 0.05.
We next examined whether Hsp72 expression affected H2O2-induced HMGB1 release. As compared with RAW 264.7 cells transfected with empty pcDNA3.1 plasmid, the H2O2-induced HMGB1 release was significantly reduced in Hsp72-expressing RAW 264.7 cells (Fig. 2C), suggest a potential role for Hsp72 as a negative regulator of oxidative stress-induced HMGB1 release. Notably, Hsp72 overexpression rendered macrophages resistant to HMGB1 release induced by hydrogen peroxide at both nontoxic (0.125 mM), and low-toxic concentrations (0.25 mM; Fig. 2C), indicating that a prior heat shock counterregulates oxidative stress-induced active secretion and passive release of HMGB1.
Overexpression of Hsp72 inhibits H2O2-induced HMGB1 cytoplasmic translocation
To gain insight into the mechanisms underlying Hsp72-mediated suppression of HMGB1 release, we determined whether overexpression of Hsp72 renders macrophages resistant to stress-induced HMGB1 cytoplasmic translocation. Hsp72 is normally localized in the cytoplasm but can be translocated to the nucleus in response to heat shock or oxidative stress. Indeed, hydrogen peroxide induced a substantial Hsp72 nuclear translocation in a time-dependent manner, beginning as early as 1 h, peaking around 3 h, and maintaining up to 6 h (Fig. 3, A and B). Consistent with previous report (33), heat shock similarly induced nuclear translocation of Hsp72 in a time-dependent manner (Fig. 3C). We recently demonstrated that hydrogen peroxide induces HMGB1 cytoplasmic translocation in macrophage cultures (13). However, the hydrogen peroxide-induced HMGB1 cytoplasmic translocation was significantly attenuated in Hsp72-expressing RAW 264.7 cells (Fig. 3D), suggesting a possibility that Hsp72 nuclear translocation may be needed to prevent HMGB1 cytoplasmic translocation.
FIGURE 3.
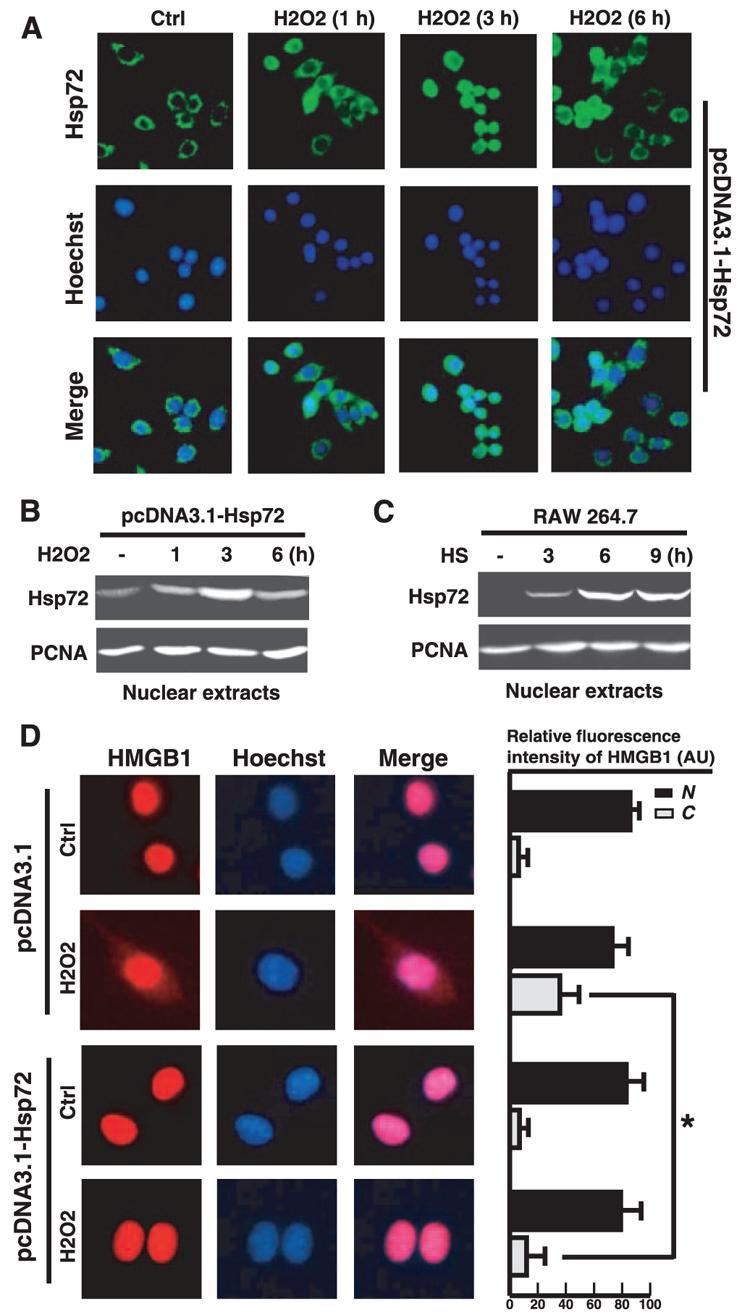
Overexpression of Hsp72 attenuates H2O2-induced HMGB1 cytoplasmic translocation in macrophage cultures. A and B, H2O2 induces transient nuclear translocation of Hsp72 in macrophages cultures. pcDNA3.1-Hsp72-transfected RAW 264.7 cells were stimulated with H2O2 (0.125 mM) for various period of time and examined for Hsp72 subcellular localization by immunocytochemistry (A) or cell fractionation/Western blot (B). Green, Hsp72; blue, nuclei. Original magnification, ×400. A nuclear protein, proliferating cell nuclear Ag, was used as a loading control. C, Heat shock induces Hsp72 expression and nuclear translocation in macrophages cultures. RAW 264.7 cells subject to mild heat shock (42.5°C, 1 h), and nuclear Hsp72 content was determined by Western blotting. D, Overexpression of Hsp72 attenuates H2O2-induced HMGB1 cytoplasmic translocation. RAW 264.7 cells transfected with empty pcDNA3.1 plasmid, or pcDNA3.1-Hsp72 construct were stimulated with H2O2 (0.125 mM) for 12 h, and the subcellular localization of HMGB1 was determined by immunocytochemical analysis (D, left). Relative fluorescence intensity of HMGB1 in the nuclear (N) and cytoplasmic (C) regions of multiple representative cells was assayed using the ImageProPlus software (D, right). Image is representative of three experiments with similar results. Red, HMGB1; blue, nuclei. Original magnification, ×1000. Values are means ± SEM (n = 50) of three experiments in duplicate. *, p < 0.05.
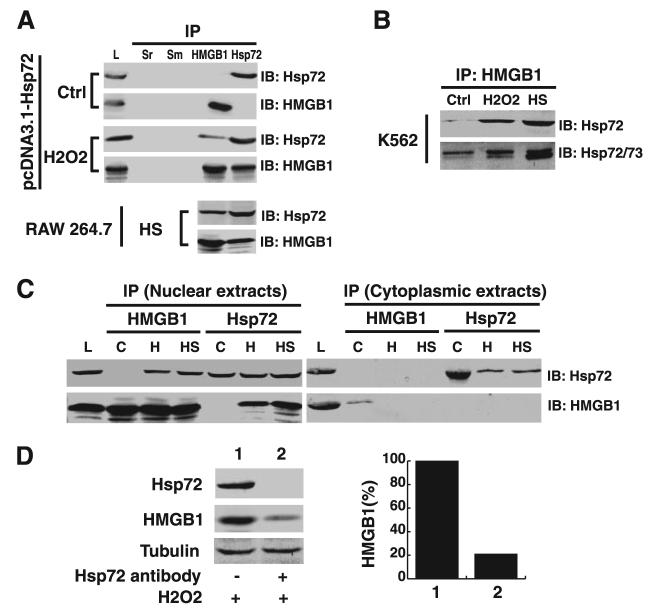
Hsp72 and HMGB1 are coimmuoprecipitated after mild heat shock or oxidative stress
To determine mechanisms underlying Hsp72-mediated suppression HMGB1 cytoplasmic translocation, we used coimmunoprecipitation techniques to evaluate potential Hsp72-HMGB1 interaction. In lysate of quiescent RAW 264.7 cells transfected with pcDNA3.1-Hsp72 construct, Hsp72 and HMGB1 were not coimmunoprecipitated, indicating a poor (if any at all) Hsp72-HMGB1 interaction (Fig. 4A). After oxidative stress (with H2O2, 0.125 mM), or mild heat shock (e.g., 42.5°C, 1 h), sustantially higher amounts of HMGB1 were coimmunoprecipitated with Hsp72 (Fig. 4A), indicating an increase in Hsp72-HMGB1 interaction in response to oxidative stress. In addition to RAW 264.7 cells, oxidative stress-induced Hsp72-HMGB1 interaction was also found in human leukemia K562 cells. In response to oxidative stress (H2O2) or heat shock, there was a strong increase in Hsp72, or Hsp72/Hsp73 content in complexes immunoprecipitated with HMGB1-specific Abs (Fig. 4B).
FIGURE 4.
Hsp72 and HMGB1 are coimmunoprecipitated after oxidative stress or heat shock. A, Coimmunoprecipitation of Hsp72 and HMGB1 from lysate of RAW 264.7 cells. RAW 264.7 cells transfected with pcDNA3.1-Hsp72 construct were stimulated with H2O2 (0.125 mM) for 3 h, and whole-cell lysate was immunoprecipitated with various Abs: nonspecific rabbit serum (Sr), nonspecific mouse serum (Sm), or Abs specific for Hsp72 or HMGB1, respectively. In parallel experiment, RAW 264.7 cells were subjected to mild heat shock (42.5°C for 1 h) and returned to 37°C for 6 h, and whole-cell lysate was immunoprecipitated with HMGB1- or Hsp72-specific Abs, respectively. The precipitated complexes were separately immunoblotted with Hsp72- or HMGB1-specific Abs. IP, immunoprecipitation; IB, Immunoblotting. Hsp72-transfected cell lysate (L) was used as a positive control. Blot is representative of three experiments with similar results. B, Coimmunoprecipitation of Hsp72 and HMGB1 from lysate of human leukemia K562 cells. K562 cells were subjected to oxidative stress (by stimulating withH2O2, 0.125 mM, for 12 h) or mild heat shock (HS, 42.5°C, 1 h, and then returned to 37°C for 6 h), and whole-cell lysate was immunoprecipitated with HMGB1-specific Abs. The precipitated complexes were then sequentially blotted with Hsp72- or HMGB1-specific Abs, respectively. IP, immunoprecipitation; IB, immunoblotting; Ctrl, control cells. Blot is representative of two experiments with similar results. C, Coimmunoprecipitation of Hsp72 and HMGB1 from nuclear and cytoplasmic fraction of RAW 264.7 cells transfected with pcDNA3.1-Hsp72. Cells were subjected to oxidative stress (H2O2, 0.125 mM for 3 h; H), or mild heat shock (42.5°C for 1 h, then returned to 37°C for 6 h; HS), and nuclear or cytoplasmic proteins were immunoprecipitated with Hsp72-or HMGB1-specific Abs. The precipitated complexes were then blotted with Hsp72- or HMGB1-specific Abs. Hsp72-transfected cell lysates was used as a positive control. C, control cells. Blot is representative of two experiments with similar results. D, Stoichiometry analysis of Hsp72-bound HMGB1 in nuclear fractions. RAW 264.7 cells transfected with pcDNA3.1-Hsp72 were stimulated with H2O2 (0.125 mM) for 3 h, and levels of HMGB1 in the whole-cell extracts before (1, set at 100%) or after (2) immunoprecipitation with excessive amount of Hsp72-specific Ab was determined by Western blotting. Blot is representative of three experiments with similar results.
To determine whether Hsp72 interacts with HMGB1 in the nucleus, we performed coimmunoprecipitation experiments with nuclear fractions of Hsp72-expressing cells. Similarly, after oxidative stress or mild heat shock, a strong Hsp72-HMGB1 interaction in the nucleus was suggested by the presence of Hsp72 or HMGB1 in complexes immunoprecipitated with HMGB1- or Hsp72-specific Abs, respectively (Fig. 4C), implicating that Hsp72-HMGB1 interaction in the nucleus may be required to interfere with oxidative stress-induced HMGB1 cytoplasmic translocation. In contrast, Hsp72 was not coimmunoprecipitated with HMGB1 in the cytoplasmic fractions (Fig. 4C), which may be partly attributable to distinct cytoplasmic localization of Hsp72 (in exosomes; Ref. 37), and HMGB1 (in endolysosomes; Ref. 38). To elucidate the mechanism for prevention of HMGB1 translocation, we further determined the Stoichiometry of nuclear HMGB1-Hsp72 interaction using Hsp72-specific Abs. Stoichiometry analysis revealed that the majority of nuclear HMGB1 was associated with Hsp72, because elimination of Hsp72 protein from the nuclear fraction with Hsp72-specific Abs concurrently removed ∼80% HMGB1 protein from the nuclear fraction (Fig. 4D).
An herb-derived antioxidant, quercetin, attenuates H2O2-induced Hsp72 nuclear translocation and Hsp72-HMGB1 interaction
As an abundant herbal flavonoid, quercetin is a powerful antioxidant capable of blocking heat shock-induced Hsp72 expression and nuclear translocation (39-41). To confirm the requirement of Hsp72 nuclear translocation for HMGB1 binding, we determined whether quercetin attenuates H2O2-induced Hsp72 nuclear translocation in RAW 264.7 cells transfected with pcDNA3.1-Hsp72 construct. By itself, quercetin was not cytotoxic even at concentrations up to 50 μM (data not shown) and did not affect the cytoplasmic localization of Hsp72 in quiescent cells (Fig. 5A). However, pretreatment of macrophage cultures with quercetin substantially inhibited H2O2-induced Hsp72 nuclear translocation, as judged by immunocytochemistry or cell fractionation/Western blot (Fig. 5, A and B). Furthermore, quercetin substantially reduced HMGB1 content in the complexes immunoprecipitated with Hsp72-specific Abs (Fig. 5C), indicating that inhibition of Hsp72 nuclear translocation prevents Hsp72-HMGB1 interaction in the nucleus. However, like other antioxidants (such as tanshinones; Ref. 42), quercetin paradoxically attenuated oxidative stress-induced HMGB1 release (data not shown), forcing us to use more powerful molecular tools to assess the importance of nuclear Hsp72-HMGB1 interaction in the regulation of oxidative stress-induced HMGB1 release.
FIGURE 5.
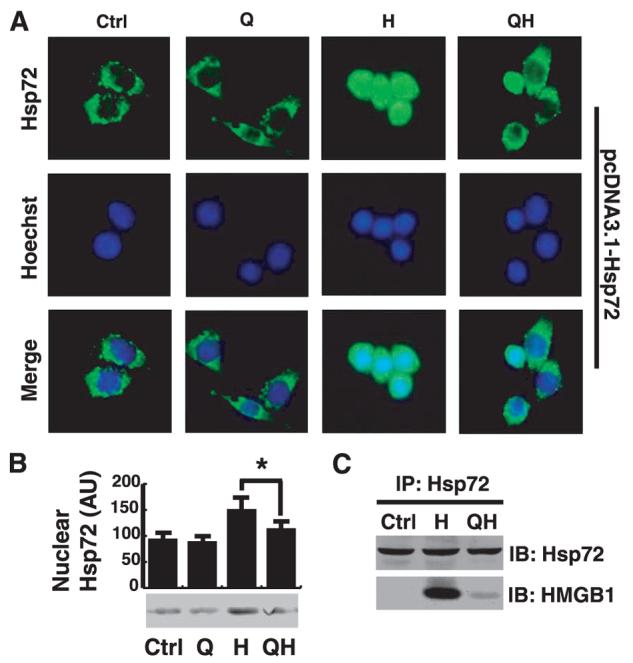
An herb-derived antioxidant, quercetin, attenuates H2O2-induced Hsp72 nuclear translocation, and Hsp72-HMGB1 interaction in macrophage cultures. RAW 264.7 cells transfected with pcDNA3.1-Hsp72 construct were pretreated with quercetin (50 μM) for 4 h and subsequently stimulated with H2O2 (0.125 mM) for 3 h. Subcellular localization of Hsp72 in cells was determined by immunocytochemistry (A) or Western blot (B). Results are representative of three experiments with similar results. Green, Hsp72; blue, nuclei. Original magnification, ×400). Ctrl, control cells; Q, + quercetin; H, + H2O2; QH, + quercetin + H2O2. In parallel experiments, whole-cell lysate was immunoprecipitated with Hsp72-specific Abs, and the precipitated complexes were immunoblotted with Hsp72- or HMGB1-specific Abs, respectively (C). IP, immunoprecipitation; IB, immunoblotting. Blot is representative of three experiments with similar results.
Identification of Hsp72 domains responsible for interacting with nuclear HMGB1
Hsp72 contains several functional domains including a bipartite NLS (aa 246–273) within the N-terminal ATPase domain, and the PBD (Fig. 6A; Refs. 43 and 44). To determine the requirement of NLS and PBD for Hsp72-HMGB1 interaction, we transiently transfected RAW 264.7 cells with expression construct encoding full-length Hsp72 (Hsp-WT) or mutants lacking the NLS motif (Hsp72-ΔNLS) or PBD region (Hsp72-ΔPBD). As expected, Myc-tagged Hsp72-WT and Hsp72-ΔPBD, but not Hsp72-ΔNLS, were found in the nuclear extract after H2O2 stimulation (Fig. 6B), confirming the requirement of NLS for H2O2-induced Hsp72 nuclear translocation. Although both Hsp72-WT and Hsp72-ΔPBD were translocated to the nucleus after oxidative stress, only the full-length Hsp72, but not Hsp72-ΔPBD, was found in the complexes immunoprecipitated with HMGB1-specific Abs (Fig. 6C), indicating that both NLS and PBD are required for Hsp72-HMGB1 interaction in the nucleus. More importantly, impairment of nuclear translocation by specific deletion of the NLS of Hsp72, or disruption of nuclear Hsp72-HMGB1 interaction by specific deletion of the PBD, uniformly lead to impairment in Hsp72-mediated suppression of oxidative stress (H2O2)-induced HMGB1 cytoplasmic translocation and release (Fig. 6D), supporting a novel role for Hsp72 as a negative regulator of HMGB1 cytoplasmic translocation and release.
FIGURE 6.

Identification of functional domains of Hsp72 for interacting with nuclear HMGB1. A, Schematic diagram of Hsp72 and two mutants lacking the NLS or PBD. B, Detection of HMGB1 and Myc-tagged Hsp72, Hsp72-ΔNLS, or Hsp72-ΔPBD by Western blotting. RAW 264.7 cells were transiently transfected with pcDNA3.1-Hsp72, pcDNA3.1-Hsp72-ΔNLS, or pcDNA3.1-Hsp72-ΔPBD and stimulated with H2O2 (0.125 mM) for 3 h; nuclear extract was assayed for HMGB1 and Myc-tagged proteins by Western blotting (Immunoblotting; IB) analysis. Blots are representative of two independent experiments with similar results. C, Coimmunoprecipitation of HMGB1 with Myc-tagged Hsp72, Hsp72-ΔNLS, or Hsp72-ΔPBD. In parallel experiments, nuclear extracts were immunoprecipitated (IP) with HMGB1-specific Abs, and the precipitated complexes were then assayed for levels of HMGB1 or Myc-tagged proteins by Western blotting. Blots are representative of two independent experiments with similar results. D, Western blotting of H2O2-induced HMGB1 release (D, left) and translocation (D, right) in RAW 264.7 cells transfected with empty plasmid, pcDNA3.1-Hsp72, pcDNA3.1-Hsp72-ΔNLS, or pcDNA3.1-Hsp72-ΔPBD. Tubulin was used as a loading control. Blots are representative of three independent experiments with similar results.
Discussion
In response to infection or injury, innate immune cells (e.g., macrophages and neutrophils) release large amounts of ROS and proinflammatory cytokines (such as TNF, IL-1, and IFN-γ). Although essential in the innate immunity against infection, an excessive production of ROS and proinflammatory cytokines may adversely contribute to the pathogenesis of various inflammatory diseases including arthritis, ischemia/reperfusion injury, and sepsis (1). A ubiquitous nuclear protein, HMGB1, can be released by activated innate immune cells in response to infection or injury (8) and is a danger signal to alert the immune system (9, 16, 45). In addition to bacterial products (such as endotoxin) or proinflammatory cytokines (such as TNF-α and IFN-γ), oxidative stress (such as H2O2) is also capable of inducing active or passive HMGB1 release (13).
Representing a universal response to a variety of stresses (including heat shock and infection), cells rapidly express stress-inducible Hsps such as Hsp90, Hsp70, Hsp60, and Hsp27 (27, 28). As a major stress-inducible protein, intracellular Hsp72 functions as a molecular chaperone to maintain cellular homeostasis and cell survival (27, 28). However, the potential regulatory role of Hsp72 in the regulation of oxidative stress-induced HMGB1 release was previously unknown. In this study, we demonstrated that mild heat shock pretreatment rendered RAW 264.7 cells resistant to hydrogen peroxide-induced HMGB1 cytoplasmic translocation and release. To elucidate the mechanism underlying heat shock-mediated suppression of HMGB1 release, we permanently transfected macrophages cells with Hsp72 expression construct, and we determined the effect of Hsp72 expression on oxidative stress-induced HMGB1 release. Similarly, we discovered that enhanced Hsp72 expression rendered RAW 264.7 cells resistant to hydrogen peroxide-induced HMGB1 cytoplasmic translocation and release, supporting a protective role for Hsp72 against adverse stresses (46, 47).
Under physiological conditions, Hsp72 is localized predominantly in the cytosol. In response to inflammatory (such as LPS) and oxidative stimuli, Hsp72 can be translocated into the nucleus, where it confers protective role against environmental stress (30-33). Accumulating evidence support the significance of Hsp72 translocation in fulfilling its cytoprotective and immunoregulatory functions (33, 48, 49). For instance, the nuclear translocation of Hsp72 is essential for its apoptosis-suppressing activities (50). Consistently, we observed that Hsp72 is predominantly localized in the cytosol of quiescent macrophages, but transiently translocated into the nucleus in response to oxidative stress. Within the nucleus, Hsp72 may interact with many nuclear proteins including HMGB1, because immunoprecipitation of macrophage nuclear (but not cytoplasmic) fraction with HMGB1-, or Hsp72-specific Abs brought down both HMGB1 and Hsp72 simultaneously.
The requirement of Hsp72 nuclear translocation for its HMGB1-binding activities was supported by the observations that pharmacological inhibition of Hsp72 nuclear translocation by an herb-derived antioxidant, quercetin (41), or genetic deletion of the NLS similarly prevented the formation of HMGB1-Hsp72 complexes after oxidative stress. Moreover, the requirement of nuclear Hsp72-HMGB1 interaction for Hsp72-mediated suppression of HMGB1 cytoplasmic translocation and release was supported by the observations that genetic deletion of the PBD from Hsp72 (51, 52) prevented nuclear HMGB1-Hsp72-PBD interaction. Furthermore, impairment of Hsp72 nuclear translocation by specific deletion of the NLS, or disruption of nuclear Hsp72-HMGB1 interaction by specific deletion of the PBD, uniformly abolished Hsp72-mediated suppression of oxidative stress-induced HMGB1 cytoplasmic translocation and release (Fig. 6D), supporting a novel role for Hsp72 as a negative regulator HMGB1 cytoplasmic translocation and release. Notably, heat shock similarly affects active HMGB1 release induced by other stimulus (such as bacterial endotoxin; Ref. 11). It thus appears that heat shock response, as an evolutionarily conserved process, is beneficial for surviving various environmental deleterious stimuli (such as oxidative stress and microbial infection).
As an important molecular chaperone, Hsp72 is capable of binding various denatured proteins (with exposed hydrophobic domains) to prevent activation of innate immune cells by these damaged proteins (so-called danger signals). Consequently, Hsp72 is essential for the maintenance of cellular homeostasis and cell survival (27). In parallel, HMGB1 can be regarded as an important DNA chaperone involved in the regulation of nuclear transactions (53, 54). Our experimental data now provided the first evidence for a potential link between oxidative stress and the activation of nuclear stress response as manifested by Hsp72-HMGB1 interaction within the nucleus.
The mechanisms underlying the regulation of active HMGB1 release are complex and still remain elusive. HMGB1 can be translocated into the cytoplasmic vesicles (such as endolysosomes) if its NLS is acetylated (14). Oxidative stress can induce acetylation of nuclear proteins (such as histones; Refs. 55 and 56), which was modestly inhibited by overexpression of Hsp72 in macrophage cultures (data not shown). It remains elusive whether nuclear Hsp72 similarly inhibits acetylation of HMGB1, although elevation of Hsp72 expression stimulated HMGB1-histone deacetylase 1 interaction (data not shown).
In summary, in the present work, we demonstrate that mild heat shock (e.g., 42.5°C, 1 h) or enhanced expression of Hsp72 (by gene transfection) similarly rendered RAW 264.7 cells resistant to hydrogen peroxide-induced HMGB1 cytoplasmic translocation and release. In response to oxidative stress, cytoplasmic Hsp72 translocated to the nucleus, where it interacted with nuclear proteins, including HMGB1, and prevented oxidative stress (H2O2)-induced HMGB1 cytoplasmic translocation and release (Fig. 7). Our discovery of Hsp72 as a negative regulator of oxidative stress-induced HMGB1 release may shed light on the development of novel therapeutic strategies for the treatment of various inflammatory diseases.
FIGURE 7.
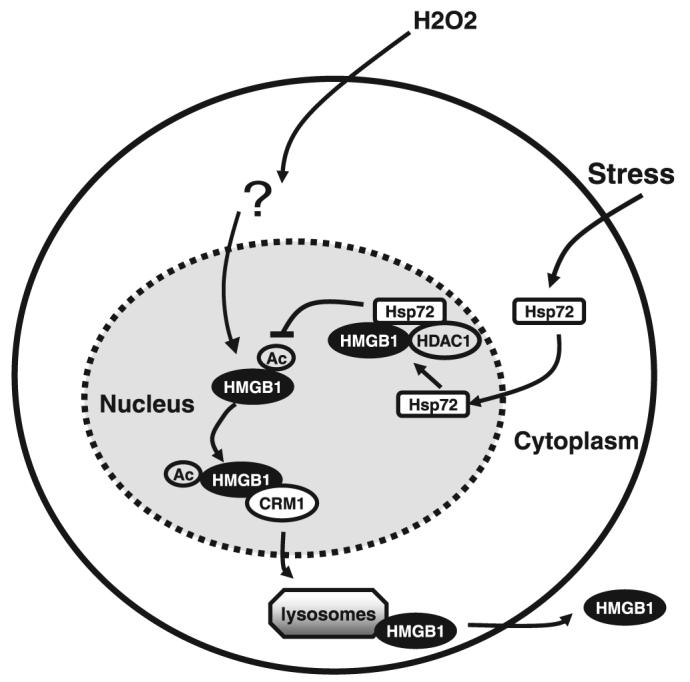
Hypothetical role of Hsp72 in the regulation of oxidative stress-induced HMGB1 cytoplasmic translocation and release. In response to stresses (e.g., heat shock), Hsp72 is produced to maintain a pool of Hsp72 in the cytoplasm. Upon stimulation with secondary oxidative stress (e.g., H2O2), Hsp72 is translocated into the nucleus, where it directly, or indirectly, interacts with various nuclear proteins (such as HMGB1 and histone deacetylase 1 (HDAC1). The intranuclear Hsp72-HMGB1 consequently prevents HMGB1 cytoplasmic translocation and subsequent release via the secretory lysosome pathway.
Acknowledgments
We thank Dr. Haibo Chen (Center Laboratory, Dalian Medical University, Lianoning, China) for help in fluorescence intensity assay. We also are grateful for the suggestions of Dr. Stuart K. Calderwood (Harvard Medical School, Cambridge, MA).
Footnotes
This work was supported by Grants 30500485 (to D.T.) and 30330280 (to X.X.) from the National Natural Sciences Foundation of China, the Major National Basic Research Program of China Grant G2000056908 (to X.X.), the Specialized Research Fund for the Doctoral Program of Higher Education of China Grant 20060533009 (to X.X.), and the Innovative Program of Central South University for Post-graduate Research Grant 2005-75239 (to D.T.), and in part by National Institutes of Health Grants (National Institute of General Medical Sciences) R01GM063075 and R01GM070817 (to H.W.).
Abbreviations used in this paper: ROS, reactive oxygen species; HMGB1, high mobility group box 1 protein; Hsp, heat shock protein; LDH, lactate dehydrogenase; NLS, nuclear localization sequence; PBD, peptide binding domain.
Disclosures
The authors have no financial conflict of interest.
References
- 1.Finkel T, Holbrook NJ. Oxidants, oxidative stress and the biology of ageing. Nature. 2000;408:239–247. doi: 10.1038/35041687. [DOI] [PubMed] [Google Scholar]
- 2.Kujoth GC, Hiona A, Pugh TD, Someya S, Panzer K, Wohlgemuth SE, Hofer T, Seo AY, Sullivan R, Jobling WA, et al. Mitochondrial DNA mutations, oxidative stress, and apoptosis in mammalian aging. Science. 2005;309:481–484. doi: 10.1126/science.1112125. [DOI] [PubMed] [Google Scholar]
- 3.Mantell LL. Introduction to serial reviews on redox signaling in immune function and cellular responses in lung injury and diseases. Free Radical Biol. Med. 2006;41:1–3. doi: 10.1016/j.freeradbiomed.2006.04.014. [DOI] [PubMed] [Google Scholar]
- 4.Mendes AF, Caramona MM, Carvalho AP, Lopes MC. Differential roles of hydrogen peroxide and superoxide in mediating IL-1-induced NF-κB activation and iNOS expression in bovine articular chondrocytes. J. Cell. Biochem. 2003;88:783–793. doi: 10.1002/jcb.10428. [DOI] [PubMed] [Google Scholar]
- 5.Suematsu N, Tsutsui H, Wen J, Kang D, Ikeuchi M, Ide T, Hayashidani S, Shiomi T, Kubota T, Hamasaki N, Takeshita A. Oxidative stress mediates tumor necrosis factor-α-induced mitochondrial DNA damage and dysfunction in cardiac myocytes. Circulation. 2003;107:1418–1423. doi: 10.1161/01.cir.0000055318.09997.1f. [DOI] [PubMed] [Google Scholar]
- 6.Muller S, Scaffidi P, Degryse B, Bonaldi T, Ronfani L, Agresti A, Beltrame M, Bianchi ME. New EMBO members' review: the double life of HMGB1 chromatin protein: architectural factor and extracellular signal. EMBO J. 2001;20:4337–4340. doi: 10.1093/emboj/20.16.4337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang H, Bloom O, Zhang M, Vishnubhakat JM, Ombrellino M, Che J, Frazier A, Yang H, Ivanova S, Borovikova L, et al. HMG-1 as a late mediator of endotoxin lethality in mice. Science. 1999;285:248–251. doi: 10.1126/science.285.5425.248. [DOI] [PubMed] [Google Scholar]
- 8.Lotze MT, Tracey KJ. High-mobility group box 1 protein (HMGB1): nuclear weapon in the immune arsenal. Nat. Rev. Immunol. 2005;5:331–342. doi: 10.1038/nri1594. [DOI] [PubMed] [Google Scholar]
- 9.Scaffidi P, Misteli T, Bianchi ME. Release of chromatin protein HMGB1 by necrotic cells triggers inflammation. Nature. 2002;418:191–195. doi: 10.1038/nature00858. [DOI] [PubMed] [Google Scholar]
- 10.Rendon-Mitchell B, Ochani M, Li J, Han J, Wang H, Yang H, Susarla S, Czura C, Mitchell RA, Chen G, et al. IFN-γ induces high mobility group box 1 protein release partly through a TNF-dependent mechanism. J. Immunol. 2003;170:3890–3897. doi: 10.4049/jimmunol.170.7.3890. [DOI] [PubMed] [Google Scholar]
- 11.Tang D, Shi Y, Jang L, Wang K, Xiao W, Xiao X. Heat shock response inhibits release of high mobility group box 1 protein induced by endotoxin in murine macrophages. Shock. 2005;23:434–440. doi: 10.1097/01.shk.0000159556.95285.df. [DOI] [PubMed] [Google Scholar]
- 12.Wang H, Vishnubhakat JM, Bloom O, Zhang M, Ombrellino M, Sama A, Tracey KJ. Proinflammatory cytokines (tumor necrosis factor and interleukin 1) stimulate release of high mobility group protein-1 by pituicytes. Surgery. 1999;126:389–392. [PubMed] [Google Scholar]
- 13.Tang D, Shi Y, Kang R, Li T, Xiao W, Wang H, Xiao X. Hydrogen peroxide stimulates macrophages and monocytes to actively release HMGB1. J. Leukocyte Biol. 2007;81:741–747. doi: 10.1189/jlb.0806540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bonaldi T, Talamo F, Scaffidi P, Ferrera D, Porto A, Bachi A, Rubartelli A, Agresti A, Bianchi ME. Monocytic cells hyper-acetylate chromatin protein HMGB1 to redirect it towards secretion. EMBO J. 2003;22:5551–5560. doi: 10.1093/emboj/cdg516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Andersson U, Wang H, Palmblad K, Aveberger AC, Bloom O, Erlandsson-Harris H, Janson A, Kokkola R, Zhang M, Yang H, Tracey KJ. High mobility group 1 protein (HMG-1) stimulates proinflammatory cytokine synthesis in human monocytes. J. Exp. Med. 2000;192:565–570. doi: 10.1084/jem.192.4.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rovere-Querini P, Capobianco A, Scaffidi P, Valentinis B, Catalanotti F, Giazzon M, Dumitriu IE, Muller S, Iannacone M, Traversari C, et al. HMGB1 is an endogenous immune adjuvant released by necrotic cells. EMBO Rep. 2004;5:825–830. doi: 10.1038/sj.embor.7400205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Messmer D, Yang H, Telusma G, Knoll F, Li J, Messmer B, Tracey KJ, Chiorazzi N. High mobility group box protein 1: an endogenous signal for dendritic cell maturation and Th1 polarization. J. Immunol. 2004;173:307–313. doi: 10.4049/jimmunol.173.1.307. [DOI] [PubMed] [Google Scholar]
- 18.Dumitriu IE, Baruah P, Valentinis B, Voll RE, Herrmann M, Nawroth PP, Arnold B, Bianchi ME, Manfredi AA, Rovere-Querini P. Release of high mobility group box 1 by dendritic cells controls T cell activation via the receptor for advanced glycation end products. J. Immunol. 2005;174:7506–7515. doi: 10.4049/jimmunol.174.12.7506. [DOI] [PubMed] [Google Scholar]
- 19.Taguchi A, Blood DC, del Toro G, Canet A, Lee DC, Qu W, Tanji N, Lu Y, Lalla E, Fu C, et al. Blockade of RAGE-amphoterin signalling suppresses tumour growth and metastases. Nature. 2000;405:354–360. doi: 10.1038/35012626. [DOI] [PubMed] [Google Scholar]
- 20.Sparatore B, Pedrazzi M, Passalacqua M, Gaggero D, Patrone M, Pontremoli S, Melloni E. Stimulation of erythroleukaemia cell differentiation by extracellular high-mobility group-box protein 1 is independent of the receptor for advanced glycation end-products. Biochem. J. 2002;363:529–535. doi: 10.1042/0264-6021:3630529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Degryse B, Bonaldi T, Scaffidi P, Muller S, Resnati M, Sanvito F, Arrigoni G, Bianchi ME. The high mobility group (HMG) boxes of the nuclear protein HMG1 induce chemotaxis and cytoskeleton reorganization in rat smooth muscle cells. J. Cell Biol. 2001;152:1197–1206. doi: 10.1083/jcb.152.6.1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Limana F, Germani A, Zacheo A, Kajstura J, Di Carlo A, Borsellino G, Leoni O, Palumbo R, Battistini L, Rastaldo R, et al. Exogenous high-mobility group box 1 protein induces myocardial regeneration after infarction via enhanced cardiac C-kit+ cell proliferation and differentiation. Circ. Res. 2005;97:E73–E83. doi: 10.1161/01.RES.0000186276.06104.04. [DOI] [PubMed] [Google Scholar]
- 23.Mitola S, Belleri M, Urbinati C, Coltrini D, Sparatore B, Pedrazzi M, Melloni E, Presta M. Cutting edge: extracellular high mobility group box-1 protein is a proangiogenic cytokine. J. Immunol. 2006;176:12–15. doi: 10.4049/jimmunol.176.1.12. [DOI] [PubMed] [Google Scholar]
- 24.Palumbo R, Sampaolesi M, De Marchis F, Tonlorenzi R, Colombetti S, Mondino A, Cossu G, Bianchi ME. Extracellular HMGB1, a signal of tissue damage, induces mesoangioblast migration and proliferation. J. Cell Biol. 2004;164:441–449. doi: 10.1083/jcb.200304135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Abraham E, Arcaroli J, Carmody A, Wang H, Tracey KJ. HMG-1 as a mediator of acute lung inflammation. J. Immunol. 2000;165:2950–2954. doi: 10.4049/jimmunol.165.6.2950. [DOI] [PubMed] [Google Scholar]
- 26.Sappington PL, Yang R, Yang H, Tracey KJ, Delude RL, Fink MP. HMGB1 B box increases the permeability of Caco-2 enterocytic monolayers and impairs intestinal barrier function in mice. Gastroenterology. 2002;123:790–802. doi: 10.1053/gast.2002.35391. [DOI] [PubMed] [Google Scholar]
- 27.Hightower LE. Heat shock, stress proteins, chaperones, and proteotoxicity. Cell. 1991;66:191–197. doi: 10.1016/0092-8674(91)90611-2. [DOI] [PubMed] [Google Scholar]
- 28.Hartl FU. Molecular chaperones in cellular protein folding. Nature. 1996;381:571–579. doi: 10.1038/381571a0. [DOI] [PubMed] [Google Scholar]
- 29.Meng X, Harken AH. The interaction between Hsp70 and TNF-α expression: a novel mechanism for protection of the myocardium against post-injury depression. Shock. 2002;17:345–353. doi: 10.1097/00024382-200205000-00001. [DOI] [PubMed] [Google Scholar]
- 30.Welch WJ, Feramisco JR. Nuclear and nucleolar localization of the 72,000-dalton heat shock protein in heat-shocked mammalian cells. J. Biol. Chem. 1984;259:4501–4513. [PubMed] [Google Scholar]
- 31.Velazquez JM, Lindquist S. hsp70: nuclear concentration during environmental stress and cytoplasmic storage during recovery. Cell. 1984;36:655–662. doi: 10.1016/0092-8674(84)90345-3. [DOI] [PubMed] [Google Scholar]
- 32.Ellis S, Killender M, Anderson RL. Heat-induced alterations in the localization of HSP72 and HSP73 as measured by indirect immunohisto-chemistry and immunogold electron microscopy. J. Histochem. Cytochem. 2000;48:321–332. doi: 10.1177/002215540004800302. [DOI] [PubMed] [Google Scholar]
- 33.Cowan KJ, Diamond MI, Welch WJ. Polyglutamine protein aggregation and toxicity are linked to the cellular stress response. Hum. Mol. Genet. 2003;12:1377–1391. doi: 10.1093/hmg/ddg151. [DOI] [PubMed] [Google Scholar]
- 34.Asea A, Kraeft SK, Kurt-Jones EA, Stevenson MA, Chen LB, Finberg RW, Koo GC, Calderwood SK. HSP70 stimulates cytokine production through a CD14-dependant pathway, demonstrating its dual role as a chaperone and cytokine. Nat. Med. 2000;6:435–442. doi: 10.1038/74697. [DOI] [PubMed] [Google Scholar]
- 35.Schreiber E, Matthias P, Muller MM, Schaffner W. Rapid detection of octamer binding proteins with ‘mini-extracts,’ prepared from a small number of cells. Nucleic Acids Res. 1989;17:6419. doi: 10.1093/nar/17.15.6419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bellocq A, Doublier S, Suberville S, Perez J, Escoubet B, Fouqueray B, Puyol DR, Baud L. Somatostatin increases glucocorticoid binding and signaling in macrophages by blocking the calpain-specific cleavage of Hsp 90. J. Biol. Chem. 1999;274:36891–36896. doi: 10.1074/jbc.274.52.36891. [DOI] [PubMed] [Google Scholar]
- 37.Lancaster GI, Febbraio MA. Exosome-dependent trafficking of HSP70: a novel secretory pathway for cellular stress proteins. J. Biol. Chem. 2005;280:23349–23355. doi: 10.1074/jbc.M502017200. [DOI] [PubMed] [Google Scholar]
- 38.Gardella S, Andrei C, Ferrera D, Lotti LV, Torrisi MR, Bianchi ME, Rubartelli A. The nuclear protein HMGB1 is secreted by monocytes via a non-classical, vesicle-mediated secretory pathway. EMBO Rep. 2002;3:995–1001. doi: 10.1093/embo-reports/kvf198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bjeldanes LF, Chang GW. Mutagenic activity of quercetin and related compounds. Science. 1977;197:577–578. doi: 10.1126/science.327550. [DOI] [PubMed] [Google Scholar]
- 40.Wei YQ, Zhao X, Kariya Y, Fukata H, Teshigawara K, Uchida A. Induction of apoptosis by quercetin: involvement of heat shock protein. Cancer Res. 1994;54:4952–4957. [PubMed] [Google Scholar]
- 41.Jakubowicz-Gil J, Pawlikowska-Pawlega B, Piersiak T, Pawelec J, Gawron A. Quercetin suppresses heat shock-induced nuclear translocation of Hsp72. Folia Histochem. Cytobiol. 2005;43:123–128. [PubMed] [Google Scholar]
- 42.Li W, Li J, Ashok M, Wu R, Chen D, Yang L, Yang H, Tracey KJ, Wang P, Sama AE, Wang H. A cardiovascular drug rescues mice from lethal sepsis by attenuating late-acting proinflammatory mediator, high mobility group box 1. J. Immunol. 2007;178:3856–3864. doi: 10.4049/jimmunol.178.6.3856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Milarski KL, Morimoto RI. Mutational analysis of the human HSP70 protein: distinct domains for nucleolar localization and adenosine triphosphate binding. J. Cell Biol. 1989;109:1947–1962. doi: 10.1083/jcb.109.5.1947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bukau B, Horwich AL. The Hsp70 and Hsp60 chaperone machines. Cell. 1998;92:351–366. doi: 10.1016/s0092-8674(00)80928-9. [DOI] [PubMed] [Google Scholar]
- 45.Andrews NW. Membrane repair and immunological danger. EMBO Rep. 2005;6:826–830. doi: 10.1038/sj.embor.7400505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hutter JJ, Mestril R, Tam EK, Sievers RE, Dillmann WH, Wolfe CL. Overexpression of heat shock protein 72 in transgenic mice decreases infarct size in vivo. Circulation. 1996;94:1408–1411. doi: 10.1161/01.cir.94.6.1408. [DOI] [PubMed] [Google Scholar]
- 47.Voss MR, Gupta S, Stice JP, Baumgarten G, Lu L, Tristan JM, Knowlton AA. Effect of mutation of amino acids 246–251 (KRKHKK) in HSP72 on protein synthesis and recovery from hypoxic injury. Am. J. Physiol. 2005;289:H2519–H2525. doi: 10.1152/ajpheart.00872.2004. [DOI] [PubMed] [Google Scholar]
- 48.Asea A. Stress proteins and initiation of immune response: chaperokine activity of hsp72. Exerc. Immunol. Rev. 2005;11:34–45. [PMC free article] [PubMed] [Google Scholar]
- 49.Welch WJ, Mizzen LA. Characterization of the thermotolerant cell, II: effects on the intracellular distribution of heat-shock protein 70, intermediate filaments, and small nuclear ribonucleoprotein complexes. J. Cell Biol. 1988;106:1117–1130. doi: 10.1083/jcb.106.4.1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kato K, Yamanaka K, Hasegawa A, Okada S. Dimethylarsinic acid exposure causes accumulation of Hsp72 in cell nuclei and suppresses apoptosis in human alveolar cultured (L-132) cells. Biol. Pharm. Bull. 1999;22:1185–1188. doi: 10.1248/bpb.22.1185. [DOI] [PubMed] [Google Scholar]
- 51.Gao T, Newton AC. The turn motif is a phosphorylation switch that regulates the binding of Hsp70 to protein kinase C. J. Biol. Chem. 2002;277:31585–31592. doi: 10.1074/jbc.M204335200. [DOI] [PubMed] [Google Scholar]
- 52.Park HS, Lee JS, Huh SH, Seo JS, Choi EJ. Hsp72 functions as a natural inhibitory protein of c-Jun N-terminal kinase. EMBO J. 2001;20:446–456. doi: 10.1093/emboj/20.3.446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bonaldi T, Langst G, Strohner R, Becker PB, Bianchi ME. The DNA chaperone HMGB1 facilitates ACF/CHRAC-dependent nucleosome sliding. EMBO J. 2002;21:6865–6873. doi: 10.1093/emboj/cdf692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zimber A, Nguyen QD, Gespach C. Nuclear bodies and compartments: functional roles and cellular signalling in health and disease. Cell. Signal. 2004;16:1085–1104. doi: 10.1016/j.cellsig.2004.03.020. [DOI] [PubMed] [Google Scholar]
- 55.Tikoo K, Lau SS, Monks TJ. Histone H3 phosphorylation is coupled to poly-(ADP-ribosylation) during reactive oxygen species-induced cell death in renal proximal tubular epithelial cells. Mol. Pharmacol. 2001;60:394–402. doi: 10.1124/mol.60.2.394. [DOI] [PubMed] [Google Scholar]
- 56.Miyata Y, Towatari M, Maeda T, Ozawa Y, Saito H. Histone acetylation induced by granulocyte colony-stimulating factor in a map kinase-dependent manner. Biochem. Biophys. Res. Commun. 2001;283:655–660. doi: 10.1006/bbrc.2001.4840. [DOI] [PubMed] [Google Scholar]