Abstract
Costimulatory pathway ligands and receptors can deliver either positive or negative signals to help determine the ultimate fate of activated T lymphocytes. Cytotoxic T lymphocyte antigen-4 (CTLA-4) represents one of the most extensively studied receptors in the costimulatory pathway and has recently been shown to function as a potent inhibitor of T cell-mediated immunity. T-cell expression of CTLA-4 indirectly facilitates tumor progression by restraining host antitumoral immunity. In contrast, administration of a monoclonal antibody to block CTLA-4 function can alleviate restraints on T-cell activity to promote immune-mediated tumor regression. We review the preclinical and clinical experience with CTLA-4 blockade as a promising immunotherapeutic approach to treat patients with advanced prostate cancer.
Keywords: Prostatic neoplasms, Immunotherapy, T-lymphocyte, Costimulation, CTLA-4
Introduction
During the last decade, there has been an explosion of new insights into the fundamental regulatory mechanisms governing host immune cell activation and function. As a result, a number of novel and relatively potent immunotherapeutic manipulations have recently emerged that show great promise for the treatment of human malignancy, including cancers occurring within the genitourinary tract. In general, immunotherapy encompasses targeted manipulation of host immune system components to promote effective antitumoral immunity. Unlike chemotherapy, which tends to be somewhat nonspecific in its action, immunotherapy shows the potential to induce tumor cell death with a relatively high degree of specificity, distinguishing between malignant and normal tissues. We summarize the preclinical and clinical experience with antibody mediated cytotoxic T lymphocyte antigen-4 (CTLA-4) blockade, a form of immunotherapy that has been developed to abrogate restrictive T-cell signaling to facilitate markedly the host’s ability to mount an effective immune response against tumors, including malignancy of the prostate.
The costimulatory pathway regulates T-lymphocyte activation
A primary goal of antitumoral immunotherapy is to generate a systemic antigen-specific T-cell response that is capable of destroying a tumor, its metastases, or even the parent tissues from which tumors occur. Under normal situations, professional antigen-presenting cells (APCs), including dendritic cells, monocytes, activated B-lymphocytes, and macrophages, play a central role in the induction of antigen-specific T-cell activation. Under the influence of proinflammatory cytokines, APCs scavenge antigens from tumor cell debris, undergo maturation, and then migrate to lymphoid tissues to encounter naïve and memory T cells. It is within these tissues that APCs can interact with CD4+ and CD8+ T cells to foster the activation of T cells capable of recognizing tumor-specific or tumor-associated antigens.
At a molecular level, T lymphocytes require 2 simultaneous but independent signals to become fully activated [1–5]. The first signal occurs from interactions between antigen/major histocompatibility complex, present on the surface of APCs, with corresponding antigen-specific T-cell receptors. The second signal occurs from B7.1 (CD80) and B7.2 (CD86) costimulatory molecules (or ligands), also present on the surface of APCs, interacting with the T cell “costimulatory” receptor, CD28 (Fig. 1). It is this second antigen-independent signal (called “costimulatory signal”) that is critical for facilitating T-cell activation, sustaining T-cell proliferation, allowing for cell-to-cell cooperation, and induction of differentiation from a naive to an effector or memory T-cell phenotype [5]. Underscoring the importance of costimulatory signaling, it has been shown that transgenic mice, lacking either the CD28 costimulatory receptor [6] or B7 (B7.1 and B7.2) costimulatory ligands [7], show severe impairments in generating T-cell responses.
Fig. 1.
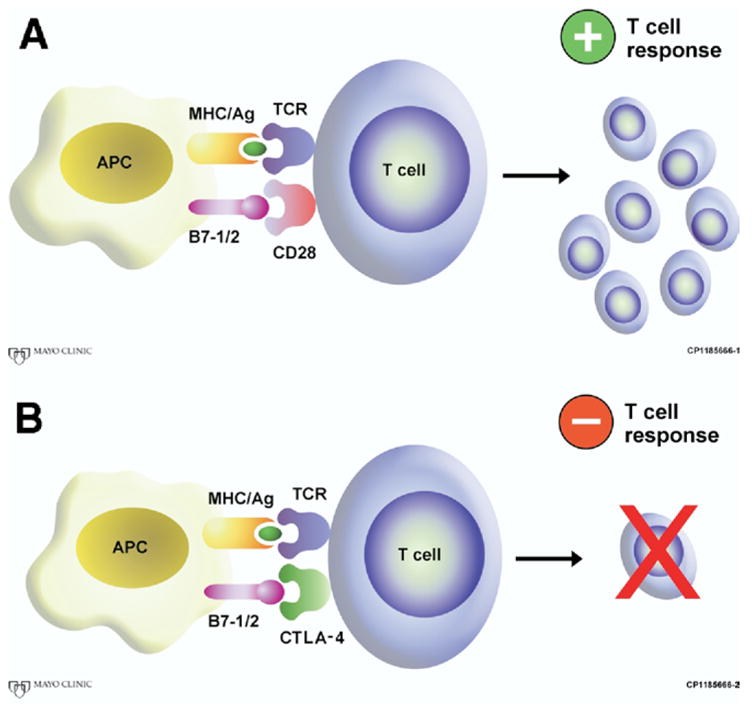
T-cell activation requires 2 independent signals. First, antigen is presented to the T-cell receptor (TCR) via an antigen/major histocompatibility complex (MHC). A second antigen independent signal, termed costimulation, is required to govern the fate of the T cell. (A) When the T-cell receptor CD28 interacts with its counter-receptor B7-1/2, a positive T-cell response is generated. (B) In the presence of CTLA-4, T-cell responses are abrogated, and tolerance of presented antigen is allowed. (Color version of figure is available online.)
However, based on very recent evidence, it appears that the ultimate fate of an activated T cell is also governed by a number of additional positive and negative signals emanating from a variety of “accessory” coregulatory T-cell receptors interacting with their cognate ligands [4]. For example, OX-40 (CD134) [8,9], 4-1BB (CD137) [10], and inducible costimulator [5] represent positive coregulators (or costimulators) that may further work to stimulate activated antigen-specific T cells (Fig. 2). In contrast, CTLA-4, B7-H1/PD-1, and B and T lymphocyte attenuator represent negative coregulators that act to inhibit T-cell function and diminish T-cell survival, presumably to thwart self-antigen recognition and the subsequent development of autoimmune disease (Fig. 2) [4].
Fig. 2.
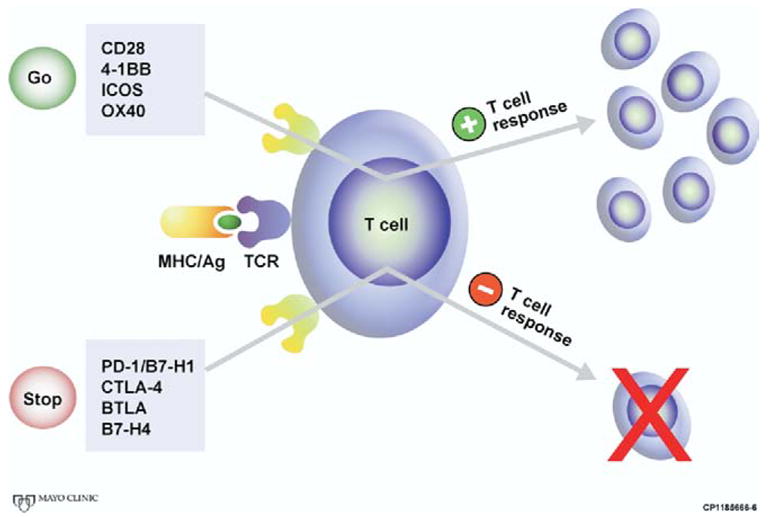
Although required for T-cell activation, the antigen-T-cell receptor interaction does not govern the fate of the T cell. Positive costimulatory molecules, such as CD28, 4-1BB, inducible costimulator, and OX40 can stimulate a T-cell response specific for the antigen presented. On the other hand, negative costimulatory molecules, such as CTLA-4, B7-H1/PD-1. B7-H4, and BLTA can inhibit T-cell activation, and may induce anergy (tolerance) to the presented antigen. (Color version of figure is available online.)
Most recently, immunotherapeutic manipulations of negative T-cell coregulators have garnered significant attention in the fields of immunology and oncology. For example, antibody mediated blockade of the CTLA-4 receptor, a negative coregulator in the T-cell costimulatory pathway that ultimately acts to deactivate or inhibit T cells, has been capable of prolonging and potentiating antitumoral T-cell responses (discussed later). Consequently, CTLA-4 blockade is being aggressively investigated as a promising immunotherapeutic approach to treat multiple forms of malignancy, including prostate cancer. In what remains, we discuss the mechanism whereby the CTLA-4 receptor regulates T-cell function, and summarize the preclinical and clinical experience with CTLA-4 blockade (anti-CTLA-4 treatment) as an immunotherapeutic approach to treat cancer.
The CTLA-4 receptor functions to inhibit T-cell responses
T-cell CD28 and CTLA-4 are among the most extensively studied costimulatory pathway receptors reported in the current literature [2,4,11]. CD28 is typically expressed on the surface of all T cells. When engaged by B7 ligands, in the presence of T-cell receptor occupancy by antigen/major histocompatibility complex, CD28 provides a vital positive signal to the T cell that stimulates its activation, proliferation, and maturation [2].
In contrast, CTLA-4 (CD 152) appears on the surface of T cells only after their activation [2]. CTLA-4 is a protein homolog of CD28 that binds to B7 ligands with 50 to 200-fold higher affinity than CD28. Also in contrast to CD28, CTLA-4 has delivered a negative or inhibitory signal to T cells, perhaps to truncate ongoing T-cell responses to abort induction of autoimmunity [2,4,11]. In support of this effect, transgenic mice lacking the CTLA-4 receptor spontaneously have an aggressive lymphoproliferative disorder develop that results in dramatic multi-organ, polyclonal lymphocytic infiltration, and lethality by 1 month of age [12–14]. However, transgenic mice lacking both CTLA-4 and CD28 fail to have a lymphoproliferative disease develop, suggesting that CTLA-4 function is intimately linked to intact CD28-mediated signaling [15]. In vivo experiments using antibodies and ligands further show that the engagement of CD28 stimulates T cells, whereas the engagement of CTLA-4 inhibits T-cell responses [2,16]. Thus, these observations collectively suggest that CTLA-4 inhibits T-cell activation by not only outcompeting CD28 for binding to B7 ligands but also by actively suppressing positive costimulatory signals that typically occur from CD28 to mediate T-cell activation.
In addition to eclipsing directly the stimulatory function of CD28, CTLA-4 may also inhibit T-lymphocyte function through a number of alternate mechanisms [2,4]. For instance, ligation of CTLA-4 has been suggested to also limit T cells by: (1) inhibiting interleukin-2 production, (2) inducing immunosuppressive transforming growth factor-beta production [17], and (3) limiting lymphocyte expansion by arresting the elaboration of numerous regulatory proteins required for G0/G1 cell-cycle progression [2,18,19]. CTLA-4 has also been reported to be present at high levels on immunosuppressive CD4+CD25+ T-regulatory cells [20–22].
It has been suggested that antibody mediated blockade of CTLA-4 can abrogate the function of T-regulatory cells, although this conclusion remains controversial [20,21]. CTLA-4 may also accelerate cell tryptophan metabolism, increasing production of toxic tryptophan catabolites, which ultimately produce impairments in APC and T-lymphocyte expansion, function, and survival [4,23–25]. In summary, T-cell expression of CTLA-4 plays a critical role in maintaining immune system homeostasis, functioning to suppress T-cell activation toward self-antigens, thereby limiting generation of autoimmune disease. Moreover, CTLA-4 is capable of inhibiting the activity of activated antitumoral T cells and, therefore, represents a highly attractive target for cancer immunotherapy.
Preclinical experience with anti-CTLA-4 treatment of prostate cancer
It was discovered in 1996 that in vivo antibody mediated blockade of the CTLA-4 receptor is capable of promoting T-cell mediated regression of solid tumors in mice [26]. Shortly before this in 1995, the laboratory of Dr. Norman M. Greenberg introduced the TRAMP mouse (transgenic adenocarcinoma of the mouse prostate), a genetically engineered mouse in which spontaneous tumors of the prostate develop and one that is particularly well suited for the comprehensive testing of novel immunotherapeutic approaches to treat prostate cancer [27]. Subsequently, using tumor cells derived from TRAMP mice, Kwon et al. [28] showed that in vivo CTLA-4 blockade (i.e., administration of a monoclonal antibody to block the CTLA-4 receptor) (Fig. 3) is capable of enhancing T-cell mediated regression of subcutaneous prostate tumors in C57BL/6 mice. In this study, in vivo CTLA-4 blockade triggered the complete or partial regression of subcutaneous TRAMP prostate tumors in nearly all tumor-bearing mice that received anti-CTLA-4 antibody. In further studies, systemic CTLA-4 blockade markedly diminished metastatic outgrowth of TRAMP tumors by roughly 50% when given as an adjunctive therapy after primary tumor extirpation with surgery [29].
Fig. 3.
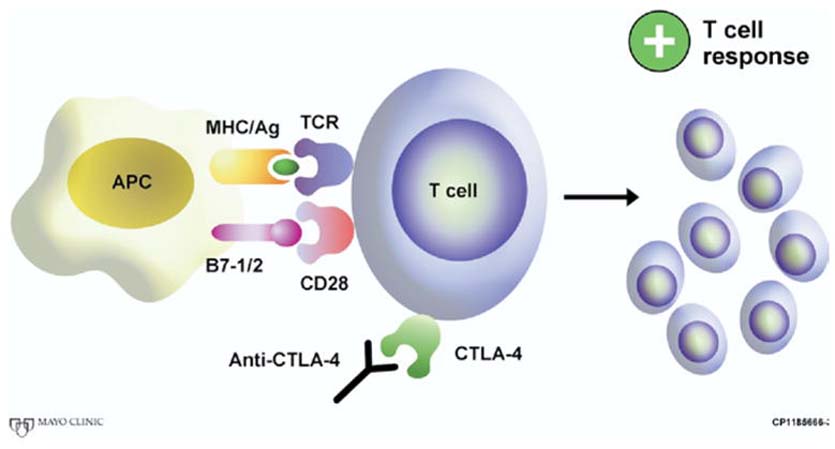
Monoclonal antibody blockade of CTLA-4 allows CD28 to interact with its B7 family counter-receptor, thus stimulating a tumor specific T-cell response. (Color version of figure is available online.)
Although CTLA-4 blockade has facilitated T-cell mediated tumor rejection in a variety of murine cancer models [30–32], it has also become evident that CTLA-4 blockade monotherapy may not be effective for the treatment of highly established and/or poorly immunogenic tumors, as has been the experience with mammary SM1 or melanoma B16 tumors in mice [33,34]. Therefore, to address this issue, a number of studies have now explored whether the effectiveness of CTLA-4 blockade could be enhanced by combining this approach with other forms of immunotherapy. In support of this, Hurwitz et al. [30] have shown that host vaccination with tumor cells modified to express granulocyte-macrophage colony-stimulating factor (GM-CSF) markedly improves the overall antitumoral effectiveness of CTLA-4 blockade. CTLA-4 blockade combined with GM-CSF modified tumor cell vaccination has been successfully used to generate potent T-cell mediated antitumoral responses against poorly immunogenic murine forms of breast carcinoma (SM1) and melanoma (B16) in mice [33,34].
In addition, this combined immunotherapeutic approach has reduced rates of autochthonous prostate tumor formation in TRAMP mice. Moreover, combined CTLA-4 blockade plus GM-CSF tumor cell vaccination is not only capable of producing potent responses against tumors but also T-cell responses against the parent tissues from which the tumors occur. For example, combined CTLA-4 blockade plus GM-CSF prostate tumor cell vaccination has led to the development of inflammatory prostatitis in normal male mice [30]. Likewise, combined CTLA-4 blockade plus GM-CSF melanoma tumor cell vaccination produces autoimmune-like hair depigmentation in black C57BL/6 mice. Mechanistically, these studies suggest that GM-CSF tumor cell vaccination functions to “trigger” an antitumoral T-cell response that is subsequently prolonged and potentiated by CTLA-4 blockade. Moreover, these studies illustrate that it is feasible to induce autoimmune-like responses and eliminate parent tissues from which tumors occur, an outcome that may be both necessary and beneficial for the effective treatment of tumors occurring in nonessential organs such as the prostate. Finally, these studies dramatically underscore the robust potency and potential for anti-CTLA-4 based immunotherapy as a treatment for cancer, including carcinoma of the prostate.
Clinical experience with anti-CTLA-4 treatment of prostate cancer
To test CTLA-4 blockade in the clinical setting, a fully humanized monoclonal anti-CTLA-4 antibody, an antibody that should not be regarded as foreign by the human immune system, was created. In general, monoclonal antibodies have long been used as immunotherapeutic reagents to diagnose and treat human disease. Specific to cancer, several monoclonal antibodies are now Food and Drug Administration approved, commercially available, and commonly used for cancer therapy, including, but not limited to, anti-CD20 (rituximab), which represents the standard-of-care treatment for diffuse large B-cell lymphoma, and anti-Her2/neu (trastizumab), which is used to treat receptor-positive advanced breast carcinoma. In distinction to these antibodies, it is noteworthy that anti-CTLA-4 antibody targets a receptor on the T cell as opposed to receptors that might be shown on a given tumor cell. Thus, by definition, anti-CTLA-4 therapy may potentially be applied to treat a broad array of human cancers. On the other hand, because of its somewhat tumor-aspecific mechanism of action, CTLA-4 blockade may trigger or exacerbate other forms of T-cell mediated inflammation that are not warranted, or desired, for tumor therapy. In support of this effect, it has been shown that CTLA-4 blockade is capable of potentiating T-cell mediated autoimmune encephalomyelitis, which is an experimental murine form of multiple sclerosis, and auto-immune diabetes in mice [35,36].
With this result in mind, humanized anti-CTLA-4 monoclonal antibody (MDX-010) was extensively tested during preclinical evaluation before initiation of human trials. In cynomolgus monkeys, repeated administration anti-CTLA-4 failed to produce any noticeable clinical or pathologic toxicity, and induction of autoimmune responses was not observed [37]. Thus, phase I trials to assess the safety of anti-CTLA-4 (MDX-010) treatment were subsequently conducted in patients with advanced prostate cancer and melanoma. Briefly, these phase I trials established that a single dose of anti-CTLA-4 antibody (3 mg/kg body weight) is generally well tolerated, producing relatively few and minor side effects. In addition, these phase I trials showed some evidence that CTLA-4 blockade is capable of generating antitumoral activity [38–40]. Thus, phase II trials to test the effectiveness of CTLA-4 blockade for the treatment of a number of forms of cancer, including prostate cancer, ovarian cancer, and melanoma, have recently been initiated.
Although many of these trials are still ongoing, Phan et al. [41] have recently reported their experience with combined melanoma peptide vaccination and repetitive anti-CTLA-4 administration (3 mg/kg intravenously every 3 weeks) as a treatment for progressive stage IV melanoma in 14 patients. Initially intended to accrue 21 patients, this trial was stopped early as a result of significant rates of grade III/IV autoimmune toxicity that were observed in treated subjects [41]. Specifically, grade III/IV toxicity developed in 6 of 14 (43%) patients during the course of therapy, including autoimmune-like enterocolitis, hypophysitis, and hepatitis. All patients with autoimmune symptomatology responded to steroids without apparent abrogation of anti-tumoral response. Nevertheless, 3 of the patients who had grade III/IV autoimmune toxicity also had an objective clinical response to treatment [41]. Based on this result, it has been suggested that to generate an effective antitumoral response during immunotherapy, it might also be necessary to induce some degree of autoimmunity against the parent tissues from which a given tumor occurs (i.e., tissue-specific autoimmunity). Certainly, it has been shown that many human tumor-associated antigens are simply over-expressed self-antigens. Thus, it remains possible that host tolerance to self-antigens must be broken or reduced to achieve an effective antitumoral response.
Alternatively, one might argue that the study by Phan et al. [41] not only underscores the profound biologic potency of CTLA-4 blockade but also the critical need for more precise and practical strategies to direct or steer the T-cell mediated antitumoral responses that are generated by anti-CTLA-4 therapy. Related to this, it is noteworthy that the autoimmune toxicities reported by Phan et al. [41] occurred after closely spaced intervals of repeated anti-CTLA-4/peptide vaccination treatment. In contrast, other groups have shown that single or reduced doses of anti-CTLA-4, given alone or in combination with other forms of immunotherapy, are associated with extremely tolerable rates of auto-immune toxicity [42]. Regardless, it is quite clear that the balance between achieving tumor regression and autoimmunity will require close scrutiny as CTLA-4 blockade continues to evolve as an immunotherapeutic treatment for cancer.
Finally, in an effort to explore more practical and precise methods to direct antitumoral T-cell mediated responses against prostate tumors during anti-CTLA-4 therapy, a new phase II trial using CTLA-4 blockade in combination with androgen withdrawal has recently been initiated at the Mayo Clinic and the University of California, San Francisco. In this trial, patients with prostate cancer will receive a short course of androgen ablation alone or in combination with a single dose of MDX-010 (3 mg/kg). The basis for this trial emanates from the observation that androgen ablation promotes infiltration of T cells into normal as well as cancerous prostate tissues [43]. In addition, it has been shown that T cells infiltrating into androgen-ablated prostate tissues typically show profiles associated with antigen-specific activation. Thus, it appears that androgen ablation causes prostate tissues to behave as an “in situ vaccine” to induce T-cell responses against prostate tumors. Consequently, it is postulated that CTLA-4 blockade will ultimately potentiate the T-cell mediated antiprostate tumor responses that are initially induced by androgen ablation. Patients eligible for enrolling in this study will be those with newly diagnosed cT3-4, N0-1 disease, with or without limited skeletal metastases. The patients who have had prior hormonal therapy, radical prostatectomy, or radiation to the prostate will not be eligible for study.
Conclusions
A number of immunotherapeutic approaches are already being exploited for the effective treatment of various forms of human malignancy. With the current explosive pace of immunologic research, it is entirely reasonable to expect substantial progress with the development of immune-based strategies to treat prostate cancer as well. Certainly, removal of the inhibitors of T-cell activation and function, as occurs with anti-CTLA-4 therapy, encompasses one extremely promising approach to treating malignancies occurring in the genitourinary tract.
References
- 1.Mueller DL, Jenkins MK, Schwartz RH. Clonal expansion versus functional clonal inactivation: A costimulatory signalling pathway determines the outcome of T cell antigen receptor occupancy. Annu Rev Immunol. 1989;7:445–80. doi: 10.1146/annurev.iy.07.040189.002305. [DOI] [PubMed] [Google Scholar]
- 2.Chambers CA, Kuhns MS, Egen JG, et al. CTLA-4-mediated inhibition in regulation of T cell responses: Mechanisms and manipulation in tumor immunotherapy. Annu Rev Immunol. 2001;19:565–94. doi: 10.1146/annurev.immunol.19.1.565. [DOI] [PubMed] [Google Scholar]
- 3.Schwartz RH. T cell anergy. Annu Rev Immunol. 2003;21:305–34. doi: 10.1146/annurev.immunol.21.120601.141110. [DOI] [PubMed] [Google Scholar]
- 4.Chen L. Co-inhibitory molecules of the B7-CD28 family in the control of T-cell immunity. Nat Rev Immunol. 2004;4:336–47. doi: 10.1038/nri1349. [DOI] [PubMed] [Google Scholar]
- 5.Frauwirth KA, Thompson CB. Activation and inhibition of lymphocytes by costimulation. J Clin Invest. 2002;109:295–9. doi: 10.1172/JCI14941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shahinian A, Pfeffer K, Lee KP, et al. Differential T cell costimulatory requirements in CD28-deficient mice. Science. 1993;261:609–12. doi: 10.1126/science.7688139. [DOI] [PubMed] [Google Scholar]
- 7.Borriello F, Sethna MP, Boyd SD, et al. B7-1 and B7-2 have overlapping, critical roles in immunoglobulin class switching and germinal center formation. Immunity. 1997;6:303–13. doi: 10.1016/s1074-7613(00)80333-7. [DOI] [PubMed] [Google Scholar]
- 8.Weinberg AD, Rivera MM, Prell R, et al. Engagement of the OX-40 receptor in vivo enhances antitumor immunity. J Immunol. 2000;164:2160–9. doi: 10.4049/jimmunol.164.4.2160. [DOI] [PubMed] [Google Scholar]
- 9.Gramaglia I, Weinberg AD, Lemon M, et al. Ox-40 ligand: A potent costimulatory molecule for sustaining primary CD4 T cell responses. J Immunol. 1998;161:6510–7. [PubMed] [Google Scholar]
- 10.Cheuk AT, Mufti GJ, Guinn BA. Role of 4-1BB:4-1BB ligand in cancer immunotherapy. Cancer Gene Ther. 2004;11:215–26. doi: 10.1038/sj.cgt.7700670. [DOI] [PubMed] [Google Scholar]
- 11.Thompson CB, Allison JP. The emerging role of CTLA-4 as an immune attenuator. Immunity. 1997;7:445–50. doi: 10.1016/s1074-7613(00)80366-0. [DOI] [PubMed] [Google Scholar]
- 12.Chambers CA, Cado D, Truong T, et al. Thymocyte development is normal in CTLA-4-deficient mice. Proc Natl Acad Sci USA. 1997;94:9296–301. doi: 10.1073/pnas.94.17.9296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tivol EA, Borriello F, Schweitzer AN, et al. Loss of CTLA-4 leads to massive lymphoproliferation and fatal multiorgan tissue destruction, revealing a critical negative regulatory role of CTLA-4. Immunity. 1995;3:541–7. doi: 10.1016/1074-7613(95)90125-6. [DOI] [PubMed] [Google Scholar]
- 14.Waterhouse P, Penninger JM, Timms E, et al. Lymphoproliferative disorders with early lethality in mice deficient in Ctla-4. Science. 1995;270:985–8. doi: 10.1126/science.270.5238.985. [DOI] [PubMed] [Google Scholar]
- 15.Mandelbrot DA, McAdam AJ, Sharpe AH. B7-1 or B7-2 is required to produce the lymphoproliferative phenotype in mice lacking cytotoxic T lymphocyte-associated antigen 4 (CTLA-4) J Exp Med. 1999;189:435–40. doi: 10.1084/jem.189.2.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Krummel MF, Sullivan TJ, Allison JP. Superantigen responses and co-stimulation: CD28 and CTLA-4 have opposing effects on T cell expansion in vitro and in vivo. Int Immunol. 1996;8:519–23. doi: 10.1093/intimm/8.4.519. [DOI] [PubMed] [Google Scholar]
- 17.Chen W, Jin W, Wahl SM. Engagement of cytotoxic T lymphocyte-associated antigen 4 (CTLA-4) induces transforming growth factor beta (TGF-beta) production by murine CD4(+) T cells. J Exp Med. 1998;188:1849–57. doi: 10.1084/jem.188.10.1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Krummel MF, Allison JP. CTLA-4 engagement inhibits IL-2 accumulation and cell cycle progression upon activation of resting T cells. J Exp Med. 1996;183:2533–40. doi: 10.1084/jem.183.6.2533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brunner MC, Chambers CA, Chan FK, et al. CTLA-4-Mediated inhibition of early events of T cell proliferation. J Immunol. 1999;162:5813–20. [PubMed] [Google Scholar]
- 20.Read S, Malmstrom V, Powrie F. Cytotoxic T lymphocyte-associated antigen 4 plays an essential role in the function of CD25(+)CD4(+) regulatory cells that control intestinal inflammation. J Exp Med. 2000;192:295–302. doi: 10.1084/jem.192.2.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Takahashi T, Tagami T, Yamazaki S, et al. Immunologic self-tolerance maintained by CD25(+)CD4(+) regulatory T cells constitutively expressing cytotoxic T lymphocyte-associated antigen 4. J Exp Med. 2000;192:303–10. doi: 10.1084/jem.192.2.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Salomon B, Lenschow DJ, Rhee L, et al. B7/CD28 costimulation is essential for the homeostasis of the CD4+CD25+ immunoregulatory T cells that control autoimmune diabetes. Immunity. 2000;12:431–40. doi: 10.1016/s1074-7613(00)80195-8. [DOI] [PubMed] [Google Scholar]
- 23.Grohmann U, Orabona C, Fallarino F, et al. CTLA-4-Ig regulates tryptophan catabolism in vivo. Nat Immunol. 2002;3:1097–101. doi: 10.1038/ni846. [DOI] [PubMed] [Google Scholar]
- 24.Fallarino F, Grohmann U, Vacca C, et al. T cell apoptosis by tryptophan catabolism. Cell Death Differ. 2002;9:1069–77. doi: 10.1038/sj.cdd.4401073. [DOI] [PubMed] [Google Scholar]
- 25.Fallarino F, Grohmann U, Hwang KW, et al. Modulation of tryptophan catabolism by regulatory T cells. Nat Immunol. 2003;4:1206–12. doi: 10.1038/ni1003. [DOI] [PubMed] [Google Scholar]
- 26.Liu B, Podack ER, Allison JP, et al. Generation of primary tumor-specific CTL in vitro to immunogenic and poorly immunogenic mouse tumors. J Immunol. 1996;156:1117–25. [PubMed] [Google Scholar]
- 27.Greenberg NM, DeMayo F, Finegold MJ, et al. Prostate cancer in a transgenic mouse. Proc Natl Acad Sci USA. 1995;92:3439–43. doi: 10.1073/pnas.92.8.3439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kwon ED, Hurwitz AA, Foster BA, et al. Manipulation of T cell costimulatory and inhibitory signals for immunotherapy of prostate cancer. Proc Natl Acad Sci USA. 1997;94:8099–103. doi: 10.1073/pnas.94.15.8099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kwon ED, Foster BA, Hurwitz AA, et al. Elimination of residual metastatic prostate cancer after surgery and adjunctive cytotoxic T lymphocyte-associated antigen 4 (CTLA-4) blockade immunotherapy. Proc Natl Acad Sci USA. 1999;96:15074–9. doi: 10.1073/pnas.96.26.15074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hurwitz AA, Foster BA, Kwon ED, et al. Combination immunotherapy of primary prostate cancer in a transgenic mouse model using CTLA-4 blockade. Cancer Res. 2000;60:2444–8. [PubMed] [Google Scholar]
- 31.Leach DR, Krummel MF, Allison JP. Enhancement of antitumor immunity by CTLA-4 blockade. Science. 1996;271:1734–6. doi: 10.1126/science.271.5256.1734. [DOI] [PubMed] [Google Scholar]
- 32.Yang YF, Zou JP, Mu J, et al. Enhanced induction of antitumor T-cell responses by cytotoxic T lymphocyte-associated molecule-4 blockade: The effect is manifested only at the restricted tumor-bearing stages. Cancer Res. 1997;57:4036–41. [PubMed] [Google Scholar]
- 33.Hurwitz AA, Yu TF, Leach DR, et al. CTLA-4 blockade synergizes with tumor-derived granulocyte-macrophage colony-stimulating factor for treatment of an experimental mammary carcinoma. Proc Natl Acad Sci USA. 1998;95:10067–71. doi: 10.1073/pnas.95.17.10067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.van Elsas A, Hurwitz AA, Allison JP. Combination immunotherapy of B16 melanoma using anti-cytotoxic T lymphocyte-associated antigen 4 (CTLA-4) and granulocyte/macrophage colony-stimulating factor (GM-CSF)-producing vaccines induces rejection of subcutaneous and metastatic tumors accompanied by autoimmune depigmentation. J Exp Med. 1999;190:355–66. doi: 10.1084/jem.190.3.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Perrin PJ, Maldonado JH, Davis TA, et al. CTLA-4 blockade enhances clinical disease and cytokine production during experimental allergic encephalomyelitis. J Immunol. 1996;157:1333–6. [PubMed] [Google Scholar]
- 36.Luhder F, Hoglund P, Allison JP, et al. Cytotoxic T lymphocyte-associated antigen 4 (CTLA-4) regulates the unfolding of autoimmune diabetes. J Exp Med. 1998;187:427–32. doi: 10.1084/jem.187.3.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Keler T, Halk E, Vitale L, et al. Activity and safety of CTLA-4 blockade combined with vaccines in cynomolgus macaques. J Immunol. 2003;171:6251–9. doi: 10.4049/jimmunol.171.11.6251. [DOI] [PubMed] [Google Scholar]
- 38.Hanahan D, Lanzavecchia A, Mihich E. Fourteenth Annual Pezcoller Symposium: The novel dichotomy of immune interactions with tumors. Cancer Res. 2003;63:3005–8. [PubMed] [Google Scholar]
- 39.Hodi FS, Mihm MC, Soiffer RJ, et al. Biologic activity of cytotoxic T lymphocyte-associated antigen 4 antibody blockade in previously vaccinated metastatic melanoma and ovarian carcinoma patients. Proc Natl Acad Sci USA. 2003;100:4712–7. doi: 10.1073/pnas.0830997100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Krejci KG, Markiewicz MA, Kwon ED. Immunotherapy for urological malignancies. J Urol. 2004;171:870–6. doi: 10.1097/01.ju.0000101161.17279.09. [DOI] [PubMed] [Google Scholar]
- 41.Phan GQ, Yang JC, Sherry RM, et al. Cancer regression and auto-immunity induced by cytotoxic T lymphocyte-associated antigen 4 blockade in patients with metastatic melanoma. Proc Natl Acad Sci USA. 2003;100:8372–7. doi: 10.1073/pnas.1533209100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sanderson K, Scotland R, Lee P, et al. Autoimmunity in a phase I trial of a fully human anti-cytotoxic T-lymphocyte antigen-4 monoclonal antibody with multiple melanoma peptides and Montanide ISA 51 for patients with resected stages III and IV melanoma. J Clin Oncol. 2005;23:741–50. doi: 10.1200/JCO.2005.01.128. [DOI] [PubMed] [Google Scholar]
- 43.Mercader M, Bodner BK, Moser MT, et al. T cell infiltration of the prostate induced by androgen withdrawal in patients with prostate cancer. Proc Natl Acad Sci USA. 2001;98:14565–70. doi: 10.1073/pnas.251140998. [DOI] [PMC free article] [PubMed] [Google Scholar]


