Abstract
The vagus nerve is thought to participate in signal transduction from the immune system to the CNS. The role of the vagus in the physiological, behavioral and neurochemical responses to intraperitoneally (ip) injected interleukin-1β (IL-1β) was studied using a wake subdiaphragmatically vagotomized rats. The rats were injected ip with saline and IL-1β (1 μg/rat) in random order. For the next 2–4 h, they were monitored for locomotor activity, body temperature via abdominally implanted telethermometers, hypothalamic norepinephrine (NE) secretion using in vivo microdialysis and blood sampled via intravenous catheters to determine concentrations of ACTH and corticosterone to assess hypothalamo–pituitary–adrenocortical (HPA) axis activation. Saline injections were followed by transient increases in locomotor activity, body temperature, dialysate NE and plasma concentrations of ACTH and corticosterone. These responses were not significantly altered by vagotomy. IL-1β injections resulted in short-lived increases in shivering and longer decreases in locomotor activity, as well as a delayed modest fever. IL-1β also induced prolonged elevations of hypothalamic microdialysate NE, as well as plasma ACTH and corticosterone. Similar responses were observed regardless of the order of the saline and IL-1β injections. Subdiaphragmatic vagotomy prevented the IL-1-induced increases in body temperature and the increase in dialysate NE, and markedly attenuated the increases in plasma ACTH and corticosterone. The results indicate close temporal relationships between the apparent release of NE and the increase in body temperature and the HPA activation. This together with the effects of vagotomy suggests that the activation of NE in turn increases body temperature and activates the HPA axis. However, because IL-1β induces a limited HPA activation in subdiaphragmatically vagotomized rats, the vagus nerve does not appear to be the only route by which ip IL-1β can activate the HPA axis. It is suggested that IL-1β-induced vagal activation of hypothalamic NE is the major mechanism of HPA activation at low doses of IL-1β. However, IL-1β can also exert direct effects on IL-1 receptors on cerebral blood vessels, activating cyclooxygenases and hence synthesis of prostaglandins which in turn can affect body temperature, behavior and HPA axis activation.
Keywords: Interleukin-1, Norepinephrine, HPA axis, Body Temperature, Behavior, Vagus, ACTH, Corticosterone
1. Introduction
There is substantial evidence for the existence of bidirectional communication between the nervous and immune systems. Changes in the activity of the immune system produced by infections or inflammation elicit neurochemical, endocrine and behavioral effects. Many of these effects have been postulated to be mediated by the production of cytokines associated with the activation of the immune system (Watkins et al., 1995b; Dunn, 2002).
Administration of interleukin-1 (IL-1) to mice and rats induces many behavioral, physiological and neurochemical responses. IL-1 induces a modest fever (Dinarello, 1994) and activates the hypothalamo–pituitary–adrenocortical (HPA) axis (Besedovsky et al., 1986), and to a lesser extent the sympathetic nervous system (Berkenbosch et al., 1989). IL-1 also induces characteristic behavioral changes, including decreases in locomotor activity, exploration, feeding and sexual activity (Dantzer et al., 2001; Larson and Dunn, 2001) and increases the time spent in slow-wave sleep (Krueger and Majde, 1995). In the central nervous system, IL-1 stimulates the activity of noradrenergic and serotonergic systems and increases brain tryptophan (Dunn, 2001).
There have been many studies of the mechanisms of these effects of IL-1, but the relationships among the various responses remain unclear (Dunn, 2002). It has been proposed that afferents of the vagus nerve participate in signal transduction to the CNS in response to systemic infections and the peripheral administration of endotoxin (lipopolysaccharide, LPS) and IL-1 (Watkins et al., 1995b). A vagal involvement has been suggested for the fever (Blatteis and Sehic, 1997; Watkins et al., 1995a), the brain noradrenergic activation (Fleshner et al., 1995), the HPA axis activation (Fleshner et al., 1995; Kapcala et al., 1996) and some of the behavioral responses (Bret-Dibat et al., 1995). However, not all studies have found evidence for a critical role of the vagus. For example, Konsman et al. (2000) reported that vagotomy failed to prevent IL-1- and LPS-induced fever, whereas it prevented the depression of social investigation in response to both IL-1 and LPS. However, other behavioral responses (e.g., feeding) are not altered by vagotomy (Porter et al., 1998; Schwartz et al., 1997).
The aim of the present study was to elucidate the mechanisms by which intraperitoneally administered IL-1 activates the HPA axis. We focussed specifically on the roles of the vagus and the activation of hypothalamic norepinephrine (NE). Thus, we studied the effects of subdiaphragmatic vagotomy (SDV) on the changes in body temperature, in behavior, in hypothalamic dialysate NE and in the activation of the HPA axis indicated by increases in the plasma concentrations of ACTH and corticosterone induced by intraperitoneal (ip) injection of IL-1β in awake rats. The expectation was that the simultaneous measurement of the various parameters would indicate the nature of the relationships between the noradrenergic activation and the fever, the behavioral responses and the HPA activation.
2. Results
Subdiaphragmatic vagotomy (SDV) increased the stomach weights of the rats from a mean control weight of 4.23 ± 0.23 g to 15.10 ± 1.08 g in vagotomized animals (P < 0.0001, Student’s t test). All rats that did not have a stomach weight more than 4% of the body weight (10.3 g) were removed from the study.
2.1. Behavioral effects of IL-1β
Locomotor activity increased transiently in the first 20 min time period after saline or IL-1β (1 μg ip) administration (Fig. 1). Subsequently, IL-1β administration decreased locomotor activity for about the following 4 h after injection (Fig. 1). Repeated measures ANOVA indicated a marginally statistically significant interaction between IL-1β and vagotomy (F(17,578) = 1.59, P < 0.06), along with significant effects of IL-1β injection (F(17,578) = 75.7, P < 0.001), and a marginally significant effect of the vagotomy (F(1,34) = 3.34, P < 0.08). Subsequent Fisher’s LSD tests indicated significant decreases in IL-1β-injected compared to saline-injected rats in each time period starting from 100 min after injection to the end of experiment (P < 0.01).
Fig. 1. Locomotor activity of vagotomized and sham-operated rats after saline and IL-1β treatments.
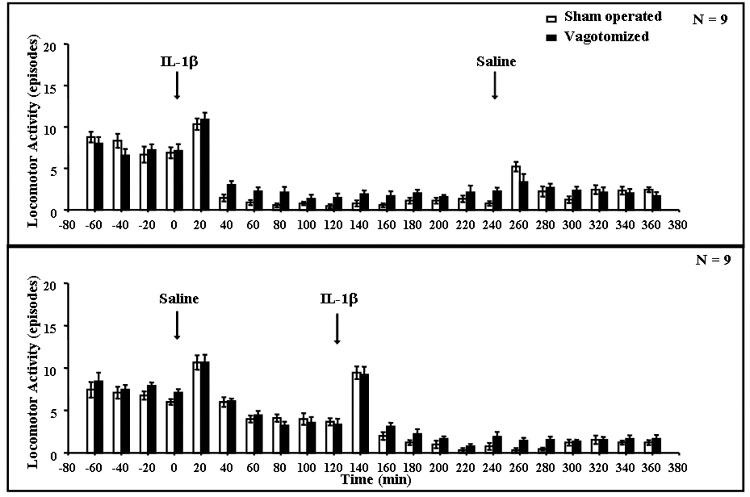
Locomotor activity was scored manually as the number of episodes observed in each 20-min period. After an 80-min period of stabilization, rats were injected (arrows) with either saline (open bars) or rIL-1β (1 μg/rat ip-solid bars) followed 4 h later by saline (upper graph), or saline followed 2 h later by rIL-1β (lower graph). IL-1β significantly depressed locomotor activity (see text); n = 9 rats per group.
When the combined locomotor activity data obtained for 2 h after saline and IL-1β were analyzed, ANOVA indicated differences between IL-1β and saline injections (F(1,68) = 8.87, P < 0.004), but there was no significant vagotomy × IL-1β interaction (F(1,68) = 2.68), and no significant differences between vagotomized and sham-operated rats (F(1,68) = 0.88). However, there were significant differences over time (F(5,340) = 217, P < 0.001) and the interaction between IL-1β and time (F(5,340) = 23.4, P < 0.001). Subsequent post hoc Fisher’s tests showed differences in the time periods after IL-1β, starting 40 min after injection until the end of experiment (P < 0.001).
IL-1β administration also induced shivering, with a maximal response in the first hour after IL-1β administration, lasting almost to the end of experiment (Fig. 2). No shivering was observed in saline-injected rats. Shivering also occurred in the IL-1β-treated rats after vagotomy but less frequently than in the sham-operated rats. Repeated measures ANOVA indicated a statistically significant interaction between vagotomy and IL-1β (F(9,306) = 32.7, P < 0.001) and significant effects of vagotomy (F(1,34) = 97.1, P < 0.001) and IL-1β (F(9,306) = 147, P < 0.001). Subsequent Fisher’s LSD tests indicated significant differences between saline- and IL-1β-injected animals in each time period from 20 to 80 min after injection (P < 0.001).
Fig. 2. Shivering episodes of vagotomized and sham-operated rats after saline (open bars) and IL-1β (solid bars) treatments.
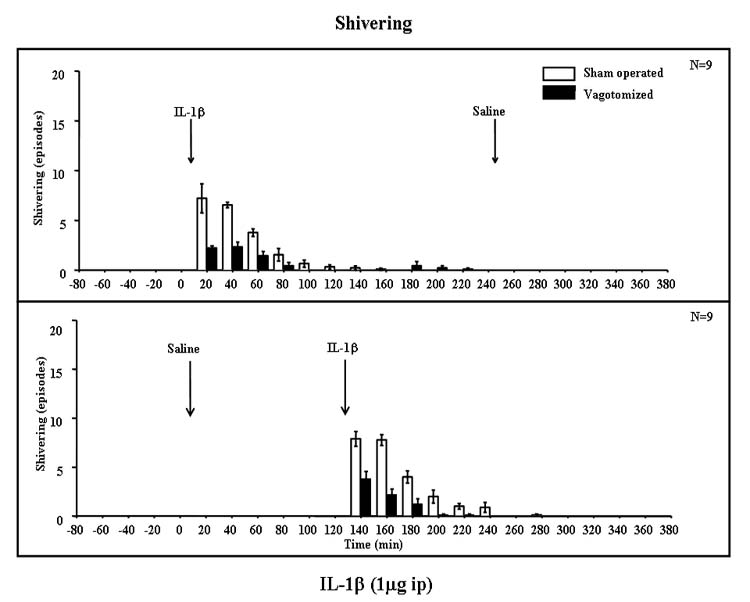
Shivering bouts were scored manually as the number of episodes observed in each 20-min period. Results are from the same rats as in Fig. 1. Shivering was observed only after IL-1β treatment and was significantly attenuated by SDV (see text); n = 9.
When the combined shivering data obtained for 2 h after saline and IL-1β were analyzed, ANOVA indicated a significant interaction between vagotomy and IL-1β (F(1,68) = 120, P < 0.001), indicating that vagotomy significantly attenuated the responses to IL-1β. There were also differences between saline and IL-1β injection (F(1,68) = 450, P < 0.001) and vagotomized and sham-operated rats (F(1,68) = 120, P < 0.001) and a significant interaction between IL-1β and time (F(5,340) = 65.2, P < 0.001). Post hoc analyses indicated differences between sham-operated and vagotomized rats from 20 to 80 min after IL-1β (P < 0.01). Significant differences between IL-1β- and saline-injected rats occurred in all time periods from 60 min after injection until the end of the experiment (P < 0.001).
2.2. Effect of IL-1β on core body temperature
IL-1β induced increases in body temperature compared to saline lasting for about 4 h after injection (Fig. 3). Maximum temperature differences (ca. 0.7–0.9 °C) appeared at around 2 h. Subdiaphragmatic vagotomy completely blocked the increases in body temperature. This was indicated by a significant interaction between vagotomy and IL-1β (F(17,578) = 10.1, P < 0.001). Repeated measures ANOVA also indicated statistically significant effects of vagotomy (F(1,34) = 12.3, P < 0.001) and IL-1β (F(17,578) = 14.0, P < 0.001). Subsequent Fisher’s LSD tests showed that there were significant differences between saline- and IL-1β-injected animals in each time period from 40 to 140 min after injections and from 200 min until the end of experiment (P < 0.01).
Fig. 3. Core temperature of vagotomized (filled circles) and sham-operated (open circles) rats after saline and IL-1β treatments.
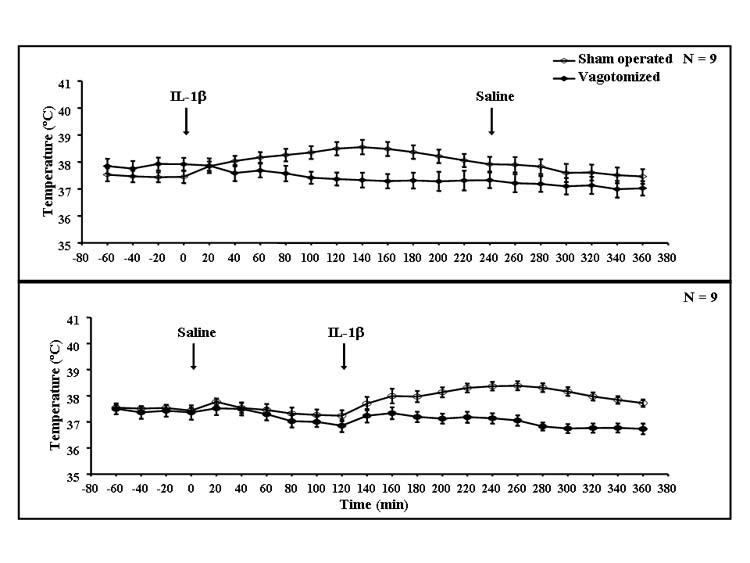
Results were obtained from the same rats as in Figs. 1 and 7. After an 80-min observation, rats were injected with rIL-1β followed 4 h later by saline (upper graph), or saline followed 2 h later by rIL-1β (lower graph). The core temperature was determined at the end of each 20-min period using Minimitters placed in the peritoneum of each rat; n = 9.
When the combined data obtained for 2 h after saline or IL-1β were analyzed, ANOVA indicated significant differences of vagotomy (F(1,68) = 11.9, P < 0.001), IL-1β (F(1,68) = 6.41, P < 0.01) and time (F(5,340) = 4.40, P < 0.001), but no significant interaction between vagotomy and IL-1β (F(1,68) = 1.20), although there was a significant IL-1β × time interaction (F(5,340) = 17.7, P < 0.001). Subsequent post hoc tests indicated differences between sham-operated and vagotomized animals from 100 until 140 min after IL-1β injection (P < 0.05). IL-1β induced significant increases compared to saline in the time periods from 60 min after IL-1β until the end of the experiment (P < 0.05).
2.3. Effect of IL-1β on microdialysate NE from the medial hypothalamus
Saline injections induced a short lasting increase of dialysate NE (maximum 20–60%) in the first one or two time periods after ip administration. SDV had little or no effect on this response. In contrast, ip IL-1β injection induced a prolonged increase in dialysate NE from the medial hypothalamus (Fig. 4). The peak (maximum 209%) appeared 1.5–2 h after IL-1β administration. Vagotomy essentially completely prevented this increase in dialysate NE. This was indicated by a significant interaction between vagotomy and IL-1β in the repeated measures ANOVA (F(17,561) = 9.37, P < 0.001), which also indicated significant effects of vagotomy (F(1,33) = 38.0, P < 0.001) and IL-1β (F(17,561) = 9.14, P < 0.001). Subsequent Fisher’s LSD tests showed that there were significant differences between saline-and IL-1β-injected animals in each time period from 40 to 120 min after injection and from 200 min until the end of the experiment (P < 0.001).
Fig. 4. Microdialysate NE from the medial hypothalamus of vagotomized and sham-operated rats after saline or IL-1β treatment.
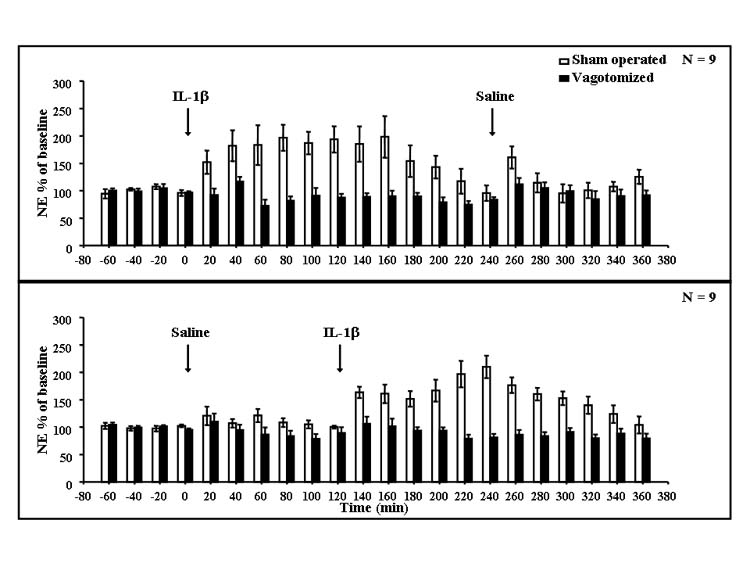
Rats were implanted with microdialysis probes in the medial hypothalamus. Results were obtained from the same rats from which the data in Figs. 1-3 were obtained. Rats were sham operated (open bars) or vagotomized (solid bars) and after an 80-min observation were injected with rIL-1β followed 4 h later by saline (upper graph), or saline followed 2 h later by IL-1β (lower graph). NE was determined by HPLC in the dialysates obtained in each 20-min period; n = 9.
When the combined data obtained for 2 h after saline and IL-1β were analyzed, ANOVA indicated a significant interaction between vagotomy and IL-1β (F(1,66) = 18.0, P < 0.00) and significant effects of vagotomy (F(1,66) = 55.6, P < 0.001), of IL-1β (F(1,66) = 15.3, P < 0.001) and of the IL-1β × time interaction (F(5,330) = 3.61, P < 0.003). Post hoc tests indicated differences between sham-operated and vagotomized animals in all analyzed samples following IL-1β (P < 0.01). IL-1β induced differences from saline in the time periods from 60 to 100 min after injection (P < 0.05).
2.4. Effect of IL-1β on plasma concentrations of ACTH and corticosterone
Injection of IL-1β increased plasma concentrations of ACTH and corticosterone. The increase in plasma ACTH lasted about 3 h, with a peak around 20 min (Fig. 5). The increase in plasma corticosterone also lasted about 3 h with a maximum around 1 h after IL-1β (Fig. 6). SDV markedly attenuated the increases in plasma ACTH and corticosterone. Repeated measures ANOVA indicated significant effects on plasma ACTH concentrations for the interaction between vagotomy and IL-1β (F(17,578) = 3.67, P < 0.001), as well as for vagotomy (F(1,34) = 80.8, P < 0.001) and IL-1β (F(17,578) = 8.05, P < 0.001). ANOVA also indicated a significant interaction between vagotomy and IL-1β (F(17,578) = 2.10, P < 0.01) and the effects of vagotomy (F(1,34) = 30.7, P < 0.001) and IL-1β (F(17,578) = 10.8, P < 0.001) for plasma corticosterone. The significant interactions between vagotomy and IL-1β indicate that the vagotomy significantly attenuated the responses of both plasma ACTH and corticosterone to IL-1β. Subsequent Fisher’s LSD tests showed that there were significant differences between saline-and IL-1β-injected animals in each time period starting from 20 min until the end of the experiment for ACTH and from 20 to 140 min after injections for corticosterone (P < 0.01).
Fig. 5. Plasma ACTH concentrations of vagotomized and sham-operated rats after saline or IL-1β treatment.
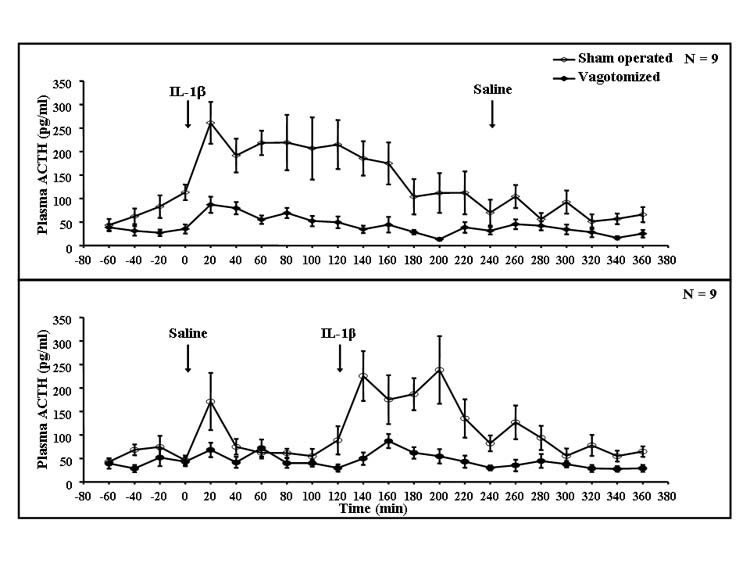
Results were obtained from the same rats from which the data in Figs. 1-4 were obtained. Rats were sham operated (open circles) or vagotomized (filled circles) and after an 80-min observation were injected with rIL-1β followed 4 h later by saline (upper graph), or saline followed 2 h later by rIL-1β (lower graph). Plasma was drawn at the end of each 20-min period and ACTH determined by radioimmunoassay; n = 9 rats.
Fig. 6. Plasma corticosterone concentrations of vagotomized and sham-operated rats after saline or IL-1β treatment.
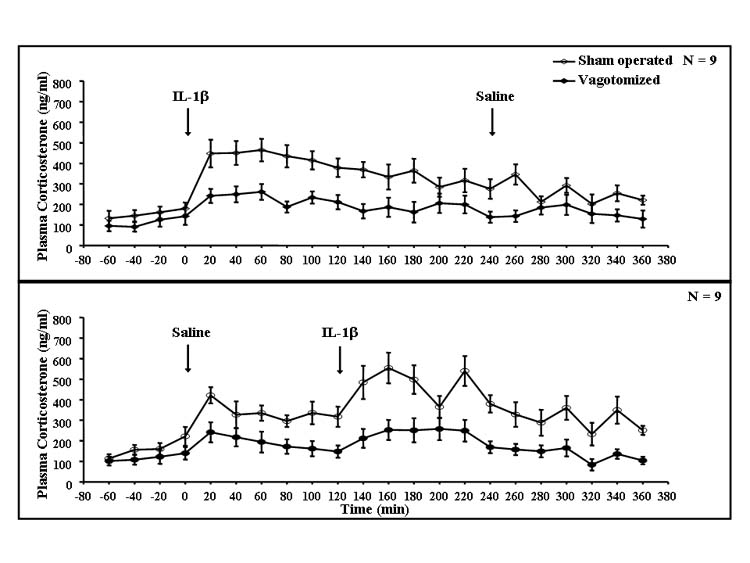
Results were obtained from the same rats from which the data in Figs. 1-5 were obtained. Rats were sham operated (open circles) or vagotomized (filled circles) and an after 80-min observation were injected with rIL-1β followed 4 h later by saline (upper graph), or saline followed 2 h later by rIL-1β (lower graph). Plasma was drawn at the end of each 20-min period and corticosterone determined by radioimmunoassay; n = 9.
When the combined plasma ACTH data obtained for 2 h after saline and IL-1β were analyzed, ANOVA indicated a significant interaction between vagotomy and IL-1β (F(1,68) = 21.2, P < 0.001), as well as effects of vagotomy (F(1,68) = 66.5, P < 0.001), IL-1β (F(1,68) = 41.4, P < 0.001) and time (F(5,340) = 4.23, P < 0.001). Subsequent post hoc tests indicated differences between saline- and IL-1β-injected animals from 20 min until the end of the observations (P < 0.01). Statistically significant differences between sham-operated and vagotomized rats also appeared in the time periods starting from 40 min after IL-1β until the end of the experiment (P < 0.01). IL-1β induced statistically significant increases in plasma ACTH in vagotomized rats (P < 0.01).
In the similar analysis for plasma corticosterone concentrations in the 2-h period after saline or IL-1β, ANOVA indicated only marginally significant interactions between vagotomy and IL-1β (F(1,68) = 3.40, P < 0.07) and IL-1β and time (F(5,340), P < 0.07). However, there were significant effects of vagotomy (F(1,68) = 42.0, P < 0.001), IL-1β (F(1,68) = 16.1, P < 0.001) and time (F(5,340) = 7.26, P < 0.001). Subsequent post hoc tests indicated differences between saline- and IL-1β-injected animals between 40 and 100 min after injection (P < 0.05). IL-1β induced statistically significant increases in plasma corticosterone in vagotomized rats (P < 0.01).
2.5. Relationships among the various responses to IL-1β
To determine the relationships among the various responses to ip IL-1β injections, we calculated Pearson’s correlation coefficients. For this analysis, we compared each behavioral, physiological, neurochemical and hormonal responses to IL-1β for each time point after injection. When saline was injected before IL-1β in sham-operated rats, we observed significant correlations between locomotor activity, temperature and dialysate NE. Also shivering episodes correlated with plasma ACTH and corticosterone, core temperature with NE, NE with ACTH and corticosterone and ACTH with corticosterone (Table 1). Similar correlations were noted in vagotomized animals. When IL-1β was injected after saline, in sham-operated rats, we also observed significant correlations like those obtained when IL-1β was injected before saline (Table 1).
Table 1.
Pearson’s correlation coefficients of the measured parameters of rats’ answer to IL-1β ip injections
| Saline then IL-1β injections | IL-1β then saline injections | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MOV | SHIV | TEMP | NOR | ACTH | CORT | MOV | SHIV | TEMP | NOR | ACTH | CORT | |
| Sham-operated rats | ||||||||||||
| MOV | ||||||||||||
| SHIV | ||||||||||||
| TEMP | −0.62,
P < 0.002 |
−0.63,
P < 0.002 |
||||||||||
| NOR | −0.54,
P < 0.01 |
0.88,
P < 0.001 |
0.85,
P < 0.001 |
|||||||||
| ACTH | 0.72,
P < 0.001 |
0.52,
P < 0.01 |
0.65,
P < 0.001 |
0.67,
P < 0.001 |
0.84,
P < 0.001 |
|||||||
| CORT | 0.66,
P < 0.001 |
0.64,
P < 0.01 |
0.68,
P < 0.001 |
0.63,
P < 0.002 |
0.74,
P < 0.001 |
0.83,
P < 0.001 |
0.87,
P < 0.001 |
|||||
| Vagotomized rats | ||||||||||||
| MOV | ||||||||||||
| SHIV | ||||||||||||
| TEMP | 0.75,
P < 0.001 |
0.78,
P < 0.001 |
||||||||||
| NOR | 0.81,
P < 0.001 |
0.43,
P < 0.04 |
0.70,
P < 0.001 |
|||||||||
| ACTH | 0.43,
P < 0.04 |
0.51,
P < 0.01 |
0.47,
P < 0.03 |
0.76,
P < 0.001 |
||||||||
| CORT | 0.69,
P < 0.001 |
0.64,
P < 0.001 |
0.62,
P < 0.002 |
|||||||||
3. Discussion
Consistent with previous studies, intraperitoneal injection of rats with IL-1β induced a modest fever (Dinarello, 1994), increased shivering (Malkinson et al., 1988), decreased locomotor activity (Avitsur et al., 1995), activated hypothalamic NE (Kabiersch et al., 1988) and elevated plasma concentrations of ACTH and corticosterone (Besedovsky et al., 1986). The present results indicate that subdiaphragmatic vagotomy prevented the ip IL-1β-induced increase in body temperature, whereas it decreased the shivering and attenuated the decrease in locomotor activity. SDV also prevented the increased release of NE in the hypothalamus and attenuated, but did not block, the increases in plasma concentrations of ACTH and corticosterone.
A role for the vagus in immune system signaling to the brain was initially proposed by Wan et al. (1994) who observed that SDV prevented the LPS-induced appearance of Fos protein in the brains of rats. Subsequent studies showed that SDV prevented the IL-1-induced fever, indicating a role for the vagus in the effects of IL-1 on body temperature (Blatteis and Sehic, 1997; Watkins et al., 1995a). Our finding that IL-1β did not increase the core temperature in subdiaphragmatically vagotomized rats is consistent with this. However, Konsman et al. (2000) did not observe any effect of vagotomy on the IL-1- or the LPS-induced fever. The discrepancy probably reflects the dose of IL-1β used because we used approximately 3 μg/kg of recombinant rat, whereas Konsman et al. used 25 μg/kg, a substantially higher dose. It is to be noted that Hansen et al. (2001) reported a dose dependence of the effect of SDV on the fever induced by recombinant human IL-1β (see below).
A vagal involvement in certain behavioral responses to peripheral administration of LPS was discovered by Bluthé et al. (1994) who showed that SDV of rats impaired the reduction in social investigation of a juvenile in response to ip LPS. Similar results were subsequently observed with IL-1β (Konsman et al., 2000). SDV also blocked the conditioned taste aversion to ip IL-1β in the rat (Goehler et al., 1995) and attenuated the reductions in food-motivated behavior in the mouse induced by relatively high intraperitoneal doses of IL-1 (750 ng/mouse) and LPS (400 μg/kg; Bret-Dibat et al., 1995). However, SDV failed to attenuate the reduction in feeding in rats induced by ip IL-1 or LPS (Porter et al., 1998; Schwartz et al., 1997), although it attenuated the decrease in sweetened milk consumption in response to IL-1β and LPS in mice (Wieczorek et al., 2005). Thus, the vagus appears to be involved in some, but not all, of the behavioral responses to IL-1 and LPS.
A vagal involvement in the hypothalamic NE response to ip administration of IL-1β was first indicated by Fleshner et al. (1995) who showed that the IL-1-induced depletion of hypothalamic NE did not occur in rats after SDV. Subsequently, Ishizuka et al. (1997) reported that NE release from the paraventricular nucleus assessed by microdialysis was attenuated in abdominally vagotomized rats. Our results are in good general agreement with these studies; the difference between the block we observed and the attenuation in Ishizuka’s study may be explained by the difference between the abdominal vagotomy they used and the subdiaphragmatic vagotomy we performed. Our results are also consistent with the observation that SDV prevented the increased firing of locus caeruleus (LC) noradrenergic neurons in response to ip LPS, peptidoglycan or poly I:C (Borsody and Weiss, 2005), although the LC contributes only modestly to the noradrenergic innervation of the hypothalamus.
Fleshner et al. (1995) also showed that the increase in plasma corticosterone elicited by ip IL-1 was attenuated by SDV. Kapcala et al. (1996) reported that SDV attenuated the ACTH response at 2 h to a high dose (20 μg/kg) of IL-1β, whereas it prevented the response to a lower dose (4 μg/ kg). Curiously however, in these studies, SDV did not significantly alter the corticosterone response. Hansen et al. (2000) also reported that SDV had no effect on the ip LPS (10, 50 and 100 mg/kg)-induced increases in plasma corticosterone in rats. Although our results are generally consistent with these findings, we observed substantial effects of SDV on the IL-1-induced increases in both ACTH and corticosterone.
Saline injections induced short-lived increases in body temperature, in locomotor activity, in dialysate NE and in plasma ACTH and corticosterone. Consistent with previous studies (Watkins et al., 1995a), the transient effect on temperature was not affected by SDV. In the present study, SDV did not appear to affect the transient increases in locomotor activity and in dialysate NE, but the increases in plasma ACTH and corticosterone were attenuated. These transient effects are most likely accounted for by the stress associated with the handling and injection, as suggested by Watkins et al. (1995a). The failure of SDV to affect the changes in body temperature, locomotor activity and hypothalamic NE associated with stress contrasts with the role of the vagus in the IL-1β-induced changes.
The ip IL-1β-induced shivering presumably contributes to the elevation of body temperature, but the limited shivering does not appear to account for the full duration of the fever. It is to be noted that some shivering occurred in the SDV rats, which did not exhibit any fever. The noradrenergic and fever responses to IL-1β parallel each other closely, and both were prevented by SDV, suggesting that the increased NE may mediate the fever. Indeed NE has been implicated in the early stages of IL-1-induced fever (Blatteis et al., 2005).
The effects of SDV we observed indicate a strong relationship between the noradrenergic and HPA activation. This complements the evidence that lesions of the noradrenergic projections to the hypothalamus in rats impair the increase in plasma corticosterone induced by IL-1 (Chuluyan et al., 1992). In the present study, the SDV-induced reduction in the hypothalamic NE response was greater than that on ACTH and corticosterone. However, the depletion of hypothalamic NE in the Chuluyan study (77%) was far from complete. Thus, it is likely that the failure of SDV to block the effects of IL-1β on ACTH and corticosterone secretion indicates that the vagus is not the only route by which systemic IL-1 signals the brain to activate the HPA axis, although the effects of IL-1 in SDV rats could involve intact vagal afferents above the diaphragm.
Goehler et al. (1997) showed that biotinylated IL-1 receptor antagonist (IL-1ra) binds to glomus cells in vagal paraganglia. Binding was found to abdominal, laryngeal, thoracic and hepatic vagal paraganglia. Thus, systemic IL-1 may bind directly to cells in the vagal paraganglia that subsequently activate the vagal afferents, but the details of the mechanism have yet to be determined.
The mechanisms of the behavioral responses to IL-1 appear to depend on the route of injection. In rats, Bluthé et al. (1996b) noted that although SDV substantially attenuated the reductions in social investigation in response to ip IL-1β, those to intracerebroventricular administration were not affected. Moreover, whereas the reductions in social investigation of mice in response to ip IL-1 were attenuated by SDV, the responses to subcutaneous and intravenous IL-1 were not (Bluthé et al., 1996b).
The route of IL-1 injection may also determine the specific vagal afferents that are stimulated. For example, whereas Watkins et al. (1995a) found that SDV attenuated the ip IL-1β-induced fever, hepatic vagotomy had no effect. However, SDV attenuated the increase in body temperature induced by intravenous IL-1β (Fleshner et al., 1998). The effects also depend on dose; Hansen et al. (2001) showed that SDV prevented the effect of ip IL-1β on body temperature at a dose of 0.1 μg/kg, attenuated it at 0.5 μg/kg but had no effect at all at 1 μg/kg. Thus, it is entirely possible that had we used a higher dose of IL-1β, vagotomy would have had a proportionally smaller effect on the body temperature and HPA responses. We suggest that low doses of intraperitoneal IL-1β exert their behavioral, noradrenergic and HPA activating effects primarily via binding to the paraganglion cells associated with the vagal afferents, and subsequent noradrenergic activation of the hypothalamus inducing the fever and the HPA axis activation, and possibly some of the behavioral effects. Higher ip doses of IL-1β would result in higher circulating concentrations and thus greater occupancy of the IL-1 receptors on cerebral blood vessels that are most likely responsible for the rapid effects of IL-1β on prostaglandin synthesis, and hence body temperature, behavior and the HPA axis (see Dunn et al., submitted for publication). Thus, at higher doses of IL-1β, we would expect proportionally lower effects of SDV.
It is interesting to compare the results of the present study in rats with the results we obtained in subdiaphragmatically vagotomized mice (Wieczorek et al., 2005). In mice, SDV decreased the effects of ip IL-1β and LPS on feeding and locomotor activity, but the effects were relatively small. SDV also attenuated the responses in plasma ACTH and corticosterone, but these effects were much smaller than we observed in the rat. Moreover, in mice, SDV had only small effects on the IL-1β- and LPS-induced increase in the metabolism of brain NE, although the reductions in the hypothalamic NE response were consistent (Wieczorek et al., 2005). There were some important differences between the experiments in the two species. In the rat, we collected repeated blood samples for determination of ACTH and corticosterone, but the assays used were not sufficiently sensitive to allow this in mice. Also, we assessed NE release in the rat by microdialysis, which would be very difficult in mice. However, in other experiments in rats we have observed that increases in the brain concentrations of MHPG parallel quite closely increases in dialysate NE, albeit without the benefits of repeated sampling in the same animal made possible by microdialysis. Thus, it seems unlikely that the methodological differences account for the greater effects of SDV in the rat. We conclude that in the rat, the vagus appears to play an important role in the ip IL-1-induced increases in body temperature, in shivering, in the activation of hypothalamic NE and in the HPA axis activation. Whereas in the mouse, the vagus plays only a modest role in the IL-1β- and LPS-induced activation of the HPA axis and brain noradrenergic systems. Thus, the contributions of the various mechanisms may differ among species, and the vagal route appears to be less important for these responses in the mouse than the rat.
The present results add to the evidence that vagal afferent activation by IL-1 plays an important role in transduction of information from the peritoneum to the brain. In the rat, the vagus appears to be a critical route for the IL-1-induced fever and the activation of hypothalamic noradrenergic systems. It also plays an important, but less critical, role in the HPA activation, and in the induction of shivering and certain other behavioral responses. The hypothalamic noradrenergic activation appears to be important, but not essential, for the HPA response but may be critical for the fever. Overall, these studies suggest that multiple mechanisms are involved in the physiological and behavioral effects of intraperitoneal IL-1. Moreover, the different mechanisms may vary in importance depending on the site of the secretion or the administration of the IL-1, the dose and the species.
4. Experimental procedures
4.1. Experimental animals
The experiments were performed using 26 male Sprague–Dawley rats, weighing 250–300 g obtained from Harlan Sprague–Dawley Inc., Houston, TX. The animals were housed individually, under controlled environmental conditions of temperature (22 ± 2 °C), humidity (55 ± 5%) and on a 12:12-h light/dark cycle (lights on at 7:00 A.M.). Water and Purina rat chow were available ad libitum.
4.2. Materials
Recombinant rat interleukin-1β (IL-1β) was purchased from R&D Systems Inc. (Minneapolis, MN). All other chemicals were analytical grade from Sigma Chemical Company (St. Louis, MO).
4.3. Microdialysis probes
Concentric microdialysis probes were used. The inner fused silica tube (outer diameter 150 μm; Polymicrotechnologies, Phoenix, AZ) was inserted through a polyethylene tube (Clay Adams PE-50) and then inserted into a stainless steel tube (C312G; Plastics One, Roanoke, VA), the upper end of which was slipped into the PE-50 tubing in such way, that the fused silica passed through it. The dialysis membrane 3 mm long and 250 μm in diameter (Cuprophane Pore Fiber, molecular weight cutoff 13,000) was attached to the end of the stainless steel tube and sealed at its tip with epoxy cement (Locktite Quickset gel). The net active length of the dialysis membrane was approximately 2.0 mm. The total length of the microdialysis probe from the bottom of the plastic sleeve on the Plastics One tube to the tip of the dialysis membrane was 19.4–19.8 mm.
4.4. Surgical procedure
All surgical procedures were performed aseptically, and all surgical instruments were sterilized before use. Animals were anesthetized using Innovar Plus (3 mg fentanyl, 210 mg droper-idol, 150 mg midazolam dissolved in 174 ml of water) at a dose of 6 μl/g body weight ip. Rats were placed on their backs and a 5-cm long incision was made in the lower abdomen. The liver was carefullymovedto the righttoexpose the esophagus. Then under a surgical microscope both branches of the vagus nerve (stomach and liver) were exposed and small fragments (about 0.7 mm long) were dissected. Then a telethermometer (Minimitter) was placed in the peritoneal cavity. The abdominal muscles were then sutured with surgical silk. The skin was sutured and topical antibiotic (Neosporin) applied to counter infection.
Next, a catheter was implanted jugular vein. A short (1.5 cm) incision was made in the neck; the jugular vein was carefully exposed and ligated with surgical silk as distally as possible to prevent bleeding. A small incision was made in the jugular vein and a catheter (4 cm long) filled with heparinized saline (80 IU) was inserted into the vein. A small amount of blood was then withdrawn to check the patency of the catheter, which was then fixed in place with surgical silk. The other end of the catheter (10 cm long, equipped with a pedestal; Plastics One) was passed beneath the skin and fixed to the skull with dental cement. The skin was sutured and Neosporin was applied.
The rats were then placed in a Kopf stereotaxic apparatus, and guide cannulae for microdialysis probes (C312G, 5.8 mm long; Plastics One) were implanted bilaterally and fixed to the skull using Cranioplastic cement (Plastics One). The coordinates for the microdialysis guide cannulae in the medial hypothalamus were A-P: −2.0 mm; L: +2.2 mm; V: −4.8 mm, tilted medially at an angle of 15.8° to prevent damage to the superior sagittal sinus. These coordinates were determined from the atlas of Paxinos and Watson (1997; see Fig. 7). Stainless steel miniature self-tapping bone screws (Fine Scientific Tools, Foster City, CA) were screwed into the skull to help mount the microdialysis probe. The screws were covered with the same cranioplastic cement. The animals were then placed in individual home cages and allowed 10 days to recover from the surgery. After all experiments were finished, the rats were killed with anover dose of Innovar Plus, their brains were removed from the skull, sectioned on a freezing microtome and stained with cresyl violet staining for determination of the microdialysis probe placement. Data are only presented from those animals in which microdialysis probes passed through the PVN of hypothalamus.
Fig. 7. Coronal section of the rat brain from the Paxinos and Watson atlas (bregma –1.80) indicating the approximate position of the microdialysis probes.
The upper part of the probe is the stainless steel tubing of the cannula guide, the middle section is the stainless steel tubing of the probe shaft and the lower (thinnest) part is the microdialysis membrane. Because the microdialysis membrane is flexible, its precise position may vary slightly from rat to rat.
These procedures were approved by the Louisiana State University Health Sciences Center Animal Care and Use Committee and conform to National Institutes of Health guidelines.
4.5. Verification of the vagotomy
The effectiveness of the vagotomy was assessed by postmortem stomach weights. At the end of the experiment, animals were euthanized with an overdose of Innovar Plus. Then, their stomachs were removed and weighed. Only rats in which the stomach weight increased to more than 4% of the body weights were considered to be properly vagotomized and were included in the data presented.
4.6. Microdialysis system
The inflow to the microdialysis probe was driven by a CMA/100 Microinjection Pump at a flow rate of 1.2 μl/min. The perfusion fluid was artificial cerebrospinal fluid (aCSF) made according to Sharp et al. (1989): 1.2 mM CaCl2, 1.2 mM Na2HPO4, 0.3 mM NaH2PO4, 3.4 mM KCl, 140 mM NaCl, pH 7.2. Samples were collected in 20-min periods directly into 0.5-ml polypropylene vials containing 50 pg of N-methyldopamine (NMDA) as an internal standard in 5 μl of 0.15 M HClO4–0.15 mM ethylene-diaminetetraacetic acid (EDTA). The samples were frozen immediately after collection and stored on dry ice in a freezer at −70 °C until analyzed.
In vitro recovery of the microdialysis probes was determined as described previously (Lavicky and Dunn, 1995). The recoveries varied between 12% and 16% for NE. Because the diffusion of materials from within brain tissue is likely to differ from that in a saline solution (Benveniste, 1989), we did not correct our data for these in vitro recoveries.
4.7. HPLC
HPLC with colorimetric detection was used to determine the content of NE and NMDA with reference to freshly diluted standards (Sigma). The system consisted of a chromatographic syringe pump (ISCO Model 100DM), an ESA Coulochem III detector (ESA, Inc., Chelmsford, MA) and a manual injector (Rheodyne 9126). Separation was performed on a Keystone microbore analytical 125 × 1 mm column C-18, particle size 5 mm (Thermo Hypersil). The mobile phase contained 50 mM sodium acetate, 0.5 mM EDTA and 2.05 mM 1-decanesulfonic acid sodium salt and 12% v/v acetonitrile. It was adjusted to pH 6.0 with acetic acid, filtered under vacuum through a 0.22-mm Nylon membrane filters 47 mm diameter filter (Sigma) and degassed with helium. The column was pumped at a flow rate of 0.1 ml/min at ambient temperature (22–24 °C).
Detection conditions were as follows: guard cell = +350 mV (ESA 5020); microdialysis cell (ESA 5014B) E1 = −150 mV, E2 = +250 mV; and a recorder range of 50 nA. The detector output was captured and analyzed using Waters Millenium32 version 3.20. The concentrations of NE in each sample were calculated from the chromatogram peak heights and expressed as a percentage of baseline release, determined from the first four microdialysate samples.
4.8. Experimental procedure
Each animal was familiarized with the experimental chamber (15-in. diameter animal enclosure; Instech Laboratories, Inc., Plymouth Meeting, PA) for 4 consecutive days for 1 h each day. The evening before the experiment (7:00–7:30 P.M.), rats were placed in the microdialysis chambers, the microdialysis probes were inserted in the cannula guides and connected to the microdialysis pump and the pump started at a flow rate of 0.5 μl/min. On the following morning (ca. 8:30 AM), the flow rate was increased to 1.2 μl/min and the system left to stabilize for 1 h. At the end of this period, collection of microdialysate samples was initiated, as well as the behavioral observations, the recording of body temperature and the collection of blood samples. Microdialysate and blood samples (0.6 ml) were collected every 20 min for the next 6 h. Body temperature was measured at the same time as the microdialysate collection tubes were changed and blood was drawn. The first four microdialysate and blood samples were collected to determine a baseline. After this (i.e., at 80 min), rats were injected with either saline (100 μl) or rIL-1β (1 μg/rat ip) followed 4 h later by saline or saline followed 2 h later by rIL-1β. Whether the rat received saline or IL-1β was decided on a random basis.
Locomotor activity was recorded manually as the number of episodes of movement involving all four paws observed, and shivering as the number of bouts of shivering in each 20-min period. Blood samples were immediately centrifuged for 10 min at 4000 rpm at 4 °C and the supernatant (plasma) removed and frozen. The blood cells were then resuspended in 4% bovine serum albumin dissolved in sterile normal saline and returned to the same donor rat every hour through the jugular vein catheter.
In most cases, each rat was tested again 1 week later, using the same protocol and procedure, but the order of the saline and IL-1β injections was reversed. Because the insertion of microdialysis probes causes tissue damage and consequent gliosis that can impair the collection of solutes from the extracellular fluid (Benveniste, 1989), a new microdialysis probe was inserted in the contralateral medial hypothalamus for this second procedure.
4.9. Statistical analysis
Statistical analyses were performed in two ways: (1) a repeated measures ANOVA was performed on all the data, using surgery (vagotomy vs. sham), IL-1β, and time as main factors; (2) the data obtained in the 2 h following IL-1β and saline treatments for both treatment orders were separately combined for each time bin, and a second repeated measures ANOVA performed, again using surgery, IL-1β, and time as the main factors. When the analyses indicated significant interactions between vagotomy and IL-1β, the data were analyzed post hoc using Fisher’s LSD test to determine whether or not there were significant effects of IL-1β (vs. saline) in sham-operated and vagotomized rats, for each 20-min time period after IL-1β or saline injections.
Pearson’s correlational coefficients were calculated to examine relationships among the various measured responses to IL-1β. The correlation was performed for each of the measured responses to IL-1β for each rat at all time points after injection.
5. Uncited reference
Acknowledgments
We thank Charles Dempsey for technical assistance with the neurochemical assays. This work was supported by a grant from the National Institutes of Health (NS35370).
References
- Avitsur R, Donchin O, Barak O, Cohen E, Yirmiya R. Behavioral effects of interleukin-1β: modulation by gender, estrus cycle, and progesterone. Brain Behav Immun. 1995;9:234–241. doi: 10.1006/brbi.1995.1022. [DOI] [PubMed] [Google Scholar]
- Benveniste H. Brain microdialysis. J Neurochem. 1989;52:1667–1679. doi: 10.1111/j.1471-4159.1989.tb07243.x. [DOI] [PubMed] [Google Scholar]
- Berkenbosch F, De Goeij DEC, del Rey A, Besedovsky HO. Neuroendocrine, sympathetic and metabolic responses induced by interleukin-1. Neuroendocrinology. 1989;50:570–576. doi: 10.1159/000125283. [DOI] [PubMed] [Google Scholar]
- Besedovsky HO, del Rey A, Sorkin E, Dinarello CA. Immunoregulatory feedback between interleukin-1 and glucocorticoid hormones. Science. 1986;233:652–654. doi: 10.1126/science.3014662. [DOI] [PubMed] [Google Scholar]
- Blatteis CM, Sehic E. Fever: how may circulating pyrogens signal the brain. News Physiol Sci. 1997;12:1–9. [Google Scholar]
- Blatteis CM, Li S, Li Z, Feleder C, Perlik V. Cytokines, PGE2 and endotoxic fever: a re-assessment. Prostaglandins Other Lipid Mediat. 2005;76:1–18. doi: 10.1016/j.prostaglandins.2005.01.001. [DOI] [PubMed] [Google Scholar]
- Bluthé R-M, Walter V, Parnet P, Layé S, Lestage J, Verrier D, Poole S, Stenning BE, Kelley KW, Dantzer R. Lipopolysaccharide induces sickness behaviour in rats by a vagal mediated mechanism. C R Acad Sci Paris. 1994;317:499–503. [PubMed] [Google Scholar]
- Bluthé R-M, Michaud B, Kelley KW, Dantzer R. Vagotomy attenuates behavioural effects of interleukin-1 injected peripherally but not centrally. NeuroReport. 1996a;7:1485–1488. doi: 10.1097/00001756-199606170-00008. [DOI] [PubMed] [Google Scholar]
- Bluthé R-M, Michaud B, Kelley KW, Dantzer R. Vagotomy blocks behavioural effects of interleukin-1 injected via the intraperitoneal route but not via other systemic routes. NeuroReport. 1996b;7:2823–2827. doi: 10.1097/00001756-199611040-00083. [DOI] [PubMed] [Google Scholar]
- Borsody MK, Weiss JM. The subdiaphragmatic vagus nerves mediate activation of locus coeruleus neurons by peripherally administered microbial substances. Neuroscience. 2005;131:235–245. doi: 10.1016/j.neuroscience.2004.09.061. [DOI] [PubMed] [Google Scholar]
- Bret-Dibat J-L, Bluthé R-M, Kent S, Kelley KW, Dantzer R. Lipopolysaccharide and interleukin-1 depress food-motivated behavior in mice by a vagal-mediated mechanism. Brain Behav Immun. 1995;9:242–246. doi: 10.1006/brbi.1995.1023. [DOI] [PubMed] [Google Scholar]
- Chuluyan H, Saphier D, Rohn WM, Dunn AJ. Noradrenergic innervation of the hypothalamus participates in the adrenocortical responses to interleukin-1. Neuroendocrinology. 1992;56:106–111. doi: 10.1159/000126215. [DOI] [PubMed] [Google Scholar]
- Dantzer R, Bluthé R-M, Castanon N, Chauvet N, Capuron L, Goodall G, Kelley KW, Konsman J-P, Layé S, Parnet P, Pousset F. Cytokine effects on behavior. In: Ader R, Felten D, Cohen N, editors. Psychoneuroimmunology. Academic Press; San Diego, CA: 2001. pp. 703–727. [Google Scholar]
- Dinarello CA. Interleukin-1. 1994;25:21–51. doi: 10.1016/s1054-3589(08)60429-9. [DOI] [PubMed] [Google Scholar]
- Dunn AJ. Effects of cytokines and infections on brain neurochemistry. In: Ader R, Felten DL, Cohen N, editors. Psychoneuroimmunology. Academic Press; New York: 2001. pp. 649–666. [Google Scholar]
- Dunn AJ. Mechanisms by which cytokines signal the brain. In: Clow A, Hucklebridge F, editors. Neurobiology of the Immune System, International Review of Neurobiology. Vol. 52. Academic Press; San Diego: 2002. pp. 43–65. [DOI] [PubMed] [Google Scholar]
- Dunn AJ, Swiergiel AH, Zhang H, Quan N. Interleukin-1 and lipopolysaccharide induce cyclooxygenase-2 in brain endothelia paralleling the hypophagia submitted for publication. [Google Scholar]
- Fleshner M, Goehler LE, Hermann J, Relton JK, Maier SF, Watkins LR. Interleukin-1β induced corticosterone elevation and hypothalamic NE depletion is vagally mediated. Brain Res Bull. 1995;37:605–610. doi: 10.1016/0361-9230(95)00051-f. [DOI] [PubMed] [Google Scholar]
- Fleshner M, Goehler LE, Schwartz BA, McGorry M, Martin D, Maier SF, Watkins LR. Thermogenic and corticosterone responses to intravenous cytokines (IL-1β and TNF-α) are attenuated by subdiaphragmatic vagotomy. J Neuroimmunol. 1998;86:134–141. doi: 10.1016/s0165-5728(98)00026-5. [DOI] [PubMed] [Google Scholar]
- Goehler LE, Busch CR, Tartaglia N, Relton J, Sisk D, Maier SF, Watkins LR. Blockade of cytokine induced conditioned taste aversion by subdiaphragmatic vagotomy: further evidence for vagal mediation of immune-brain communication. Neurosci Lett. 1995;185:163–166. doi: 10.1016/0304-3940(95)11251-q. [DOI] [PubMed] [Google Scholar]
- Goehler LE, Relton JK, Dripps D, Kiechle R, Tartaglia N, Maier SF, Watkins LR. Vagal paraganglia bind biotinylated interleukin-1 receptor antagonist (IL-1ra) in the rat: a possible mechanism for immune-to-brain communication. Brain Res Bull. 1997;43:357–364. doi: 10.1016/s0361-9230(97)00020-8. [DOI] [PubMed] [Google Scholar]
- Hansen MK, Daniels S, Goehler LE, Gaykema RP, Maier SF, Watkins LR. Subdiaphragmatic vagotomy does not block intraperitoneal lipopolysaccharide-induced fever. Autonom Neurosci. 2000;85:83–87. doi: 10.1016/S1566-0702(00)00224-1. [DOI] [PubMed] [Google Scholar]
- Hansen MK, O’Conner KA, Goehler LE, Watkins LR, Maier SF. The contribution of the vagus nerve in interleukin-1beta-induced fever is dependent on dose. Am J Physiol. 2001;280:R929–R934. doi: 10.1152/ajpregu.2001.280.4.R929. [DOI] [PubMed] [Google Scholar]
- Ishizuka Y, Ishida Y, Kunitake T, Kato K, Hanamori T, Mitsuyama Y, Kannan H. Effects of area postrema lesion and vagotomy on interleukin-1β-induced norepinephrine release in the hypothalamic paraventricular nucleus region in the rat. Neurosci Lett. 1997;223:57–60. doi: 10.1016/s0304-3940(97)13388-2. [DOI] [PubMed] [Google Scholar]
- Kabiersch A, del Rey A, Honegger CG, Besedovsky HO. Interleukin-1 induces changes in norepinephrine metabolism in the rat brain. Brain Behav Immun. 1988;2:267–274. doi: 10.1016/0889-1591(88)90028-1. [DOI] [PubMed] [Google Scholar]
- Kapcala LP, He JR, Gao Y, Pieper JO, DeTolla LJ. Subdiaphragmatic vagotomy inhibits intra-abdominal interleukin-1β stimulation of adrenocorticotropin secretion. Brain Res. 1996;728:247–254. doi: 10.1016/0006-8993(96)00511-2. [DOI] [PubMed] [Google Scholar]
- Konsman JP, Luheshi GN, Bluthé R-M, Dantzer R. The vagus nerve mediates behavioural depression, but not fever, in response to peripheral immune signals; a functional anatomical analysis. Eur J Neurosci. 2000;12:4346–4434. doi: 10.1046/j.0953-816x.2000.01319.x. [DOI] [PubMed] [Google Scholar]
- Krueger JM, Majde JA. Microbial products and cytokines in sleep and fever regulation. Crit Rev Immunol. 1995;14:355–379. doi: 10.1615/critrevimmunol.v14.i3-4.70. [DOI] [PubMed] [Google Scholar]
- Larson SJ, Dunn AJ. Behavioral effects of cytokines. Brain Behav Immun. 2001;15:371–387. doi: 10.1006/brbi.2001.0643. [DOI] [PubMed] [Google Scholar]
- Lavicky J, Dunn AJ. Endotoxin administration stimulates cerebral catecholamine release in freely moving rats as assessed by microdialysis. J Neurosci Res. 1995;40:407–413. doi: 10.1002/jnr.490400316. [DOI] [PubMed] [Google Scholar]
- Malkinson TJ, Cooper KE, Veale WL. Physiological changes during thermoregulation and fever in urethan-anesthetized rats. Am J Physiol. 1988;255:R73–R81. doi: 10.1152/ajpregu.1988.255.1.R73. [DOI] [PubMed] [Google Scholar]
- Porter MH, Hrupka BJ, Langhans W, Schwartz GJ. Vagal and splanchnic afferents are not necessary for the anorexia produced by peripheral IL-1beta, LPS, and MDP. Am J Physiol. 1998;275:R384–R389. doi: 10.1152/ajpregu.1998.275.2.R384. [DOI] [PubMed] [Google Scholar]
- Schwartz GJ, Plata-Salaman CR, Langhans W. Subdiaphragmatic vagal deafferentation fails to block feeding-suppressive effects of LPS and IL-1 beta in rats. Am J Physiol. 1997;273:R1193–R1198. doi: 10.1152/ajpregu.1997.273.3.R1193. [DOI] [PubMed] [Google Scholar]
- Sharp BM, Matta SG, Peterson PK, Newton R, Chao C, McAllen K. Tumor necrosis factor-a is a potent ACTH secretagogue: comparison to interleukin-1β. Endocrinology. 1989;124:3131–3133. doi: 10.1210/endo-124-6-3131. [DOI] [PubMed] [Google Scholar]
- Wan W, Wetmore L, Sorensen CM, Greenberg AH, Nance DM. Neural and biochemical mediators of endotoxin and stress-induced c-fos expression in the rat brain. Brain Res Bull. 1994;34:7–14. doi: 10.1016/0361-9230(94)90179-1. [DOI] [PubMed] [Google Scholar]
- Watkins LR, Goehler LE, Relton JK, Tartaglia N, Silbert L, Martin D, Maier SF. Blockade of interleukin-1 induced hyperthermia by subdiaphragmatic vagotomy: evidence for vagal mediation of immune-brain communication. Neurosci Lett. 1995a;183:27–31. doi: 10.1016/0304-3940(94)11105-r. [DOI] [PubMed] [Google Scholar]
- Watkins LR, Maier SF, Goehler LE. Cytokine-to-brain communication: a review and analysis of alternative mechanisms. Life Sci. 1995b;57:1011–1026. doi: 10.1016/0024-3205(95)02047-m. [DOI] [PubMed] [Google Scholar]
- Wieczorek M, Pournajafi-Nazarloo H, Swiergiel AH, Dunn A. Physiological and behavioral responses to interleukin-1β and LPS in vagotomized mice. Physiol Behav. 2005;84:500–511. doi: 10.1016/j.physbeh.2005.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]