Abstract
Purpose:
Clinical trials of antiangiogenic agents used alone for advanced malignancy have been disappointing but preclinical studies suggest that the addition of radiation therapy could improve anti-tumor efficacy. To test the hypothesis that antiangiogenic therapy combined with radiation therapy can overcome the limitations of antiangiogenic monotherapy, we studied the effects of endostatin combined with radiation on the growth and vascularization of A431 human epidermoid carcinomas growing intramuscularly in the legs of mice.
Methods and Materials:
Mice with established A431 human epidermoid leg tumors were treated with radiation, endostatin, both radiation and endostatin, or vehicle control. The experiment was repeated and mice from each group were killed at 2, 7, and 10 days after irradiation so that tumor tissue could be obtained to further analyze the kinetics of the anti-tumor, anti-vascular, and antiangiogenic response to therapy.
Results:
Endostatin enhanced the anti-tumor effects of radiation and prolonged disease free survival was observed in the combined treatment group. Endothelial cell proliferation was increased in tumors after irradiation but was blocked by the concurrent administration of endostatin, and the combination of endostatin with radiation enhanced endothelial cell apoptosis within 48 hours after irradiation. Expression of vascular endothelial growth factor, interleukin-8, and matrix metalloproteinase-2 were increased in tumors after irradiation, and this increase was blocked by concurrent administration of endostatin.
Conclusions:
These data indicate that endostatin can block tumor revascularization after radiation therapy and thereby augment radioresponse.
Keywords: Endostatin, Radiation, Revascularization, Angiogenesis, Mice
INTRODUCTION
Under physiological conditions that require neovascularization, angiogenesis is tightly regulated (1). In malignant angiogenesis, new vessels are markedly disordered (2) and are characterized by a lack of pericytes and other support cells, impaired flow (3), the presence of plasma proteins that function as a neostroma (4), and increased vascular permeability. Many antiangiogenic agents have been described and several are now in clinical trials in cancer patients (5) and a few have been approved for clinical use (6). However, although antiangiogenic agents offer great therapeutic potential, preclinical and early clinical trial results suggest that these agents will have a delayed onset of activity and may induce stabilization of disease, and not regression, in patients with advanced disease (7-10). For example, preclinical studies with antiangiogenic agents demonstrate that antiangiogenic monotherapy is associated with a delayed onset of activity with tumors progressing by as much as 400% over a period of several days prior to response to therapy (9). Given that the doubling time of murine tumors is several folds higher than is observed in the presentation of human cancer, this delay in onset of activity could translate to several months in patients. For patients with advanced malignancies, this delay in onset of activity may make the use of these agents impractical. Further, microscopic residual disease persists in tumor-bearing mice treated with antiangiogenic monotherapy even after prolonged administration at high dose (10) and recent studies suggest that angiogenesis in various capillary beds may be differentially regulated, suggesting that antiangiogenic therapy may require organ-specific optimization (11, 12). Thus, before antiangiogenic agents can be successfully incorporated into clinical strategies to treat cancer and other angiogenesis dependent diseases, a greater understanding of the process of angiogenesis and of the interaction between the endothelial cell and its microenvironment is required.
Angiogenesis provides both a perfusion effect and a paracrine effect to a growing tumor (5) and in addition to providing nutrients and oxygen and removing catabolites, proliferating endothelial cells produce multiple growth factors that can promote tumor cell growth, invasion, and survival (13). These observations led Folkman to propose a two-compartment model of malignancy consisting of endothelial and tumor cell populations (14) and it will be prudent to target both by combining radiation and other conventional therapies with antiangiogenic agents. Although traditional therapies for cancer are associated with a number of toxicities and therapeutic challenges, their limitations are distinct from those that have been observed and might be expected with angiogenesis inhibition. Thus, we hypothesized that the use of angiogenesis inhibitors in combination with other modalities could help to overcome the limits of each and that angiogenesis inhibitors will block tumor revascularization after radiation therapy. To test this hypothesis, we studied the effects of endostatin (8), an endogenous angiogenesis inhibitor that is highly specific for microvascular endothelial cells and has not caused any significant toxicity in animal models or clinical use, combined with radiation on the growth and revascularization of A431 human epidermoid carcinoma xenografts in the legs of nude mice.
METHODS AND MATERIALS
Cell lines and reagents
A431 cells were maintained in Eagle's minimum essential medium supplemented with 10% fetal bovine serum, sodium pyruvate, nonessential amino acids, L-glutamine, vitamin solution, and penicillin-streptomycin at 37°C in 5% CO2 and 95% air. Tumor cells were free of Mycoplasma and pathogenic murine viruses. Human recombinant endostatin was provided by EntreMed, Inc. (Rockville, MD).
Tumor radioresponse studies
Male 6 – 8 week old athymic nu-nu/Ncr (nu/nu) mice were maintained in the specific pathogen-free mouse colony of the Department of Experimental Radiation Oncology approved by the American Association for Accreditation of Laboratory Animal Care in accordance with current regulations and standards of the U.S. Department of Agriculture and the Department of Health and Human Services. The musculature of the right proximal hind legs of the mice were injected with 1 × 106 A431 cells. When tumors reached a diameter of 6 mm, mice were randomly assigned to receive sham treatment, a single fraction of radiation (15 Gy), endostatin (50 mg/kg subcutaneously once daily for 21 days), or the combination of radiation and endostatin. Radiation was delivered to mice that were immobilized in a jig with the tumor centered in a circular 3 cm diameter radiation field using a dual-source Cs137 irradiator at a dose rate of 6 Gy/min. Endostatin treatment was initiated 2 days prior to irradiation to allow for an effective plasma concentration prior to the delivery of irradiation. Three mutually orthogonal tumor diameters were measured every 2 days with a vernier caliper. Regression and regrowth of tumors were followed until tumor diameter reached 14 mm, at which point mice were sacrificed in accordance with institutional guidelines. The treatment experiment was repeated, and mice from each group were sacrificed at 2 days, 7 days, or 10 days after irradiation (4 mice from each group for each of the three time points) to provide tumor tissues for immunohistochemical analyses.
Immunohistochemistry
For vascular endothelial growth factor (VEGF), basic fibroblast growth factor (bFGF), interleukin-8 (IL-8), matrix metalloproteinase-2 (MMP-2), and matrix metalloproteinase-9 (MMP-9) staining, sections of formalin-fixed, paraffin-embedded tissue specimens were deparaffinized in xylene, rehydrated in graded alcohol, and transferred to PBS. For VEGF, bFGF, and IL-8 staining, the slides were treated with pepsin for 20 minutes at room temperature. For CD31 staining, sections of frozen tissues (8 μm) were fixed with cold acetone and transferred to PBS. The slides were then rinsed with PBS, and endogenous peroxidase was blocked by use of 3% hydrogen peroxide in PBS (IL-8 and MMP-9) or methanol (VEGF, bFGF, MMP-9, and CD31). The samples were then washed with PBS and incubated for 10 minutes at room temperature with a protein-blocking solution consisting of PBS (pH 7.5) containing 5% normal horse serum and 1% normal goat serum. Excess blocking solution was drained, and the samples were incubated for 18 hours at 4°C with a 1:400 dilution of rabbit polyclonal anti-VEGF antibody (Santa Cruz Biotechnology, Santa Cruz, CA), 1:1000 dilution of rabbit anti-human bFGF antibody (Sigma, St. Louis, MO), 1:25 dilution of rabbit polyclonal anti-IL-8 antibody (Biosource, Camarillo, CA), 1:100 dilution of rabbit polyclonal anti-MMP-2 antibody (Chemicon International, Temecula, CA), 1:400 dilution of rabbit polyclonal anti-MMP-9 antibody (Chemicon International) or 1:700 dilution of monoclonal rat anti-CD31 antibody (PharMingen, San Diego, CA). The samples were then rinsed with PBS and incubated for 60 minutes at room temperature with the appropriate dilution of peroxidase-conjugated anti-rabbit IgG or anti-rat IgG. For MMP-2 and MMP-9, slides were incubated with biotinylated anti-rabbit IgG (Biocare Medical, Walnut Creak, CA) for 30 minutes and then washed and incubated with streptavidin HRP (DAKO, Carpinteria, CA) for 30minutes. The slides were rinsed with PBS and incubated for 5 minutes with diaminobenzidine (Research Genetics, Huntsville, AL). The sections were then washed three times with distilled water and counterstained with Gill's hematoxylin. Sections (4 μm thick) of formalin-fixed, paraffin-embedded tumors were also stained with hematoxylin-eosin for routine histologicexamination.
Immunofluorescence double staining for endothelial cell proliferation and apoptosis
For CD31 and bromodeoxyuridine (BrdU) double staining, mice were injected intravenously with 250 μg of BrdU. One hour later, the mice were killed, and tumors were collected and cut into two pieces, which were placed into either buffered 10% formalin solution or OCT compound (Miles Laboratories, Pittsburg, PA) to be snap-frozen in liquid nitrogen. Frozen tissues were sectioned (8 μm), and then monoclonal rat anti-CD31 and secondary goat anti-rat antibody conjugated to Texas Red were used for CD31 staining. For BrdU staining, the slides were incubated with 1% Triton X-100 for 8 minutes. After washing, the slides were treated with 2 N HCl in PBS for 30 minutes at 37°C. The slides were washed twice with 0.1 M Tris for 5 minutes, then treated with protein-blocking solution and incubated for 18 hours at 4°C with monoclonal mouse anti-BrdU Ab (Becton, Dickinson, Franklin Lakes, NJ). The samples were then incubated with goat anti-mouse antibody conjugated to Alexa488. The TUNEL assay was performed using an apoptosis detection kit (Promega Corp., Madison, WI) following the manufacturer's instructions. After CD31 staining with Texas Red, samples were fixed with 4% paraformaldehyde (methanol free) for 10 minutes at room temperature, washed twice with PBS for 5 minutes, and then incubated with 0.2% Triton X-100 for 15 minutes at room temperature. After again being washed twice with PBS for 5 minutes, the samples were incubated with equilibration buffer (from kit) for 10 minutes at room temperature. The equilibration buffer was drained, and reaction buffer containing equilibration buffer, nucleotide mix, and terminal deoxynucleotidyl transferase enzyme was added to the tissue sections. The sections were incubated in a humid atmosphere at 37°C for 1 hour in the dark. The reaction was terminated by immersing the samples in 2X standard saline citrate for 15 minutes. Samples were washed 3 times for 5 minutes to remove unincorporated fluorescein-dUTP. For quantification of endothelial cells, the samples were incubated with 300 μg/ml Hoechst stain for 10 minutes at room temperature. Immunofluorescence microscopy was performed using an × 20 or × 40 objective to individually select for green and red fluorescence. Endothelial cells were identified by red fluorescence and proliferating and apoptotic cells were identified by localized green nuclear staining. The number of proliferating endothelial cells was determined in 18-25 random fields at × 200 magnification and the number of apoptotic events was counted in 7-9 random fields at × 400 magnification.
Quantification of microvessel density, vascular area and immunoreactivity
Blood vessels in solid tumors were counted under a light microscope after immunostaining for CD31. Areas containing the highest number of capillaries and small venules were identified by scanning the tumor sections at low power (× 40). Individual vessels were counted in 10 random fields at x100 magnification (0.159-mm2/field). Vascular area was determined by summing the whole vascular areas in 10 random 0.159-mm2 fields at × 100 magnification.
For quantification of the immunohistochemical reaction intensity, the absorbance of 100 bFGF-, VEGF-, IL-8, MMP2-, and MMP9-positive cells from each of the differently treated tumor tissues was measured in 10 random fields at ×200 magnification using Optimas Image Analysis software (Bioscan, Washington, DC). The cytoplasmic immunoreactivity was evaluated by computer-assisted image analysis and was expressed as a ratio of tumor cell expression to normal muscle cell expression multiplied by 100.
Data Analysis
Statistical significance was determined using Student's t test. P<0.05 was considered to be statistically significant.
RESULTS
Endostatin treatment improves tumor radioresponse
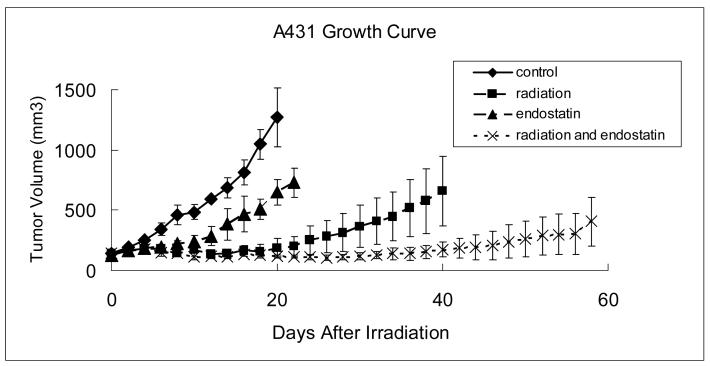
To determine the effect of endostatin on tumor radioresponse, A431 tumor xenografts of 6 mm in diameter were treated with 15 Gy single dose local tumor irradiation alone, endostatin at a modest dose of 50 mg/kg administered subcutaneously once daily for 21 consecutive days or the combination of endostatin and irradiation. For the combined treatments, endostatin therapy was initiated 2 days before irradiation to the tumor. Tumor growth delay was the primary treatment endpoint. Each treatment was effective in delaying tumor growth, as compared to control (p < 0.005), with the combined treatment with endostatin and irradiation being the most highly effective (Fig. 1). In the group treated with endostatin and irradiation together, 2 of 5 mice still had locally controlled tumors 185 days after irradiation at which time the experiment was terminated.
Figure 1.
Growth of A431 tumor xenografts. The musculature of the right proximal hind legs of male nude mice were injected with A431 human epidermoid carcinoma cells and when tumors reached a diameter of 6 mm, mice were randomly assigned to receive sham treatment (control), a single fraction of radiation (15 Gy), endostatin (50mg/kg subcutaneously daily for 21 days), or the combination of radiation and endostatin. Each group consisted of 5 mice and data were expressed as mean +/− standard error.
To further quantify the anti-tumor effects of the different treatments and to determine the enhancement factor for tumor radioresponse, we calculated the time in days for tumors needed to triple in volume (Table 1). This time was 8.0 +/− 1.4 days for the control (untreated) group, 50.4 +/− 14.8 days for the irradiation group, 16.0 +/− 2.2 days for the endostatin group, and greater than 118 days for the endostatin plus irradiation group (p < 0.05). Endostatin greatly enhanced radioresponse of A431 xenografts with an enhancement factor of more than 2.4 calculated by dividing the normalized tumor growth delay (the time for tumors treated with both endostatin and radiation to grow from 8 to 12 mm in diameter minus the time in days for tumors treated with endostatin alone to reach the same size) in mice treated with endostatin plus irradiation with the absolute tumor growth delay in mice treated with irradiation alone (see Table 1).
Table 1.
| Treatment | Time required for tumor to volume to triple (days) |
Absolute growth delay (days) |
Normalized growth delay (days) |
Enhancement factor |
|---|---|---|---|---|
| Control | 8 ± 1.4 | |||
| XRT | 50.4 ± 14.8 | 42.4 ± 14.8 | ||
| Endostatin | 16 ± 2.2 | 8 ± 2.2 | ||
| Endostatin + XRT |
NA (>118) | >110 | >102 | >2.4 |
Endostatin treatment enhances tumor cell apoptosis and inhibits tumor cell proliferation after irradiation
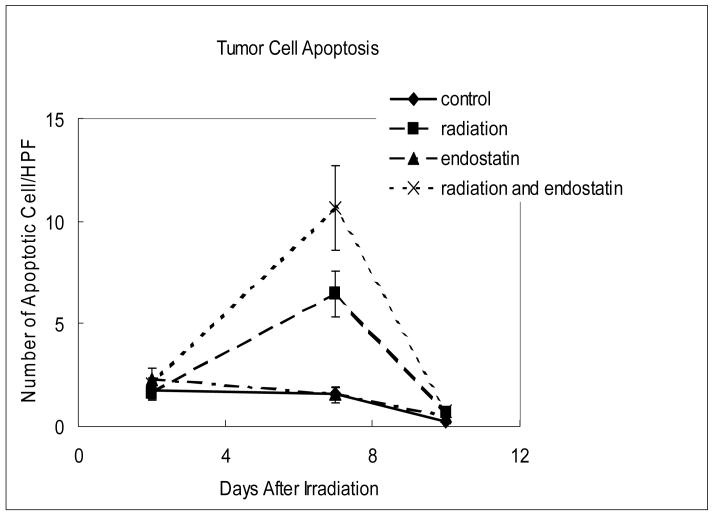
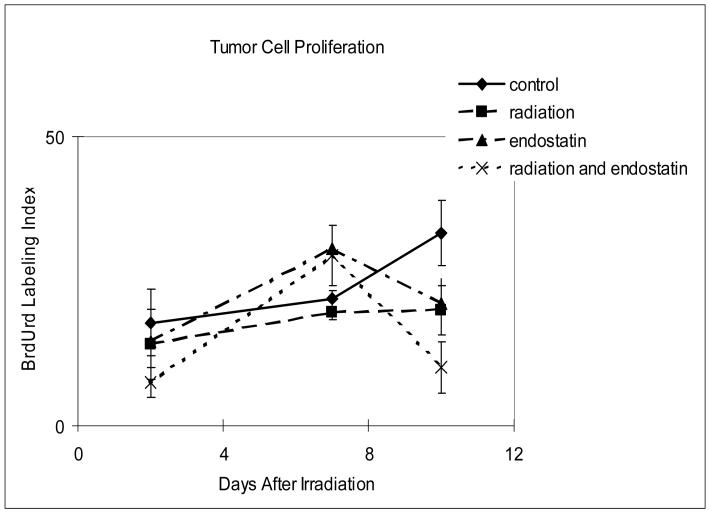
The anti-tumor effects of irradiation at the cellular level and its modification by endostatin were assessed by quantifying tumor cell apoptosis and proliferation at 2, 7, and 10 days after irradiation delivered in a single fraction. As shown in Figure 2, tumor cell apoptosis was relatively low (Fig. 2a) while proliferation was relatively high (Fig. 2b) in tumors from untreated mice. Endostatin alone had little or no effect upon tumor cell apoptosis at the time points studied whereas radiation alone significantly increased (p < 0.001) tumor cell apoptosis as compared to the control at day 7 that was increased by a factor of about 2 when radiation was combined with endostatin (Fig. 2a).
Figure 2.
Time course analyses of tumor cell apoptosis and proliferation. (a) Tumor cell apoptosis as evaluated by the TUNEL assay. Apoptotic tumor cells were counted in 7-9 random fields at ×400 magnification, excluding necrotic areas. (b) Tumor cell proliferation as evaluated by BrdU incorporation. Mice were injected intravenously with 250 μg of BrdU and killed 1 hour after injection. The number of proliferating tumor cells was determined in 3-6 random fields at ×200 magnification.
During the observation period of growth of untreated tumors tumor cell proliferation was constant until day 10 after irradiation when it was increased (Fig. 2b). At this time point, tumor cell proliferation, assessed by the labeling index with BrdU, was reduced by endostatin or radiation as compared to control and especially by the combination of these two treatments (p < 0.02).
Microvessel density and vascular area do not predict for tumor response
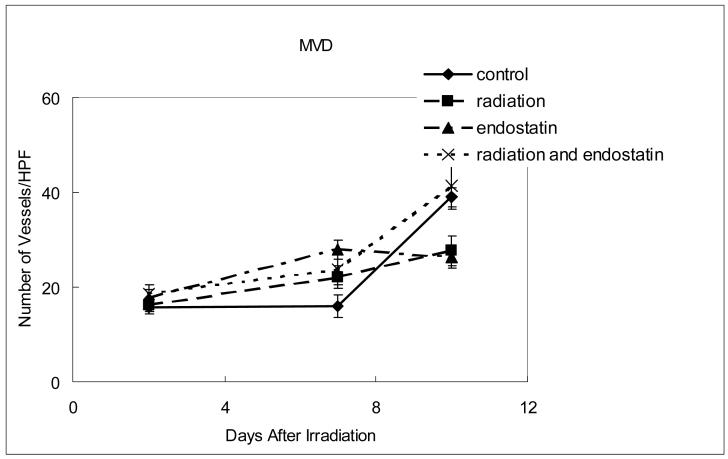
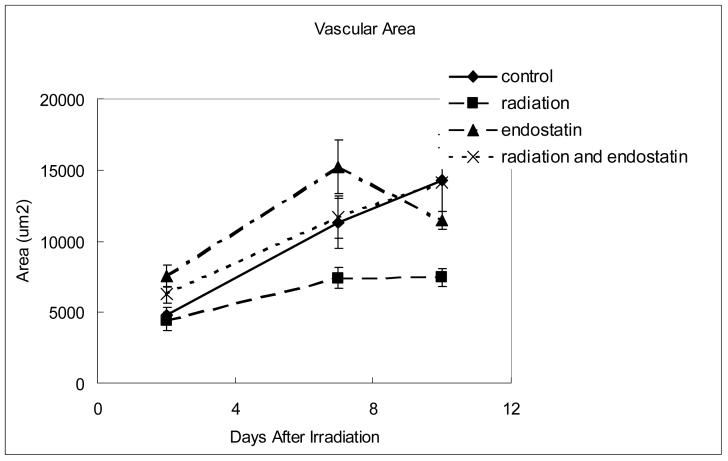
High microvessel density (MVD) correlates with poor prognosis in many different types of cancer (15, 16) and the efficacy of antiangiogenic therapy is frequently evaluated by determining MVD after tumor response to therapy. However, MVD can vary as a function of the rate of tumor response and other variables and does not always correlate with tumor response to therapy (17). To study the feasibility of using MVD as a surrogate marker for response to combined radiation therapy and antiangiogenic therapy, MVD was sequentially measured in tumor tissues obtained from mice 2, 7, or 10 days after treatment with endostatin and/or irradiation (Fig. 3a and b). MVD in the control group increased from 15.7 ± 1.3 per high-power field at day 2 to 39 ± 2.0 per high-power field at day 10. At day 10, the endostatin group had significantly lower (p < 0.001) MVD than the control group yet there was no significant difference in MVD between the endostatin plus radiation group and the control group. Vascular area was also measured (Fig. 3c) and no significant difference between the control and endostatin treatment groups was observed and as with MVD there was no significant difference between the endostatin plus radiation group and the control group. The radiation alone group had a significantly lower vascular area than the control group at day 10 (p < 0.01). When taken together, these data suggest that even though MVD is useful as a prognostic indicator for the risk of metastatic disease in patients with early stage cancer (15, 16), it is not necessarily useful as a surrogate for predicting tumor response to combined antiangiogenic and radiation therapies.
Figure 3.
Time course analyses of tumor MVD and vascular area. (a) Representative CD31 staining in tumor tissue harvested from mice killed 7 days after irradiation. (b) MVD (CD31 staining) was determined in 10 random 0.0159 mm2 fields at ×100 magnification. (c) Vascular area was determined by summing the whole vascular area in 10 random 0.159-mm2 fields at ×100 magnification.
Endostatin blocks tumor revascularization after irradiation
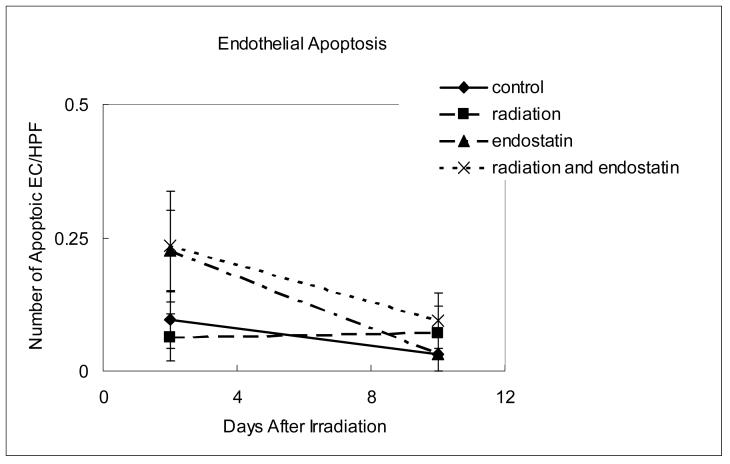
It has recently been reported (18) that endothelial cell apoptosis precedes crypt stem cell apoptosis in a gastrointestinal radiation damage model and that the endothelial cell may be a primary target of irradiation (19) in some tumor models where endothelial cell apoptosis precedes tumor cell apoptosis after radiation. To characterize the effects of endostatin upon tumor revascularization after irradiation in A431 tumors we sequentially assessed both endothelial cell apoptosis and proliferation using immunofluorescence double staining for endothelial cells (CD31) and apoptosis (TUNEL) or proliferation (BrdU incorporation). Within 2 days of irradiation given in a single fraction endothelial cell apoptosis was increased in the endostatin and endostatin plus radiation groups (p < 0.05 versus control). However, 10 days after tumor irradiation, endothelial cell apoptosis was not increased in any of the treatment groups (Fig. 4a).
Figure 4.
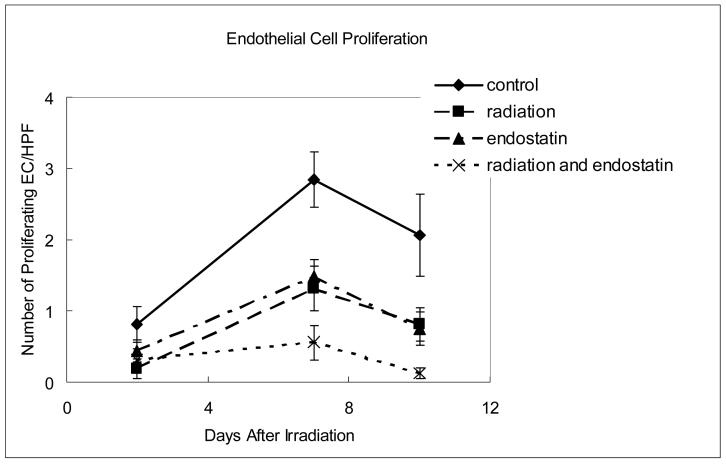
Time course analyses of endothelial cell apoptosis and proliferation. (a) Endothelial cell apoptosis as determined by CD31-TUNEL double-staining. The number of apoptotic endothelial cells was counted in 7-9 random fields at ×400 magnification. (b) Endothelial cell proliferation as determined by CD31-BrdU double-staining. The number of proliferating endothelial cells was determined in 18-25 random fields at ×200 magnification. (c) Representative staining for CD31 (red fluorescence), BrdU (green fluorescence), or both CD31 and BrdU (yellow fluorescence) in tumor tissue harvested from mice killed 7 days after irradiation.
In contrast to apoptosis, endothelial cell proliferation in treated tumors increased with time having the highest level at day 7 (Fig. 4b and c) after irradiation. Endostatin alone inhibited endothelial cell proliferation throughout the observation period and when endostatin and radiation were combined, the inhibition of endothelial cell proliferation was virtually complete (p < 0.005 relative to control) suggesting that the effect of endostatin is particularly prominent in inhibiting endothelial cell proliferation.
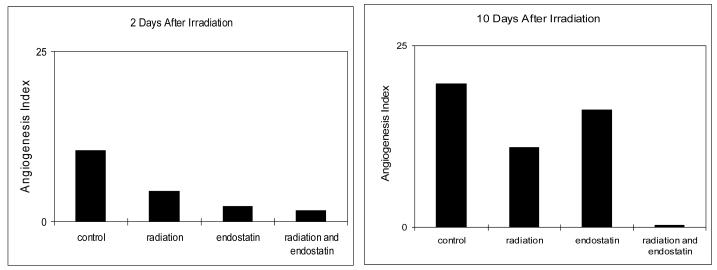
To further define the impact of endostatin and/or irradiation upon tumor revascularization, we calculated the ratio of endothelial cell proliferation and apoptosis as an angiogenesis index. As shown in Figure 5, the angiogenesis index was lowest for the combined endostatin and radiation treatment group and was close to 0 at day 10. Given that tumor angiogenesis is directly dependent upon both endothelial cell proliferation and apoptosis it will be prudent to include both parameters as part of the assessment of the impact of antiangiogenic agents as part of the combined modality treatment of cancer.
Figure 5.
The ratio of endothelial cell proliferation and apoptosis.
Endostatin suppresses the upregulation of proangiogenic and invasive molecules induced by irradiation
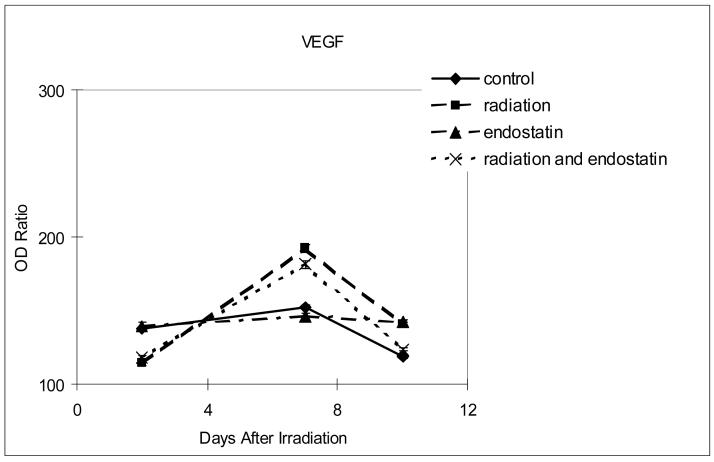
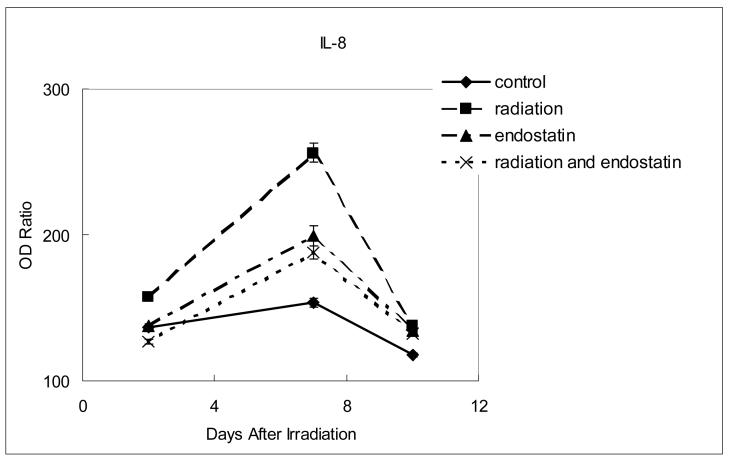
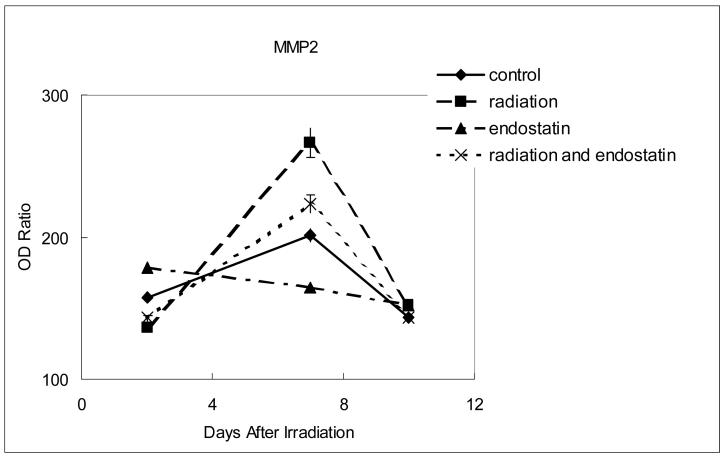
To further characterize the inhibition of tumor revascularization that was observed after irradiation in mice treated with concurrent endostatin, the expression of proangiogenic and invasive molecules was sequentially studied by optical density analysis of stained tissue sections. For each of the time points studied (2, 7, and 10 days after the single 15 Gy fraction of irradiation in the radiation group) no significant difference in the expression of basic fibroblast growth factor or matrix metalloproteinase 9 was observed between the different treatment groups (data not shown). However, within 7 days of radiation therapy VEGF, IL-8, and MMP-2 were all upregulated in the irradiation group as compared to control (Fig. 6, p < 0.0001). The increased expression of VEGF after irradiation was offset only marginally (Fig. 6a) by the addition of endostatin (p < 0.005) whereas the increased expression of both IL-8 (Fig. 6b) and MMP-2 (Fig. 6c) after irradiation was substantially offset by the addition of endostatin (p < 0.001). These data show that endostatin can selectively offset proangiogenic and invasive factors that are increased in response to radiation therapy of tumors.
Figure 6.
Time course analyses of the expression of proangiogenic and invasive molecules. The expression of VEGF, IL-8, or MMP-2 was determined by the absorbance intensity for each of the differently treated tumor tissues was measured in 10 random fields at ×200 magnification using Optimas Image Analysis software (Bioscan). The cytoplasmic immunoreactivity was evaluated by computer-assisted image analysis and was expressed as a ratio of tumor cell expression to normal muscle cell expression multiplied by 100. (a) Expression of VEGF. (b) Expression of IL-8. (c) Expression of MMP-2.
DISCUSSION
It had long been assumed that an angiogenesis inhibitor would impair the effect of ionizing radiation by inducing tumor hypoxia until Teicher and colleagues (20) observed that antiangiogenic agents given in combination with radiation therapy improved tumor oxygenation and anti-tumor efficacy. Hansen-Algenstaedt and colleagues studied androgen-dependent Shinogi male mouse mammary carcinomas treated with anti-VEGFR2 antibodies, hormone ablation and/or chemotherapy (21). They found that in the groups that received combined modality therapy, pO2 initially decreased with therapy but then subsequently increased and tumor growth arrest was observed. Enhancement of tumor response to radiation therapy has been demonstrated when it is combined with anti-VEGF agents (22) even for tumors that were markedly hypoxic (23, 24) with an increase in tumor growth delay and an augmentation of tumor curability in the combined modality groups. More recently, it has been shown that the combination of agents that prevent VEGF signaling and radiotherapy reduced tumor resistance to radiation response and improved response (25) and SU5416, a small molecule antagonist of VEGFR signaling, had an additive effect when combined with irradiation and permanent vascular changes were observed after combined treatment resulting in complete remission (26). An increase in tumor growth delay and an augmentation of tumor curability was observed when radiation therapy was combined with a selective inhibitor of the cyclooxygenase-2 enzyme (27) providing the basis for clinical trials. In patients with poor prognosis lung cancer, celecoxib at high dose was administered concurrently with thoracic radiotherapy. The treatment resulted in actuarial local progression-free survival of 66.0% at 1 year and 42.2% at 2 years (28). However, given the cardiac toxicity that has now been observed with this class of agents, further assessment and additional studies are required and more specific inhibitors of angiogenesis may be needed to minimize the potential of toxicity.
In the current study, the highly selective inhibitor of angiogenesis endostatin significantly enhanced radioresponse of A431 human epidermoid carcinoma xenografts in mice and was associated with long term tumor control in some animals. Weichselbaum and colleagues also demonstrated an enhancement of the effects of radiotherapy when the angiostatin (29) or endostatin (30) and ionizing radiation were combined using low doses of each modality. In our studies, we found that the enhancement of the effects of radiotherapy by endostatin is due in part to a prevention of the tumor revascularization that was observed shortly after irradiation mediated by sustained endothelial cell apoptosis and a prevention of endothelial cell proliferation. In clinical trials, endostatin therapy was associated with increased endothelial cell apoptosis (31, 32). In the current study, endostatin increased endothelial cell apoptosis and the effect was enhanced by radiation. These data provide the basis for a strategy to use endostatin and other antiangiogenic agents to maximize and sustain the endothelial apoptosis that can occur within hours of radiation therapy (19) and to then prevent endothelial cell proliferation driven by proangiogenic factors secreted by tumors after radiation therapy (22).
The increased expression of proangiogenic factors after irradiation may function to protect the tumor vasculature and thereby mediate a rapid recovery of endothelial cell proliferation accounting for the rapid regrowth of tumors that can be observed after radiation therapy. The current studies indicate that revascularization after radiation therapy can be blocked by endostatin and that the combination of antiangiogenic therapy and radiation therapy may therefore overcome the limitations of each therapy resulting in superior and more durable anti-tumor activity. Prior reports have shown that endostatin can impact the endothelial cell at multiple molecular levels (33) and endostatin treatment can decrease tumor cell expression of several proangiogenic and invasive molecules (34-37). To further analyze the mechanism of the blockade of tumor revascularization by endostatin after radiation therapy we evaluated changes in expression of the proangiogenic or invasive molecules VEGF, bFGF, and IL-8 and MMP-2 and MMP-9. In our studies, endostatin only marginally offset VEGF expression but had a substantial impact upon both IL-8 and MMP-2.
In conclusion, our studies show that multimodality therapy that includes radiation therapy and angiogenesis inhibitors offers the potential for improved efficacy in the treatment of cancer and other angiogenesis dependent diseases. However, additional work is still needed to provide a rational basis for the combination of angiogenesis inhibitors with other modalities and to identify potential problems that might arise from these combinations so that these agents will be successfully incorporated into existing therapy in a timely and rational manner.
Acknowledgments
Grant Support: Supported in part by NIH Research Grant CA-06294, M.D. Anderson Cancer Center Physician Scientist Program Awards (M.S.O. and R.S.H.), and a grant from the Department of Defense (DAMD 17-02-1-0706)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest Notification: There are no actual or potential conflicts of interest related to this manuscript.
REFERENCES
- 1.Folkman J. Clinical applications of angiogenesis research. N Engl J Med. 1995;333:1757–1763. doi: 10.1056/NEJM199512283332608. [DOI] [PubMed] [Google Scholar]
- 2.Jain RK. Normalizing tumor vasculature with anti-angiogenic therapy: a new paradigm for combination therapy. Nature Med. 2001;7:987–989. doi: 10.1038/nm0901-987. [DOI] [PubMed] [Google Scholar]
- 3.Konerding MA, Malkusch W, Klapthor B, et al. Evidence for characteristic vascular patterns in solid tumours: quantitative studies using corrosion casts. Br J Cancer. 1999;80:724–732. doi: 10.1038/sj.bjc.6690416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dvorak HF. Tumors: wounds that do not heal. N Engl J Med. 1999;315:1650–1659. doi: 10.1056/NEJM198612253152606. [DOI] [PubMed] [Google Scholar]
- 5.O'Reilly MS. Antiangiogenesis: basic principles. In: Rosenberg SA, editor. Principles and Practice of the Biologic Therapy of Cancer. Third edition Lippincott Williams & Wilkins; Philadelphia: 2000. pp. 827–843. [Google Scholar]
- 6.Hurwitz HI, Fehrenbacher L, Hainsworth JD, et al. Bevacizumab in combination with Fluorouracil and leucovorin: an active regimen for first-line metastatic colorectal cancer. J Clin Oncol. 2005;23:3502–3508. doi: 10.1200/JCO.2005.10.017. [DOI] [PubMed] [Google Scholar]
- 7.O'Reilly MS, Holmgren L, Shing Y, et al. Angiostatin: a novel angiogenesis inhibitor that mediates the suppression of metastases by a Lewis lung carcinoma. Cell. 1994;79:315–328. doi: 10.1016/0092-8674(94)90200-3. [DOI] [PubMed] [Google Scholar]
- 8.O'Reilly MS, Boehm T, Shing Y, et al. Endostatin: an endogenous inhibitor of angiogenesis and tumor growth. Cell. 1997;88:277–285. doi: 10.1016/s0092-8674(00)81848-6. [DOI] [PubMed] [Google Scholar]
- 9.O'Reilly MS, Holmgren L, Chen C, et al. Angiostatin induces and sustains dormancy of human primary tumors in mice. Nature Med. 1996;2:689–692. doi: 10.1038/nm0696-689. [DOI] [PubMed] [Google Scholar]
- 10.Boehm T, Folkman J, Browder T, et al. Antiangiogenic therapy of experimental cancer does not induce acquired drug resistance. Nature. 1997;390:404–407. doi: 10.1038/37126. [DOI] [PubMed] [Google Scholar]
- 11.Arap W, Pasqualini R, Ruoslahti E. Cancer treatment by targeted drug delivery to tumor vasculature in a mouse model. Science. 1998;279:377–380. doi: 10.1126/science.279.5349.377. [DOI] [PubMed] [Google Scholar]
- 12.Lund EL, Bastholm L, Kristjansen PE. Therapeutic synergy of TNP-470 and ionizing radiation: effects on tumor growth, vessel morphology, and angiogenesis in human glioblastoma multiforme xenografts. Clin Cancer Res. 2000;6:971–978. [PubMed] [Google Scholar]
- 13.Rak J, Filmus J, Kerbel RS. Reciprocal paracrine interactions between tumour cells and endothelial cells: the ‘angiogenesis progression’ hypothesis. Euro J Cancer. 1996;32A:2438–2450. doi: 10.1016/s0959-8049(96)00396-6. [DOI] [PubMed] [Google Scholar]
- 14.Folkman J. Tumor angiogenesis and tissue factor. Nature Med. 1996;2:167–168. doi: 10.1038/nm0296-167. [DOI] [PubMed] [Google Scholar]
- 15.Weidner N. Intratumor microvessel density as a prognostic factor in cancer. Am J Pathol. 1995;147:9–19. [PMC free article] [PubMed] [Google Scholar]
- 16.Weidner N, Semple JP, Welch WR, et al. Tumor angiogenesis and metastasis - correlation in invasive breast carcinoma. N Engl J Med. 1991;324:1–8. doi: 10.1056/NEJM199101033240101. [DOI] [PubMed] [Google Scholar]
- 17.Kerbel RS, Folkman J. Clinical translation of angiogenesis inhibitors. Nat Rev Cancer. 2002;2:727–739. doi: 10.1038/nrc905. [DOI] [PubMed] [Google Scholar]
- 18.Paris F, Fuks Z, Kang A, et al. Endothelial apoptosis as the primary lesion initiating intestinal radiation damage in mice. Science. 2001;293:293–297. doi: 10.1126/science.1060191. [DOI] [PubMed] [Google Scholar]
- 19.Garcia-Barros M, Paris F, Cordon-Cardo C, et al. Tumor response to radiotherapy regulated by endothelial cell apoptosis. Science. 2003;300:1155–1159. doi: 10.1126/science.1082504. [DOI] [PubMed] [Google Scholar]
- 20.Teicher BA, Dupuis N, Kusomoto T, et al. Antiangiogenic agents can increase tumor oxygenation and response to radiation therapy. Rad Oncol Invest. 1995;2:269–276. [Google Scholar]
- 21.Hansen-Algenstaedt N, Stoll BR, Padera TP, et al. Tumor oxygenation in hormone-dependent tumors during vascular endothelial growth factor receptor-2 blockade, hormone ablation, and chemotherapy. Cancer Res. 2000;60:4556–4560. [PubMed] [Google Scholar]
- 22.Gorski DH, Beckett MA, Jaskowiak NT, et al. Blockage of the vascular endothelial growth factor stress response increases the antitumor effects of ionizing radiation. Cancer Res. 1999;59:3374–3378. [PubMed] [Google Scholar]
- 23.Lee CG, Heijn M, di Tomaso E, et al. Anti-Vascular endothelial growth factor treatment augments tumor radiation response under normoxic or hypoxic conditions. Cancer Res. 2000;60:5565–5570. [PubMed] [Google Scholar]
- 24.Hess C, Vuong V, Hegyi I, et al. Effect of VEGF receptor inhibitor PTK787/ZK222584 combined with ionizing radiation on endothelial cells and tumour growth. Brit J Cancer. 2001;85:2010–2016. doi: 10.1054/bjoc.2001.2166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Geng L, Donnelly E, McMahon G, et al. Inhibition of vascular endothelial growth factor receptor signaling leads to reversal of tumor resistance to radiotherapy. Cancer Res. 2001;61:2413–2419. [PubMed] [Google Scholar]
- 26.Schuuring J, Bussink J, Bernsen HJJA, et al. Irradiation combined with SU5416: Microvascular changes and growth delay in a human xenograft glioblastoma tumor line. Int J Rad Oncol Biol Phys. 2005;61:529–534. doi: 10.1016/j.ijrobp.2004.09.063. [DOI] [PubMed] [Google Scholar]
- 27.Milas L, Kishi K, Hunter N, et al. Enhancement of tumor response to gamma-radiation by an inhibitor of cyclooxygenase-2 enzyme. J Natl Cancer Inst. 1999;91:1501–1504. doi: 10.1093/jnci/91.17.1501. [DOI] [PubMed] [Google Scholar]
- 28.Liao Z, Komaki R, Milas L, et al. A phase I clinical trial of thoracic radiotherapy and concurrent celecoxib for patients with unfavorable performance status inoperable/unresectable non-small cell lung cancer. Clin Cancer Res. 2005;11:3342–3348. doi: 10.1158/1078-0432.CCR-04-1741. [DOI] [PubMed] [Google Scholar]
- 29.Mauceri HJ, Hanna NN, Beckett MA, et al. Combined effects of angiostatin and ionizing radiation in antitumour therapy. Nature. 1998;394:287–291. doi: 10.1038/28412. [DOI] [PubMed] [Google Scholar]
- 30.Hanna NN, Seetharam S, Mauceri HJ, et al. Antitumor interaction of short-course endostatin and ionizing radiation. Cancer J. 2000;6:287–293. [PubMed] [Google Scholar]
- 31.Herbst RS, Mullani NA, Davis DW, et al. Development of biologic markers of response and assessment of antiangiogenic activity in a clinical trial of human recombinant endostatin. J Clin Oncol. 2002;20:3804–3814. doi: 10.1200/JCO.2002.05.102. [DOI] [PubMed] [Google Scholar]
- 32.Herbst RS, Hess KR, Tran HT, et al. Phase I study of recombinant human endostatin in patients with advanced solid tumors. J Clin Oncol. 2002;20:3792–3803. doi: 10.1200/JCO.2002.11.061. [DOI] [PubMed] [Google Scholar]
- 33.Abdollahi A, Hahnfeldt P, Maercker C, et al. Endostatin's antiangiogenic signaling network. Mol Cell. 2004;13:649–663. doi: 10.1016/s1097-2765(04)00102-9. [DOI] [PubMed] [Google Scholar]
- 34.Kim YM, Jang JW, Lee OH, et al. Endostatin inhibits endothelial and tumor cellular invasion by blocking the activation and catalytic activity of matrix metalloproteinase. Cancer Res. 2000;60:5410–5413. [PubMed] [Google Scholar]
- 35.Raut CP, Takamori RK, Davis DW, et al. Direct effects of recombinant human endostatin on tumor cell IL-8 production are associated with increased endothelial cell apoptosis in an orthotopic model of human pancreatic cancer. Cancer Biol Ther. 2004;3:679–687. doi: 10.4161/cbt.3.7.967. [DOI] [PubMed] [Google Scholar]
- 36.Zhang M, Yang Y, Yan M, et al. Downregulation of vascular endothelial growth factor and integrinbeta3 by endostatin in a mouse model of retinal neovascularization. Exp Eye Res. 2006;82:74–80. doi: 10.1016/j.exer.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 37.Hajitou A, Grignet C, Devy L, et al. The antitumoral effect of endostatin and angiostatin is associated with a down-regulation of vascular endothelial growth factor expression in tumor cells. Faseb J. 2002;16:1802–1804. doi: 10.1096/fj.02-0109fje. [DOI] [PubMed] [Google Scholar]