Abstract
The recreational drug 3,4-methylenedioxymethamphetamine (MDMA, ecstasy) increases locomotor activity when administered to rats. Although the published pharmacology of MDMA has focused almost exclusively on the roles of serotonin and dopamine, in vitro studies indicate that MDMA induces serotonin and norepinephrine release with equal potency. The present experiments tested the hypothesis that blockade of α1-adrenoceptors with systemic or local administration of the antagonist prazosin would attenuate the locomotor response to systemic administration of (±)-MDMA. Pretreatment with systemic prazosin (0.5 mg/kg) or microinjections into either the prefrontal cortex or ventral tegmental area completely blocked the locomotor stimulant effects of 5 mg/kg (±)-MDMA, assessed using a computerized Behavioral Pattern Monitor. Prazosin was more potent in blocking the locomotor stimulant effects of (±)-MDMA than a 2 mg/kg dose of (+)-amphetamine that produced a similar locomotor activity increase. These results indicate that activation of α1-adrenoceptors in both the prefrontal cortex and ventral tegmental areas modulate the locomotor response to MDMA.
Keywords: MDMA, locomotor activity, prazosin, α1-adrenoceptor, rats
1. Introduction
MDMA (3,4-methylenedioxymethamphetamine; ecstasy) is a substituted amphetamine derivative that has become a popular recreational drug (Strote et al. 2002; Vollenweider et al. 2002). Neurochemically, MDMA elevates central extracellular monoamine levels by releasing terminal stores through a mechanism that involves a reversal of reuptake transporters. In vitro data indicate that MDMA is equally potent at increasing the outflow of both [3H]5-HT (serotonin) and [3H]NE (norepinephrine) from rat brain synaptosomes, and somewhat less potent in releasing [3H]DA (dopamine) (Steele et al. 1987; Johnson et al. 1991; Rothman et al. 2001). The effect of MDMA on 5-HT and NE also has been demonstrated in hippocampal brain slices (Fitzgerald and Reid 1993).
In spite of the potent ability of MDMA to induce release of NE, previous studies of the mechanism of action of MDMA have focused almost exclusively on its ability to affect brain serotonergic and dopaminergic systems (e.g. Cole and Sumnall 2003; Green et al. 2003; Simantov 2004). Recently, however, Sprague et al. (2003) have shown that α1 adrenoceptors are involved in the hyperthermic response to MDMA, and Fantegrossi et al. (2004) reported that the α1-adrenoceptor antagonist prazosin blocked the hyperthermia and locomotor increase induced by MDMA in mice. No microdialysis data have been reported to indicate whether MDMA enhances central extracellular NE concentrations.
In rats, acute administration of MDMA produces hyperlocomotion (Gold et al. 1988; Callaway and Geyer 1992a; Callaway and Geyer 1992b; Callaway et al. 1992; Callaway et al. 1990; McNamara et al. 1995). Gold et al. (1988) described this motor response as being more similar to hallucinogen-induced locomotor activity than that induced by amphetamine. The main focus of investigations into neurochemical mechanisms underlying MDMA-induced hyperactivity has been on the contribution of serotonergic neurotransmission, despite the observation that the locomotor response to MDMA is sensitive to 6-OHDA (6-hydroxydopamine)-induced lesions of the nucleus accumbens (NAc) (Gold et al. 1989) and dopamine receptor antagonists (Kehne et al. 1996),.
In studies of drug-induced increases in locomotor activity, local administration of prazosin into the prefrontal cortex (PFC) decreased the locomotor response to amphetamine and morphine (Blanc et al. 1994; Darracq et al. 1998; Drouin et al. 2001), indicating that NE may modulate stimulant-induced locomotor activity through activation of α1-adrenoceptors in this region. α1-Adrenoceptors in the VTA also facilitate the ability of stimulant drugs to regulate the mesolimbic system. Amphetamine modulates burst activity of VTA neurons and induces DA release in the NAc through the activation of VTA α1-receptors (Pan et al. 1996; Shi et al. 2000). This evidence supports a role for tegmental α1-adrenoceptors in the neurochemical enhancing effects of psychostimulant drugs, but to our knowledge, it is not yet known whether activation of α1-adrenergic receptors in the VTA produces a behavioral effect.
Other studies have shown that systemic administration of prazosin can attenuate the locomotor response to cocaine, the NMDA receptor antagonist MK-801, and the DA reuptake inhibitor GBR 12783 (Arnt 1995; Dickinson et al. 1988; Drouin et al. 2002a; Harkin et al. 2001; Mathe et al. 1996; Snoddy and Tessel 1985; Svensson et al. 1995; Wellman et al. 2002). Thus, the experiments described herein tested the hypothesis that activation of α1-adrenoceptors in the prefrontal cortex and ventral tegmental area of the rat modulate the locomotor response to MDMA.
2. Materials and methods
2.1. Animals
Male Sprague-Dawley rats (Harlan Labs, Indianapolis, IN) weighing approximately 300-350 g at the time of testing were housed in groups of 2 - 3 with food and water available ad libitum. For the microinjection studies, animals were housed individually in clear Plexiglas shoe box cages after cannula implantation. A 12/12 hour light/dark cycle was used with lights on at 0700. All experiments were conducted between 0900 and 1700. Animals used in these studies were maintained in accordance with U.S. Public Health Service Policy on Humane Care and Use of Laboratory Animals as amended August 2002, and experiments were carried out in accordance with protocols approved by the Purdue Animal Care and Use Committee Care.
2.2. Apparatus
The Behavioral Pattern Monitor (Photobeam Activity System with Flex Field; San Diego Instruments, Inc., San Diego, CA) used for this experiment consisted of eight rectangular “flex field” chambers. Each chamber measured 38 cm high × 30 cm wide × 60 cm long and was comprised of four clear Plexiglas sides set atop an opaque Plexiglas base that measured 44 cm × 72 cm. The bottom frame was located 3 cm from the base and contained eight photo beams along its length and four across the ends. The sides of each chamber were covered with a black curtain and illumination came from a 15 watt bulb hanging directly above the chamber. The Flex Field apparatus collected individual photobeam breaks in “real time.” The field of each chamber was partitioned into 32 equal measured zones, and the number of entries an animal made into a particular zone was recorded through an interface that relayed data to a personal computer in an adjacent room. The number of entries into the 20 zones located along the walls was defined as peripheral activity and the number of entries into the 12 centrally-located zones was recognized as central activity.
2.3. Surgery
After one week of handling and acclimation to the lab environment, animals were stereotaxically implanted with bilateral 23-gauge guide cannulae (Plastics One, Roanoke, VA.) into the PFC (A = +2.2 mm, L = ±0.5 mm, V = -3.2 mm, from bregma) or the VTA (A = -6.0 mm, L = ±1.0 mm, V = -5.5 mm, from bregma). Stylets were inserted into the guide cannulae and animals were allowed one week to recover before beginning the microinjection experiments.
2.4. Microinjections
During the microinjection procedure, animals were gently restrained by hand, the stylet was removed from the guide cannula and replaced with an internal injection cannula (Plastics One, Roanoke, VA) attached by Teflon tubing to a 250 μL Hamilton syringe (Fisher Scientific, Chicago, IL). A syringe pump (Instech model 2000, Instech Laboratories, Inc., Plymouth Meeting, PA) was used to infuse bilaterally a 0.5 μL volume containing 0.25 μg (0.6 nmol) of prazosin at a rate of a 0.5 μL/minute/side into either the PFC or VTA. Following injection, the cannula was left in place for approximately 30 sec to prevent backflow of fluid.
2.5. Histology
At the completion of the behavioral experiments, animals were overdosed with Nembutal (Abbott Labs, North Chicago, IL) and transcardially perfused with 4% formaldehyde. Brain sections 40 μm thick were cut on a freezing microtome and floated onto gelatin coated slides. After drying overnight, sections were stained with cresyl violet and coverslipped with DPX (Sigma-Aldrich Chemical, St. Louis, MO). Cannula placement was verified, and data were discarded for animals that did not have correct placement.
2.6. Drugs
The (±)-MDMA hydrochloride used in this study was synthesized in our laboratory (Nichols et al. 1986) and dissolved in 0.9% saline. (+)-Amphetamine hydrochloride (Sigma, St. Louis) was also dissolved in saline. Prazosin hydrochloride (Tocris, Ellisville, MO) was dissolved in distilled water with brief sonication. MDMA, amphetamine, and the 0.5 mg/kg (1.2 μmol/kg) dose of prazosin were administered intraperitoneally (IP) in a volume of 1 mL/kg. The 1 mg/kg (2.4 μmol/kg) dose of prazosin was administered IP in a volume of 2 mL/kg. The doses of prazosin were selected based on the results of Darracq et al. (1998).
2.7. Behavioral Procedures
2.7.1. Effect of prazosin on amphetamine
Animals were handled and habituated to laboratory conditions for one week, during which they were placed without injection into the Behavioral Pattern Monitor (BPM) for one hour each day to familiarize them with the testing apparatus. In experiment one, eighteen animals were divided into the following three test groups, with six animals per group: salineamphetamine (2 mg/kg); prazosin (0.5 mg/kg)-amphetamine (2 mg/kg); and prazosin (1 mg/kg)-amphetamine (2 mg/kg). On the test day animals were again habituated to the test chamber for one hour while photo beam breaks were recorded to establish baseline activity. After habituation, animals received an intraperitoneal injection of saline or prazosin (0.5 or 1.0 mg/kg) and locomotor activity was monitored for 30 min. Once the 30 min interval was completed, all animals were injected intraperitoneally IP with 2 mg/kg amphetamine, and motor activity was monitored for another 120 min.
2.7.2. Effect of systemic prazosin on MDMA locomotor activity
Animals were handled and habituated as described above. The four test groups for this second experiment included saline-saline (n = 14), saline-prazosin (n = 6), saline-MDMA (n = 6), and prazosin 0.5-MDMA (n = 6). After the one-hour habituation phase, animals were injected IP with saline or 0.5 mg/kg, prazosin. Following the 30-minute pretreatment interval, animals were injected with saline or MDMA (5 mg/kg), returned to the test chamber, and activity counts were recorded for 120 minutes.
2.7.3. Effect of intra-PFC and intra-VTA prazosin on MDMA locomotor activity
In the third experiment, microinjections of prazosin into the prefrontal cortex (n = 8 [14 - 6 discarded after histological evaluation]) or ventral tegmental area (n = 6 [12 - 6 discarded after histology]) were given to block activation of α1-receptors. Following recovery from surgery, during the four days preceding the test day, animals were habituated to the Behavioral Pattern Monitor for one hour each day. At this time, animals also were exposed to the microinjection procedure by gently restraining them and handling them around the cannula implantation site. On the test day, animals were placed without injection into the BPM chambers for 30 min. Microinjections of saline or prazosin were then administered and animals were returned to the Behavioral Pattern Monitor and locomotor activity was recorded for 30 min to establish baseline activity. After this 30-minute interval, animals were injected intraperitoneally with saline or 5 mg/kg of MDMA and locomotor activity was recorded for 120 min.
2.7.4 Topographical analysis of locomotor activity pattern after (+)-amphetamine or MDMA administration
Data collected for experiments 2.7.1 and 2.7.2 were used for comparison of topographical components of the locomotor activity pattern. For each group of animals (determined by the treatment regimen) the mean of the number of photobeam crosses along the peripheral zones was compared with the mean of entries into the central zones. One animal with the number of entries closest to the mean for each group (saline-saline, saline-2 mg/kg (+)-amphetamine, saline-5 mg/kg MDMA, and 0.5 mg/kg prazosin-5 mg/kg MDMA) was chosen for graphical representation of the locomotor activity pattern for that treatment in the behavioral pattern monitor.
2.8 Data Analysis
Because of the variability in the individual 5 min time points, data were averaged for 15 min blocks and then normalized to provide data as percent of baseline photo beam breaks. Baseline is defined as the mean number of photo beam breaks during the two 15-minute intervals just prior to the treatment phase. For overall comparisons, the summed total of normalized activity counts for each rat was used as an approximation to the area under the curve (AUC). Significant differences between groups in Experiments 1 and 3 were determined using a one-way analysis of variance followed by a Newman-Keuls post hoc test. A two-way analysis of variance with a Newman-Keuls post hoc test was implemented to detect differences between groups in Experiment 2. The effect of systemic prazosin administration on spontaneous locomotor activity was analyzed using a one-way ANOVA. The difference between central and peripheral activity was compared using a paired Student’s t test. The level of significance was set at p < 0.05. Statistical analyses were conducted using Statistica (StatSoft, Tulsa OK) and graphs were generated using GraphPad© Prism software (San Diego, CA).
3. Results
3.1. Effect of prazosin on amphetamine
The complete time course for experiment 1 is illustrated in Figure 1 for the effect of (+)-amphetamine. Increases observed in activity counts at time points -30 and 0 minutes result from handling and injection. The normalized data from this experiment are shown in Figure 3A.
Figure 1.
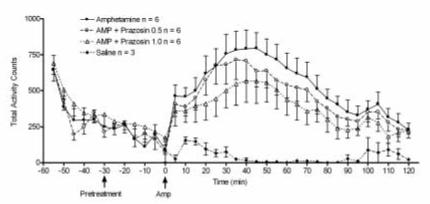
Illustration of the time course of effect of 2 mg/kg (+)-amphetamine hydrochloride on rat total locomotor activity (n = 6) and effect of systemic prazosin (0.50 or 1.0 mg/kg; n = 6 each) pretreatment. Data were recorded every five minutes and are shown as raw untransformed activity counts.
Figure 3.
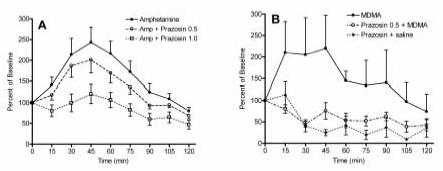
Effect of prazosin pretreatment (1 mg/kg) on (A.) 2 mg/kg (+)-amphetamine- (n = 6) and, (B.) 5 mg/kg MDMA-induced (n = 6) increased locomotor activity. Photo beam breaks were recorded in 5 minute intervals, averaged over 15 min bins, and normalized to percent of baseline (see methods).
Photo beam breaks during the pretreatment phase of each experiment were recorded to determine the effect of prazosin alone on spontaneous motor activity. The data from animals used in experiments 1 and 2 were pooled for this assessment. As shown in Figure 2, systemic prazosin alone had no significant effect on locomotor behavior. Prazosin injected into the PFC likewise had no significant effect on spontaneous locomotor activity in comparison to intra-PFC saline injections (data not shown). Similarly, intra-VTA injections of prazosin had no statistically significant effect on motor activity (data not shown).
Figure 2.
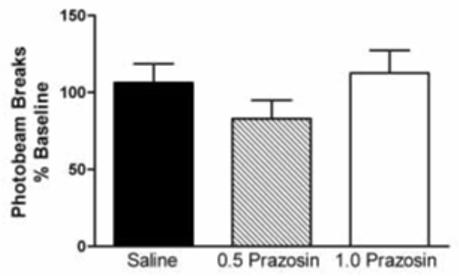
The effect of systemic prazosin administration on rat spontaneous locomotor activity. Photo beam breaks were recorded during the 30 minutes following saline or prazosin treatment, and prior to administration of amphetamine or MDMA. Thus, the pretreatment data are pooled, and are expressed as a percent of baseline locomotor activity. Saline n = 30, 0.5 mg/kg prazosin n = 21, 1.0 mg/kg prazosin n = 6. The effect of prazosin alone on locomotor activity was not significant (p > 0.05).
Systemic pretreatment with prazosin attenuated the locomotor response to 2 mg/kg (+)-amphetamine (Figures 1, 3A, and 4). The results of this experiment are consistent with the findings of Darracq et al. (1998), who had previously reported that prazosin blocked the locomotor stimulant effect of amphetamine. A one-way ANOVA indicated an overall significant effect of prazosin administration on the locomotor response to 2 mg/kg amphetamine (p < 0.01). All data are expressed as the percentage of baseline, which was generated by the saline-saline group; and thus this plot is not presented in figures 3A or 4. The magnitude of the (+)-amphetamine-induced increase of locomotor activity was significantly reduced in animals pretreated with either 0.5 mg/kg (p < 0.05) or 1.0 mg/kg prazosin (p < 0.01).
Figure 4.
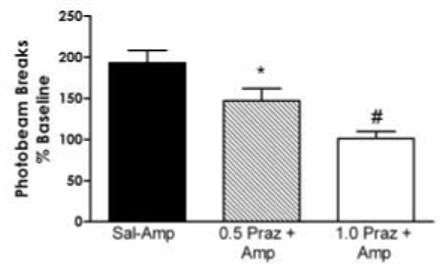
The effect of systemic prazosin administration on the locomotor response to 2 mg/kg (+)-amphetamine. Pretreatment with 0.5 or 1.0 mg/kg prazosin produced a significant overall effect on the locomotor response to (+)-amphetamine (F(2,12) = 12.63; p < 0.01). Significant deceases in (+)-amphetamine-induced hyperactivity were observed in animals pretreated with 0.5 mg/kg (* p < 0.05) and 1.0 mg/kg (**p < 0.01) of prazosin. Number of animals used for each treatment n = 6.
3.2. Effect of systemic prazosin on MDMA locomotor activity
As shown in Figures 3B and 5, prazosin (0.5 mg/kg) significantly (p < 0.001) decreased the hyperlocomotion generated by acute administration of 5 mg/kg MDMA. A two-way ANOVA with a post hoc comparison of the saline-MDMA and prazosin-MDMA groups revealed a significant interaction of pretreatment and treatment (p < 0.05). Although we also initially pretreated with 1.0 mg/kg prazosin, this dose gave no further reduction in locomotor activity (data not shown).
Figure 5.
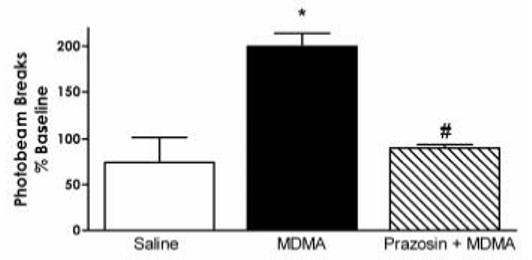
The effect of systemic prazosin administration on the locomotor response to MDMA. In animals pretreated with 0.5 mg/kg prazosin, the hyperlocomotion generated by 5 mg/kg MDMA was significantly reduced (*p < 0.01), and this effect was not significantly different from prazosin-saline alone; saline-MDMA n = 14, prazosin-MDMA n = 6. * p<0.05 vs saline.
3.3. Effect of intra-PFC and intra-VTA prazosin on MDMA locomotor activity
Histological examination of infusion sites intended for the prefrontal cortex confirmed cannulae traces in the PFC between 2.70 to 4.20 mm anterior to bregma, in the prelimbic, medial optical, and motor cortex regions. Four animals from the prazosin-MDMA group with cannulae implanted into this region were removed from the study because the internal cannulae could not be completely inserted.
Histological analysis of animals with cannulae implanted into the VTA showed traces from -4.25 to -6.04 mm posterior to bregma. Eight animals from the prazosin-MDMA group were dropped from statistical analysis because of incorrect cannula placement.
Intra-PFC injections of prazosin produced a significant overall effect on the locomotor response to MDMA (Figure 6A). Systemic MDMA administration increased animals’ locomotor activity to 181 ± 7.6% of baseline (p < 0.001 vs saline), but after intra-PFC prazosin pretreatment the effect of MDMA was not significantly different from baseline (90 ± 10%). Thus, blockade of cortical α1-adrenergic receptors significantly reduced (p < 0.01) the locomotor response to systemic administration of 5 mg/kg MDMA. Pretreatment with intra-VTA injections of prazosin likewise produced an overall significant effect (p < 0.001) on MDMA-induced locomotor activity (Figure 6B).
Figure 6.
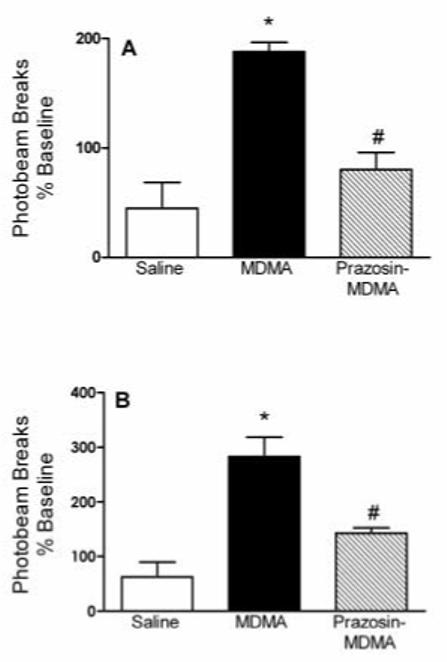
Figure 6A. Locomotor activity was significantly increased by systemic administration of 5 mg/kg MDMA compared to saline (p < 0.001). The response to MDMA was significantly reduced in animals receiving intra-PFC injections of prazosin (*p < 0.01). Number of animals used for each treatment: n = 11 for saline-saline, n = 6 for saline-MDMA and prazosin-MDMA.
Figure 6B. Locomotor activity was significantly increased by 5 mg/kg MDMA compared to saline (p < 0.001). The locomotor response elicited by MDMA was significantly attenuated by intra-VTA injections of prazosin (*p < 0.01). Number of animals used for each treatment: n = 9 for saline-saline, N = 6 for saline-MDMA and prazosin-MDMA.
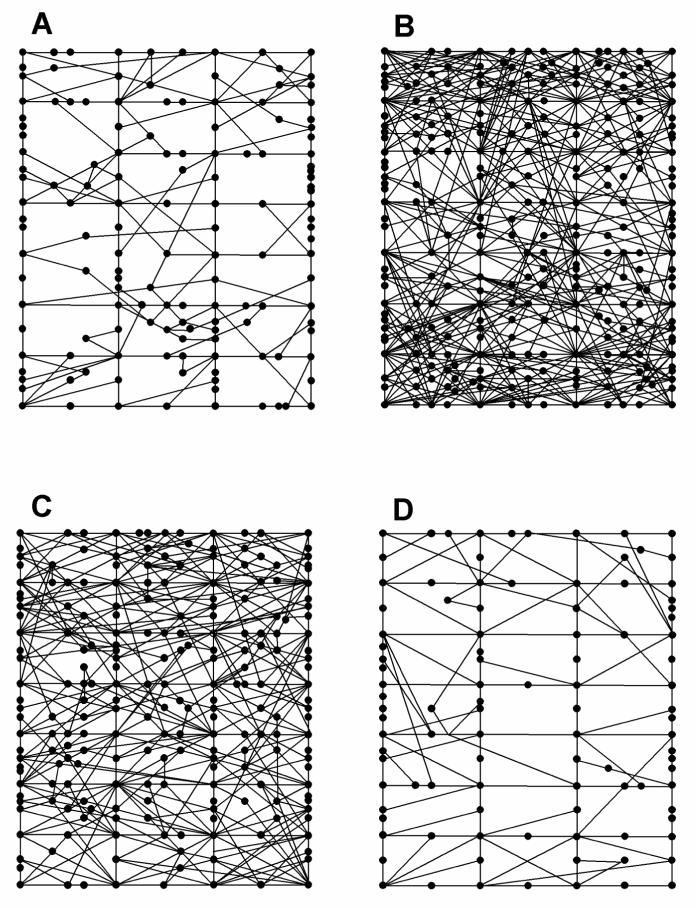
3.4. Topographical analysis of locomotor activity pattern after (+)-amphetamine or MDMA administration
The Behavioral Pattern Monitor activity data were analyzed to compare the time animals spent in the central versus the peripheral area of the test chamber after administration of saline, 2 mg/kg (+)-amphetamine, and MDMA (5 mg/kg) (Figures 7 and 8). Systemic (+)-amphetamine administration (Figure 7B) increased central activity to 263 ± 23% of baseline, but gave only a 166 ± 12% increase of peripheral activity. Consistent with the findings of Geyer et al. (1987), the present data show that activity in the central part of the chamber was significantly greater (*p < 0.01) than peripheral activity after amphetamine administration.
Figure 7.
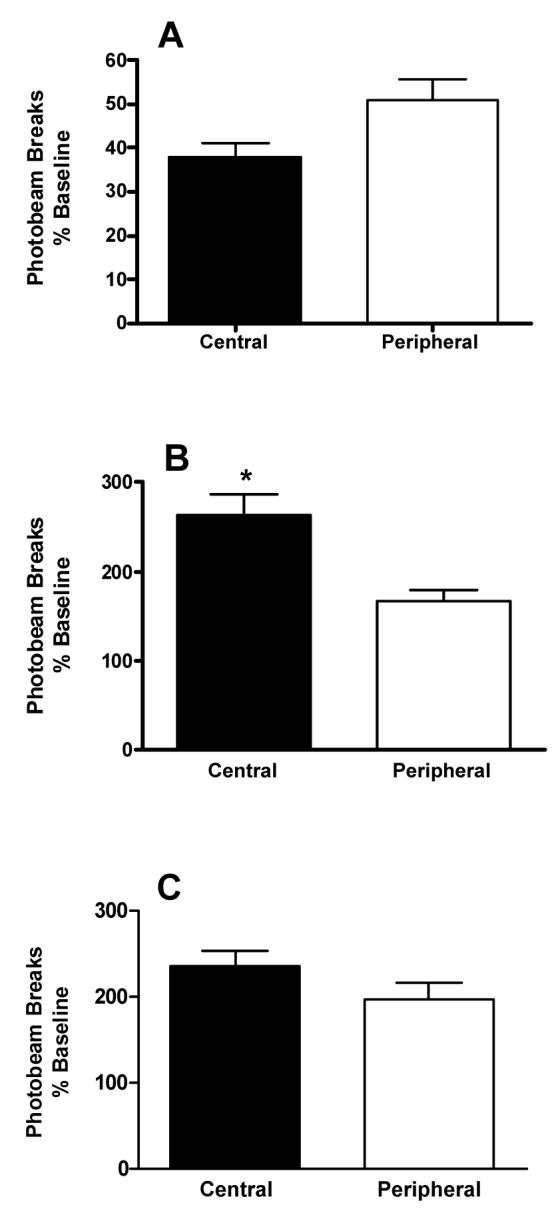
Comparison of locomotor activity in central versus peripheral areas of the activity chamber (see methods). (A.) The effect of saline on central vs peripheral activity. There was no significant difference between the time spent in the central vs peripheral part of the chamber, although there was a trend to spend more time in the peripheral region of the chamber (p = 0.06). (B.) The effect of (+)-amphetamine on central versus peripheral activity. Following administration of 2 mg/kg of (+)-amphetamine, animals spent significantly more time in the central area of the test chamber than in the peripheral area (*p < 0.01). Number of animals for each treatment n = 6. (C.) The effect of MDMA on central versus peripheral activity. There was no significant difference (p > 0.05) between time spent in central versus peripheral areas of the chamber after administration of 5 mg/kg MDMA (n = 5).
Figure 8.
Representative locomotor activity patterns in the behavioral pattern monitor for (A) saline, (B) 2 mg/kg (+)-amphetamine, (C) 5 mg/kg MDMA, and (D) 5 mg/kg MDMA after pretreatment with 0.5 mg/kg prazosin.
Racemic MDMA increased locomotor activity in both the central (240 ± 17% of baseline) and peripheral (200 ± 19% of baseline) areas of the test chamber (Figures 7C and 8). No significant difference (p > 0.05) was detected, however, between peripheral and central locomotor activity after racemic MDMA treatment. These results are in contrast to the previously reported activity profile of (+)-MDMA (Callaway et al. 1990; Gold et al. 1988).
4. Discussion
The present experiments tested the hypothesis that the hyperactivity produced by MDMA in the rat is modulated by activation of α1-adrenoceptors in the prefrontal cortex and ventral tegmental area. The main finding is that blockade of α1-adrenoceptors by prazosin attenuates the locomotor response to MDMA. Prazosin binds nonselectively to the α1A-, α1B-, and α1D-adrenergic receptors, with 100-fold lower affinity for α2B- and α2C-receptors (Bylund et al., 1994). Nevertheless, α1B-deficient mice demonstrate markedly attenuated locomotor responses to (+)-amphetamine and cocaine (Drouin et al 2002b), suggesting that this α1 adrenergic receptor subtype is the likely target of prazosin in these studies.
The effect of prazosin observed here is similar to the effects reported in other studies examining the role of the α1-adrenergic receptor in the locomotor response to amphetamine or cocaine (Arnt 1995; Blanc et al. 1994; Darracq et al. 1998; Dickinson et al. 1988; Drouin et al. 2002a; Drouin et al. 2002b; Snoddy et al. 1985; Wellman et al. 2002). In fact, our data indicate that MDMA-induced motor behavior is more sensitive to α1-adrenoceptor blockade than that produced by (+)-amphetamine. Although a higher dose of MDMA (5 mg/kg) was used than (+)-amphetamine (2 mg/kg), at these doses both drugs produced comparable increases in locomotor activity: a 200% increase over baseline following MDMA and a 193% increase after (+)-amphetamine. After pretreatment with 0.5 mg/kg prazosin, MDMA-induced activity was reduced by 55%, but this dose of prazosin produced only a 21% reduction in (+)-amphetamine-induced behavior. Whereas the larger 1.0 mg/kg dose of prazosin further attenuated the (+)-amphetamine response, the same dose of prazosin produced no further reduction of the locomotor increase by MDMA (data not shown). It seems possible that (+)-amphetamine, which is more potent than MDMA at increasing NE levels in the brain (Steele et al. 1987; Rothman et al. 2000), may produce greater α1-adrenoceptor activation and is therefore less responsive to prazosin than MDMA.
Electrophysiology studies in rat PFC slices show that prazosin blocks NE-induced burst firing of cortical neurons, and α1-adrenoceptor agonists increase neuronal activity (Bradshaw et al. 1981; Bradshaw et al. 1983; Marek and Aghajanian 1999), indicating that excitation of cortical α1-adrenoceptors can regulate neuronal activity in the cortex. Excitation of PFC neurons modulates mesolimbic transmission through projections to the VTA and nucleus accumbens, with the tegmental projection playing a more dominant role (Karreman et al. 1996; Karreman and Moghaddam 1996). Thus, noradrenergic-dopaminergic interactions may occur through activation of α1-adrenergic receptors in the PFC. Consistent with these in vitro data, microinjections of phenylephrine into the PFC of rats increased locomotor activity (Smialowska et al. 1994), whereas microinjections of prazosin into this area abolished the hyperactivity induced by localized or systemic injections of (+)-amphetamine, as well as systemic injections of the opiate morphine (Blanc et al. 1994; Darracq et al. 1998; Drouin et al. 2001).
In light of these results and the role of the PFC in regulating locomotor activity, we examined whether MDMA-induced locomotor activity was sensitive to the blockade of cortical α1-adrenoceptors. We observed a significantly decreased locomotor response to MDMA in animals that had received intra-PFC injections of prazosin compared to those injected with saline. Thus, the results of the present microinjection experiments indicate that α1-adrenoceptors in the PFC modulate the locomotor response to MDMA.
Locus coeruleus-mediated burst firing of VTA neurons is also sensitive to α1-adrenoceptor blockade (Grenhoff et al. 1993), and α1-adrenoceptors regulate amphetamine-induced neuronal activity in the VTA and DA release in the nucleus accumbens (Pan et al. 1996). Additionally, the α1-adrenergic receptor agonist phenylephrine depolarized VTA neurons in rat brain slices (Grenhoff et al. 1995). In the present experiments prazosin microinjections into the VTA of rats prior to systemic administration of MDMA decreased the locomotor response to MDMA, suggesting that activation of α1-adrenoceptors in this region plays a role in the behavioral consequences of MDMA administration. Finally, it is proposed that the intra-VTA prazosin-induced blockade of the locomotor hyperactivity produced by MDMA is linked to the previously demonstrated regulatory role of noradrenergic neurons on cortical DA transmission (e.g. Gresch et al. 1995; Pozzi et al. 1994).
It might be noted that immediately after injection into the PFC or VTA the local concentration of prazosin may approach 1 millimolar at the injection site. This high concentration would allow some diffusion from the site in both brain areas, as well as potentially allowing prazosin to interact with nonadrenergic sites where it has much lower affinity. This problem occurs in any microinjection study unless the drug concentration is very low, is given very slowly, or both. As a practical matter, the use of awake and unrestrained rats does not allow prolonged administration times. Nevertheless, our results are consistent with other microinjection studies and we believe the effects observed after local administration are best explained by adrenergic receptor blockade in the specific brain areas targeted.
We also examined the pattern of locomotor activity produced by administration of both (+)-amphetamine and racemic MDMA. Consistent with previous reports (Geyer et al. 1987), we observed that animals spent more time (>40%) in central than peripheral regions of the chamber following administration of 2 mg/kg (+)-amphetamine. By contrast, several studies from different laboratories have reported observing more peripheral than central activity following (+)-MDMA administration (McCreary et al. 1999; Bankson and Cunningham 2002; Gold et al. 1988; Callaway et al. 1990). In the present experiments, however, locomotor activity was nearly evenly distributed between the central and peripheral areas of the chamber following administration of 5 mg/kg (±)-MDMA.
In light of these previous studies, our results were somewhat surprising. Nevertheless, there are several important methodological differences between the present and earlier studies, such as habituation and time of activity measurement. Rodents tend to display a natural preference for the perimeters (thigmotaxis) of an environment and avoidance of open areas. In the open field paradigm, avoidance of central areas is used as an indicator of anxiety and is sensitive to anxiolytic drugs (Treit and Fundytus 1988). In an effort to reduce the effect of a novel environment on locomotor activity, animals used in the present experiments were repeatedly exposed to the test chamber. Most importantly, subjects were allowed one hour to habituate to the measurement arena prior to drug administration. Thus, the lack of effect of MDMA on central versus peripheral activity in the present study could be caused by a reduction in anxiety-like behaviors during the test period.
Perhaps one of the most important differences may be the fact that we used (±)-MDMA, whereas all of the cited previous studies used (+)-MDMA. Racemic MDMA is the only form of the drug found on the illicit market; its (+) enantiomer has never appeared on the street. Although (+)-MDMA is more “potent” in psychostimulant-like behavioral effects in animals than the racemic mixture, the (-) isomer is certainly not inert and probably contributes to the overall pharmacological properties of racemic (±)-MDMA (e.g. Nash et al. 1994; Anderson, III et al. 1978). We suggest that if the psychopharmacology of MDMA in humans is to be fully understood, the substance that is actually available to human users, racemic (±)-MDMA, is the one that should be studied.
In conclusion, the present experiments have demonstrated that blockade of α1-receptors in the prefrontal cortex and ventral tegmental area abolishes the motor stimulant effects of the recreational drug 3,4-methylenedioxymethamphetamine (MDMA, ecstasy) in rats. The motor activating effect of dependence-producing drugs is believed to require an increase in DA transmission in the mesolimbic projection from the midbrain VTA to the forebrain NAc (Bozarth and Wise 1981). Thus, the results of the present study, together with the findings of previous work, suggest that α1-adrenoceptors are essential for a regulatory role of noradrenergic neurons in cortical dopamine transmission, and that noradrenergic-dopaminergic interactions may underlie the stimulant mechanisms of recreational drugs. We further suggest that additional research is warranted into the pharmacological relevance of noradrenergic pathways to the psychopharmacology of MDMA.
Acknowledgments
This research was funded by NIH grants DA04758 and DA02189 from the National Institute on Drug Abuse. The authors express appreciation to Dr. Danuta Marona-Lewica for her very helpful discussions and careful reading of the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Anderson GM, III, Braun G, Braun U, Nichols DE, Shulgin AT. Absolute configuration and psychotomimetic activity. NIDA Res Monogr. 1978:8–15. [PubMed] [Google Scholar]
- Arnt J. Differential effects of classical and newer antipsychotics on the hypermotility induced by two dose levels of (+)-amphetamine. Eur J Pharmacol. 1995;283:55–62. doi: 10.1016/0014-2999(95)00292-s. [DOI] [PubMed] [Google Scholar]
- Bankson MG, Cunningham KA. Pharmacological studies of the acute effects of (+)-3,4-methylenedioxymethamphetamine on locomotor activity: role of 5- HT(1B/1D) and 5-HT(2) receptors. Neuropsychopharmacology. 2002;26:40–52. doi: 10.1016/S0893-133X(01)00345-1. [DOI] [PubMed] [Google Scholar]
- Blanc G, Trovero F, Vezina P, Herve D, Godeheu AM, Glowinski J, Tassin JP. Blockade of prefronto-cortical alpha 1-adrenergic receptors prevents locomotor hyperactivity induced by subcortical (+)-amphetamine injection. Eur J Neurosci. 1994;6:293–298. doi: 10.1111/j.1460-9568.1994.tb00272.x. [DOI] [PubMed] [Google Scholar]
- Blendy JA, Perry DC, Pabreza LA, Kellar KJ. Electroconvulsive shock increases alpha 1b- but not alpha 1a-adrenoceptor binding sites in rat cerebral cortex. J Neurochem. 1991;57:1548–1555. doi: 10.1111/j.1471-4159.1991.tb06350.x. [DOI] [PubMed] [Google Scholar]
- Bradshaw CM, Pun RY, Slater NT, Szabadi E. Comparison of the effects of methoxamine with those of noradrenaline and phenylephrine on single cerebral cortical neurones. Br J Pharmacol. 1981;73:47–54. doi: 10.1111/j.1476-5381.1981.tb16770.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradshaw CM, Stoker MJ, Szabadi E. Comparison of the neuronal responses to 5-hydroxy-tryptamine, noradrenaline and phenylephrine in the cerebral cortex: effects of haloperidol and methysergide. Neuropharmacology. 1983;22:677–685. doi: 10.1016/0028-3908(83)90090-4. [DOI] [PubMed] [Google Scholar]
- Bylund DB, Eikenberg C, Hieble JP, Langer Sz, Lefkowitz RJ, Minneman KP, Molinoff PB, Ruffolo RR, Jr, Trendelenburg U. IV International Union of Pharmacology: nomenclature of adrenoreceptors. Pharmacol Rev. 1994;46:121–128. [PubMed] [Google Scholar]
- Bymaster FP, Calligaro DO, Falcone JF, Marsh RD, Moore NA, Tye NC, Seeman P, Wong DT. Radioreceptor binding profile of the atypical antipsychotic olanzapine. Neuropsychopharmacology. 1996;14(a):87–96. doi: 10.1016/0893-133X(94)00129-N. [DOI] [PubMed] [Google Scholar]
- Bymaster FP, Hemrick-Luecke SK, Perry KW, Fuller RW. Neurochemical evidence for antagonism by olanzapine of dopamine, serotonin, alpha 1-adrenergic and muscarinic receptors in vivo in rats. Psychopharmacology (Berl) 1996;124(b):87–94. doi: 10.1007/BF02245608. [DOI] [PubMed] [Google Scholar]
- Callaway CW, Geyer MA. Stimulant effects of 3,4-methylenedioxymethamphetamine in the nucleus accumbens of rat. Eur J Pharmacol. 1992;214(a):45–51. doi: 10.1016/0014-2999(92)90094-k. [DOI] [PubMed] [Google Scholar]
- Callaway CW, Geyer MA. Tolerance and cross-tolerance to the activating effects of 3,4-methylenedioxymethamphetamine and a 5-hydroxytryptamine1B agonist. J Pharmacol Exp Ther. 1992;263(b):318–326. [PubMed] [Google Scholar]
- Callaway CW, Rempel N, Peng RY, Geyer MA. Serotonin 5-HT1-like receptors mediate hyperactivity in rats induced by 3,4-methylenedioxymethamphetamine. Neuropsychopharmacology. 1992;7:113–127. [PubMed] [Google Scholar]
- Callaway CW, Wing LL, Geyer MA. Serotonin release contributes to the locomotor stimulant effects of 3,4-methylenedioxymethamphetamine in rats. J Pharmacol Exp Ther. 1990;254:456–464. [PubMed] [Google Scholar]
- Cole JC, Sumnall HR. Altered states: the clinical effects of Ecstasy. Pharmacol Ther. 2003;98:35–58. doi: 10.1016/s0163-7258(03)00003-2. [DOI] [PubMed] [Google Scholar]
- Darracq L, Blanc G, Glowinski J, Tassin JP. Importance of the noradrenaline-dopamine coupling in the locomotor activating effects of (+)-amphetamine. J Neurosci. 1998;18:2729–2739. doi: 10.1523/JNEUROSCI.18-07-02729.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickinson SL, Gadie B, Tulloch IF. Alpha 1- and alpha 2-adrenoreceptor antagonists differentially influence locomotor and stereotyped behaviour induced by (+)-amphetamine and apomorphine in the rat. Psychopharmacology (Berl) 1988;96:521–527. doi: 10.1007/BF02180034. [DOI] [PubMed] [Google Scholar]
- Drouin C, Blanc G, Trovero F, Glowinski J, Tassin JP. Cortical alpha 1-adrenergic regulation of acute and sensitized morphine locomotor effects. Neuroreport. 2001;12:3483–3486. doi: 10.1097/00001756-200111160-00022. [DOI] [PubMed] [Google Scholar]
- Drouin C, Blanc G, Villegier AS, Glowinski J, Tassin JP. Critical role of alpha1-adrenergic receptors in acute and sensitized locomotor effects of (+)-amphetamine, cocaine, and GBR 12783: influence of preexposure conditions and pharmacological characteristics. Synapse. 2002;43(a):51–61. doi: 10.1002/syn.10023. [DOI] [PubMed] [Google Scholar]
- Drouin C, Darracq L, Trovero F, Blanc G, Glowinski J, Cotecchia S, Tassin JP. Alpha1b-adrenergic receptors control locomotor and rewarding effects of psychostimulants and opiates. J Neurosci. 2002;22(b):2873–2884. doi: 10.1523/JNEUROSCI.22-07-02873.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fantegrossi WE, Kiessel CL, Leach PT, Martin CV, Karabenick RL, Chen X, Ohizumi Y, Ullrich T, Rice KC, Woods JH. Nantenine: an antagonist of the behavioral and physiological effects of MDMA in mice. Psychopharmacology (Berl) 2004 doi: 10.1007/s00213-003-1741-2. [DOI] [PubMed] [Google Scholar]
- Fitzgerald JL, Reid JJ. Interactions of methylenedioxymethamphetamine with monoamine transmitter release mechanisms in rat brain slices. Naunyn Schmiedebergs Arch Pharmacol. 1993;347:313–323. doi: 10.1007/BF00167451. [DOI] [PubMed] [Google Scholar]
- Geyer MA, Russo PV, Segal DS, Kuczenski R. Effects of apomorphine and amphetamine on patterns of locomotor and investigatory behavior in rats. Pharmacol Biochem Behav. 1987;28:393–399. doi: 10.1016/0091-3057(87)90460-6. [DOI] [PubMed] [Google Scholar]
- Gold LH, Hubner CB, Koob GF. A role for the mesolimbic dopamine system in the psychostimulant actions of MDMA. Psychopharmacology (Berl) 1989;99:40–47. doi: 10.1007/BF00634450. [DOI] [PubMed] [Google Scholar]
- Gold LH, Koob GF, Geyer MA. Stimulant and hallucinogenic behavioral profiles of 3,4-methylenedioxymethamphetamine and N-ethyl-3,4-methylenedioxyamphetamine in rats. J Pharmacol Exp Ther. 1988;247:547–555. [PubMed] [Google Scholar]
- Green AR, Mechan AO, Elliott JM, O′Shea E, Colado MI. The pharmacology and clinical pharmacology of 3,4-methylenedioxymethamphetamine (MDMA, ″ecstasy″) Pharmacol Rev. 2003;55:463–508. doi: 10.1124/pr.55.3.3. [DOI] [PubMed] [Google Scholar]
- Grenhoff J, Nisell M, Ferre S, Aston-Jones G, Svensson TH. Noradrenergic modulation of midbrain dopamine cell firing elicited by stimulation of the locus coeruleus in the rat. J Neural Transm Gen Sect. 1993;93:11–25. doi: 10.1007/BF01244934. [DOI] [PubMed] [Google Scholar]
- Grenhoff J, North RA, Johnson SW. Alpha1-adrenergic effects on dopamine neurons recorded intracellularly in the rat midrain slice. Eur J Neurosci. 1995;7:1707–1713. doi: 10.1111/j.1460-9568.1995.tb00692.x. [DOI] [PubMed] [Google Scholar]
- Gresch PJ, Sved AF, Zigmond MJ, Finlay JM. Local influence of endogenous norepinephrine on extracellular dopamine in rat medial prefrontal cortex. J Neurochem. 1995;65:111–116. doi: 10.1046/j.1471-4159.1995.65010111.x. [DOI] [PubMed] [Google Scholar]
- Harkin A, Morris K, Kelly JP, O′Donnell JM, Leonard BE. Modulation of MK-801-induced behaviour by noradrenergic agents in mice. Psychopharmacology (Berl) 2001;154:177–188. doi: 10.1007/s002130000630. [DOI] [PubMed] [Google Scholar]
- Ipsen M, Zhang Y, Dragsted N, Han C, Mulvany MJ. The antipsychotic drug sertindole is a specific inhibitor of alpha1A-adrenoceptors in rat mesenteric small arteries. Eur J Pharmacol. 1997;336:29–35. doi: 10.1016/s0014-2999(97)01242-9. [DOI] [PubMed] [Google Scholar]
- Johnson MP, Conarty PF, Nichols DE. [3H]monoamine releasing and uptake inhibition properties of 3,4-methylenedioxymethamphetamine and p-chloroamphetamine analogues. Eur J Pharmacol. 1991;200:9–16. doi: 10.1016/0014-2999(91)90659-e. [DOI] [PubMed] [Google Scholar]
- Karreman M, Moghaddam B. The prefrontal cortex regulates the basal release of dopamine in the limbic striatum: an effect mediated by ventral tegmental area. J Neurochem. 1996;66:589–598. doi: 10.1046/j.1471-4159.1996.66020589.x. [DOI] [PubMed] [Google Scholar]
- Karreman M, Westerink BH, Moghaddam B. Excitatory amino acid receptors in the ventral tegmental area regulate dopamine release in the ventral striatum. J Neurochem. 1996;67:601–607. doi: 10.1046/j.1471-4159.1996.67020601.x. [DOI] [PubMed] [Google Scholar]
- Kehne JH, Ketteler HJ, McCloskey TC, Sullivan CK, Dudley MW, Schmidt CJ. Effects of the selective 5-HT2A receptor antagonist MDL 100,907 on MDMA- induced locomotor stimulation in rats. Neuropsychopharmacology. 1996;15:116–124. doi: 10.1016/0893-133X(95)00160-F. [DOI] [PubMed] [Google Scholar]
- Marek GJ, Aghajanian GK. 5-HT2A receptor or alpha1-adrenoceptor activation induces excitatory postsynaptic currents in layer V pyramidal cells of the medial prefrontal cortex. Eur J Pharmacol. 1999;367:197–206. doi: 10.1016/s0014-2999(98)00945-5. [DOI] [PubMed] [Google Scholar]
- Mathe JM, Nomikos GG, Hildebrand BE, Hertel P, Svensson TH. Prazosin inhibits MK-801-induced hyperlocomotion and dopamine release in the nucleus accumbens. Eur J Pharmacol. 1996;309:1–11. doi: 10.1016/0014-2999(96)00315-9. [DOI] [PubMed] [Google Scholar]
- McCreary AC, Bankson MG, Cunningham KA. Pharmacological studies of the acute and chronic effects of (+)-3, 4- methylenedioxymethamphetamine on locomotor activity: role of 5- hydroxytryptamine(1A) and 5-hydroxytryptamine(1B/1D) receptors. J Pharmacol Exp Ther. 1999;290:965–973. [PubMed] [Google Scholar]
- McNamara MG, Kelly JP, Leonard BE. Some behavioural and neurochemical aspects of subacute (+/-)3,4- methylenedioxymethamphetamine administration in rats. Pharmacol Biochem Behav. 1995;52:479–484. doi: 10.1016/0091-3057(95)00206-c. [DOI] [PubMed] [Google Scholar]
- Mogilnicka E, Zazula M, Wedzony K. Functional supersensitivity to the alpha 1-adrenoceptor agonist after repeated treatment with antidepressant drugs is not conditioned by beta-down-regulation. Neuropharmacology. 1987;26:1457–1461. doi: 10.1016/0028-3908(87)90163-8. [DOI] [PubMed] [Google Scholar]
- Nalepa I, Kreiner G, Kowalska M, Sanak M, Zelek-Molik A, Vetulani J. Repeated imipramine and electroconvulsive shock increase alpha 1A-adrenoceptor mRNA level in rat prefrontal cortex. Eur J Pharmacol. 2002;444:151–159. doi: 10.1016/s0014-2999(02)01660-6. [DOI] [PubMed] [Google Scholar]
- Nash JF, Roth BL, Brodkin JD, Nichols DE, Gudelsky GA. Effect of the R(-) and S(+) isomers of MDA and MDMA on phosphatidyl inositol turnover in cultured cells expressing 5-HT2A or 5-HT2C receptors. Neurosci Lett. 1994;177:111–115. doi: 10.1016/0304-3940(94)90057-4. [DOI] [PubMed] [Google Scholar]
- Pan WH, Sung JC, Fuh SM. Locally application of amphetamine into the ventral tegmental area enhances dopamine release in the nucleus accumbens and the medial prefrontal cortex through noradrenergic neurotransmission. J Pharmacol Exp Ther. 1996;278:725–731. [PubMed] [Google Scholar]
- Pozzi L, Invernizzi R, Cervo L, Vellebuona F, Samanin R. Evidence that extracellular concentrations of dopamine are regulated by noradrenergic neurons in the frontal cortex of rats. J Neurochem. 1994;63:195–200. doi: 10.1046/j.1471-4159.1994.63010195.x. [DOI] [PubMed] [Google Scholar]
- Rogoz Z, Margas W, Skuza G, Solich J, Kusmider M, Dziedzicka-Wasylewska M. Effect of repeated treatment with reboxetine on the central alpha 1-adrenergic and dopaminergic receptors. Pol J Pharmacol. 2002;54:593–603. [PubMed] [Google Scholar]
- Rothman RB, Baumann MH, Dersch CM, Romero DV, Rice KC, Carroll FI, Partilla JS. Amphetamine-type central nervous system stimulants release norepinephrine more potently than they release dopamine and serotonin. Synapse. 2001;39:32–41. doi: 10.1002/1098-2396(20010101)39:1<32::AID-SYN5>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- Rothman RB, Partilla JS, Baumann MH, Dersch CM, Carroll FI, Rice KC. Neurochemical neutralization of methamphetamine with high-affinity nonselective inhibitors of biogenic amine transporters: a pharmacological strategy for treating stimulant abuse. Synapse. 2000;35:222–227. doi: 10.1002/(SICI)1098-2396(20000301)35:3<222::AID-SYN7>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- Shi WX, Pun CL, Zhang XX, Jones MD, Bunney BS. Dual effects of (+)-amphetamine on dopamine neurons mediated by dopamine and nondopamine receptors. J Neurosci. 2000;20:3504–3511. doi: 10.1523/JNEUROSCI.20-09-03504.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simantov R. Multiple molecular and neuropharmacological effects of MDMA (Ecstasy) Life Sci. 2004;74:803–814. doi: 10.1016/j.lfs.2003.08.002. [DOI] [PubMed] [Google Scholar]
- Smialowska M, Gastol-Lewinska L, Tokarski K. The role of alpha-1 adrenergic receptors in the stimulating effect of neuropeptide Y (NPY) on rat behavioural activity. Neuropeptides. 1994;26:225–232. doi: 10.1016/0143-4179(94)90074-4. [DOI] [PubMed] [Google Scholar]
- Snoddy AM, Tessel RE. Prazosin: effect on psychomotor-stimulant cues and locomotor activity in mice. Eur J Pharmacol. 1985;116:221–228. doi: 10.1016/0014-2999(85)90156-6. [DOI] [PubMed] [Google Scholar]
- Sprague JE, Banks ML, Cook VJ, Mills EM. Hypothalamic-pituitary-thyroid axis and sympathetic nervous system involvement in hyperthermia induced by 3,4-methylenedioxymethamphetamine (Ecstasy) J Pharmacol Exp Ther. 2003;305:159–166. doi: 10.1124/jpet.102.044982. [DOI] [PubMed] [Google Scholar]
- Steele TD, Nichols DE, Yim GK. Stereochemical effects of 3,4-methylenedioxymeth-amphetamine (MDMA) and related amphetamine derivatives on inhibition of uptake of [3H]monoamines into synaptosomes from different regions of rat brain. Biochem Pharmacol. 1987;36:2297–2303. doi: 10.1016/0006-2952(87)90594-6. [DOI] [PubMed] [Google Scholar]
- Strote J, Lee JE, Wechsler H. Increasing MDMA use among college students: results of a national survey. J Adolesc Health. 2002;30:64–72. doi: 10.1016/s1054-139x(01)00315-9. [DOI] [PubMed] [Google Scholar]
- Svensson TH, Mathe JM, Andersson JL, Nomikos GG, Hildebrand BE, Marcus M. Mode of action of atypical neuroleptics in relation to the phencyclidine model of schizophrenia: role of 5-HT2 receptor and alpha 1-adrenoceptor antagonism [corrected] J Clin Psychopharmacol. 1995;15:11S–18S. doi: 10.1097/00004714-199502001-00003. [DOI] [PubMed] [Google Scholar]
- Treit D, Fundytus M. Thigmotaxis as a test for anxiolytic activity in rats. Pharmacol Biochem Behav. 1988;31:959–962. doi: 10.1016/0091-3057(88)90413-3. [DOI] [PubMed] [Google Scholar]
- Vollenweider FX, Liechti ME, Gamma A, Greer G, Geyer M. Acute psychological and neurophysiological effects of MDMA in humans. J Psychoactive Drugs. 2002;34:171–184. doi: 10.1080/02791072.2002.10399951. [DOI] [PubMed] [Google Scholar]
- Wellman P, Ho D, Cepeda-Benito A, Bellinger L, Nation J. Cocaine-induced hypophagia and hyperlocomotion in rats are attenuated by prazosin. Eur J Pharmacol. 2002;455:117–126. doi: 10.1016/s0014-2999(02)02616-x. [DOI] [PubMed] [Google Scholar]