Abstract
This clinical trial evaluated a contingency management intervention designed to improve medication adherence among HIV-positive methadone maintenance patients. After a 4-week baseline observation phase, eligible participants (N = 66) were randomly assigned to: (a) medication coaching sessions every other week to assist with adherence strategies (comparison group) or (b) medication coaching plus voucher reinforcement for opening electronic medication caps on time (voucher group). Baseline adherence (percent doses taken/percent total possible doses) was 51% using electronic measurement, 75% using self-report and 75% using pill count. The intervention was provided for 12 weeks, with a 4-week follow-up. The primary outcome results of the clinical trial indicated effectiveness during the intervention, with significant mean adherence differences between voucher and comparison groups using electronic measurement (78% vs. 56%), pill count (86% vs. 75%), and self-report (87% vs. 69%). Differences between groups faded after vouchers were discontinued. Contingency management shows promise as a strategy to promote antiretroviral medication adherence in this population.
Keywords: randomized clinical trial, opioid use, contingency management, voucher reinforcement, methadone treatment, substance abuse treatment
1. Introduction
Research has demonstrated that HIV-positive substance users have difficulty adhering to complex highly active antiretroviral therapy (HAART) regimens, particularly when they are actively using substances (Batki and Ferrando, 1996; Freeman et al., 1996; Fogarty et al., 2002). Cocaine use is associated with a 41% decline in median antiretroviral adherence and is a strong predictor of failure to maintain viral load (Arnsten et al., 2002). Golin et al. (2002) found that active substance and alcohol users take significantly fewer doses of HAART medication than substance-free clients. Similarly, Gebo et al. (2003) showed drug users are more than twice as likely to be non-adherent to antiretroviral regimens as non-drug users.
A considerable clinical need exists to assist substance users in taking HIV medications on time and as directed. Adherence to HAART has been associated with relative improvements in immunologic and virologic markers (Low-Beer et al., 2000; Paterson et al., 2001), increased body weight (Shikuma et al, 2004), and less rapid progression to Acquired Immunodeficiency Syndrome (AIDS; Bangsberg et al., 2001). Despite the seriousness of problems related to nonadherence, empirical studies on HAART adherence interventions for HIV-positive substance users are lacking (Simoni et al., 2005).
A significant body of work indicates that contingency management is an effective treatment for patients with substance use disorders, including those receiving methadone treatment (for example, Hall et al., 1977; Higgins et al., 1986; Calsyn et al., 1994). Voucher-based contingency management helps patients achieve and maintain abstinence from drugs by providing a voucher incentive for each drug-free urine sample. The voucher has monetary value and can be exchanged for goods and services consistent with the goals of treatment. Initially the value of the vouchers is low, but the value increases with the number of consecutive drug-free urine specimens. This approach has been successful with methadone maintenance patients in encouraging sustained abstinence from cocaine (Silverman et al., 1996a; Silverman et al., 2004) and opiates (Silverman et al., 1996b) as well as increasing full-day attendance at treatment (Jones et al., 2001).
For patients who have difficulty taking medications as directed, contingency management strategies can be used to promote adherence. Previous studies show improved isoniazid (INH) compliance with tuberculosis treatment (Elk et al., 1993, 1995) by dispensing methadone contingent upon INH ingestion. Similarly, Bickel et al. (1988–89) reported on a contingency management program for alcohol-dependent patients in which continuation in methadone treatment was contingent upon daily disulfiram ingestion. Several studies have shown efficacy with opioid-dependent patients using voucher incentives to reinforce naltrexone ingestion (Carroll et al., 2001; Carroll et al., 2002; Preston et al., 1999). Contingency management may also be an efficacious intervention for substance-using HIV patients with poor HAART compliance.
The first published investigation of contingency management to improve adherence to HAART medications (Rigsby et al., 2000) was a pilot that compared three conditions: control training (i.e., encouragement), cue-dose training (i.e., identifying cues) and cue-dose training plus cash reinforcement. This randomized, controlled study (N = 55) tracked adherence using the Medication Events Monitoring System (MEMS). MEMS is an electronic monitor that registers the openings and closings of a medication bottle by using a cap containing a micro-electronic circuit. Events stored in the MEMS cap memory are transferred through a desktop communicator to a computer program that reads and stores the data, calculates results, presents visual displays, and prints reports based on patient data. In this study reinforcement was based on one “primary” medication placed in the MEMS bottle, and patients were encouraged to cue other medications to this one. Cash reinforcement was given to participants at weekly meetings for each dose of the primary medication taken within 2 hours of the prescribed dosing time. Reinforcement began at $2 per dose and increased with each consecutive dose to a maximum of $10 per day (possible earnings = $280 for 4 weeks). The reinforcement was reset to $2 if a dose was not taken within 2 hours of the set dosing time. Study results demonstrated that the group receiving cue-dose training plus monetary reinforcement had significantly higher adherence during the intervention period compared to the other two groups. The mean adherence in the reinforced group increased from 70% at baseline to 88% at week 1. The authors suggest that this rapid improvement was due to the motivating effect of reinforcement rather than new skill acquisition from cue-dose training. Four weeks of contingency management resulted in an average of 92% of doses taken on time in comparison with 70% in the non-contingency management controls. These adherence improvements were not sustained during the follow-up period, and the study did not show an effect of improved adherence on viral load. The study demonstrated the feasibility and efficacy of behavioral strategies for improving HAART adherence among HIV-positive clients using an electronic method to measure adherence.
In the present study we adapted the use of voucher-based contingency management to reinforce taking HAART medications as prescribed. This research represents a novel application of contingency management as the first reported study to apply the use of voucher incentives to HIV medication adherence. We posited that, relative to a comparison group, participants randomly assigned to receive voucher-based contingency management would show better medication adherence (measured by MEMS cap openings, pill count, and self-report), as well as relative improvement in biological and behavioral measures of health (HIV-RNA levels, CD4+ count, and self-report).
2. Methods
The current study was a two-arm randomized controlled experiment that included a 4-week baseline phase, a 12-week intervention phase in which participants were randomized to receive or not receive voucher incentives, and a 4-week follow-up phase.
2.1. Participants
Participants were recruited between April 2001 and March 2004 from among HIV-positive patients enrolled in methadone maintenance treatment at two urban clinics in San Francisco, California: The San Francisco General Hospital Opiate Treatment Outpatient Program (OTOP) and Bay Area Addiction Research and Treatment (BAART), Market Street Clinic. OTOP, a hospital-based program supported by the San Francisco Department of Public Health, provides substance abuse, medical, and psychiatric treatment to approximately 600 opioid-dependent patients. Nearly 33% of OTOP patients are HIV-positive. BAART, a private network of twelve clinics in five California counties, offers substance abuse treatment and medical care. The BAART Market Street Clinic provides services to 600 patients, with 10% diagnosed as HIV-positive. Research records were not revealed to clinic staff – for example results of urine toxicology screens were not shared with the drug treatment program so that participation in the research would not affect the patient’s level of clinic privileges.
2.1.1. Inclusion and exclusion criteria
All participants met the following criteria for study participation: (a) enrolled in outpatient methadone maintenance treatment, (b) HIV antibody seropositive as indicated by clinic records, and (c) prescribed an antiretroviral medication for treatment of HIV/AIDS for at least 1 month, evidenced by prescription or prescription bottle. Participants were excluded from the study if they were: (a) participating in other adherence-improvement research or clinical programs or (b) living in a controlled environment that dispensed residents’ HIV medications. Although it was not an inclusion criterion, all of the study participants were on twice-daily HAART dosing regimens.
The University of California, San Francisco Institutional Review Board approved all study procedures. A recruitment flyer was distributed to clinics and counselors, who encouraged interested patients to contact the research staff. Research staff met with potential participants in a private room and administered a screening form assessing whether inclusion/exclusion criteria were met.
After screening, the research interviewer administered a written informed consent process, explaining that throughout the study, medication adherence results would not be given to the methadone clinic staff. The intake assessment battery was then administered, and in a second session the participant completed a diagnostic research interview, typically within 1 week of intake. Participants were told that after four weeks they would learn whether or not they could continue in the study based on information gained in the first four weeks in the study.
2.2. 4-week baseline phase
After study intake procedures, participants were enrolled in a 4-week baseline observation phase to record adherence to HAART medications, in preparation for the clinical trial.
2.2.1. Assignment of electronic medication-cap (MEMS Cap)
At the beginning of the baseline phase participants received a standard plastic vial with a MEMS cap for one of their HAART medications (e.g., protease inhibitor, nucleoside analog reverse transcriptase inhibitor, or non-nucleoside reverse transcriptase inhibitor). For patients taking multiple medications, the one with the most frequent pill-taking schedule or the one that was determined as most difficult to take was selected as the target. Patients were told that the cap would register the date and time when the bottle was opened and serve as a measure of adherence. When the MEMS data were downloaded to the computer, patients were given feedback on their adherence over the past week by viewing a printed copy of their results.
2.2.2. Medication coaching
All participants met with a registered nurse or trained research assistant who provided medication coaching once during the baseline phase and then every two weeks throughout the study. The research assistant communicated current adherence to the medication coach by providing a hard copy of MEMS data. The medication coaching intervention included 10 strategies to improve adherence and was adapted from an approach used in the Adult AIDS Clinical Trials Group (AACTG; Chesney, 2003). The role of the medication coach was to provide support with the medication regimen and to work in partnership with the client to improve medication adherence. The medication coach assessed current antiretroviral medications prescribed for the patient and generated a personalized schedule, taking into account lifestyle variations in sleep and eating patterns. As a general guideline, the baseline meeting assessed the client’s current medication-taking habits, whereas sessions in the intervention phase built skills needed for improved medication adherence, and the follow-up meetings promoted future adherence. A treatment manual (Haug et al., in press) describes the medication coaching and other interventions of the study.
2.2.3. Twice-weekly assessments with research assistants
During baseline, participants met with a research assistant twice per week to provide results of medication-cap openings. The visits were scheduled to coincide with the patient’s methadone dosing days. At each session the patient had an opportunity to view the display of medication-cap openings recorded since the last visit with the research assistant. The first interview of the week lasted 10–15 minutes per client and involved downloading information on medication-cap opening, counting pills remaining in the patient’s prescription bottle, and asking patients to provide self-report of medication adherence and side effects. The second appointment of the week simply downloaded information on medication-cap openings and usually lasted no more than 5 minutes. At the end of the 4-week baseline phase the research assistant assessed the participant’s interest in continuing in the clinical trial phase of the study, reassessed eligibility criteria, and recruited eligible participants.
2.2.4. Selection for clinical trial and random assignment
At the end of the 4-week baseline phase the study team reviewed the patients’ eligibility for the intervention phase of the study. Participants were not eligible for the clinical trial if their records indicated more than 80% medication adherence during the baseline phase, as measured by the medication-cap openings. Participants who were not eligible due to high adherence rates were thanked for their participation and debriefed about the study. This exclusion criterion was implemented to generalize to the population that a clinic would want to target for an adherence intervention and to avoid ceiling effects that could unduly minimize the effects of the intervention. In debriefing we tactfully explained if they were excluded from the clinical trial because of their high medication adherence rates. For the excluded participants the medication coach provided additional referral resources for help and support in taking their medication. Ethically it was incumbent on the investigators to explain why they were ineligible for the intervention. Most participants excluded due to high rates of medication-bottle opening were disappointed about not being able to participate in the remainder of the study, but they accepted the rationale. It is possible that disappointment of these participants negatively affected clinic recruitment or promoted low rates of medication bottle-opening among participants motivated to get into the clinical trial phase of the study. Yet no data indicated that clinic recruitment in later phases of the study was affected negatively by the exclusion of participants after the baseline period.
Eligible participants were randomly assigned to receive vouchers or a comparison intervention (medication coaching only) according to a computer-generated list. Assignment was stratified by baseline CD4 lymphocyte count (i.e., greater than or equal to 200 cells/UL versus less than 200 cells/UL) because more medically compromised patients were likely to have more severe medical problems during the study. The research assistant opened a sealed envelope of intervention group assignments generated by the project statistician.
2.3. 12-week intervention phase
During the intervention phase, each participant met twice weekly with a research assistant, for a total of 24 visits. All participants received medication coaching every two weeks.
2.3.1. Voucher intervention
In addition to receiving medication coaching, participants in the voucher intervention condition who opened medication caps as scheduled received vouchers that were exchangeable for goods and services in the community. MEMS confirmed when the medication cap was opened, and participants earned a voucher for each instance that the medication cap was opened within 2 hours plus or minus the scheduled dosing time. On Day 1 each participant could earn up to $1 per day (e.g., $.50 per dose for medications taken twice daily). If the cap was not opened within the scheduled timeframe, a voucher was not earned for that dose. A key feature of the protocol was that the value of a voucher increased for each consecutive day that medication bottle openings were on time but was reset to the original amount when the medication bottle was not opened on time for a scheduled dose. In the first five days the participant had opportunity to earn $1.40 more with each successive day if all doses were taken as scheduled.
Our consultant Kenneth Silverman, an expert in voucher incentive systems, suggested this quick ramping-up of the reinforcement value as a strategy to quickly promote consistent opening of the medication bottles. From Day 6 on, the increase for taking all doses within schedule was $.20 per day (from $7.00 for Day 7 to $7.20 for Day 8, etc.). This relative flattening of the reinforcement curve was intended to keep the total available reinforcement close to the $1155 used in previous studies (Silverman et al. 1996a, b). On any day a participant did not take a dose on schedule, the earnings level reset to $1 per day, and the value of subsequent vouchers increased as in the schedule beginning on Day 2. After two weeks of perfect adherence, the highest level previously earned could be reinstated. The time period required for reinstatement was also designed to be the same as that used in previous studies, on which the present study based its voucher values. Silverman’s group collected urine specimens three times a week, while our study measured adherence twice daily. Thus the time-course for a reset was the same, but the number of consecutive compliances was much higher in our study (28 consecutive on-time openings of bottles versus 6 consecutive opiate-free urine samples). A participant could earn as much as $1172.40 in vouchers if he/she took all medication doses as scheduled through the 12-week intervention period.
2.3.2. Comparison intervention
Patients in the comparison condition received medication coaching every other week like the voucher participants, but they did not receive vouchers. Several of the first eight comparison participants, however, expressed dissatisfaction with their treatment relative to the voucher participants. Two participants discontinued study participation because, even though they were receiving compensation for attending study visits and assessments, they were not receiving voucher compensation. To promote retention in the comparison group the project implemented a fishbowl prize system described by Petry and Martin (2002), which reinforced attendance at scheduled interviews. The fishbowl was not tied to adherence or other study outcomes but rather targeted attendance at research appointments and medication coach sessions.
The fishbowl prize system awarded small ($1–$2) and large prizes (approximately $80). For each completed research visit, the participant had the opportunity to pick a number from the fishbowl. Participants had a 1 in 3 chance of winning a small prize (snacks, soaps, lotions, nutritional drinks, socks, etc.) and a 1 in 350 chance of winning a large prize. As part of implementing the fishbowl prize system, the researchers polled participants to determine their preference for prizes, and during the study participants were asked what prizes they wanted to have replenished. Comparison group attendance improved after we implemented the fishbowl prize system (Veluz et al., 2003). Specifically, the attrition rate dropped from 25% to 0%, and the total number of missed interviews decreased from an average of 4.38 to 0.47.
2.4. 4-week follow-up phase
During the 4-week follow-up phase, vouchers were discontinued, but visits with the medication coach continued. All participants continued to meet with the research assistant and download MEMS results. To promote retention in both study groups, the fishbowl reinforcement system continued in weeks 17–20 in the comparison group and was initiated in the voucher group, rewarding participants’ attendance to research visits with the opportunity to draw a number from a bowl of small and large prizes.
2.5. Self-report measures
2.5.1. Addiction Severity Index (ASI)
The ASI is a semi-structured clinical interview that measures psychosocial problems in seven domains of functioning: medical, employment, alcohol, drug, legal, family/social and psychiatric (McLellan et al., 1992).
2.5.2. Beck Depression Inventory–II (BDI-II)
The BDI-II is a widely used, 21-item measure of severity of depressive symptoms in the past 2 weeks (Beck et al., 1996).
2.5.3. Computerized Diagnostic Interview Schedule–IV (C-DIS)
The C-DIS is a structured interview yielding diagnoses according to the Diagnostic and Statistical Manual of Mental Disorders (4th edition; DSM-IV; American Psychiatric Association, 1994). The current study administered only the Mood and Substance Abuse/Dependence Modules (Robins et al., 1995).
2.6. Medication adherence measures
2.6.1. Medication Event Monitoring System V TrackCap (MEMS Cap)
MEMS is a measure of medication adherence that uses a medication cap containing a microelectronic chip to record bottle opening dates and times (Aprex, Inc.). These data are downloaded to a computer using PowerView software (Aardex Ltd.) that stores the information in a database. In this study, we monitored one selected HAART medication as the primary outcome measure of adherence, as research has shown it to be more sensitive than other methods (Arnsten et al., 2001; McNabb et al., 2003). On-time MEMS cap openings were expressed as the percentage of scheduled dosing times when the cap was opened “on-time,” defined as taking place in a 4-hour window (2 hours before or after) around the scheduled dosage time, that is, percent observed openings/percent expected openings. We used MEMS records to calculate on-time openings (i.e., adherence) and the longest documented days of continuous on-time openings (i.e., continuous adherence) because continuous adherence to medications is important to suppressing viral load and avoiding the development of drug resistance.
2.6.2. Medication adherence and side effects questionnaire
Adapted from AACTG Adherence Instruments (Chesney et al., 2000), this questionnaire is a self-report of patient’s adherence for the previous day and the past 3 days for each medication in the current regimen. The measure also includes questions about medication side effects and was administered weekly throughout the study. The mean value of the weekly 3-day measurements was used in the analysis for each study phase.
2.6.3. Pill count
Pills were counted for the one selected HAART medication at baseline to reflect the initial stock, then weekly to track adherence, and after refills to account for new pills added to the bottle. We calculated the number of pills taken (difference between current and previous pill count), which was divided by the number of pills the patient was expected to take based on their prescribed regimen. In the few cases where the calculation exceeded 100% (9% of all the weekly assessments in the voucher condition and 10% in the comparison condition), it was capped at 100%. This practice is consistent with other studies using pill count as an outcome measure (Bangsberg et al., 2002). Percent adherence over each study phase was used in the analysis.
2.7. Health indices
2.7.1. Medical outcomes study short-form (SF-36)
The SF-36 is a 36-item self-administered instrument that evaluates health-related quality of life in eight dimensions, including: physical functioning, role functioning, bodily pain, general health, vitality, social functioning, mental health, and reported health transition (Ware, 1996). It provides two summary measures; a Mental Health Summary Scale and a Physical Health Summary Scale, which were utilized as the primary outcome measures of self-reported health. The SF-36 was administered monthly (baseline, weeks 4, 8, 12, 16, and 20). 2.7.2. Plasma HIV-1 RNA (viral load). Viral load was quantified from monthly blood draws using the Quantiplex bDNA Assay Version 3.0 (Bayer Corp), which has a dynamic range of 75 – 500,000 copies/ml. Because viral load results typically are widely variable, we present raw results and log-10 transformed values. A decrease of at least .5 in log10 viral load is considered consistent with improvement and not within the range of lab error.
2.7.3. CD4+ lymphocyte counts
CD4, a type of white blood cells or T-cells vital to immune function, was obtained at baseline, weeks 12 and 20 from blood samples sent to the hematology section of San Francisco General Hospital clinical laboratories. HIV infection leads to a progressive reduction in the number of T-cells with CD4 receptors, and the CD4 count is used as a staging indicator for treatment decisions. When there are fewer than 200 CD4+ T-cells per microliter (μL) of blood, a person is diagnosed with AIDS.
2.7.4. Weight
Weight was measured in pounds on a physician mechanical balance beam scale at baseline and at 20-week follow-up.
2.7.5. Drug use
Urine specimens were collected monthly and analyzed by Quest Diagnostics, Inc. using Enzyme Multiplied Immunoassay Technique (EMIT), and positive screens were re-analyzed using Thin-layer Chromatography (TLC). Reported results included alcohol, amphetamines, barbiturates, benzodiazepines, cannabinoids, cocaine, and opiates; however, due to low frequencies of positive tests for most substances only cocaine and opiates were analyzed in the current report.
2.8. Monetary compensation
All participants were compensated for completing scheduled assessments and other research activities: $10 for baseline assessments; $10 for the C-DIS; $8 for MEMS cap assignment; $2 for each twice-weekly download interviews (40 interviews); $2 for each weekly survey interview (20 interviews); $2 for each medication coach visit (10 visits); $10 for each monthly blood draw (6 blood draws); $3 for each monthly urine sample (6 urine samples); and $10 for final return of the MEMS cap. The maximum amount paid to participants was $256.00.
2.9. Data analysis
2.9.1. Models tested
As the primary focus was average levels of medication adherence within study phase, and to accommodate both missing data and data collected on differing schedules, for adherence measures from instruments collected weekly or more (MEMS, self-report, pill count), a single measure (per phase) was constructed by taking the mean of all assessments within each of the baseline, intervention, and follow-up phases. Measures assessed only monthly (i.e., SF-36, plasma HIV-1 RNA levels, urine toxicology screens) and CD4 counts, assessed only three times, were not averaged across assessment points. The main analysis was a mixed-effects linear model estimated via maximum likelihood by Proc MIXED in SAS version 9.1.3 with treatment condition, assessment phase and the interaction of the two as effects in the model using the Kenward-Roger’s adjustment to the degrees of freedom (Kenward and Roger, 1997). The treatment condition term in the model tested the mean difference between the conditions, collapsed across time. The phase term tested for change in the outcome over time, collapsed across group. The interaction term, the test of primary interest, tested whether the change across phases differed between the conditions. The viral RNA level, which displayed both an abundance of values below the level of detection and skew in the detected levels, was tested using a two-part model (Tooze et al., 2002) where one of the parts modeled the presence versus absence of detectable viral levels and the second part modeled the mean of the log-transformed values of those levels above detection. This model was estimated twice, once using all assessment points as an outcome and a second time using the two baseline phase assessments (weeks 0 and 4) as covariates. Secondary analysis included an examination of side effects using standard two-group comparisons. In addition, we correlated viral load with adherence measures as increased adherence should eventually lead to a decreased viral load, an important health indicator.
2.9.2. Missing data
The amount of missing data was limited due to two factors. First, as noted in section 3.1.1, subject attrition was relatively low, with between 75% and 100 % of subjects in each condition at each assessment providing data. Second, as data for the measures collected weekly or more (e.g., the MEMS cap and the self-reported adherence) were aggregated by study phase, an outcome measure could still be constructed even if a participant was not assessed at all weeks within a phase.
The use of aggregated data, however, raised the question of whether the amount of data aggregated varied by condition and whether the aggregated totals were based on small amounts of data. The amount of all possible data points available for aggregation did vary by measure and condition. With the exception of the pill counts, the missing data was less than 10% in the voucher condition and less than 20% in the comparison condition. Individual pill counts in the comparison condition were missing at a greater rate (31% during the Baseline phase, 15% during the Intervention phase, and 26% during the Follow-up phase). No evidence was found that these differences biased the findings. Finally, using maximum likelihood estimation in statistical testing allowed for all collected data to be used in the analysis. Thus, data from all participants who started the intervention were used in the analysis.
3. Results
3.1. Cohort description
A total of 181 people were screened during a 31-month recruitment period. Out of those screened, 78 were ineligible. Reasons for ineligibility were: n = 7 did not have sufficient clinic records to indicate that they were HIV antibody seropositive, n = 36 were not being prescribed an antiretroviral medication for treatment of HIV/AIDS for at least one month, n = 9 were participating in other adherence-improvement research or clinical programs, n = 5 were living in a controlled environment that dispensed residents’ HIV medications and n = 21 were ineligible due to other reasons (i.e., too ill, incarcerated, psychiatric impairment). Among the 103 eligible clients, retention from eligibility to consent was 83%, with 86 people enrolling in the study and beginning the baseline phase. Of the 86 participants who began the baseline phase, 66 were randomized and began the intervention phase. Reasons for not being randomized are as follows: adherence greater than 80% (n = 8); illness (n = 1); not interested (n = 7); clinic discharge (n = 2); another adherence study (n = 1); physician recommendation (n = 1).
3.1.1. Subject attrition
Attrition from the study during the intervention and follow-up phases was minimal. For the analysis of the primary outcomes, 28 of 32 (87.5%) of the comparison condition and 32 of 34 (94%) of the voucher condition provided data. At the end of the follow-up phase, the respective retention percentages were 81% and 91%. Of participants who dropped out of the intervention and follow-up phases, one was discharged from the methadone maintenance program, four dropped out due to group assignment or time commitment, two discontinued HAART medications, and two died.
3.1.2. Participant characteristics
A total of 66 study participants were randomized to the two treatment conditions; comparison (n = 32) and voucher (n = 34). Table 1 displays summary statistics of the participants at study intake. The majority were heterosexual men and women who were unemployed and had limited income, with an average age of 43 years and taking an average dose of 80 mg of methadone when they entered the study. The mean BDI-II score at baseline was 19.7 (SD = 11.05), indicative of moderate depression, and 32 of 63 (51%) assessed participants met the DSM-IV criteria for lifetime major depressive episode on the C-DIS. The ASI composite scores, reflecting a general measure of patient status in specific domains of function, indicated highest scores (i.e. more problems) in the medical, employment, and psychiatric areas. The most frequent antiretroviral medications prescribed to participants included nelfinavir, lopinavir, lamivudine and zidovudine combination, and nevirapine. Participants from the two methadone clinics were compared on the background characteristics listed in Table 1 revealing no differences; consequently the clinics were combined in outcome analyses. A previously published report examined gender differences of the sample and found that women did not differ from men on measures of adherence (Haug et al., 2005).
Table 1.
Participant Characteristics by Group Assignment.
| Variable | % Voucher (n = 34) | % Comparison (n = 32) | % Total Sample (N = 66) |
|---|---|---|---|
| Gender | |||
| Men | 62 | 44 | 53 |
| Women | 35 | 47 | 41 |
| Transgendera | 3 | 9 | 6 |
| Ethnicity | |||
| Caucasian | 44 | 28 | 36 |
| African-American | 26 | 37 | 32 |
| Latino | 15 | 9 | 12 |
| Other/Mixed | 17 | 26 | 20 |
| Sexual orientation | |||
| Heterosexual | 71 | 69 | 70 |
| Homosexual | 12 | 12 | 12 |
| Bisexual | 17 | 19 | 18 |
| Marital status - Married | 24 | 3 | 14 |
| Education - high school or less | 65 | 69 | 67 |
| Employed (full or part-time) | 9 | 0 | 5 |
| Yearly income < $10,000 | 85 | 81 | 83 |
| Unstable living situationb | 35 | 41 | 38 |
| Opiate (positive urine screen) | 35 | 41 | 38 |
| Cocaine (positive urine screen) | 53 | 50 | 52 |
| Mean (SD)
|
Mean (SD)
|
Mean (SD)
|
|
| Age (years) | 44.0 (7.84) | 42.6 (7.29) | 43.3 (7.55) |
| Weight | 157.8 (30.27) | 176.1 (41.36) | 166.5 (37.10) |
| Methadone dose (mg.) | 85.4 (30.7) | 73.3 (34.14) | 79.8 (32.62) |
| ASI Medical Composite | 0.55 (0.32) | 0.56 (0.308) | 0.55 (0.312) |
| ASI Drug Composite | 0.21 (0.11) | 0.21 (0.105) | 0.21 (0.107) |
| ASI Psychiatric Composite | 0.45 (0.287) | 0.42 (0.271) | 0.44 (0.277) |
| ASI Employment Composite | 0.83 (0.244) | 0.92 (0.148) | 0.87 (0.207) |
| ASI Alcohol Use Composite | 0.09 (0.199) | 0.04 (0.104) | 0.07 (0.161) |
| ASI Legal Status Composite | 0.07 (0.143) | 0.06 (0.128) | 0.06 (0.135) |
| ASI Family/Social Relations | 0.10 (0.18) | 0.21 (0.219) | 0.15 (0.206) |
| Beck Depression Inventory | 21.6 (12.54) | 17.8 (8.99) | 19.7 (11.05) |
| SF-36 Physical Health Summary | 24.6 (1.2) | 24.3 (1.51) | 24.5 (1.36) |
| SF-36 Mental Health Summary | 22.1 (1.88) | 23.0 (1.93) | 22.5 (1.94) |
| Plasma HIV-1 RNA [copies] | 4209.9 (13416.05) | 22325.4 (90107.33) | 12984.6 (63569.14) |
| Log Plasma HIV-1 RNA [copies | 3.5 (3.81) | 4.31 (4.54) | 3.9 (4.2) |
| CD4+ (cells/UL) | 301.71 (197.51) | 298.8 (230.35) | 300.3 (211.81) |
| Number of HAART pills taken | 5.0 (4.02) | 4.0 (3.52) | 4.6 (3.83) |
Male-to-female
Self-reported as homeless or living in room/hotel/motel
3.2. Primary outcomes
Means of the primary outcomes by phase and treatment condition are presented in Table 2 and Figures 1–4. Baseline comparisons on the measures listed in Table 1 indicated that the only difference between groups was marital status: the comparison group had more never married than the intervention group (44% versus 29%, respectively, p < .05 ).
Table 2.
Means (and Standard Deviations) and Percentages of Primary Outcomes by Study Phase and Treatment Condition
| Study Phase | Baseline Phase Weeks 0–4) | Intervention Phase Weeks 5–16) | Follow-up Phase Weeks 17–20) | |||
|---|---|---|---|---|---|---|
| Voucher
|
Comparison
|
Voucher
|
Comparison
|
Voucher
|
Comparison
|
|
| MEMS On-time Openings (% adherence, SD) | 50.1 (17.21) | 51.9 (19.99) | 77.6 (17.49) | 55.5 (23.09) | 66.0 (23.91) | 53.1 (29.22) |
| MEMS Longest Days Continuous On-time Openings (M, SD) | 3.6 (3.25) | 4.4 (2.68) | 21.1 (16.74) | 8.9 (10.07) | 7.3 (5.03) | 5.2 (4.80) |
| Pill count (% adherence, SD) | 70.5 (20.92) | 79.1 (1879) | 85.9 (11.42) | 75.4 (21.30) | 80.9 (18.31) | 78.4 (28.35) |
| Self-Report (% adherence, SD) | 75.0 (22.40) | 75.9 (24.97) | 87.3 (13.98) | 68.7 (17.73) | 80.6 (21.16) | 71.6 (25.54) |
| Plasma HIV-1 RNA (copies) (M, [Median]) | 8885.0 [57] | 21063.3 [0] | 6880.4 [0] | 5549.6 [0] | 15932.6 [0] | 2908.0 [0] |
| CD4+ (cells/UL) (M, SD) | 301.7 (197.51) | 298.8 (330.35) | 302.4 (194.29) | 314.0 (164.89) | 323.6 (216.20) | 360.5 (195.13) |
| SF-36 Physical Health (M, SD) | 36.1 (9.46) | 35.6 (11.02) | 39.7 (8.69) | 35.2 (8.09) | 37.2 (10.01) | 35.3 (9.54) |
| Opioid (% positive) | 47 | 47 | 42 | 36 | 50 | 37 |
| Cocaine (% positive) | 50 | 63 | 55 | 61 | 50 | 59 |
Figure 1.
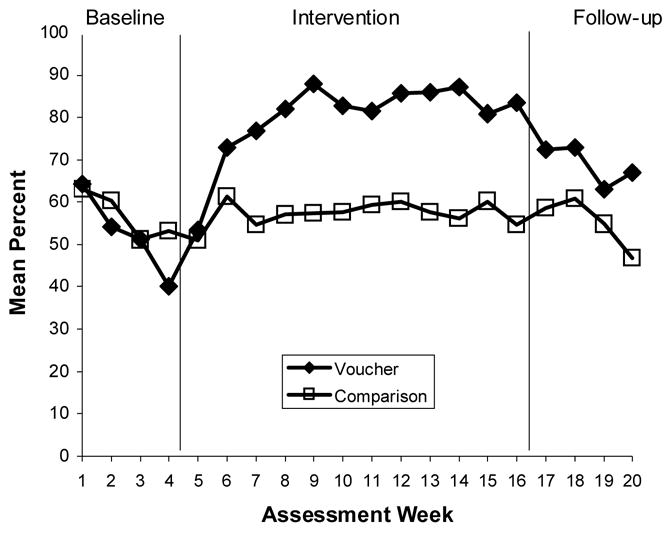
Medication Adherence: Weekly On-Time Medication-Cap Openings
Figure 4.
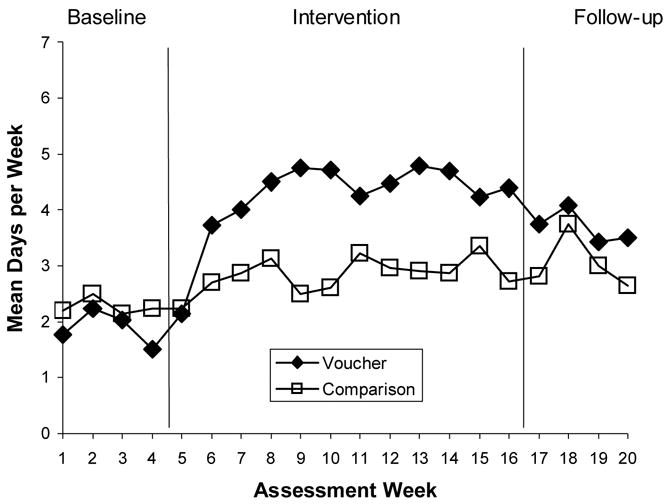
Medication Adherence: Longest Days of Continuous Medication-Cap Openings by Phase
3.2.1. Medication adherence
Results indicated clear improvement in medication adherence in the voucher group from baseline phase through the intervention phase. The mean percent of MEMS-cap adherence did not differ in the baseline period, (50% in voucher participants, 52% in comparison participants). During the intervention period the voucher participants averaged 78% MEMS-cap adherence, while the comparison participants showed 56%, p < .0001. Significant intervention condition-by-assessment interaction effects were found for all four of the adherence measures; MEMS-cap adherence (F(2, 187) = 5.11, p = .0069), pill count adherence (F(2, 190) = 4.06, p = .0187), self-reported adherence (F(2, 192) = 3.43, p = .0345), and MEMS cap longest days of continuous adherence (F(2, 179) = 9.34, p = .0001). As Figure 1 illustrates, adherence declined during the 4-week baseline. During the intervention phase (Weeks 5–16), the voucher participants quickly exceeded the comparison condition in their on-time MEMS-cap openings, and they continued through the 12-week intervention. During the follow-up phase, the voucher reinforcement was discontinued, and the groups converged again. During the follow-up period, the voucher participants averaged 66% on-time openings, which represented considerable improvement over the baseline phase, but was not significantly different from the non-voucher participants 53%. (p = .07).
Voucher participants could have earned $1172.40 if they displayed a perfect record of MEMS cap openings over 12 weeks. One participant achieved this perfect record, and the mean earning for the voucher group was $378.47 (31% of possible earnings).
3.2.2. Health indices
No significant effects for condition or change over time were seen in the three measures of health: HIV-1 RNA levels, CD4 lymphocyte count, SF-36 Physical Component summary scale, or for weight at the end of the study. 3.2.3. Drug use. Similarly, no significant differences between groups were found in the results of the urine toxicology screens for opiate or cocaine use, with anywhere from 32% to 47% of participants testing positive for opiates and 47% to 63% testing positive for cocaine at any one of the six assessment points where urine samples were tested.
3.3. Secondary analyses
3.3.1. Side effects
No significant differences were observed for the summary side effects measure. On examination of the residuals from the statistical model, one participant had markedly greater residual values than the others. After confirming that the entered data matched the original instrument (consistently high side effects were reported at all assessments), we re-estimated the model for this variable without that case. The p-value for the effect measuring change over time went from p = .06 to p = .02 indicating a significant decrease in reported side effects for both conditions. The most commonly reported side effects by participants were: fatigue/loss of energy (60%), pain, numbness, tingling (52%), muscle/joint aches (50%), fever, chills, sweat (49%), bloating/gas (48%), and loss of appetite (47%). The other two effects of treatment condition and the interaction in the model remained non-significant.
3.3.2. Correlations
Viral load was significantly associated with the percentage of medication-cap openings at Week 4 (r = −0.27, p = .03), Week 8 (r = −0.43, p = .0006) and Week 20 (r = −0.37, p = .005). At Week 16 the correlation was negative (r = −.26) but not statistically significant (p = .0518). The negative sign indicates that the greater the level of MEMS adherence, the lower the viral load. No statistically significant similar relationships were found with the CD4 count or the SF-36 Physical Health summary scale.
4. Discussion
This study assessed whether participation in voucher reinforcement was associated with improved HIV medication adherence in methadone maintenance patients. Relative to the comparison group, participants randomly assigned to receive voucher reinforcement showed better medication adherence, measured by MEMS cap, pill count, and self-report. Differences faded after the voucher contingencies were removed. Viral load was significantly and negatively associated with the percentage of on-time MEMS cap openings, reflecting a positive/desired change in viral load associated with medication adherence, although relative improvement did not appear in biological and behavioral outcome measures of health. The results provide preliminary support for the development of voucher reinforcement as a strategy to improve medication adherence among HIV-positive methadone maintenance patients. Voucher reinforcement was associated with a difference in opening of medication caps from 56% to 78%. Thus this study builds upon randomized trials of voucher reinforcement aimed at decreasing drug use (Jones et al., 2001; Silverman et al., 1996a; Silverman et al., 1996b; Silverman et al., 2004) as well as the application of behavioral approaches to improve adherence to HIV medication regimens (Rigsby et al., 2000). Specifically, this study extends these findings, indicating that voucher reinforcement can be applied successfully to reinforcing adherence to medications in HIV-positive methadone maintenance patients and providing a clinical trial in addition to Rigsby et al. (2000) indicating the utility of contingency management in promoting adherence to HAART medications.
In the present study the adherence differences faded after the voucher contingencies were removed. In short, the voucher intervention did not build long-lasting adherence habits that endured without reinforcement, and the results may speak to the desirability of a longer intervention. Recently Silverman et al. (2004) demonstrated that reinforcing contingencies can retain their effectiveness over periods as long as a year if continuously applied. The contingencies used in the present study may have greater effectiveness in a longer trial and more opportunity to improve participants’ health. Furthermore, this kind of intervention could be applied to address medication adherence issues among patients with diseases such as tuberculosis or hepatitis C, which involve time-limited medication regimens.
The improvements in medication adherence were not sufficient to produce changes in health. The lack of differences may reflect a limitation of the intervention, in that improved adherence was still not at the 95% level that may be necessary for the medications to suppress viral load and raise CD4 counts. Also, the intervention phase lasted only 12 weeks, the sample size of the trial was relatively small, and a larger, multi-site trial might uncover significant differences in measures of health.
Urine drug screens to detect use of opiates or cocaine also indicated no differences between intervention conditions. This result is not surprising, since the voucher incentives reinforced medication cap openings rather than abstinence from illicit drugs. Future research could devise more complex reinforcement schedules to address both medication-taking and drug use.
The study has several limitations. First, rigorous initial exclusion criteria may limit the generalizability of the results. Second, after the baseline period, 8 participants were excluded due to high adherence. The debriefing of these participants included an explanation that they were excluded because of high medication adherence. We had no indication that under-reporting of adherence or deliberate non-adherence actually occurred so that patients could be eligible for the clinical trial phase of the study. Because the study involved only HIV-positive patients, it is our impression that patients did not talk about the study among each other the way they might in other types of clinical trials because they did not want other patients to know their HIV status. Nonetheless, this is a possible source of contamination, in that other clinic participants might have understood that to stay in the trial they would need to show poor adherence during baseline. Third, we note that use of the MEMS cap was necessary to reinforce medication cap opening but was awkward for many patients, who preferred use of pill boxes or “medisets” that put their medications in compartments to be taken at times indicated. As noted by Samet et al. (2001), a computerized mediset would be a useful addition to the adherence-measurement armamentarium. Further, the study did not observe actual medication-taking, but rather measured the opening of medication bottles.
For practitioners considering applications of this work, perhaps more flexible procedures could be instituted. In particular, 36 potential participants were screened out because they had not been on antiretroviral medications for a month before the trial began. In fact, patients new to HIV medications may be ideal for mounting clinical adherence interventions. The addition of a fishbowl lottery procedure to promote retention in the comparison condition may have attenuated differences between the voucher and comparison interventions, and in future work the procedure might be used with voucher participants as well. It would also be useful to align the measurement of side effects to capture more of the time period under study, perhaps by asking about side effects twice weekly rather than capturing only a three-day window before a weekly interview.
The level of the reinforcement was guided by what had been accomplished in the work of studies that were reinforcing abstinence from substance use rather than medication adherence. In the present study the number of consecutive events needed to reinstate pre-reset reinforcement levels was much higher than in the studies on which we based the reinforcement procedures, which may have limited the efficacy of the intervention in the present study. Future research would benefit by testing reinforcement ratios, procedures, and sizes that are designed specifically for medication adherence.
We also note that there are broader solutions to non-adherence, such as educating patients about treatment, working with patients to improve practical life supports, treating co-occurring psychiatric disorders that prevent optimal adherence to medications, and simplifying medication regimens (see Gathe, 2003).
This study successfully applied the voucher reinforcement approach to adherence to medications for HIV/AIDS. Voucher participants improved their adherence relative to a medication-coaching comparison group. The intervention had limited efficacy in that it did not improve physical health of participants. Future studies may explore a longer-term and more comprehensive intervention, higher cash values for vouchers, and larger sample sizes. There were clear limitations that include a 12-week intervention period (HIV medications are taken for much longer) and focusing on adherence to a single HIV medication (while HIV/AIDS characteristically requires multiple medications). It is possible that adherence levels could be improved to the levels needed to show improvement on immunologic and virologic markers if vouchers had higher reinforcement values or better delivery systems. The authors note, however, that methadone patients with HIV/AIDS have significant psychosocial problems that clearly interfere with adhering to medications, including psychiatric difficulties and homelessness, so contingency management procedures will need to be combined with additional services to have a lasting effect on management of HIV/AIDS. This initial trial of voucher-based contingency management demonstrates the clear positive impact of this intervention, demonstrating proof of concept.
Figure 2.
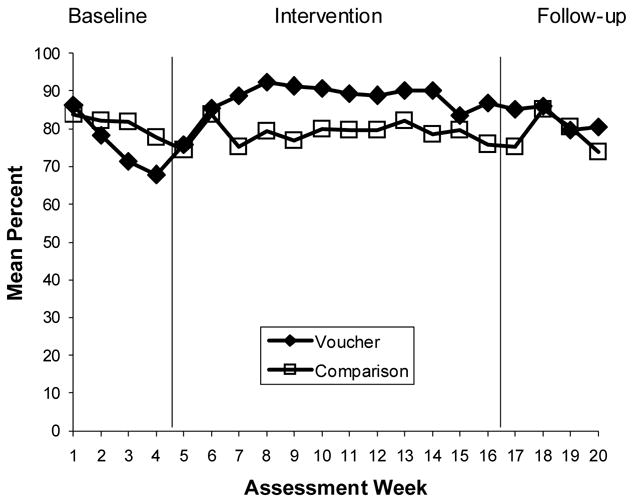
Medication Adherence: Pill Counts
Figure 3.
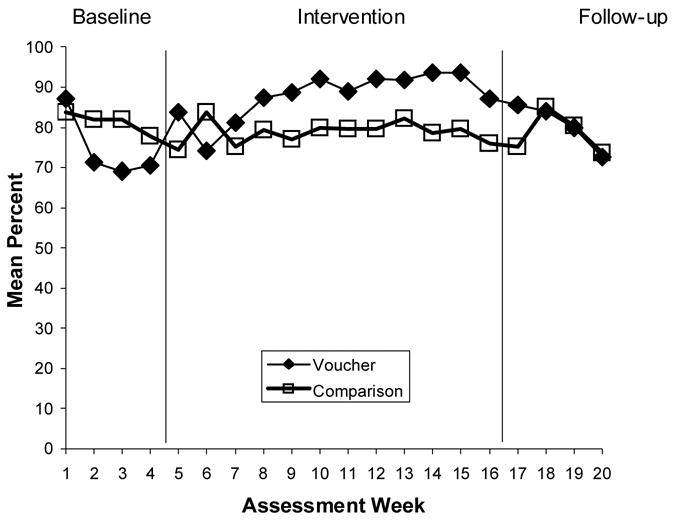
Medication Adherence: Self-Reported Adherence
Acknowledgments
This project was supported by NIH Research Grants, primarily P50DA09253 (San Francisco Treatment Research Center) as well as U10DA15815, K0516752, and R01DA11344 where the medication coaching intervention was developed. The authors express gratitude to the staff and patients of the Opiate Treatment Outpatient Program (Division of Substance Abuse and Addiction Medicine, San Francisco General Hospital) and the Market Street Clinic of the Bay Area Addiction Research and Treatment Programs. We are grateful for the participation Anne Park, Anna Veluz, Jennifer Reeve, Kevin Ahern, Nicole Lollo, Wynnie Wong, Mary Ann Hauf, Robin Sera, Gregory Roth, and TeChieh Chen in carrying out the study.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4. Washington, DC: American Psychiatric Association; 1994. [Google Scholar]
- Arnsten JH, Demas PA, Farzadegan H, Grant RW, Gourevitch MN, Chang C, Buono D, Eckholdt H, Howard AA, Schoenbaum EE. Antiretroviral therapy adherence in HIV-infected drug users: Comparison of self-report and electronic monitoring. Clin Infect Dis. 2001;33:1417–1423. doi: 10.1086/323201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnsten JH, Demas PA, Grant RW, Gourevitch MN, Farzadegan H, Howard AA, Schoenbaum Impact of active drug use on antiretroviral therapy adherence and viral suppression in HIV-infected drug users. JGIM. 2002;17:377–381. doi: 10.1046/j.1525-1497.2002.10644.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bangsberg DR, Perry S, Charlebois ED, Clark RA, Roberston M, Zolopa AR, Moss A. Non-adherence to highly active antiretroviral therapy predicts progression to AIDS. AIDS. 2001;15:1181–1183. doi: 10.1097/00002030-200106150-00015. [DOI] [PubMed] [Google Scholar]
- Bangsberg DR, Bronstone A, Hofman R. A computer-based assessment detects regimen misunderstandings and nonadherence for patients on HIV antiretroviral therapy. AIDS Care. 2002;14(1):3–15. doi: 10.1080/09540120220097892. [DOI] [PubMed] [Google Scholar]
- Batki SL, Ferrando SJ. Diagnosis and treatment of substance use disorders in patients with HIV infection. International Review of Psychiatry. 1996;8(2–3):242–252. [Google Scholar]
- Beck AT, Steer RA, Brown GK. BDI-II manual. The Psychological Corporation; San Antonio: 1996. [Google Scholar]
- Bickel WK, Rizzuto P, Zielony RD, Klobas JPangiosonlis P, Merit R, Knight WF. Combined behavioral and pharmacological treatment of alcoholic methadone patients. J Subst Abuse. 1988–1989;1(2):161–171. doi: 10.1016/s0899-3289(88)80019-1. [DOI] [PubMed] [Google Scholar]
- Calsyn DA, Wells EA, Saxon AJ, Jackson TR, Wrede AF, Stanton V, Fleming C. Contingency management of urinalysis results and intensity of counseling services have an interactive impact on methadone maintenance treatment outcome. J Addict Dis. 1994;13:47–63. doi: 10.1300/j069v13n03_05. [DOI] [PubMed] [Google Scholar]
- Carroll KM, Ball SA, Nich C, O’Connor PG, Eagan DA, Frankforter TL, Triffleman EG, Shi J, Rounsaville BJ. Targeting behavioral therapies to enhance naltrexone treatment of opioid dependence. Arch Gen Psychiatry. 2001;58:755–761. doi: 10.1001/archpsyc.58.8.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll KM, Sinha R, Nich C, Babuscio T, Rounsaville BJ. Contingency management to enhance naltrexone treatment of opioid dependence: A randomized clinical trial of reinforcement magnitude. Exp Clin Psychopharmacol. 2002;10(1):54–63. doi: 10.1037//1064-1297.10.1.54. [DOI] [PubMed] [Google Scholar]
- Chesney M. Adherence to HAART regimens. AIDS Patient Care and STDS. 2003;17:169–177. doi: 10.1089/108729103321619773. [DOI] [PubMed] [Google Scholar]
- Chesney MA, Ickovics JR, Chamber DB, Gifford AL, Neidig J, Zwickl B, Wu AW. Self-reported adherence to antiretroviral medications among participants in HIV clinical trials: the AACTG Adherence Instruments. AIDS Care. 2000;12:255–266. doi: 10.1080/09540120050042891. [DOI] [PubMed] [Google Scholar]
- Elk R, Grabowski J, Rhoades H, Spiga R, Schmitz J, Jennings W. Compliance with tuberculosis treatment in methadone-maintained patients: Behavioral interventions. J Subst Abuse Treat. 1993;10:371–382. doi: 10.1016/0740-5472(93)90022-t. [DOI] [PubMed] [Google Scholar]
- Elk R, Schmitz J, Spiga R, Rhoades H, Andres R, Grabowski J. Behavioral treatment of cocaine-dependent pregnant women and TB-exposed patients. Addict Behav. 1995;20:533–542. doi: 10.1016/0306-4603(94)00076-b. [DOI] [PubMed] [Google Scholar]
- Fogarty L, Rotter D, Larson S, Burke J, Gillespie J, Levy R. Patient adherence to HIV medication regimens: A review of published and abstract reports. Patient Education and Counseling. 2002;42:93–108. doi: 10.1016/s0738-3991(01)00219-1. [DOI] [PubMed] [Google Scholar]
- Freeman RC, Rodriguez GM, French JF. Compliance with AZT treatment regimen of HIV-seropositive injection drug users: A neglected issue. AIDS Educ Prev. 1996;8:58–71. [PubMed] [Google Scholar]
- Gathe J. Adherence and potency with antiretroviral therapy: A combination for success. AIDS. 2003;34(s2):S118–S122. doi: 10.1097/00126334-200310012-00004. [DOI] [PubMed] [Google Scholar]
- Gebo KA, Keruly J, Moore RD. Association of social stress, illicit drug use, and health beliefs with nonadherence to antiretroviral therapy. JGIM. 2003:18,104–111. doi: 10.1046/j.1525-1497.2003.10801.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golin CE, Liu H, Hays RD. A prospective study of predictors of adherence to combination antiretroviral medication. JGIM. 2002;17:756–765. doi: 10.1046/j.1525-1497.2002.11214.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall SM, Cooper JL, Burmaster S, Polk A. Contingency contracting as a therapeutic tool with methadone maintenance clients: six single subject studies. Behav Res Ther. 1977;15:438–441. doi: 10.1016/0005-7967(77)90049-3. [DOI] [PubMed] [Google Scholar]
- Haug NA, Sorensen JL, Gruber VA, Lollo N, Roth G. Medication adherence strategies for HIV-positive methadone clients: A treatment manual for implementing voucher incentives and medication coaching. Behav Modif. doi: 10.1177/0145445506288229. in press. [DOI] [PubMed] [Google Scholar]
- Haug NA, Sorensen JL, Lollo ND, Gruber VA, Delucchi KL, Hall SM. Gender differences among HIV-positive methadone maintenance patients enrolled in a medication adherence trial. AIDS Care. 2005;17:1022–1029. doi: 10.1080/09540120500100882. [DOI] [PubMed] [Google Scholar]
- Higgins ST, Stitzer ML, Bigelow GE, Liebson IA. Contingent methadone delivery: Effects on illicit-opiate use. Drug Alcohol Depend. 1986;17(4):311–322. doi: 10.1016/0376-8716(86)90080-3. [DOI] [PubMed] [Google Scholar]
- Jones HE, Haug N, Silverman K, Stitzer M, Svikis D. The effectiveness of incentives in enhancing treatment attendance and drug abstinence in methadone-maintained pregnant women. Drug Alcohol Depend. 2001;61(3):297–306. doi: 10.1016/s0376-8716(00)00152-6. [DOI] [PubMed] [Google Scholar]
- Kenward MG, Roger JH. Small sample inference for fixed effects from restricted maximum likelihood. Biometrics. 1997;53:983–997. [PubMed] [Google Scholar]
- Low-Beer S, Yip B, O’Shaughnessy MV, Hogg RS, Montaner JS. Adherence to triple therapy and viral load response. Journal of Acquired Immune Deficiency Syndrome. 2000;23:360–361. doi: 10.1097/00126334-200004010-00016. [DOI] [PubMed] [Google Scholar]
- McLellan AT, Kushner H, Metzger D, Peters R, Smith I, Grissom G, Pettinati H, Argerious M. The fifth edition of the Addiction Severity Index. J Subst Abuse Treat. 1992;9:199–213. doi: 10.1016/0740-5472(92)90062-s. [DOI] [PubMed] [Google Scholar]
- McNabb JJ, Nicolau DP, Stoner JA, Ross J. Patterns of adherence to antiretroviral medications: The value of electronic monitoring. AIDS. 2003;17(12):1763–1767. doi: 10.1097/00002030-200308150-00005. [DOI] [PubMed] [Google Scholar]
- Paterson DL, Swindells S, Mohr J, Brester M, Vergis EN, Squier C, Wagner MM, Singh N. Adherence to protease inhibitor therapy and outcomes in patients with HIV infection. Ann Intern Med. 2001;133:21–30. doi: 10.7326/0003-4819-133-1-200007040-00004. [DOI] [PubMed] [Google Scholar]
- Petry NM, Martin B. Low-cost contingency management for treating cocaine- and opioid-abusing methadone patients. J Consult Clin Psychol. 2002;70(2):398–405. doi: 10.1037//0022-006x.70.2.398. [DOI] [PubMed] [Google Scholar]
- Preston KL, Silverman K, Umbricht A, DeJesus A, Montoya I, Schuster C. Improvement in naltrexone compliance with contingency management. Drug Alcohol Depend. 1999;54:127–135. doi: 10.1016/s0376-8716(98)00152-5. [DOI] [PubMed] [Google Scholar]
- Rigsby MO, Rosen MI, Beauvais JE, Cramer JA, Rainey PM, O’Malley SS, Dieckhause KD, Rounsaville BJ. Cue-dose training with monetary reinforcement: Pilot study of an antiretroviral adherence intervention. JGIM. 2000;15:841–847. doi: 10.1046/j.1525-1497.2000.00127.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robins L, Cottler L, Bucholz K, Compton W. Department of Psychiatry. Washington University School of Medicine; St. Louis: 1995. Diagnostic Interview Schedule for DSM-IV (DIS-IV) [Google Scholar]
- Samet JH, Sullivan LM, Traphagen ET, Ickovics JR. Measuring adherence among HIV-infected persons: Is MEMS consummate technology? AIDS and Behavior. 2001;5:21–30. [Google Scholar]
- Shikuma CM, Zackin R, Sattler F, Mildvan D, Nyangweso P, Alston B, Evans S, Mulligan K the AIDS Clinical Trial Group 892 Team. Changes in weight and lean body mass during highly active antiretroviral therapy. Clin Infect Dis. 2004;39(8):1223–1230. doi: 10.1086/424665. [DOI] [PubMed] [Google Scholar]
- Silverman K, Higgins ST, Brooner RK, Montoya ID, Cone EJ, Schuster CR, Preston KL. Sustained cocaine abstinence in methadone maintenance patients through voucher-based reinforcement therapy. Arch Gen Psychiatry. 1996a;53:409–415. doi: 10.1001/archpsyc.1996.01830050045007. [DOI] [PubMed] [Google Scholar]
- Silverman K, Robles E, Mudric T, Bigelow G, Stitzer M. A randomized trial of long-term reinforcement of cocaine abstinence in methadone-maintained patients who inject drugs. J Consult Clin Psychol. 2004;72:839–854. doi: 10.1037/0022-006X.72.5.839. [DOI] [PubMed] [Google Scholar]
- Silverman K, Wong CJ, Higgins ST, Brooner RK, Montoya ID, Contoreggi C, Umbricht-Schneiter A, Schuster CR, Preston KL. Increasing opiate abstinence through voucher-based reinforcement therapy. Drug Alcohol Depend. 1996b;41:157–165. doi: 10.1016/0376-8716(96)01246-x. [DOI] [PubMed] [Google Scholar]
- Simoni JM, Pantalone DW, Firck PA, Turner BJ. Enhancing antiretroviraladherence: A review of published reports of randomized controlled trials and on-going NIH-funded research. In: Trafton J, Gordon W, editors. Best practices in the behavioral management of chronic diseases. II. Institute for Brain Potential; Los Altos: 2005. pp. 70–95. [Google Scholar]
- Tooze JA, Grunwald GK, Jones RH. Analysis of repeated measures data with clumping at zero. Statistical Methods in Med Res. 2002;11(4):341–355. doi: 10.1191/0962280202sm291ra. [DOI] [PubMed] [Google Scholar]
- Veluz AK, Gruber V, Park AM, Lin C, Ramos L, Sorensen JL. Contingency management: Using a fish-bowl lottery to increase research visit attendance. Poster presented at the 131st Annual Meeting of the American Public Health Association; San Francisco, CA. 2003. [Google Scholar]
- Ware JE. The MOS 36-Item Short-Form Health Survey (SF-36) In: Sederer LI, Dickey B, editors. Outcomes Assessment in Clinical Practice. Williams and Wilkins; Baltimore: 1996. pp. 61–64. [Google Scholar]


