Abstract
Standard light microscope histological evaluation of peripheral nerve lesions has been used routinely to assess peripheral nerve demyelination; however, the development of magnetic resonance (MR) methodology for assessing peripheral nerve may provide complimentary information, with less expense and in less time than nerve histology methods. In this study, the utility of multicomponent NMR T2 relaxation analysis for assessing myelin injury in toxicology studies was examined using two dithiocarbamates, N,N-diethyldithiocarbamate (DEDC) and pyrrolidine dithiocarbamate (PDTC), known to produce myelin injury and elevate copper in the nervous system. T2 analysis was used in conjunction with standard histological methods to assess myelin injury and determine if dithiocarbamate-mediated copper accumulation in peripheral nerve was associated with more severe myelin lesions. Male Sprague-Dawley rats were administered i.p. DEDC for 8 weeks and maintained on either a diet containing normal (13 ppm) or elevated (200 ppm) copper. Another group of male Sprague-Dawley rats was administered oral PDTC and a 200 ppm copper diet, with controls given only the 200 ppm copper diet, for 47 weeks. Following exposures, the morphology of sciatic nerve was evaluated using light microscopy and multicomponent T2 analysis of excised fixed nerves; and copper levels in sciatic nerve were determined using ICP-AES. Light microscopy demonstrated the presence of a primary myelinopathy in dithiocarbamate-exposed rats characterized by intramyelinic edema, demyelination, and secondary axonal degeneration. Both the nerve copper level and number of degenerated axons, as ascertained by ICP-AES and microscopy respectively, were augmented by dietary copper supplementation in conjunction with administration of DEDC or PDTC. T2 analysis revealed a decreased contribution from the shortest T2 component in multicomponent T2 spectra obtained from animals administered DEDC or PDTC, consistent with decreased myelin content; and the decrease of the myelin water component was inversely correlated to the levels of nerve copper and myelin lesion counts. Also, the T2 analysis showed reduced variability compared to histological assessment. These studies support multicomponent T2 analysis as a complementary method to light microscopic evaluations that may also be applicable to in vivo assessments.
Keywords: Dithiocarbamates, copper, neurotoxicity, demyelination, multicomponent T2 analysis
Introduction
Microscopic examination of peripheral nerve is the predominant method used to characterize structural changes produced in neurotoxicity studies. Various methods of morphometry have been used including automated morphometry and manual quantitation (Campadelli et al., 1999; Guena et al., 2001; Kennedy et al., 2004; Santoro et al., 1992). Both microscopic methods are valuable tools, but have limitations. The strength of automated analysis derives from quantifying the characteristics of normal structures, e.g., axon diameter, compact myelin, and axon density and has the advantage of overcoming observer error and nerve profile bias inherent in manual methods. On the other hand, one study reported an increase in false positives and missed detections using automatic morphometry software as compared to manual measurement (Romero et al., 2000). Automated morphometry is less amenable, however, for identifying and quantifying some specific lesions, e.g. intramyelinic edema. Both morphometry methods also involve histological preparation of nerve, which requires more expertise and time compared to most other tissues, in addition to the time required to review or quantitatively analyze the sections. Microscopic methods using plastic embedded thick sections (1μm) also typically evaluate a relatively small volume of peripheral nerve.
The development of MR methods to augment the study of nervous system disease has increased the number of modalities available for assessment of peripheral nerve pathology. Quantitative MR methods, including magnetization transfer, diffusion anisotropy, and T1 and T2 relaxation times have been explored to determine whether their changes correlate with changes in the microstructure of peripheral nerve. Although changes have been observed using all of these MR methods following transection or crush injury models, T2 relaxation times have arguably demonstrated the greatest potential for this application. The transverse decay of water proton magnetization in nerve containing myelinated axons has been shown to be multiexponential with at least three components (Vasilescu et al., 1978). The individual T2 components have been interpreted in terms of different water compartments that are separated by diffusion barriers resulting in relatively slow exchange rates on the MR time scale. The current prevailing interpretation of these components assigns the short, intermediate and long T2 components to myelin, intra-axonal and extra-axonal water, respectively (Menon et al., 1992), although some studies have suggested opposite assignment of the intermediate and long T2 components (Peled et al., 1999). Changes in the properties of these components have been most extensively evaluated in sciatic nerve undergoing demyelination following crush or transection injury using either excised unfixed nerve or in vivo imaging (Does and Snyder, 1996), and correlate well with quantitative microscopic assessment of myelin (Bendszus et al., 2004; Webb et al., 2003). The most consistent change observed is a decreased contribution from water in the short T2 component, which has been interpreted to represent a decrease in myelin content. The results of these studies suggest that although the interpretation of T2 spectral changes has not evolved to the level that specific lesions can be quantified, T2 analysis may provide an index of myelin content in many toxic peripheral neuropathies.
The use of dithiocarbamates as pesticides, in industrial applications and in medicine has resulted in human exposures through inhalation, ingestion and dermal exposure (Alexeeff et al., 1994; Berry et al., 1990; Brewer, 1993; Eneanya et al., 1981; Kreutzer et al., 1994; Lang et al., 1988; Vettorazzi et al., 1995; WHO, 1988). In particular, human exposure to the dithiocarbamate disulfide, disulfiram (Antabuse®), used in alcohol aversion therapy has resulted in reports of a neuropathy dating back at least half a century (Child et al., 1951). Two types of neuropathies due to dithiocarbamate exposure have been identified: an axonopathy and a myelinopathy. The type of neuropathy manifested depends upon the route of exposure and acid lability of the dithiocarbamate. Whereas the dithiocarbamate axonopathy appears to be mediated through a CS2 metabolite (Johnson et al., 1998; Valentine et al., 1995), the mechanism of myelin injury induced by the parent compound dithiocarbamate remains to be determined. Dithiocarbamates reported to produce demyelination in animal models include disulfiram (Tonkin et al., 2000), diethyldithiocarbamate (DEDC) (Edington and Howell, 1969; Tonkin et al., 2004), and pyrrolidine dithiocarbamate (PDTC) (Valentine et al., 2006).
Previous animal studies have also demonstrated the ability of demyelinating dithiocarbamates to increase copper levels and lipid peroxidation products in peripheral nerve (Calviello et al., 2005; Delmaestro and Trombetta, 1995; Tonkin et al., 2004). This suggests that dithiocarbamate-mediated demyelination may result from dithiocarbamate-mediated copper accumulation in peripheral nerve accompanied by copper promoted oxidative stress and lipid peroxidation within the membranes of myelin. Previous in vitro studies using primary rat astrocytes or thymocytes have associated intracellular transport of copper and enhanced oxidative stress to be accompanied by increased cytotoxicity (Chen et al., 2000; Orrenius et al., 1996; Wilson and Trombetta, 1999). In addition, aberrant copper regulation has also been proposed as a contributing mechanism in the development of certain neurodegenerative diseases including Alzheimer’s disease, amylotrophic lateral sclerosis, Wilson’s disease, Menke’s disease, Parkinson’s disease and prion diseases (Brown and Sassoon, 2002; Multhaup et al., 2002; Rotilio et al., 2002; Strausak et al., 2001).
Two studies involving the administration of neurotoxic dithiocarbamates are presented in this paper. In the first study, rats were administered DEDC by i.p. osmotic pumps and fed either a normal diet or diet containing elevated copper. In the second study, rats were either chronically dosed with PDTC in the drinking water and fed an elevated copper diet or given an elevated copper diet alone. Following the exposures, the severity of sciatic nerve lesions was assessed by light microscopy and multicomponent T2 analysis. The results obtained from both methods were compared to determine if similar conclusions could be drawn and statistical comparisons performed to evaluate the relative power of each method to discriminate between treatment groups. To determine if peripheral nerve copper accumulation was correlated with the production of peripheral nerve lesions, copper levels in sciatic nerve were determined by inductively coupled plasma-atomic emission spectroscopy (ICP-AES) analysis.
Materials and Methods
Chemicals
Glutaraldehyde was obtained from Electron Microscopy Sciences (Ft. Washington, PA). Laboratory rodent diet (Purina Laboratory Diet, 5001) containing 13 ppm copper and the same diet supplemented to 200 ppm copper using copper sulfate were obtained from Purina Mills (Richmond, IN). Pyrrolidine was purchased from Alfa Aesar (Ward Hill, PA). 2ML4 Alzet® osmotic pumps were obtained from Durect Corporation (Cupertino, CA). Sodium N,N-Diethyldithiocarbamate (DEDC) was obtained from Alfa Aesar (Ward Hill, MA).
Chemical Synthesis
Sodium pyrrolidine dithiocarbamate (PDTC) was synthesized as previously described (Valentine et al., 2006). The identity and purity (> 99%) of pyrrolidine dithiocarbamate was verified by NMR spectroscopy and by UV Spectrophotometry.
Animals and Exposures
All exposures were conducted in accordance with the National Institutes of Health Guide for Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committee. Male Sprague-Dawley rats obtained from Harlan Bioproducts (Indianapolis, IN) were given food and water ad libitum and caged in a room on a diurnal light cycle. Body weights were determined prior to the start of the study and then weekly during the course of the experiments.
For DEDC exposures, three groups were used: two DEDC-exposed groups receiving 0.35 mmol/kg/d for 8 weeks and one control group. The average weight of the animals at the time of the initial surgery was 300 ± 5 g. The control group (n = 4) and one of the DEDC groups (n = 4) were fed normal rodent diet containing 13 ppm copper, while the other DEDC group (n = 5) was fed rodent diet containing 200 ppm copper. The 200 ppm copper diet was chosen for copper loading after literature review indicated no significant clinical, chemical, histopathological or hematological toxicity at this level when chronically administered (Cristofori et al., 1992; Everling et al., 1990; Fuentealba et al., 2000; Milanino et al., 2000), while still affording a 15 fold increase in dietary copper. Animals were exposed to DEDC via 2ML4 Alzet® osmotic pumps surgically implanted in the abdomen under anesthesia (90 mg/kg ketamine HCl with 7.5 mg/kg xylazine i.p.). After 4 weeks, the pumps were replaced by the same procedure to extend the exposure period to 8 weeks. DEDC was delivered as an aqueous solution of NaDEDC, the concentration of which was determined from the UV absorbance at 282 nm (ε = 13,000 M−1cm−1) prior to filling the pumps. Control pumps contained deionized water. Solutions were sterilized prior to filling the pumps by filtering through a 0.22 μm-syringe filter. At the end of the exposure period, animals were perfused (see next section) and sciatic nerve, brain and liver tissues were collected. The sciatic nerves were held in 0.1M phosphate buffer (pH 7.4) at 4°C for 2 years prior to MR analysis.
Additional animals were exposed to PDTC by delivery in the drinking water. A control group (n=3), given tap water ad libitum and a PDTC-exposed group (n=3) were given Purina rodent lab diet 5001 containing 200 ppm copper sulfate. This group of animals was included because a previous study in this laboratory had shown PDTC with elevated copper produced the most severe myelin changes in any of the animal models we have used. The average starting weight of all the animals was 401 ± 6g. PDTC was solubilized in deionized water; and the concentration was determined from the UV absorbance at 277 nm (ε = 13,540 M−1cm−1). The dosing of PDTC in the drinking water was accomplished gradually to avoid rejection of the solution by increasing the concentration from 4 mM to 8 mM over a period of 21 days. A concentration of PDTC above 8mM was unacceptable to the animals, thus making 8mM the maximum tolerated oral dose. The average dose of PDTC taken in the water was determined by measuring water volume intake every 2 days per group over 10 days beginning at week 16. An average of 11.0 ml of 8 mM PDTC/100 g BW was obtained on rats given PDTC eating a 200 ppm copper diet. This was calculated as a dose of 0.88 mmol/kg/day. At 47 weeks, 2 of the 3 animals in the PDTC group had significant body weight loss and paresis of their hind limbs necessitating euthanasia of all PDTC exposed and control animals. All animals were deeply anesthetized (100 mg/Kg pentobarbital i.p.) and exsanguinated by cardiac puncture. Sciatic nerves were removed after dissection and placed into 4% glutaraldehyde in 0.1M phosphate buffer, pH 7.4 for tissue copper analysis and morphologic examination. The sciatic nerves were then transferred to 0.1 M phosphate buffer (pH 7.4) after 48 hours in glutaraldehyde; and MR measurements were made on the sciatic nerves after 2 weeks storage in phosphate buffer at 4°C.
Preparation of Tissue for Morphological Assessment
Animals dosed with DEDC for 8 weeks were perfused through the left ventricle of the heart under deep anesthesia (100 mg/kg ketamine HCL plus 15 mg/kg xylazine i.p.) with a solution of 0.8% NaCl, 0.025% KCl, 0.05% NaHCO3, in 0.01M phosphate buffer followed by 4% glutaraldehyde in 0.1M phosphate buffer, pH 7.4. Animals dosed with PDTC for 47 weeks were exsanguinated by cardiac puncture under deep anesthesia. After perfusion or exsanguination, sciatic nerves were dissected out and immersed in 4% glutaraldeyde in 0.1M phosphate buffer, pH 7.4. Sciatic nerve sections were post-fixed with osmium tetroxide and embedded in Epon; thick sections were cut and stained with toluidine blue. Thick (1 μm) sections of peripheral nerve tissue were evaluated by light microscopy on an Olympus BX41 microscope equipped with an Optronics Microfire digital camera. Four different fields at 40X (total area = 260 μm2) were photographed from 1–2 fascicles of each sciatic nerve and the total number of lesions counted by two observers and averaged for the four fields. The lesions quantified were: degenerated axons, intramyelinic edema and demyelinated axons. Thin (70 nm) sections were prepared from sciatic nerves and evaluated using a Phillips CM-12 electron microscope, 120 keV with a high resolution CCD camera system.
Analysis of Sciatic Nerve Copper Levels
Sections of sciatic nerve were analyzed by inductively coupled plasma-atomic emission spectroscopy (ICP-AES) at the Diagnostic Center for Population and Animal Health at Michigan State University (East Lansing, MI). One sciatic nerve measuring ≈10mm was taken from each animal and pooled with all other members of the same treatment group to provide adequate tissue for ICP-AES analysis; and the pooled samples were each injected twice generating two values per group.
NMR Measurements
One glutaraldehyde-fixed sciatic nerve (≈10 mm) from each animal was placed into a 5mm (o.d.) NMR tube. The tube was filled with perfluorocarbon solution (fomblin) to prevent tissue drying without contributing proton signal; and the nerve was held below the fluid surface with a teflon plug. NMR data were acquired at 300 MHz, at room temperature (≈ 20 °C), using a 16-cm bore 7T horizontal MRI system with a home-built, 10-mm diameter loop gap resonator. For each sample, a Carr Purcell Meiboom Gill (CPMG) pulse sequence using ≈ 40 μs refocusing pulses, 1-ms echo spacing, 4096 echoes and a 10 s predelay was used to acquire data, corresponding to a total acquisition time of approximately 5 minutes per nerve. From these data, even echo magnitudes were extracted and T2 spectra were computed by fitting a sum of 128 decaying exponential functions with time constants logarithmically distributed between 2 ms and 2 s and smoothed with a minimum energy constraint (Whittall and Mackay, 1989). The spectra generally exhibited three distinct T2 components, consistent with the aforementioned studies. For DEDC-exposed nerves and controls, the domains of these spectral components were defined as 5–26 ms, 26–86 ms, and >86 ms; and for PDTC-exposed nerves and controls they were 5–46 ms, 46–149 ms, and > 149 ms. The sum of total signal over each range was divided by the sum of signal of all the ranges for each spectrum to give the percent of total area for each T2 component.
Statistical Analysis
For lesion counts and T2 data, comparisons among the DEDC exposure groups were performed by one-way analysis of variance (ANOVA) and Tukey-Kramer’s Multiple Comparisons Test using InStat (Graphpad Software, Inc.). The PDTC exposure group and the 200 ppm Cu control were compared by the unpaired one-tailed t-test also using InStat. Lesion counts were square root transformed to normalize their distribution and equalize variances prior to statistical comparisons. For two group comparisons where equal variances were not obtained as determined by the F test, the t-test corrected for unequal variance was used. Statistical significance was taken to be p < 0.05 unless otherwise noted. Pearson correlation tests were performed using Prism 4 (Graphpad Software, Inc.). Significant differences in the trend for incidence of animals with lesions for the control 13 ppm Cu, DEDC 13 ppm Cu, and DEDC 200 ppm Cu were determined using chi-square test for trend with Prism 4. Significant differences in incidence of lesions for the control 200 ppm Cu and PDTC 200 ppm Cu were performed using the Fisher’s exact test with Prism 4.
Results
Analysis of Sciatic Nerve Copper Levels
Copper levels in sciatic nerves determined by ICP-AES are shown in Figure 1. Both DEDC exposure groups had elevated sciatic nerve copper as compared to the normal diet (13 ppm) controls; and the DEDC 200 ppm Cu group had a significant increase over the DEDC 13 ppm Cu group. Exposure to PDTC and the 200 ppm Cu diet resulted in the highest sciatic nerve copper levels of all groups and was significantly increased compared to the 200 ppm Cu controls.
Figure 1.
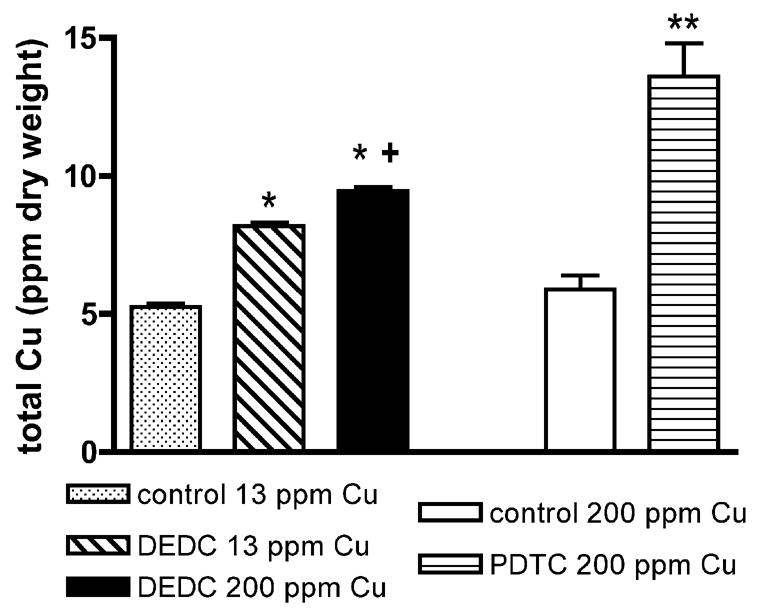
Total sciatic nerve copper levels. Values were determined by ICP-AES and are reported as mean ppm dry weight of tissue. Error bars represent SEM. Sciatic nerves were pooled (n = 3, except for Controls 13 ppm Cu (n = 4) and DEDC 200 ppm Cu (n = 5)) for each treatment group and run in duplicate. *p < 0.01 by one-way ANOVA and Tukey-Kramer post hoc test as compared to Controls 13 ppm Cu; +p < 0.01 by one-way ANOVA and Tukey-Kramer post hoc test as compared to DEDC 13 ppm Cu;** p < 0.01 by unpaired, one-tailed t test as compared to Controls 200 ppm Cu.
Peripheral Nerve Morphology
Sections of sciatic nerve obtained from controls and animals exposed to DEDC and PDTC were examined by light microscopy and electron microscopy. Normal distributions of small and large myelinated axons were present, as well as normal myelin architecture in the control groups (Figure 2A,C). Lesions in DEDC-exposed animals were consistent with a primary myelinopathy characterized by the presence of intramyelinic edema, thinning myelin, and demyelination. Decreased axon density as a result of secondary axonal degeneration was also evident (Figure 2B). The chronically exposed PDTC animals also displayed axonal regeneration and remyelination, as evidenced by clusters of axonal sprouts (Figure 2D) and onion bulbs. Electron microscopy (Figure 3) revealed evidence of demyelination, myelin regeneration, and the presence of probable macrophages in the proximity of demyelinating axons.
Figure 2.
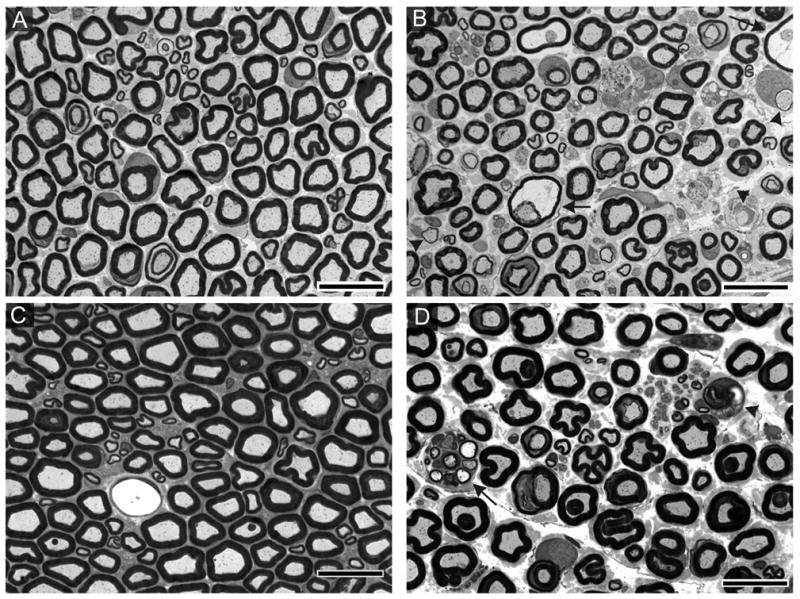
Morphology of sciatic nerve cross-sections stained with toluidine blue. Sciatic nerve from (A) Control 13 ppm Cu animal and (C) Control 200 ppm Cu animal depicting the normal distribution of small and large myelinated axons. (B) Sciatic nerve from an animal exposed to 0.35 mmol/kg/d DEDC i.p. for 8 weeks demonstrating the presence of demyelinated and thinly myelinated axons (arrowheads) and intramyelinic edema (arrows). (D) Sciatic nerve from an animal exposed to 0.88 mmol/kg/d oral PDTC and 200 ppm Cu for 47 weeks exhibiting a degenerating axon (arrow head) and axonal sprouting (arrow).
Figure 3.
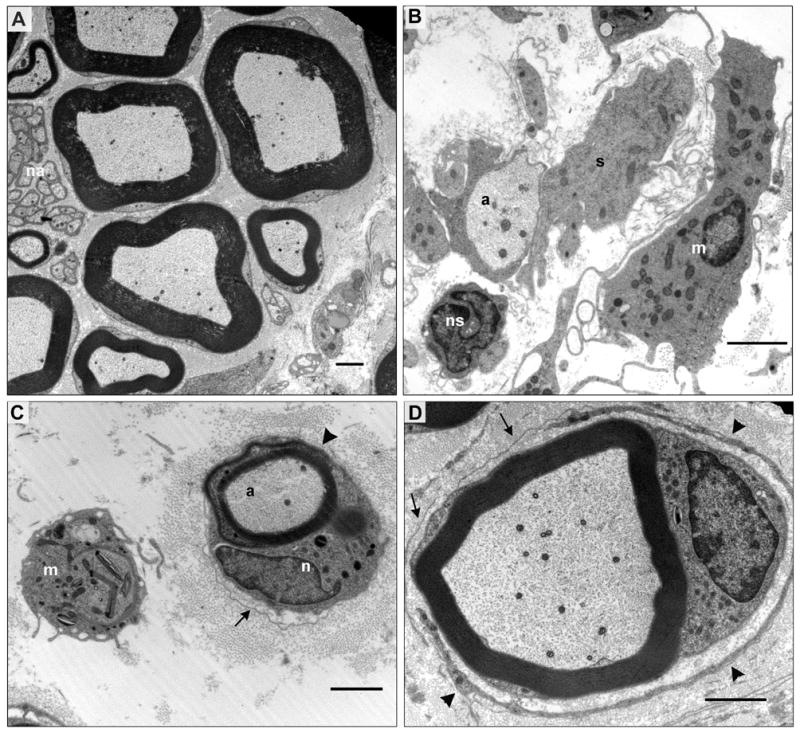
Electron micrographs of sciatic nerve cross-sections from control and PDTC treated rats. (A) Large and small myelinated axons and nonmyelinated axons in a control. The myelinated axons are surrounded by compact myelin, Schwann cell cytoplasm and basal lamina. Several small clusters of nonmyelinated axons (na) are present on the left border with each group of axons encircled by the basal lamina of a nonmyelinating Schwann cell. (B-D) Sections from a rat exposed to oral pyrrolidine dithiocarbamate and maintained on a 200 ppm copper diet for 47 weeks. (B) A demyelinated axon (a) surrounded by the processes of an associated Schwann cell (s) possessing a basal lamina. A nonmyelinated axon and its associated Schwann cell (ns) that has been sectioned through its nucleus are also present. A large elongated nucleated cell (m) with pseudopodia and no basal lamina is consistent with an activated macrophage. (C) A thinly myelinated axon (a) is surrounded by persistent basal lamina (arrow). The associated nucleated cell (n) appears to be either a myelinating Schwann cell sectioned through its nucleus and a Schmidt-Lanterman incisure (arrowhead) or a macrophage that has infiltrated under the basal lamina ensheathment and is inserting a process between the myelin lamellae (arrowhead). (D) A remyelinating axon and its current Schwann cell is surrounded by persistant basal lamina (arrows) of previous Schwann cells and the process of a supernumerary Schwann cell (arrowheads).
Values obtained from quantitation of peripheral nerve lesions by light microscopy in all groups are shown in Figure 4. Demyelinated axons were observed in all dithiocarbamate-treated groups; and none were observed in the controls. Only the PDTC 200 ppm Cu group was statistically increased over the 200 ppm Cu controls, as the variance was considerable in the DEDC 13 ppm Cu group. Intramyelinic edema was seen in all dithiocarbamate-treated groups, but not observed in the controls, and only significantly increased in the PDTC 200 ppm Cu group relative to the 200 ppm Cu controls due to the large variance seen in the DEDC groups. Degenerated axons were observed in all treatment and control groups, with PDTC high copper animals having the greatest number. Degenerated axons were found to be significantly increased in the PDTC 200 ppm Cu group over the 200 ppm Cu controls. The incidence of animals with intramyelinic edema and demyelinated axons showed a significant positive linear trend (p < 0.01 by chi-square trend analysis) for the control 13 ppm Cu (0/0), DEDC 13 ppm Cu (3/4) and DEDC 200 ppm Cu (4/4) groups. Similarly, there was a significant increase (p < 0.05 by Fisher’s exact test) in the incidence of animals with intramyelinic edema and demyelinated axons in the PDTC 200 ppm Cu group (3/3) relative to the control 200 ppm Cu group (0/0).
Figure 4.
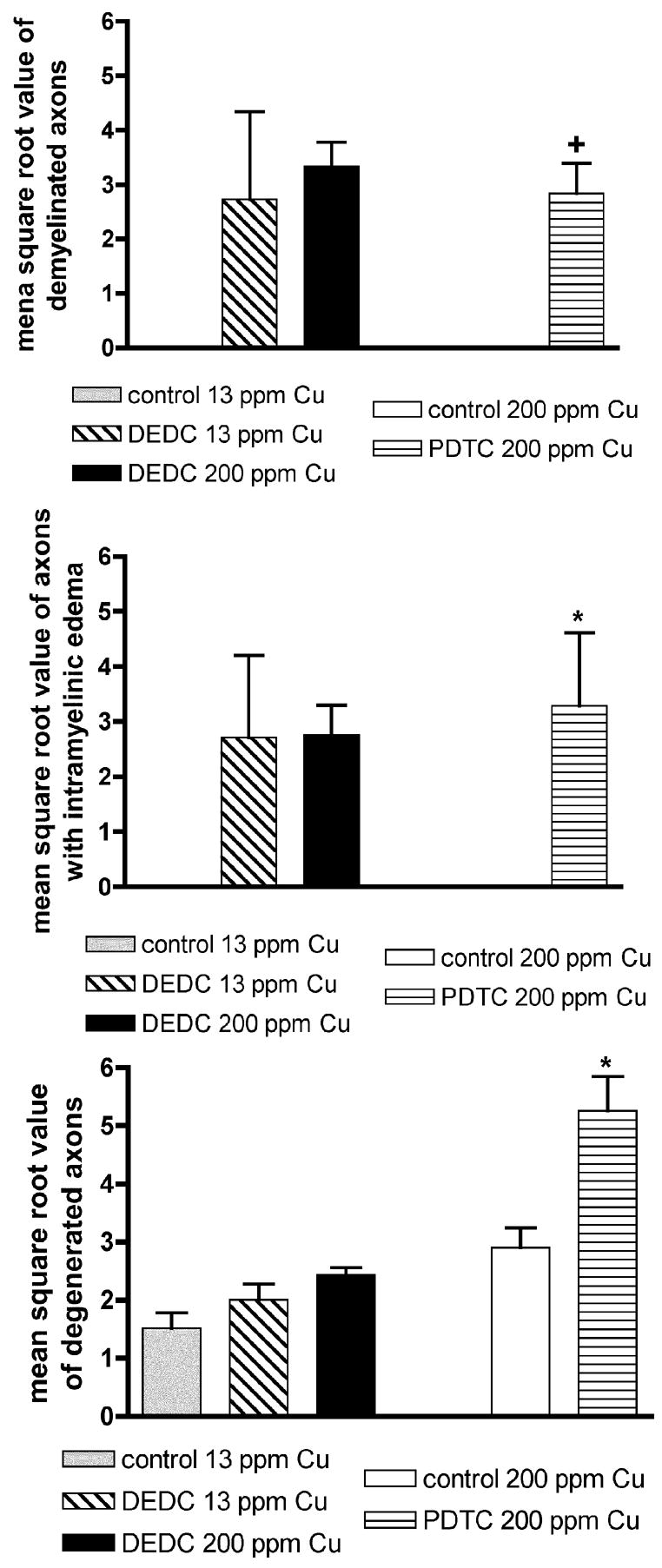
Severity of sciatic nerve lesions as determined by light microscopy. Lesions were scored by two observers (HLV and WMV). Four 40 X fields of a sciatic nerve fascicle were examined and lesions were quantified. The mean square root value of axons with lesions (SEM) was determined. n = 4 except for Control and PDTC 200 ppm Cu (n = 3) and DEDC 200 ppm Cu (n = 5). +p < 0.01 by unpaired, one-tailed t test as compared to Controls 200 ppm Cu. *p < 0.05 by unpaired t test as compared to Controls 200 ppm Cu.
T2 Spectra Analysis
Representative T2 relaxation spectra data for fixed sciatic nerves obtained from a 13 ppm Cu control and a DEDC 13 ppm Cu treated animal are shown in Figure 5A and for a 200 ppm Cu control and a PDTC 200 ppm Cu treated animal in Figure 5B. The overlays of the T2 spectra of the treated and control nerves illustrate a decrease in relative peak area of the shortest T2 component associated with myelin as a result of dithiocarbamate exposure and was seen in all dithiocarbamate treated animals. In nerves of animals co-administered an elevated copper diet and PDTC or DEDC, the percent peak area of the longest T2 component was increased. Although the spectra shown here of one of the PDTC 200 ppm Cu exposed animals shows a shift to longer T2 values of the middle and longest T2 component relative to the 200 ppm Cu controls, this was seen in only two of the three PDTC exposed nerves examined. In addition, all three T2 components in the spectra obtained from the immersion fixed 200 ppm Cu and PDTC 200 ppm Cu nerves were shifted to longer times relative to the perfusion-fixed nerves obtained from the 13 ppm Cu and DEDC groups. The percent of total area for the individual spectra components of the sciatic nerves are shown in Figure 6. Significant decreases in the percent of myelin-water were seen in both the DEDC 200 ppm Cu group and the PDTC 200 ppm Cu group relative to their 13 ppm Cu and 200 ppm Cu controls, respectively. The greatest decrease was observed for the PDTC group. The T2 values associated with axonal-water showed a trend of increased percentage in dithiocarbamate treated animals, but were not statistically significant. Extra-axonal water measurements were found to significantly increase in the DEDC 200 ppm Cu and PDTC 200 ppm Cu groups relative to their 13 ppm Cu and 200 ppm Cu controls, but not in the DEDC 13 ppm Cu group.
Figure 5.
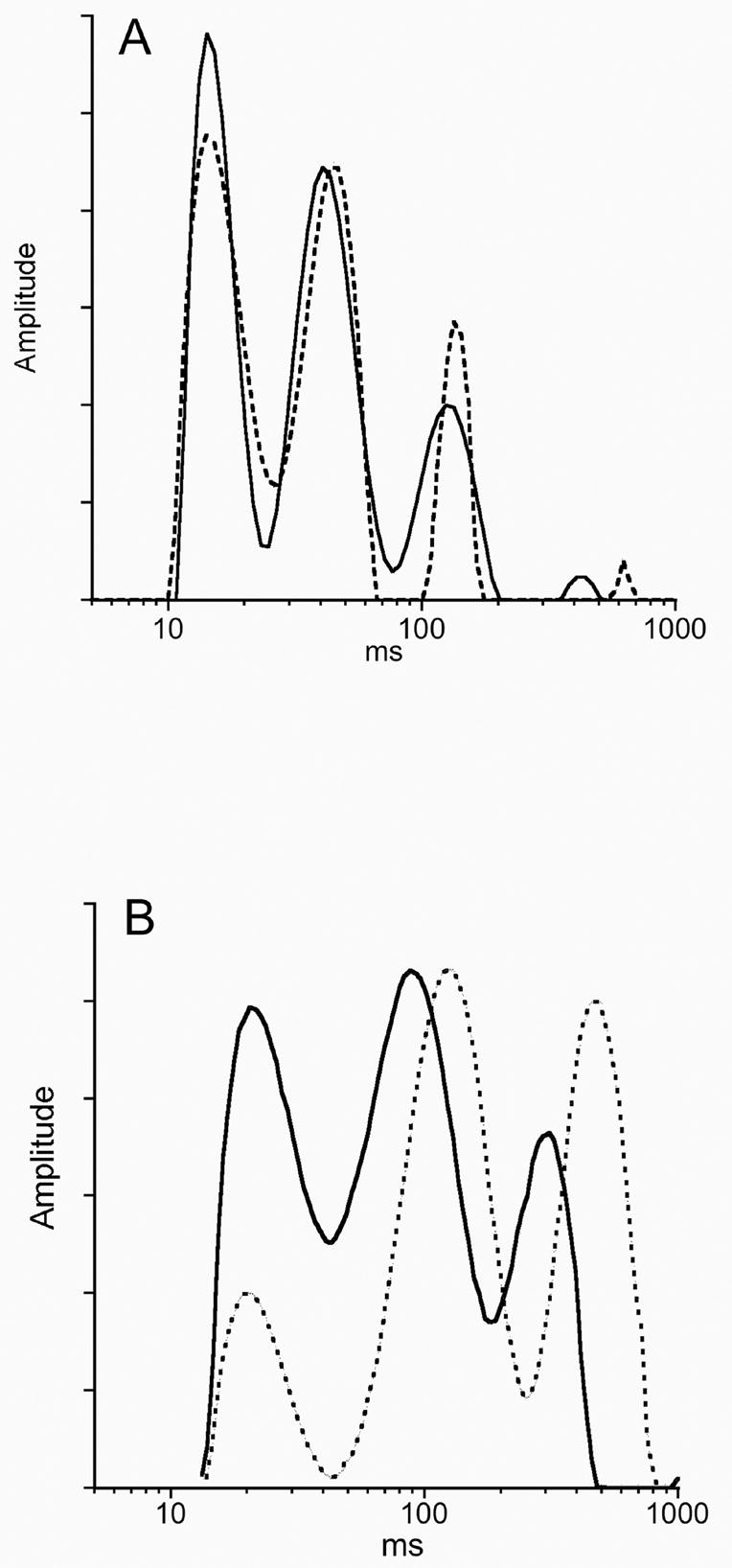
Representative spectra of sciatic nerve water T2 relaxation times. The three nerve-water components are shown as peaks from left to right: myelin, axonal and extra-axonal water. (A) The spectra from a Control 13 ppm Cu rat (solid line) and a DEDC 13 ppm Cu animal (dotted line) showing a relative decrease in the area of the first component of the DEDC exposed nerve. (B) The spectra obtained from a Control 200 ppm Cu nerve (solid line) and a PDTC 200 ppm Cu nerve (dotted line) showing a relatively greater decrease in the area of the first component and an increase in the area of the third component for the PDTC exposed nerve. Additionally, the three components for the PDTC and 200 ppm Cu Control nerves are shifted to longer T2 times relative to those in (A).
Figure 6.
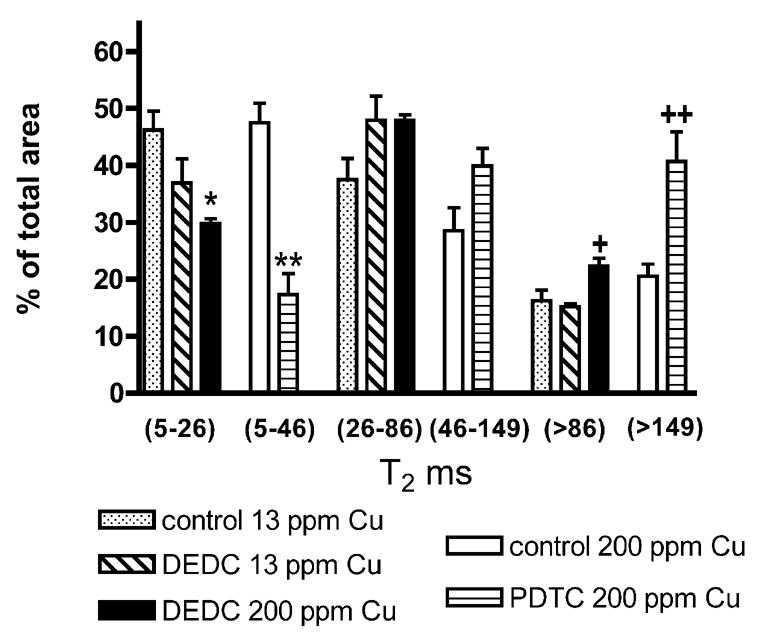
T2 sciatic nerve relaxation times in milliseconds represented as percent of total peak area. The 5–26 ms and 5–46 ms times are due to myelin-water, the 26–86 ms and 46–149 to axonal-water and >86 ms and >149 to extra-axonal water. n = 4 except for PDTC and control 200 ppm Cu (n=3) and DEDC 200 ppm Cu (n=5). *p < 0.01 by one-way ANOVA and Tukey-Kramer post hoc test as compared to Control 13 ppm Cu; ** p < 0.01 by one-way ANOVA and Tukey-Kramer post hoc test as compared to Control 200 ppm Cu; +p < 0.05 by one-way ANOVA and Tukey Kramer post hoc test as compared to Control 13 ppm Cu and DEDC 13 ppm Cu; ++p < 0.05 by unpaired, one-tailed t test as compared to Control 200 ppm Cu.
Relative Variability of Lesion Counts and T2 Measurements
To compare the variability of data obtained for microscopic lesion counts and T2 analysis, the coefficients of variation (CV) were calculated. The ranges and means of the CVs for lesion counts after data transformation for the groups exhibiting lesions were: demyelination, range 34–117 %, mean 59 %; intramyelinic edema, range 39–117%, mean 72 %; axonal degeneration, range 9–34 %, mean 22 %. The ranges and means of CVs obtained for the T2 measurements were: myelin water, range 7–43 %, mean 20 %; axonal water, range 5–28 %, mean 17 %; endoneurial water, range 7–26 %, mean 18 %.
Discussion
In contrast to light microscopy that identifies specific structural changes, such as demyelination and intramyelinic edema, multicomponent T2 analysis primarily reflects the relative contribution to the total water signal of individual water populations that differ in their transverse relaxation times and does not appear to differentiate between intact myelin and myelin debris (Webb et al., 2003). Additionally, T2 analysis has the potential to yield insight into changes of dynamic processes such as demyelination that may influence the observed transverse relaxation times of each water population. In the present study, significant treatment associated changes were observed as decreases in the contribution of the shortest T2 component and as increases in the contributions of the longest T2 component. Based upon previous investigations, the decreases observed in the shortest T2 component suggest a corresponding decrease in total myelin within the nerve (Webb et al., 2003). Consistent with this interpretation, there was a significant negative correlation between the shortest myelin water T2 contribution and the lesion counts for demyelinated axons (p < 0.05, r2 = 0.68) and intramyelinic edema (p < 0.05, r2 = 0.80). Similar decreases have been reported for the demyelination produced by Te (Pun et al., 2005) and appear consistent with the demyelination and intramyelinic edema observed here.
Interpretation of the significant increase observed in the longest T2 component and the increased contribution from the middle T2 component (although not statistically significant) in dithiocarbamate treated rats is less straightforward. A simple interpretation would be that if there is a decrease in the relative contribution from myelin, then there is a required compensatory increase in the relative contributions from the other two components. In addition to compensatory changes, early axonal degeneration resulting in a decrease in axon diameter or complete loss of axons with a subsequent decrease in axon density, may also contribute to the magnitude of the signals obtained for the middle and long T2 components in the dithiocarbamate treated animals. Alternatively, the observed lesions may also produce new populations of water with T2 times that coincide with either the middle or longest T2 component resulting in an apparent increased contribution from either of these components. For example, intramyelinic edema was a significant finding that may create a unique water compartment not present within control nerve.
Another feature of the T2 spectra obtained here was the delay in the relaxation time of the last two T2 components of two of the PDTC-exposed animals as compared to their controls. Interestingly, individual lesion counts of degenerated axons, intramyelinic edema, and demyelinated axons were much higher in these two animals as compared to the other PDTC exposed animal (individual rat data not shown), indicating greater injury. In a previous study (Stanisz et al., 2004), experimentally induced inflammation using in vivo injections of tumor necrosis factor-alpha into rat sciatic nerve was followed by T2 measurements in vitro. The resulting inflammation, as determined by immunohistologic microscopy, resulted in an influx of inflammatory cells without demyelination, and with a microscopically increased distance between axons (increased extra-axonal fraction). In that study, T2 measurements revealed a shift of the middle and longest T2 components in these inflamed nerves to longer T2 values. Since previous work supports the role of both resident and recruited macrophages in the removal of myelin in peripheral nerve injury (Perry et al., 1987; Stoll et al., 1989), inflammation may have contributed to the changes in T2 spectra observed in the present study. Because evaluation of inflammatory cells using toluidine blue stain is not reliable, they were not quantified in the present study. However, evidence of inflammation was obtained from electron micrographs of affected nerves that demonstrated numerous macrophages within the endoneurium of dithiocarbamate treated nerves. Schwann cells, containing vacuoles and whorls of myelin debris in the cytoplasm, were also observed encircling demyelinated axons. Further MR studies focused on how the influx of macrophages in the peripheral nervous system influences T2 relaxation times are required to better understand the influence of inflammation on MR parameters.
Interestingly, there was a substantial lengthening in all of the T2 values observed in the nerves that were immersion fixed relative to those that were perfusion fixed. Since this difference was observed in both the PDTC 200 ppm Cu treated and 200 ppm Cu control animals, this difference does not appear to be compound or lesion related. One explanation could be that the perfusion fixed nerves may have had a decrease in water mobility from more complete fixation resulting in shortened T2 times. A recent study (Thelwall et al., 2006) using human erythrocyte ghosts immobilized in agarose and immersed in formaldehyde and glutaraldehyde-based fixatives observed a greatly reduced sample T2 time as compared to ghosts imaged in a buffer suspension. Although the samples examined in this paper were removed from glutaraldehyde and immersed in PBS prior to MR measurement, it is possible that prolonged storage or perfusion fixation of the DEDC samples and their controls resulted in decreased T2 values as compared to the immersion fixed PDTC samples and their controls that were stored for a shorter duration. Decreases in T2 relaxation have also been reported after formalin fixation of rat and human cervical spinal cord (Carvlin et al., 1989) and in human brain tissue (Blamire et al., 1999; Tovi and Ericsson, 1992; Yong-Hing et al., 2005).
By comparing the coefficients of variation between microscopic lesion counts and T2 peak areas in this study, we found that myelin lesion counts demonstrated greater variability than T2 measurements. In the case of myelin, lesions are typically limited to individual internodes resulting in potentially different counts depending on the region of nerve sampled. In addition, observer error may be a contributing factor in lesion count variability. This level of variability decreases the power of the analysis requiring a larger number of animals or greater differences in lesion counts to detect treatment related differences. In contrast, the lesser variability of the peak areas seen in the three components of the T2 spectra in this study may reflect the greater volume of nerve being analyzed relative to plastic embedded sections, i.e. 10 mm vs. 1 μm long nerve sections.
Previous studies have suggested a role for copper-promoted oxidative stress in dithiocarbamate-mediated neurotoxicity based upon associated elevations in markers for lipid peroxidation (Calviello et al., 2005; Delmaestro and Trombetta, 1995; Tonkin et al., 2004). As copper is tightly regulated in biological systems with very limited amounts of free copper present (Rae et al., 1999), even a small magnitude of change in sciatic nerve copper could have substantial biological effects. Copper analysis of sciatic nerves in this study showed a significant increase in copper in all DEDC and PDTC treated animals relative to controls, with similar nerve copper levels observed in both the 13 ppm Cu and 200 ppm Cu controls. In contrast to the controls, dietary copper supplementation did elevate nerve copper levels in dithiocarbamate treated animals with the DEDC 200 ppm Cu group being significantly greater than the DEDC 13 ppm Cu group and the PDTC 200 ppm Cu group exhibiting the highest levels. Using the one-tailed Pearson correlation test, a significant negative correlation was observed between the myelin-water T2 contribution and the total nerve copper levels (p < 0.01, r2=0.97) suggesting that copper levels may be correlated to myelin loss. Similarly, there was a significant (p < 0.05) correlation between copper levels and lesion counts for degenerated axons (r2 = 0.71) and intramyelinic edema (r2 = 0.76).
In conclusion, this study in dithiocarbamate treated animals showed that in peripheral nerve the so-called myelin water component in a multicomponent T2 analysis decreased along with the loss and integrity of myelin and inversely correlated with tissue copper levels. Currently, microscopic evaluation remains the best method of characterizing lesions; however, it requires significant time and resources and is performed postmortem. Although MR cannot yet quantify specific lesions, it demonstrates promise for quantifying total myelin and may be more amenable to statistical evaluations than histological assessments. Previous studies (Bendazus et al., 2004; Does and Snyder, 1995, 1996; Titelbaum et al., 1989) also provide promise for the use of MR for in vivo assessments that may enable longitudinal studies in the same animal of peripheral nerve injury and repair for investigating peripheral neuropathies.
Acknowledgments
Experiments were performed in part through the use of the VUMC Research EM Resource (sponsored by NIH Grants DK20539 and DK58404) and the Vanderbilt University Institute of Imaging Science. This study was supported by NIEHS Grant ES06387, and NIH Grant EB001744.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alexeeff GV, Shusterman DJ, Howd RA, Jackson RJ. Dose-response assessment of airborne methyl isothiocyanate (MITC) following a metam sodium spill. Risk Anal. 1994;14:191–8. doi: 10.1111/j.1539-6924.1994.tb00044.x. [DOI] [PubMed] [Google Scholar]
- Bendszus M, Wessig C, Solymosi L, Reiners K, Koltzenburg M. MRI of peripheral nerve degeneration and regeneration: correlation with electrophysiology and histology. Exp Neurol. 2004;188:171–7. doi: 10.1016/j.expneurol.2004.03.025. [DOI] [PubMed] [Google Scholar]
- Berry JM, Jacobs C, Sikic B, Halsey J, Borch RF. Modification of cisplatin toxicity with diethyldithiocarbamate. J Clin Oncol. 1990;8:1585–90. doi: 10.1200/JCO.1990.8.9.1585. [DOI] [PubMed] [Google Scholar]
- Blamire AM, Rowe JG, Styles P, McDonald B. Optimising imaging parameters for post mortem MR imaging of the human brain. Acta Radiologica. 1999;40:593–97. doi: 10.3109/02841859909175593. [DOI] [PubMed] [Google Scholar]
- Brewer C. Long-term, high-dose disulfiram in the treatment of alcohol abuse. Br J Psychiatry. 1993;163:687–9. doi: 10.1192/bjp.163.5.687. [DOI] [PubMed] [Google Scholar]
- Brown DR, Sassoon J. Copper-dependent functions for the prion protein. Mol Biotechnol. 2002;22:165–78. doi: 10.1385/MB:22:2:165. [DOI] [PubMed] [Google Scholar]
- Calviello G, Filippi GM, Toesca A, Palozza P, Maggiano N, Nicuolo FD, Serini S, Azzena GB, Galeotti T. Repeated exposure to pyrrolidine-dithiocarbamate induces peripheral nerve alterations in rats. Toxicol Lett. 2005;158:61–71. doi: 10.1016/j.toxlet.2005.02.008. [DOI] [PubMed] [Google Scholar]
- Campadelli P, Gangai C, Pasquale F. Automated morphometric analysis in peripheral neuropathies. Comp Biol Med. 1999;29:147–56. doi: 10.1016/s0010-4825(98)00051-1. [DOI] [PubMed] [Google Scholar]
- Carvlin JJ, Asato R, Hackney DB, Kassab E, Joseph PM. High-resolution MR of the spinal cord in humans and rats. Am J Neuroradiol. 1989;10:13–17. [PMC free article] [PubMed] [Google Scholar]
- Chen SH, Liu SH, Liang YC, Lin JK, Lin-Shiau SY. Death signaling pathway induced by pyrrolidine dithiocarbamate-Cu(2+) complex in the cultured rat cortical astrocytes. Glia. 2000;31:249–61. doi: 10.1002/1098-1136(200009)31:3<249::aid-glia60>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- Child GP, Osinski W, Bennett RE, Davidoff E. Therapeutic results and clinical manifestations following the use of tetraethylthiuram disulfide (antabuse) Am J Psychiatry. 1951;107:774–80. doi: 10.1176/ajp.107.10.774. [DOI] [PubMed] [Google Scholar]
- Cristofori P, Terron A, Marella M, Moretti U, Pasqualicchio M, Velo GP, Milanino R. Copper supplementation in the rat: preliminary observations on the clinical, hematological and histopathological profile. Agents Actions. 1992;(Spec No):C118–20. [PubMed] [Google Scholar]
- Delmaestro E, Trombetta LD. The effects of disulfiram on the hippocampus and cerebellum of the rat brain: a study on oxidative stress. Toxicol Lett. 1995;75:235–43. doi: 10.1016/0378-4274(94)03187-c. [DOI] [PubMed] [Google Scholar]
- Does MD, Snyder RE. T2 relaxation of peripheral nerve measured in vivo. Magn Reson Med. 1995;13:575–80. doi: 10.1016/0730-725x(94)00138-s. [DOI] [PubMed] [Google Scholar]
- Does MD, Snyder RE. Multiexponential T2 relaxation in degenerating peripheral nerve. Magn Reson Med. 1996;35:207–13. doi: 10.1002/mrm.1910350212. [DOI] [PubMed] [Google Scholar]
- Edington N, Howell JM. The neurotoxicity of sodium diethyldithiocarbamate in the rabbit. Acta Neuropathol. 1969;12:339–47. doi: 10.1007/BF00809130. [DOI] [PubMed] [Google Scholar]
- Eneanya DI, Bianchine JR, Duran DO, Andresen Gd. The actions and metabolic fate of disulfiram. Ann Rev Pharmacol Toxicol. 1981;21:575–96. doi: 10.1146/annurev.pa.21.040181.003043. [DOI] [PubMed] [Google Scholar]
- Everling WE, Haywood S, Elmes ME, Jasani B, Trafford J. Histochemical and immunocytochemical evaluation of copper and metallothionein in the liver and kidney of copper-loaded rats. J Pathol. 1990;160:305–12. doi: 10.1002/path.1711600406. [DOI] [PubMed] [Google Scholar]
- Fuentealba IC, Mullins JE, Aburto EM, Lau JC, Cherian GM. Effect of age and sex on liver damage due to excess dietary copper in Fischer 344 rats. J Toxicol Clin Toxicol. 2000;38:709–17. doi: 10.1081/clt-100102384. [DOI] [PubMed] [Google Scholar]
- Guena S, Tos P, Guglielmone R, Battiston B, Giacobini-Robecchi M. Methodological issues in size estimation of myelinated nerve fibers in peripheral nerves. Anat Embryol. 2001;204:1–10. doi: 10.1007/s004290100188. [DOI] [PubMed] [Google Scholar]
- Johnson DJ, Graham DG, Amarnath V, Amarnath K, Valentine WM. Release of carbon disulfide is a contributing mechanism in the axonopathy produced by N,N-diethyldithiocarbamate. Toxicol Appl Pharmacol. 1998;148:288–96. doi: 10.1006/taap.1997.8344. [DOI] [PubMed] [Google Scholar]
- Kennedy W, Wendelshafer-Crabb G, Polydefkis M, McArthur J. Pathology and quantitation of cutaneous innervation. In: Dyck P, Thomas P, editors. Peripheral Neuropathy. Philadelphia: Elsevier Saunders; 2005. pp. 876–9. [Google Scholar]
- Kreutzer RA, Hewitt DJ, Draper WM. An epidemiological assessment of the cantara metam sodium spill: Acute health effects of methylisothiocyanate exposure. Adv Chem Series. 1994;241:209–30. [Google Scholar]
- Lang JM, Touraine JL, Trepo C, Choutet P, Kirstetter M, Falkenrodt A, Herviou L, Livrozet JM, Retornaz G, Touraine F. Randomised, double-blind, placebo-controlled trial of ditiocarb sodium ('Imuthiol') in human immunodeficiency virus infection. Lancet. 1988;2:702–6. doi: 10.1016/s0140-6736(88)90184-5. [DOI] [PubMed] [Google Scholar]
- Menon RS, Rusinko MS, Allen PS. Proton relaxation studies of water compartmentalization in a model neurological system. Magn Reson Med. 1992;28:264–74. doi: 10.1002/mrm.1910280208. [DOI] [PubMed] [Google Scholar]
- Milanino R, Marrella M, Crivellente F, Benoni G, Cuzzolin L. Nutritional supplementation with copper in the rat. I. Effects on adjuvant arthritis development and on some in vivo- and ex vivo-markers of blood neutrophils. Inflamm Res. 2000;49:214–23. doi: 10.1007/s000110050582. [DOI] [PubMed] [Google Scholar]
- Multhaup G, Dieter HH, Beyrreuther K, Bayer T. Role of copper and other transition metal ions in the pathogenesis of Parkinson's disease, prion diseases, familial amyotrophic lateral sclerosis, and Alzheimer's disease. In: Massaro E, editor. Handbook of Copper Pharmacology and Toxicology. Totowa: Humana Press; 2002. pp. 297–317. [Google Scholar]
- Orrenius S, Nobel CS, van den Dobbelsteen DJ, Burkitt MJ, Slater AF. Dithiocarbamates and the redox regulation of cell death. Biochem Soc Trans. 1996;24:1032–8. doi: 10.1042/bst0241032. [DOI] [PubMed] [Google Scholar]
- Peled S, Cory DG, Raymond SA, Kirschner DA, Jolesz FA. Water diffusion, T(2), and compartmentation in frog sciatic nerve. Magn Reson Med. 1999;42:911–8. doi: 10.1002/(sici)1522-2594(199911)42:5<911::aid-mrm11>3.0.co;2-j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry VH, Brown MC, Gordon S. The macrophage response to central and peripheral nerve injury. J Exp Med. 1987;165:1218–23. doi: 10.1084/jem.165.4.1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pun TW, Odrobina E, Xu QG, Lam TY, Munro CA, Midha R, Stanisz GJ. Histological and magnetic resonance analysis of sciatic nerves in the tellurium model of neuropathy. J Periph Nerv Syst. 2005;10:38–46. doi: 10.1111/j.1085-9489.2005.10107.x. [DOI] [PubMed] [Google Scholar]
- Rae TD, Schmidt PJ, Pufahl FA, Culotta VC, O'Halloran TV. Undetectable intracellular free copper: The requirement of a copper chaperone for superoxide dismutase. Science. 1999;284:805–08. doi: 10.1126/science.284.5415.805. [DOI] [PubMed] [Google Scholar]
- Romero E, Cuisenaire O, Denef JF, Delbeke J, Macq B, Veraart C. Automatic morphometry of nerve histological sections. J Neurosci Methods. 2000;97:111–22. doi: 10.1016/s0165-0270(00)00167-9. [DOI] [PubMed] [Google Scholar]
- Rotilio G, Ciriolo MR, Carri MT, Rossi L. Disturbances of copper homeostasis and brain function. In: Massaro E, editor. Handbook of Copper Pharmacology and Toxicology. Totowa: Humana Press; 2002. pp. 277–96. [Google Scholar]
- Santoro V, Trombetta G, Leonardi L, Messina C. A computer-assisted automatic method for myelinated nerve fiber morphometry. Acta Neurol Scand. 1992;85:18–22. doi: 10.1111/j.1600-0404.1992.tb03990.x. [DOI] [PubMed] [Google Scholar]
- Stanisz GJ, Webb S, Munro CA, Pun T, Midha R. MR properties of excised neural tissue following experimentally induced inflammation. Magn Res Med. 2004;51:473–9. doi: 10.1002/mrm.20008. [DOI] [PubMed] [Google Scholar]
- Stoll G, Trapp BD, Griffin JW. Macrophage function during wallerian degeneration of rat optic nerve: clearance of degeneating myelin and la expression. J NeuroSci. 1989;9:2327–35. doi: 10.1523/JNEUROSCI.09-07-02327.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strausak D, Mercer JF, Dieter HH, Stremmel W, Multhaup G. Copper in disorders with neurological symptoms: Alzheimer's, Menkes, and Wilson diseases. Brain Res Bull. 2001;55:175–85. doi: 10.1016/s0361-9230(01)00454-3. [DOI] [PubMed] [Google Scholar]
- Thelwall PE, Shepherd TM, Stanisz GJ, Blackband SJ. Effects of temperature and aldehyde fixation on tissue water diffusion properties, studied in an erythrocyte ghost tissue model. Magn Reson Med. 2006;56:282–89. doi: 10.1002/mrm.20962. [DOI] [PubMed] [Google Scholar]
- Titelbaum DS, Frazier JL, Grossman RI, Joseph PM, Yu LT, Kassab EA, Hickey WF, LaRossa D, Brown MJ. Wallerian degeneration and inflammation in rat peripheral nerve detected by in vivo MR imaging. Am J Neuroradiol. 1989;10:741–46. [PMC free article] [PubMed] [Google Scholar]
- Tonkin EG, Erve JC, Valentine WM. Disulfiram produces a non-carbon disulfide-dependent schwannopathy in the rat. J Neuropathol Exp Neurol. 2000;59:786–97. doi: 10.1093/jnen/59.9.786. [DOI] [PubMed] [Google Scholar]
- Tonkin EG, Valentine HL, Milatovic DM, Valentine WM. N,N-diethyldithiocarbamate produces copper accumulation, lipid peroxidation, and myelin injury in rat peripheral nerve. Toxicol Sci. 2004;81:160–71. doi: 10.1093/toxsci/kfh190. [DOI] [PubMed] [Google Scholar]
- Tovi M, Ericsson A. Measurements of T1 and T2 over time in formalin-fixed human whole-brain specimens. Acta Radiologica. 1992;33:400–04. [PubMed] [Google Scholar]
- Valentine HL, Amarnath K, Amarnath V, Valentine WM. Dietary copper enhances the peripheral myelinopathy produced by oral pyrrolidine dithiocarbamate. Toxicol Sci. 2006;89:485–94. doi: 10.1093/toxsci/kfj047. [DOI] [PubMed] [Google Scholar]
- Valentine WM, Amarnath V, Amarnath K, Rimmele F, Graham DG. Carbon disulfide mediated protein cross-linking by N,N-diethyldithiocarbamate. Chem Res Toxicol. 1995;8:96–102. doi: 10.1021/tx00043a013. [DOI] [PubMed] [Google Scholar]
- Vasilescu V, Katon E, Simplaceanu V, Demco D. Water compartments in the myelinated nerve. III. Pulsed NMR results. Experientia. 1978;34:1443–44. doi: 10.1007/BF01932339. [DOI] [PubMed] [Google Scholar]
- Vettorazzi G, Almeida WF, Burin GJ, Jaeger RB, Puga FR, Rahde AF, Reyes FG, Schvartsman S. International safety assessment of pesticides: dithiocarbamate pesticides, ETU, and PTU--a review and update. Teratog Carcinog Mutagen. 1995;15:313–37. doi: 10.1002/(SICI)1520-6866(1996)15:6<313::AID-TCM7>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- Webb S, Munro CA, Midha R, Stanisz GJ. Is multicomponent T2 a good measure of myelin content in peripheral nerve? Magn Reson Med. 2003;49:638–45. doi: 10.1002/mrm.10411. [DOI] [PubMed] [Google Scholar]
- Whittall KP, MacKay AL. Quantitative interpretation of NMR relaxation data. J Magn Reson. 1989;84:134–52. [Google Scholar]
- WHO. Dithiocarbamate pesticides, ethylenethiourea, and propylenethiourea: a general introduction. 1988;78:11–68. [Google Scholar]
- Wilson A, Trombetta LD. The protective effects of zinc on diethyldithiocarbamate cytotoxicity on rat astrocytes in vitro. Toxicol Lett. 1999;105:129–40. doi: 10.1016/s0378-4274(98)00392-0. [DOI] [PubMed] [Google Scholar]
- Yong-Hing CJ, Obenaus A, Stryker R, Tong K, Sarty GE. Magnetic resonance imaging and mathematical modeling of progressive formalin fixation of the human brain. Magn Reson Med. 2005;54:324–32. doi: 10.1002/mrm.20578. [DOI] [PubMed] [Google Scholar]


