Abstract
This review seeks to give an overview of the current approaches to drug delivery from scaffolds for neural tissue engineering applications. The challenges presented by attempting to replicate the three types of nervous tissue (brain, spinal cord, and peripheral nerve) are summarized. Potential scaffold materials (both synthetic and natural) and target drugs are discussed with the benefits and drawbacks given. Finally, common methods of drug delivery, including degradable/diffusion-based delivery systems, affinity-based delivery systems, immobilized drug delivery systems, and electrically controlled drug delivery systems, are examined and critiqued. Based on the current body of work, suggestions for future directions of research in the field of neural tissue engineering are presented.
Keywords: controlled release, neurotrophins, polymers, regenerative medicine
1. Introduction
The complexity of the nervous system allows for information to be received and transmitted through the body. As a result, brain, spinal cord, and peripheral nerve tissue pose unique challenges when designing drug delivery scaffolds to serve as replacements for injured or diseased tissue. Many requirements must be met when designing such scaffolds, including creating a permissible, biocompatible environment that allows for cell infiltration and restoration of neuronal connections lost to injury. The scaffolds should also deliver appropriate cues for promoting nerve regeneration in a controlled, localized manner. By following this guidance, engineered tissues can be produced that promote regeneration while becoming fully integrated into the existing healthy tissue. This paper will describe the challenges that need to be overcome and summarize the previous approaches to scaffold design and methods of drug delivery for neural tissue engineering applications.
1.1 Challenges in engineering scaffolds for brain tissue repair
Implantable scaffolds can be used to treat a variety of issues associated with the brain injury and disease, including replacing tissue lost to traumatic brain injury (TBI), delivering drugs to help treat neurological diseases such as Parkinson’s and Alzheimer’s, as well as serving as coatings for brain-implanted devices to limit inflammation. TBI affects over 1.5 million people per year in the United States [1]. Current treatment for TBI focuses on preserving the healthy tissue remaining after injury as opposed to attempting to regenerate damaged tissue. Replacing damaged tissue with scaffolds containing drugs could help promote regeneration and functional recovery. Parkinson’s and Alzheimer’s disease affect 1 million and 4.5 million people in the United States respectively [2, 3]. Delivery of therapeutics from scaffolds could potentially help limit damage to neurons while helping to preserve function that would normally be lost to these diseases. Finally, drug releasing coatings for brain-implanted devices can improve their function by allowing them to record signals from neurons for longer time periods. Such work is important for the design of brain-computer interfaces (BCIs), as well as for studying mammalian cognition and behavior.
Despite this wide range of potential applications for neural tissue engineering, the brain presents similar obstacles when designing scaffolds. Considerations include minimizing cell death and inflammation after scaffold implantation by choosing biocompatible materials, controlling drug release over an appropriate time course to prevent multiple surgeries or injections, and making the whole process minimally invasive to limit damage done to healthy brain tissue and to preserve the integrity of the blood-brain barrier (BBB).
Each of the aforementioned applications also poses challenges unique to that purpose. Scaffolds designed as treatment for TBI should allow for cell infiltration and eventually degradation, as well as promote tissue regeneration. The brain does not possess the regenerative capacity present in other tissues, such as the peripheral nervous system (PNS). Scaffolds produced to deliver drugs as treatment for disease should be small and minimally invasive while still being able to consistently release drugs over an extended time course due to the ongoing nature of these diseases. Scaffolds used to coat brain-implanted devices must focus on promoting neuronal survival while reducing the inflammatory response to prevent glial scarring. They should also not affect electrode function or alter its impedance. Based on the appropriate design considerations for each application, the scaffold material, therapeutic drug, and delivery method can be selected to produce the desired function.
1.2 Challenges in engineering scaffolds for spinal cord repair
Approximately 253,000 people in the U.S. live with the effects of spinal cord injury (SCI) and each year that number grows by an estimated 11,000 people [4]. Current treatment options for SCI are limited. The injured spinal cord produces a complex inhibitory environment that poses many challenges when trying to promote regeneration. After injury, a fluid filled cavity forms at the site of injury, which becomes surrounded by a dense glial scar. Reactive astrocytes, glycosaminoglycans and other inhibitory molecules prevent neurons and other cells from infiltrating the injury site, resulting in a loss of axonal connections and a loss of motor function.
To successfully design a scaffold to be used as treatment for SCI, many considerations must be taken into account. The scaffold should help lessen glial scar formation while containing sites for cell adhesion to allow regenerating neurons to extend axons into the injury site. Additionally, the scaffold should serve as a bridge to help guide the regenerating axons across the injury site and to restore connections with the target of innervation to promote functional recovery. The time course of drug delivery should be selected to promote and maintain long term functional recovery. Such scaffolds provide an alternative means of long term drug delivery compared to other methods, such as the use of osmotic pumps.
Ideally, potential treatments for SCI should be studied in chronic injury models with appropriate assessments of morphological and functional recovery, such as track tracing, locomotor function assessment, and quantification of regenerating neural fibers and immune response, to accurately gauge their promise. One of the main challenges in finding potential treatments for SCI is to find consistent, quantitative, and replicable methods of evaluating the effect of a treatment method on recovery after injury in preclinical trials. More rigorous testing at the preclincal level will allow for a better determination of which therapies to test in clinical trials.
1.3 Challenges in engineering scaffolds for peripheral nerve repair
According to various studies, peripheral nerve injury affects 2.8% of all trauma patients and approximately 200,000 nerve repair procedures are performed annually in the United States [5, 6]. The major challenge for tissue engineering in the PNS is to create an alternative to the autograft, the clinical standard of care. Currently, the treatment for long nerve injuries requires removing a donor nerve from a different location and using it to join together the severed nerve. This process currently serves as the “gold standard” for regeneration, but its limitations include requiring two incisions (for donor harvest and repair), creating donor site morbidity, and not always resulting in full functional recovery. The current challenge in peripheral nerve tissue engineering is to produce an implantable scaffold capable of bridging long gaps that will produce results similar to an autograft without requiring the harvest of autologous donor tissue. The end goal would be to produce biodegradable drug delivery scaffolds that will integrate with the damaged tissue to promote consistent fully functional recovery. Such scaffolds must be tailored to the exact specifications of the nerve injury site and should remain intact until the nerve fibers have restored connections and no longer need the support of the scaffold. Additionally, care must be taken to select drugs appropriate for promoting the desired type of neuron regeneration (sensory or motor).
2. Design Considerations
2.1 Scaffold Materials and Properties
One of the first considerations when designing a scaffold for neural tissue engineering is the choice of material. A wide range of materials have been developed for such applications. These materials must be able to conform to the dimensions of the implantation site and maintain an appropriate shape after implantation. The last consideration is especially important when designing nerve guidance conduits (NGCs) for PNS repair. Additional points to consider include how to sterilize the material prior to implantation and if the material will cause an immune response. After these issues have been considered, an appropriate scaffold can be selected based on degradation rate, ability to provide controlled release, and other chemical and physical properties. This section will highlight the novel features of a range of potential scaffold materials, as they all meet to some degree the aforementioned properties. Table 1 summarizes potential scaffold materials and their applications.
Table One.
Potential Scaffold Materials For Neural Tissue Engineering
| Material | TBI | NDD | NI | SCI | PNI | Citations |
|---|---|---|---|---|---|---|
| Synthetic Materials | ||||||
| PEG/PEO | x | x | x | [11, 12, 95, 116–118] | ||
| EVAC | x | x | x | [17–27] | ||
| PLA/PGA/PLGA | x | x | x | x | [23, 85, 99, 101, 119–125] | |
| PHEMA/PHEMA-M M A | x | x | [31–34, 62, 107, 126, 127] | |||
| Ppy | x | x | [36–41, 128–130] | |||
| Natural Materials | ||||||
| Agarose/Alginate | x | x | x | [43–50, 54, 89] | ||
| Chitosan/Nitrocellulose/Methylcellulose | x | x | x | x | [45, 49, 51–54, 75, 131, 132] | |
| Collagen | x | x | x | x | [6, 32, 57, 133] | |
| Dextran | x | x | [60–62] | |||
| Fibrin | x | x | [31–33, 64–68, 81–83, 85, 104, 106, 134, 135] | |||
| Fibronectin | x | x | [50, 69–74] | |||
| Hyaluronan/Hyaluronic acid | x | x | x | [136–140] | ||
Abbreviations: Traumatic brain injury (TBI), Neural Degenerative Disorders (NDD), Neural implants (NI), Spinal Cord Injury (SCI), Peripheral nerve injury (PNI)
2.1.1 Synthetic materials
Synthetic materials have many advantages for use as scaffolds. These polymers can be tailored to produce a wide range of mechanical properties and degradation rates. They also have known compositions and can be designed to minimize the immune response. Finally, synthetic polymers can be reacted together to combine the properties that are unique to each.
Poly (ethylene glycol)/Poly (ethylene oxide)
Poly (ethylene glycol) (PEG), also known as poly (ethylene oxide) (PEO), resists protein adsorption and cell adhesion [7]. These characteristics help minimize the immune response after implantation. Additionally, this polymer can also help seal cell membranes after injury, making it useful for limiting cell death. Hydrophilic PEG hydrogels can be made through a variety of crosslinking schemes to create scaffolds with varying degradation rates and rates of drug release [8–12]. Further chemistry can be used to modify these gels to add sites for cell adhesion or extracellular matrix (ECM) molecules to allow cells to infiltrate into these scaffolds, extending their potential applications [8, 13–16].
Poly(ethylene-co-vinylacetate)
Poly (ethylene-co-vinyl acetate) (EVA), a non-degradable, biocompatible, commercially available polymer, is often used to as a controlled drug delivery scaffold. The stability of EVA makes it desirable material choice for delivering drugs over an extended time period. It is commonly used to make microspheres for drug delivery [17–23], while more recently it has been investigated for other neural tissue engineering applications, such as repair of peripheral nerve injury [24–27].
Poly (glycolic acid)/Poly (lactic acid)/Poly (lactic-co-glycolic acid)
Poly (glycolic acid) (PGA), poly (lactic acid) (PLA) are biodegradable synthetic polymers, which can reacted to form the copolymer poly (lactic-co-glycolic acid) [28–30]. After implantation, the ester bonds that make up the backbone of the polymer can be hydrolyzed, causing the scaffold to degrade into metabolite byproducts. These byproducts can be absorbed by the body and may cause pH changes around the implantation site. The degradation rate of the scaffolds can be altered by varying the ratio of PGA to PLA in the scaffold.
Poly (2-hydroxyethyl methacrylate) and Poly (2-hydroxyethyl methacrylate-co-methyl methacrylate)
Similar to EVA, poly (2-hydroxyethyl methacrylate) (pHEMA) and poly (2-hydroxyethyl methacrylate-co-methyl methacrylate) (pHEMA-MMA) polymers are not degradable. As result, scaffolds made from pHEMA and pHEMA-MMA remain stable after implantation [31–33]. Such scaffolds can be molded into a variety shapes, including being modified to contain channels that can be filled with therapeutic drugs or ECM proteins [32]. The mechanical properties can also be modulated by adding multiple layers of pHEMA to strengthen the scaffold [34].
Polypyrrole
Polypyrroles (Ppys) are polymers that are made up of connected pyrrole ring structures [35]. In addition to being biocompatible, Ppy also is highly conductive, making it convenient for use in neural prosthetic applications [36]. It can be molded into a variety of shapes and allows for cell adhesion [37]. A wide range of work has been published describing how Ppy can be modified in different ways to improve its functionality as a scaffold for neural tissue engineering [38–41].
2.1.2 Natural materials
Natural materials possess many properties that make them attractive for neural tissue applications. Many of these materials contain sites for cell adhesion, allowing for cell infiltration. These materials also exhibit similar properties to the soft tissues they are replacing. Since these materials are obtained from natural sources, they must be purified to ensure that no foreign body response occurs after implantation. Homogeneity of product between lots can be an issue with natural materials.
Agarose/Alginate
Agarose and alginate are naturally derived, linear polysaccacharides obtained from seaweed and algae respectively. These materials are easily obtained and can be crosslinked to form three dimensional scaffolds [42–50]. Alginate scaffolds are formed by calcium crosslinking and can be degraded by calcium chelation, while agarose forms a gel based on its thermodynamic properties above a certain temperature. Both polysaccharides must undergo extensive purification to prevent immune responses after implantation. Previous work has shown a relationship between the stiffness of the agarose gel and neurite extension, suggesting an optimal concentration for neural tissue applications [42, 43].
Chitosan/Methylcellulose/Nitrocellulose
Chitosan, methylcellulose, and nitrocellulose are all polysaccharides that possess similar properties and have been characterized for use in tissue engineering applications. Cellulose is the most abundant polysaccharide found in nature and chitin, which can be deactylated to form chitosan, is the second most abundant. Methylcellulose can form thermoresponsive scaffolds, allowing it to be delivered in an injectable form [51, 52]. Though not as widely used, nitrocellulose binds proteins non-specifically, making it convenient for drug loading [53]. Due to its pH sensitive nature, chitosan can induced to form gels at neutral pH and injectable versions of chitosan scaffolds have been produced. Chitosan can be functionalized using the appropriate chemistry to further modify these scaffolds [54, 55].
Collagen
Collagen, one of the most common ECM proteins, has been extensively characterized as a potential scaffolds for neural tissue engineering. Collagen can be isolated from mammals, including rats, bovines, and humans. By changing the pH of collagen solutions, gel formation can be induced. Denatured collagen, known as gelatin, has also been evaluated for use as a potential scaffold [56]. Collagen gels are natural materials but an immune response could arise if cross species transplantation is used. These scaffolds contain sites for cell adhesion and can be covalently modified [6, 57–59]. Additionally, the scaffold properties can be varied by using different concentrations of collagen.
Dextran
Dextran, a complex polysaccharide derived from bacteria, consists of glucose subunits and possesses anti-thrombotic properties. Scaffolds made from dextran are resistant to protein and cell adhesion and have been investigated for use as coatings for neural implants. Dextran can be chemically modified to add selective cell adhesion sites and growth factors [60, 61]. Recent fabrication techniques have made it possible to create macroporous dextran scaffolds that can allow for cellular infiltration [62].
Fibrin
Fibrin serves as the natural wound healing matrix that forms after injury and its precursor, fibrinogen, can be obtained from pooled plasma. Fibrin scaffolds form when thrombin cleaves fibrinogen into fibrin monomers that assemble to form a noncovalent scaffold. Factor XIIIa then creates covalent crosslinks, which stabilize the scaffold. Similar to collagen, fibrin scaffolds contain sites for cell adhesion and the scaffold properties vary depending on the concentration of fibrin used [63]. Additionally, fibrin can also be covalently modified to further alter its properties [64–68].
Fibronectin
Fibronectin, obtained from bovine or human plasma, is a high molecular weight glycoprotein that can bind collagen, fibrin and heparin. It is found in its soluble form in blood and participates in wound healing process. It can be aggregated to form mats, which can be used as scaffolds for the repair of neural tissue [69–74]. These mats contain pores that all orient in the same direction to allow for guidance of regenerating neurons, provide cell adhesion sites, and can absorb growth factors, storing them as reservoir.
Hyaluronan/Hyaluronic acid
Hyaluronan, also known as hyaluronic acid, is a linear glycosaminoglycan found in brain ECM, making it an attractive choice for central nervous system (CNS) tissue engineering. Despite being generally non-adhesive to cells, it does contain some sites for cell adhesion and is non-immunogenic. It also helps promote wound healing, which is important when promoting tissue repair. One disadvantage of using hyaluronan is that is water soluble, making it difficult to develop an injectable version without adding additional components to help crosslink it into a stable scaffold [75].
2.2 Drug Selection
Selection of an appropriate therapeutic drug or combination of drugs should be based on the type of tissue to be regenerated or the particular application of the scaffold. Care should also be taken to ensure that the target drug retains its activity after incorporation into the scaffold. This section will highlight some of the most commonly used drugs for neural tissue engineering. Table 2 summarizes potential therapeutic drugs and their applications.
Table Two.
Potential Therapeutic Drugs For Neural Tissue Engineering
| Therapeutic Drug | TBI | NDD | NI | SCI | PNI | Citations |
|---|---|---|---|---|---|---|
| NGF | x | x | x | x | x | [19–21, 23, 25–27, 38, 57, 65, 93–95, 100, 103–106] |
| NT-3 | x | x | [12, 26, 32, 39, 41, 81–83, 94] | |||
| BDNF | x | x | [26, 46, 85] | |||
| CNTF | x | x | x | [11, 49] | ||
| aFGF/bFGF | x | x | [31, 32] | |||
| GDNF | x | x | [24, 27, 99] | |||
| Anti-inflammatory drugs | x | [40, 53, 89] |
Abbreviations: Traumatic brain injury (TBI), Neural Degenerative Disorders (NDD), Neural implants (NI), Spinal Cord Injury (SCI), Peripheral nerve injury (PNI)
2.2.1 Neurotrophins
Some of the most common growth factors used to promote neural tissue regeneration are neurotrophins. Neurotropins are a family of growth factors, which includes nerve growth factor (NGF), neurotrophin-3 (NT-3), neurotrophin-4/5 and brain derived neurotrophic factor (BNDF). They perform crucial roles in the development and maintenance of the nervous system. To activate their downstream signaling pathways, each neurotrophin has a specific high affinity Trk receptor with NGF, BDNF, and NT-3 signaling through the TrkA, TrkB, and TrkC receptors, respectively. Often neurons not receiving appropriate quantities of neurotrophins undergo apoptosis. Neutrotrophins also can signal through their low affinity receptor, the p75 neurotrophin receptor. This receptor can activate apoptosis in response to NGF when the TrkA receptor is not expressed [76].
NGF plays crucial roles in sympathetic and sensory neuron survival and maintenance [77]. Schwann cells secrete NGF and other factors after peripheral nerve injury and as a result, NGF has been investigated as a treatment for treat peripheral nerve injuries. Additionally, NGF enhances survival of cholinergic neurons, making it a potentially effective therapeutic for the treatment of neurodegenerative disorders such as Alzheimer’s disease and Huntingtion’s disease [78]. Although NGF has been explored as a potential drug for treatment of SCI, it can induce unwanted sensory neural fiber sprouting that can result in chronic pain [79].
NT-3 plays an important role in neurogenesis by promoting the differentiation of new neurons and promotes the formation of the corticospinal tract during development [80]. Based on this role, studies have shown that NT-3 can promote survival and outgrowth of motor neurons after injury to the spinal cord [12, 81–83]. In other studies, NT-3 has also been shown to improve peripheral nerve regeneration [26].
Originally isolated from the brain, BDNF also helps direct normal neural development, and it possesses neuroprotective properties similar to NGF and NT-3. It is involved with synaptic transmission and maintaining plasticity [84]. Although its use is not as common as NT-3, its potential to treat SCI has been studied [46, 85].
2.2.2 Other growth factors
Other growth factors that have been studied for their ability to promote nerve regeneration include ciliary neurotrophic factor (CNTF), fibroblast growth factors (acidic and basic, aFGF and bFGF), transforming growth factor β (TGF-β), and glial derived neurotrophic factor (GDNF). CNTF plays a role in motor neuron survival and outgrowth [86]. As result, it has been studied as possible therapeutic to treat peripheral nerve injury and SCI. However, studies suggest that CNTF contributes to glial scar formation after SCI, making it undesirable as a therapeutic in the CNS [87]. Members of the FGF family have also been studied to determine their effect they have on nerve injury. These growth factors can promote recovery indirectly, by inducing angiogenesis to the injury site, and directly by promoting cell proliferation and axon outgrowth at the injury site [88]. GDNF is another neuroprotective growth factor that is secreted by Schwann cells after injury, making it a potential therapeutic for peripheral nerve injury. It also has a potent trophic effect on dopaminergic neurons and could potential serve as treatment for neurodegenerative diseases [78]. GDNF is structurally similar to the transforming growth factors, including TGF-β. TGF-β reduces astrocyte proliferation in vitro and may be useful for coating neural implants [61].
2.2.3 Anti-Inflammatory Drugs
One of the drugs commonly used to reduce the chronic inflammation and the immune response that accompanies neural implants is dexamethasone, a synthetic steroid. Dexamethasone is commonly used to treat other inflammatory diseases such as arthritis and multiple sclerosis, and now it shows promise for neural tissue engineering applications [40, 89]. Another anti-inflammatory drug, α-melanocyte stimulating hormone (aMSH), has also been investigated for use in conjunction with neural implants. It works by inhibiting production of inflammatory cytokines that induce nuclear transcription factor ?B activation [90]. Other anti-inflammatory drugs could also be explored as potential candidate drugs for delivery from scaffolds that coat neural implants.
3. Methods of Drug Delivery
Many methods of drug delivery from scaffolds have been developed for use in engineering the different tissues of the nervous system. The method of drug delivery should be selected based on the application, scaffold material, and target drug. Not all of the following delivery methods are compatible with every scaffold material and target drug described in this review, so care should be taken when designing new types of scaffolds. Each of these four methods has advantages and disadvantages, which are discussed below.
3.1 Degradation/Diffusion-based delivery systems
One of the most common methods of creating controlled release is to utilize the physical properties of the scaffold material to regulate the amount of drug delivered. To create controlled release, the target drug is mixed with the scaffold precursors during fabrication. In such systems, the properties of the scaffold, such as pore size or crosslinking density, regulate release by diffusion. The rate of scaffold degradation also can affect how much drug is released over time. This section will summarize previous work that took this approach to drug delivery.
3.1.1 Scaffold based delivery systems
Incorporating growth factors into scaffolds during fabrication provides controlled release over a time course that varies based on the properties of the material. For the treatment of peripheral nerve injury, these drug delivery scaffolds are usually fabricated into nerve guidance conduits (NGCs) that can then be surgically implanted for testing in animal models. One study compared the ability of three different scaffold materials, including methylcellulose, collagen, and a commercial available laminin-containing ECM preparation (Biomatrix), to deliver platelet derived growth factor-BB (PDGF-BB) and insulin-like growth factor-1 (IGF-1) that were incorporated during scaffold fabrication [91]. It was hypothesized that these scaffolds could help limit protein loss to diffusion by trapping the growth factors inside of the scaffolds. The results of this study suggested that methylcellulose was the most suitable of these three materials for use in drug delivery for peripheral nerve repair. Aebischer and coworkers have done extensive work characterizing the controlled release of a variety of growth factors, including NGF, NT-3, BDNF, and GDNF, from EVA rods [24, 26, 27]. The drug delivery from these rods was characterized as an initial burst followed by sustained release for 30 days. These growth factor containing rods were incorporated into pure EVA scaffolds and implanted in different peripheral nerve injury models to test their efficacy. They found that incorporation of GDNF containing rods into nerve guidance conduits increased both the number of myelinated axons and axons present in the gap following injury. Bellamkonda and coworkers incorporated growth factors into microtubules, providing a method of controlled release from agarose scaffolds to promote peripheral nerve regeneration [92]. Using these scaffolds containing NGF and laminin as a treatment for peripheral nerve injury, the resulting regeneration was comparable to that observed for a nerve autograft.
Similar approaches have been developed as potential treatment for SCI. Collagen scaffolds were tested for their ability to deliver NT-3 to the injury site [93]. The animals receiving the collagen scaffold containing NT-3 showed improved functional recovery compared to animals that only received the collagen scaffold without growth factor. The corticospinal tract fibers only grew into the collagen scaffold containing NT-3, suggesting that the drug released from the collagen scaffold retained its biological activity. A pair of recent studies investigated the photopolymerized PEG and PEG-PLA scaffolds as a means of drug delivery for treatment of SCI [11, 12]. The first study demonstrated that these scaffolds that were more densely crosslinked delivered drugs over a longer time course of up to 50 days. The release time course also corresponded to the lifetime of the scaffold. In the second study, PEG-PLA scaffolds delivering NT-3 were tested in a model of SCI. This study showed that these scaffolds released NT-3 for 14 days in vitro, and that in in vivo they promoted axonal sprouting. Experimental animals that receiving the PEG-PLA scaffolds containing NT-3 showed increased functional recovery compared to untreated animals as assayed by the Basso, Beattie and Breshahan scale assessing locomotor function. Bellamkonda and coworkers have also used their microtubule-based delivery system to administer BDNF from agarose scaffolds in a dorsal hemisection model of SCI [46]. Axons were able to infiltrate into scaffolds and the immune response to the injury was decreased, suggesting the establishment of a permissive environment for regeneration.
Shoichet and coworkers have developed scaffolds that contain gradients of their target drug by trapping them inside of non-degradable scaffolds. They have been able to generate gradients of NGF and NT-3 inside of pHEMA scaffolds in vitro using the device shown in Figure 1 [94]. This gradient maker works by placing the growth factor solution in channel A and the prepolymer mixture in channel B. The initial polymer solution containing no growth factor starts filling the channel. Then the growth factor solution mixes with the prepolymer solution to start making the gradient. As the volume of prepolymer solution decreased, the amount of growth factor beginning mixed increases, creating a uniform gradient. These devices can be used to fabricate gradients in a polymer that creates a scaffold with small enough pores to prevent loss of drug to diffusion.
Figure 1.
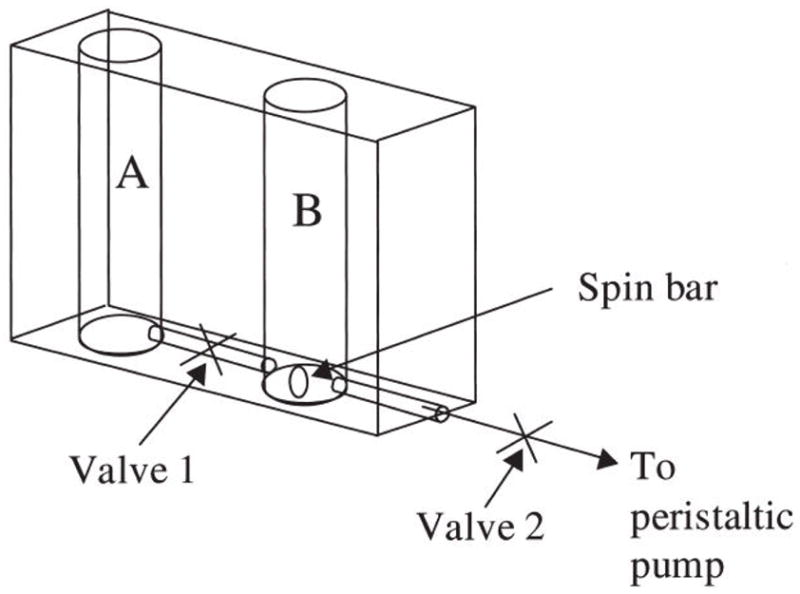
Schematic of the gradient maker used to create NGF concentration gradients in p(HEMA) gels using chambers A and B separated by a conduit. With the center valve closed, a known concentration of NGF and prepolymer solution were added to chamber A while an NGF-free prepolymer solution was added to chamber B. The center valve was opened and the peristaltic pump and stir bar were activated. The volumes in both chambers were equally depleted and mixed, and the resulting solution was delivered into a mold and allowed to polymerize, resulting in a gradient of entrapped or immobilized NGF in p(HEMA). Reproduced from reference [94], Copyright (2006), Mary Ann Liebert.
Scaffolds containing degradation/diffusion based drug delivery systems have also been used as coatings for neural implants. Bellamkonda and coworkers tested two different methods for controlled release of aMSH from nitrocellulose scaffolds, including a reservoir delivery method and a matrix delivery method (Figure 2A and B) [53]. For the reservoir delivery method, aMSH was evaporated directly onto the silicon electrode and then the nitrocellulose scaffold was added on top. For the matrix delivery method, nitrocellulose was mixed with the aMSH and then the resulting mixture used to coat the electrodes, leading to distribution of the drug throughout the scaffold. Figure 2C shows the effect of delivery method on release over an 18 day time course. The reservoir system releases drug rapidly while the matrix delivery method creates more linear release, delivering 80% of the drug over the entire time course. The release of aMSH from these scaffolds prevented nitric oxide production by microglia stimulated with lipopolysaccharide (LPS), indicating that the fabrication process does not affect the protein’s biological activity. In another study, PEG-PLA scaffolds were investigated for their ability to create controlled release of NGF [95]. The ratio of PEG to PLA was varied to determine the effect on release rate. Higher PEG to PLA ratios resulted in a longer drug delivery time course (20 days) because the PLA serves a degradable linker, thus increasing the PLA ratio should reduce scaffold stability. The prolonged time course of drug delivery with lower PLA ratios suggests that the controlled release was being regulated by the degradation of the scaffold. These scaffolds were able to deliver biologically active NGF in vitro as indicated by neurite extension of PC12 cells.
Figure 2.

Comparison of two different fabrication of nitrocellulose scaffolds for delivery of a-MSH. A) Schematic of the reservoir delivery method where a-MSH was allowed to bind the electrode followed by a coating of nitrocellulose. B) Schematic of the matrix delivery method where the a-MSH was premixed with the nitrocellulose before coating the electrode. C) Diagram showing the effect of fabrication method on the cumulative controlled release profile. The percent a-MSH loading for Reservoir produced by adding 100 ng, Reservoir produced by adding 400 ng, Matrix produced by adding 100 ng and Matrix produced by adding 400 ng. Data shown are the average ± S.E.M. (n = 3). Reproduced from reference [53], Copyright (2005), Elsevier.
When designing degradable/diffusion-based delivery scaffolds, it is important to consider that most protein drugs have sizes in the order of nanometers and thus to create controlled release will require pore sizes in that order of magnitude. Cells are much larger (on the scale of microns) and thus larger pore sizes are needed to allow for cellular infiltration into scaffolds. One way of addressing these issues is to use microspheres with relatively small pores to control drug release and incorporating the microspheres into a scaffold with micro-scale pores that allows cellular infiltration.
3.1.2 Microsphere based delivery systems
Another method of creating controlled release is to incorporating drugs into microspheres. Microspheres can be fabricated through a variety of techniques including solvent evaporation, spray freeze drying, and other methods [96]. Additional components, such as bovine serum albumin, can be added along with the target drug to help preserve biological activity of the drug during the fabrication process. The rate of drug release from microspheres is regulated by diffusion and the release kinetics of the target drug can be altered by changing the polymer used, the amount of protein loaded and the size of the microsphere [97]. They can either be injected directly into the desired location or incorporated into scaffolds made from other materials. In the later case, the scaffolds are made of a different material that contains pores large enough to allow axons and/or cells to migrate into the scaffold.
Saltzman and colleagues have done extensive work developing and characterizing release of NGF from microspheres and disks produced under a variety of conditions [19–23, 57]. They successfully encapsulated NGF into EVA and PLGA and demonstrated the ability of these particles to create controlled release in vitro and in vivo [23]. The particles made from EVA delivered NGF over a 2 month time course while microspheres made from PLGA delivered NGF over 2 weeks in vitro. Extended release was also observed when these particles were implanted in vivo. Another study by Cleland and coworkers showed that the presence of zinc during encapsulation into microspheres helped stabilize NGF [98]. These microspheres also released NGF over a two week time course, consistent with Saltzman’s results. Another study compared the influence of the material used to fabricate the microspheres on release kinetics of CNTF by comparing alginate and chitosan/PLGA [49]. The alginate microspheres produced sustained release for up to 12 days while the chitosan/PLGA microspheres were still releasing CNTF after 24 days. GDNF has also been successfully encapsulated into PLGA microspheres [99]. These studies have provided the framework for implementing such strategies as treatment for neurodegenerative disorders.
Although the size of microspheres makes them ideal for injecting into the brain, they can also be incorporated into traditional polymer scaffolds for use in other applications, such as NGCs. Tranquillo and coworkers engineered magnetically aligned collagen conduits containing NGF releasing microspheres for the treatment of peripheral nerve injuries [100]. Their study showed that PLGA microspheres incorporated into collagen scaffolds produced sustained release for 75 days while providing a mathematical model of the NGF distribution in the system. Shea and colleagues also observed long term release of NGF from PLGA microspheres molded into porous PLGA scaffolds [101]. These scaffolds allowed for cellular infiltration and maintained their structure in vivo. Similar long term kinetics have also been produced by incorporating PLGA microspheres into chitin/chitosan composite scaffolds, resulting in release of model drugs (BSA and epidermal growth factor) for up to 56 days in vitro [102]. One group investigated this combination approach using PLGA particles containing dexamethasone embedded into alginate scaffolds to create a novel coating for neural implants [89]. Electrodes coated with this scaffold maintained their impedance for 3 weeks in vivo compared to uncoated electrodes whose impedance began increasing after two weeks. All of these studies suggest that microspheres can be used for a range of neural tissue engineering applications by incorporating them into scaffolds.
3.2 Affinity based delivery systems
As an alternative to relying on a material’s physical properties to regulate release, affinity-based delivery systems utilize non-covalent interactions between the desired drug and the scaffold to provide controlled release. One of the main examples of an affinity based delivery system is a heparin binding delivery system (HBDS), which can be used to deliver any protein drug that binds to heparin. Such delivery systems were initially designed to release basic fibroblast growth factor (bFGF) [103], but recently HBDSs have been used in conjunction with fibrin scaffolds to treat peripheral and central nervous system injuries [65, 81–83, 104]. The HBDS consists of four components, a scaffold (e.g. fibrin), a bi-domain peptide, heparin, and a growth factor as seen in Figure 3. The peptide consists of a factor XIIIa substrate, which allows the covalent incorporation into fibrin scaffolds, and a heparin binding domain derived from antithrombin III. This peptide binds to heparin, sequestering it inside of the scaffold. The heparin, in turn, can bind the desired growth factor. Growth factors delivered using the HBDS showed the ability to enhance neurite extension in vitro compared to fibrin scaffolds without the delivery system [65, 66, 81]. In vivo delivery of NGF from fibrin scaffolds produced similar amounts of nerve fibers distal to a NGC and at the midline of an NGC, when compared to isograft controls [104]. This system has also been extensively characterized for its ability to deliver NT-3 as a potential treatment for SCI. In a short term study, fibrin scaffolds containing the HBDS and NT-3 promoted neuronal fiber sprouting when compared to animals treated with fibrin scaffolds without the delivery system. However, in a long term study, this approach did not lead to an increase in functional recovery [82].
Figure 3.
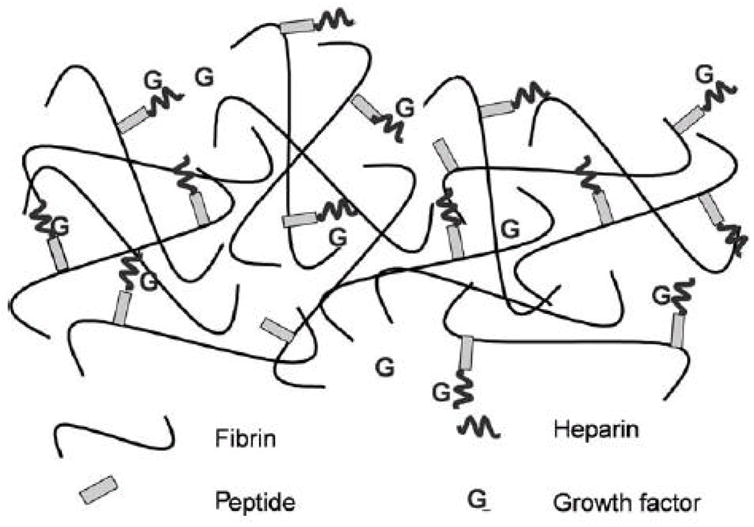
Schematic diagram showing the components of the heparin-binding delivery system. The bi-domain peptide is cross-linked into the fibrin gel via the transglutaminase activity of Factor XIIIa; heparin can bind to the peptide by electrostatic interactions. NT-3 can bind to the bound heparin, creating a gel-bound, non-diffusible complex. NT-3 can exist in the diffusible state, alone, or in a complex with free heparin. Reproduced from reference [81], Copyright (2004), Elsevier.
Other affinity based delivery systems have been developed using a rational approach combined with combinatorial screening to identify heparin or NGF binding peptides of varying affinity. Maxwell et al. screened a phage display library against heparin-conjugated chromatography resin to identify peptide domains with varying affinities for heparin [105]. These domains were then tested using the HBDS with the newly identified heparin-binding affinity domain replacing the amino acid sequence derived from antithrombin III. These newly identified peptides showed the ability to release heparin and NGF at different rates based on affinity. A similar selection method was used to find peptide domains with varying affinities for NGF by screening a phage display library against NGF-conjugated chromatography resin [106]. This delivery system also showed that NGF-binding affinity of the peptides can modulate the release rate of NGF from an affinity-based delivery system. Similar methods can be used to develop peptide binding domains for any target protein drug, regardless of affinity for heparin. Additionally, many crosslinking methods exist to allow such domains to be incorporated into a variety of scaffolds [8, 59, 68].
3.3: Immobilized drug delivery systems
A different approach to drug delivery involves covalently attaching the target drug into the scaffold material. In this manner, the drug will not be lost to diffusion and the time course of release would be comparable to the lifetime of the scaffold. An important consideration when designing such drug delivery systems is to ensure the process of immobilizing the drug onto the scaffold does not affect the efficacy or biological activity of the target drug. Loss of activity could result from using chemistry that results in denaturation, blocks the receptor binding site on the drug or binds the drug to the scaffold in a manner that prevents access to the active site of enzymes. Additionally, it is important to ensure that the target drug does not need to be internalized by the cell to produce the desired effect.
A variety of chemistries have been developed to produce immobilized drug delivery systems. One way of covalently tethering proteins to the desired scaffold is through the use of photochemistry, as shown in Figure 4A. This method has been used by several researchers to tether NGF to variety of materials, including pHEMA, PPy, and polydimethylsiloxane (PDMS) [38, 107, 108]. In a different approach, Leong and coworkers thiolated NGF so it could react with a PEO-PPO (poly (phenylene oxide))-PEO linker that could in turn bind to their desired scaffold material [109]. This chemistry is shown in Figure 4B. Using a different type of chemistry, Caplan and coworkers activated dextran with sodium metaperiodate, allowing it to form covalent linkages with TGF-β [61]. For covalently crosslinking proteins into fibrin scaffolds, a recombinant version of NGF containing a Factor XIIIa substrate allowed the protein to become incorporated upon scaffold formation [67].
Figure 4.

A) Chemistry schematic of the NGF immobilization process using azido chemistry. PAA was conjugated to an azido compound (PAA-azido). This conjugate was cast twice on PPy, followed by casting of NGF. UV light exposure promoted the formation of covalent bonds via the azido groups, immobilizing NGF to PPy. Reproduced from reference [38], Copyright (2006), Wiley.
B) Chemical schematic of thiolization of NGF and subsequent conjugation to the PEO-PPO-PEO linker. Reproduced from reference [109], Copyright (2005), Koninklijke Brill NV.
3.4 Electrically controlled drug delivery systems
Electrically controlled drug delivery systems have been investigated for use as coatings of neural electrodes. Such systems release target drugs upon electrical stimulation, which often is used during recording via such implants. In one study by Wadhwa et al., an ionic form of dexamethosone was incorporated into Ppy films grown on top of gold films through the use of electropolymerization [40]. Figure 5 shows the general chemistry scheme for incorporating molecules into PPy. Controlled release of dexamethosome from the Ppy films occurs after the application of a voltammetic stimulus. In vitro studies showed that the release of dexamethosome reduced the amount of reactive astrocytes present while having no negative effect on the viability of neurons. Additionally, the coating did not alter the impedance of the electrode.
Figure 5.

Schematic of chemical oxidation used to dope polypyrrole with NT-3. A) Synthesis of polypyrrole showing the incorporation of the dopant A−. (B) Release of the dopant A− during redox cycling of the polypyrrole. Reproduced from reference [41], Copyright (2006), Elsevier.
Using a similar strategy, a two step process was used to incorporate NT-3 into Ppy coatings. In this study, the Ppy was first doped using p-toulene sulphonate (pTS) to create a Ppy- pTS coating on gold electrodes using galvanistic methods [41]. A second layer was then formed using a mixture of Ppy, pTS, and NT-3 in same fashion. Application of pulsed voltage, pulsed current, and cyclic voltammetry promoted increased release of NT-3 when compared to controls with no current applied (diffusion only). Further studies showed that these coatings promoted neurite extension in vitro, indicating that the NT-3 retained biological activity after the polymerization process [39]. These strategies are useful for designing coatings for neural implants and may also have applications in promoting regeneration for other injuries to the nervous system.
4. Conclusions and Future Directions
Many innovative methods for creating sustained release of drugs from scaffolds for neural tissue engineering have been developed, as demonstrated by the body of work reviewed. These studies help illustrate the effectiveness of such strategies as potential treatments for injury to the nervous system and give insight into new potential strategies. Some of the more recently developed methods, such as affinity-based, immobilization-based, and electrically controlled drug delivery, still need to be explored for all types of neural tissue engineering to determine where they can be best applied. By highlighting the advantages of different scaffold materials, drugs, and methods of creating controlled release, new drug releasing scaffolds can be developed for use in neural tissue engineering applications.
Although many promising strategies have been developed for creating controlled release of drugs from scaffolds, many issues still need to be addressed for these scaffolds to serve as successful treatments. For certain applications such as designing coatings for neural implants and peripheral nerve injury, a successful scaffold may require combining multiple materials and drugs to obtain the desired mechanical properties and combination of regenerative effects. Other applications, such as treating TBI or SCI, drug delivering scaffolds may need to be combined with cell transplantation to obtain functional recovery. In addition to selecting an appropriate cell source to use as treatment, such work may require studying the effect of including cells on drug release rate and determining the amounts of drug that is consumed by the cells.
Another area for future investigation includes incorporating other potential therapeutic drugs into scaffolds for controlled delivery. Potential drugs, such as chondroitinase ABC and antibodies against Nogo, have shown promise in reducing the inhibitory environment created in the central nervous system after injury. Chondroitinase ABC digests chondroitin sulfate proteoglycans that are found in the CNS ECM and inhibit axonal growth [110, 111]. By breaking down these molecules, a more permissive environment for regeneration can be created. Rats receiving injections of chondroitionase ABC showed improved functional recovery on variety of tests including beam and grid walking assessments [110]. Nogo-A and other inhibitory myelin-associated molecules bind to the Nogo-66 receptor, activating a signaling cascade that limits regeneration [112]. Antibodies or other inhibitors of the Nogo receptor can serve as a therapeutic by blocking the ability of those proteins to signal through the Nogo receptor. This strategy has been used to achieve functional recovery in preclincial studies involving a SCI model [113]. In combination with other regeneration promoting strategies, small molecules drugs, such as cyclic AMP, that decrease the inhibitory effects of Nogo have been used to promote recovery after SCI [114, 115]. To successfully deliver each of these drugs will require novel delivery methods that maintain their activity. Overall, regaining function to the injured nervous system is complex problem and drug delivering scaffolds provide a way of restoring the lost tissue.
Acknowledgments
Financial support for this work was provided by the National Institute of Health (R01 NS051454).
Abbreviations used
- aMSH
α-melanocyte stimulating hormone
- BBB
Blood-brain barrier
- BCI
Brain-computer interface
- BDNF
Brain derived neurotrophin factor
- CNS
Central nervous system
- CNTF
Ciliary neurotrophic factor
- EVA
ethylene-co-vinyl acetate
- ECM
Extracellular matrix
- FGF
Fibroblast growth factor
- GDNF
Glial derived neurotrophic factor
- IGF-1
Insulin-like growth factor-1
- NGF
Nerve growth factor
- NGC
Nerve guidance conduit
- NT-3
Neurotrophin-3
- PNS
Peripheral nervous system
- PDGF
Platelet derived growth factor
- PEG
Poly (ethylene glycol)
- PEO
Poly (ethylene oxide)
- PGA
Poly (glycolic acid)
- pHEMA
Poly (2-hydroxyethyl methacrylate)
- pHEMA-MMA
Poly (2-hydroxyethyl methacrylate-co-methyl methacrylate)
- PLA
Poly (lactic acid)
- PLGA
Poly (lactic-co-glycolic acid)
- Ppy
Polypyrrole
- SCI
Spinal cord injury
- TBI
Traumatic brain injury
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
• Indicates landmark paper in the field of neural tissue engineering.
- 1.Sosin DM, Sniezek JE, Thurman DJ. Incidence of mild and moderate brain injury in the United States, 1991. Brain Inj. 1996;10(1):47–54. doi: 10.1080/026990596124719. [DOI] [PubMed] [Google Scholar]
- 2.Hebert LE, Scherr PA, Bienias JL, Bennett DA, Evans DA. Alzheimer disease in the US population: prevalence estimates using the 2000 census. Arch Neurol. 2003;60(8):1119–22. doi: 10.1001/archneur.60.8.1119. [DOI] [PubMed] [Google Scholar]
- 3.Fact Sheet about Parkinson’s Disease: Parkinson’s Disease Foundation; 2006.
- 4.National Spinal Cord Injury Statistical Center Fact Sheet.: National Spinal Cord Injury Center; June 2006.
- 5.Noble J, Munro CA, Prasad VS, Midha R. Analysis of upper and lower extremity peripheral nerve injuries in a population of patients with multiple injuries. J Trauma. 1998;45(1):116–22. doi: 10.1097/00005373-199807000-00025. [DOI] [PubMed] [Google Scholar]
- 6.Archibald SJ, Krarup C, Shefner J, Li ST, Madison RD. A collagen-based nerve guide conduit for peripheral nerve repair: an electrophysiological study of nerve regeneration in rodents and nonhuman primates. J Comp Neurol. 1991;306(4):685–96. doi: 10.1002/cne.903060410. [DOI] [PubMed] [Google Scholar]
- 7.Alcantar NA, Aydil ES, Israelachvili JN. Polyethylene glycol-coated biocompatible surfaces. J Biomed Mater Res. 2000;51(3):343–51. doi: 10.1002/1097-4636(20000905)51:3<343::aid-jbm7>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 8.Elbert DL, Hubbell JA. Conjugate addition reactions combined with free-radical cross-linking for the design of materials for tissue engineering. Biomacromolecules. 2001;2(2):430–41. doi: 10.1021/bm0056299. [DOI] [PubMed] [Google Scholar]
- 9.Peppas NA, Keys KB, Torres-Lugo M, Lowman AM. Poly(ethylene glycol)-containing hydrogels in drug delivery. J Control Release. 1999;62(1–2):81–7. doi: 10.1016/s0168-3659(99)00027-9. [DOI] [PubMed] [Google Scholar]
- 10.Nguyen KT, West JL. Photopolymerizable hydrogels for tissue engineering applications. Biomaterials. 2002;23(22):4307–14. doi: 10.1016/s0142-9612(02)00175-8. [DOI] [PubMed] [Google Scholar]
- 11.Burdick JA, Ward M, Liang E, Young MJ, Langer R. Stimulation of neurite outgrowth by neurotrophins delivered from degradable hydrogels. Biomaterials. 2006;27(3):452–9. doi: 10.1016/j.biomaterials.2005.06.034. [DOI] [PubMed] [Google Scholar]
- 12.Piantino J, Burdick JA, Goldberg D, Langer R, Benowitz LI. An injectable, biodegradable hydrogel for trophic factor delivery enhances axonal rewiring and improves performance after spinal cord injury. Exp Neurol. 2006;201(2):359–67. doi: 10.1016/j.expneurol.2006.04.020. • This paper characterized controlled release of NT-3 in vitro and in vivo and demonstrated that scaffolds for drug delivery can promoted functional recovery in SCI. [DOI] [PubMed] [Google Scholar]
- 13.Elbert DL, Pratt AB, Lutolf MP, Halstenberg S, Hubbell JA. Protein delivery from materials formed by self-selective conjugate addition reactions. J Control Release. 2001;76(1–2):11–25. doi: 10.1016/s0168-3659(01)00398-4. [DOI] [PubMed] [Google Scholar]
- 14.Groll J, Fiedler J, Engelhard E, Ameringer T, Tugulu S, Klok HA, Brenner RE, Moeller M. A novel star PEG-derived surface coating for specific cell adhesion. J Biomed Mater Res A. 2005;74(4):607–17. doi: 10.1002/jbm.a.30335. [DOI] [PubMed] [Google Scholar]
- 15.Benoit DS, Anseth KS. Heparin functionalized PEG gels that modulate protein adsorption for hMSC adhesion and differentiation. Acta Biomater. 2005;1(4):461–70. doi: 10.1016/j.actbio.2005.03.002. [DOI] [PubMed] [Google Scholar]
- 16.Tae G, Scatena M, Stayton PS, Hoffman AS. PEG-cross-linked heparin is an affinity hydrogel for sustained release of vascular endothelial growth factor. J Biomater Sci Polym Ed. 2006;17(1–2):187–97. doi: 10.1163/156856206774879090. [DOI] [PubMed] [Google Scholar]
- 17.During MJ, Freese A, Sabel BA, Saltzman WM, Deutch A, Roth RH, Langer R. Controlled release of dopamine from a polymeric brain implant: in vivo characterization. Ann Neurol. 1989;25(4):351–6. doi: 10.1002/ana.410250406. [DOI] [PubMed] [Google Scholar]
- 18.Freese A, Sabel BA, Saltzman WM, During MJ, Langer R. Controlled release of dopamine from a polymeric brain implant: in vitro characterization. Exp Neurol. 1989;103(3):234–8. doi: 10.1016/0014-4886(89)90047-2. [DOI] [PubMed] [Google Scholar]
- 19.Krewson CE, Klarman ML, Saltzman WM. Distribution of nerve growth factor following direct delivery to brain interstitium. Brain Res. 1995;680(1–2):196–206. doi: 10.1016/0006-8993(95)00261-n. [DOI] [PubMed] [Google Scholar]
- 20.Krewson CE, Saltzman WM. Transport and elimination of recombinant human NGF during long-term delivery to the brain. Brain Res. 1996;727(1–2):169–81. doi: 10.1016/0006-8993(96)00378-2. [DOI] [PubMed] [Google Scholar]
- 21.Mahoney MJ, Saltzman WM. Millimeter-scale positioning of a nerve-growth-factor source and biological activity in the brain. Proc Natl Acad Sci U S A. 1999;96(8):4536–9. doi: 10.1073/pnas.96.8.4536. • This paper defined the minimum distance NGF releasing microspheres needed to be implanted to have an effect on target cells. It also proposed a method for delivering NGF to large irregularly shaped volumes inside the brain based on mathematical modeling the gradients created by the implants. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Powell EM, Sobarzo MR, Saltzman WM. Controlled release of nerve growth factor from a polymeric implant. Brain Res. 1990;515(1–2):309–11. doi: 10.1016/0006-8993(90)90612-f. [DOI] [PubMed] [Google Scholar]
- 23.Saltzman WM, Mak MW, Mahoney MJ, Duenas ET, Cleland JL. Intracranial delivery of recombinant nerve growth factor: release kinetics and protein distribution for three delivery systems. Pharm Res. 1999;16(2):232–40. doi: 10.1023/a:1018824324275. [DOI] [PubMed] [Google Scholar]
- 24.Barras FM, Pasche P, Bouche N, Aebischer P, Zurn AD. Glial cell line-derived neurotrophic factor released by synthetic guidance channels promotes facial nerve regeneration in the rat. J Neurosci Res. 2002;70(6):746–55. doi: 10.1002/jnr.10434. [DOI] [PubMed] [Google Scholar]
- 25.Hoffman D, Wahlberg L, Aebischer P. NGF released from a polymer matrix prevents loss of ChAT expression in basal forebrain neurons following a fimbria-fornix lesion. Exp Neurol. 1990;110(1):39–44. doi: 10.1016/0014-4886(90)90049-x. [DOI] [PubMed] [Google Scholar]
- 26.Bloch J, Fine EG, Bouche N, Zurn AD, Aebischer P. Nerve growth factor- and neurotrophin-3-releasing guidance channels promote regeneration of the transected rat dorsal root. Exp Neurol. 2001;172(2):425–32. doi: 10.1006/exnr.2001.7778. [DOI] [PubMed] [Google Scholar]
- 27.Fine EG, Decosterd I, Papaloizos M, Zurn AD, Aebischer P. GDNF and NGF released by synthetic guidance channels support sciatic nerve regeneration across a long gap. Eur J Neurosci. 2002;15(4):589–601. doi: 10.1046/j.1460-9568.2002.01892.x. [DOI] [PubMed] [Google Scholar]
- 28.Athanasiou KA, Niederauer GG, Agrawal CM. Sterilization, toxicity, biocompatibility and clinical applications of polylactic acid/polyglycolic acid copolymers. Biomaterials. 1996;17(2):93–102. doi: 10.1016/0142-9612(96)85754-1. [DOI] [PubMed] [Google Scholar]
- 29.Gunatillake PA, Adhikari R. Biodegradable synthetic polymers for tissue engineering. Eur Cell Mater. 2003;5:1–16. doi: 10.22203/ecm.v005a01. discussion 16. [DOI] [PubMed] [Google Scholar]
- 30.Nomura H, Tator CH, Shoichet MS. Bioengineered strategies for spinal cord repair. J Neurotrauma. 2006;23(3–4):496–507. doi: 10.1089/neu.2006.23.496. [DOI] [PubMed] [Google Scholar]
- 31.Nomura H, Katayama Y, Shoichet MS, Tator CH. Complete spinal cord transection treated by implantation of a reinforced synthetic hydrogel channel results in syringomyelia and caudal migration of the rostral stump. Neurosurgery. 2006;59(1):183–92. doi: 10.1227/01.NEU.0000219859.35349.EF. discussion 183–92. [DOI] [PubMed] [Google Scholar]
- 32.Tsai EC, Dalton PD, Shoichet MS, Tator CH. Matrix inclusion within synthetic hydrogel guidance channels improves specific supraspinal and local axonal regeneration after complete spinal cord transection. Biomaterials. 2006;27(3):519–33. doi: 10.1016/j.biomaterials.2005.07.025. [DOI] [PubMed] [Google Scholar]
- 33.Tsai EC, Dalton PD, Shoichet MS, Tator CH. Synthetic hydrogel guidance channels facilitate regeneration of adult rat brainstem motor axons after complete spinal cord transection. J Neurotrauma. 2004;21(6):789–804. doi: 10.1089/0897715041269687. [DOI] [PubMed] [Google Scholar]
- 34.Carone TW, Hasenwinkel JM. Mechanical and morphological characterization of homogeneous and bilayered poly(2-hydroxyethyl methacrylate) scaffolds for use in CNS nerve regeneration. J Biomed Mater Res B Appl Biomater. 2006;78(2):274–82. doi: 10.1002/jbm.b.30483. [DOI] [PubMed] [Google Scholar]
- 35.Masuda H, Asano DK. Preparation and properties of polypyrrole. Synthetic Metals. 2003;(135–6):43–44. [Google Scholar]
- 36.George PM, Lyckman AW, LaVan DA, Hegde A, Leung Y, Avasare R, Testa C, Alexander PM, Langer R, Sur M. Fabrication and biocompatibility of polypyrrole implants suitable for neural prosthetics. Biomaterials. 2005;26(17):3511–9. doi: 10.1016/j.biomaterials.2004.09.037. [DOI] [PubMed] [Google Scholar]
- 37.Wang X, Gu X, Yuan C, Chen S, Zhang P, Zhang T, Yao J, Chen F, Chen G. Evaluation of biocompatibility of polypyrrole in vitro and in vivo. J Biomed Mater Res A. 2004;68(3):411–22. doi: 10.1002/jbm.a.20065. [DOI] [PubMed] [Google Scholar]
- 38.Gomez N, Schmidt CE. Nerve growth factor-immobilized polypyrrole: Bioactive electrically conducting polymer for enhanced neurite extension. J Biomed Mater Res A. 2006 doi: 10.1002/jbm.a.31047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Richardson RT, Thompson B, Moulton S, Newbold C, Lum MG, Cameron A, Wallace G, Kapsa R, Clark G, O’Leary S. The effect of polypyrrole with incorporated neurotrophin-3 on the promotion of neurite outgrowth from auditory neurons. Biomaterials. 2007;28(3):513–23. doi: 10.1016/j.biomaterials.2006.09.008. [DOI] [PubMed] [Google Scholar]
- 40.Wadhwa R, Lagenaur CF, Cui XT. Electrochemically controlled release of dexamethasone from conducting polymer polypyrrole coated electrode. J Control Release. 2006;110(3):531–41. doi: 10.1016/j.jconrel.2005.10.027. • This paper utilized the electrical currents delivered by neural implants to create controlled release of a model anti-inflammatory drug. [DOI] [PubMed] [Google Scholar]
- 41.Thompson BC, Moulton SE, Ding J, Richardson R, Cameron A, O’Leary S, Wallace GG, Clark GM. Optimising the incorporation and release of a neurotrophic factor using conducting polypyrrole. J Control Release. 2006 doi: 10.1016/j.jconrel.2006.09.004. [DOI] [PubMed] [Google Scholar]
- 42.Balgude AP, Yu X, Szymanski A, Bellamkonda RV. Agarose gel stiffness determines rate of DRG neurite extension in 3D cultures. Biomaterials. 2001;22(10):1077–84. doi: 10.1016/s0142-9612(00)00350-1. [DOI] [PubMed] [Google Scholar]
- 43.Bellamkonda R, Ranieri JP, Bouche N, Aebischer P. Hydrogel-based three-dimensional matrix for neural cells. J Biomed Mater Res. 1995;29(5):663–71. doi: 10.1002/jbm.820290514. [DOI] [PubMed] [Google Scholar]
- 44.Cao X, Shoichet MS. Defining the concentration gradient of nerve growth factor for guided neurite outgrowth. Neuroscience. 2001;103(3):831–40. doi: 10.1016/s0306-4522(01)00029-x. [DOI] [PubMed] [Google Scholar]
- 45.Dillon GP, Yu X, Sridharan A, Ranieri JP, Bellamkonda RV. The influence of physical structure and charge on neurite extension in a 3D hydrogel scaffold. J Biomater Sci Polym Ed. 1998;9(10):1049–69. doi: 10.1163/156856298x00325. [DOI] [PubMed] [Google Scholar]
- 46.Jain A, Kim YT, McKeon RJ, Bellamkonda RV. In situ gelling hydrogels for conformal repair of spinal cord defects, and local delivery of BDNF after spinal cord injury. Biomaterials. 2006;27(3):497–504. doi: 10.1016/j.biomaterials.2005.07.008. [DOI] [PubMed] [Google Scholar]
- 47.Kataoka K, Suzuki Y, Kitada M, Hashimoto T, Chou H, Bai H, Ohta M, Wu S, Suzuki K, Ide C. Alginate enhances elongation of early regenerating axons in spinal cord of young rats. Tissue Eng. 2004;10(3–4):493–504. doi: 10.1089/107632704323061852. [DOI] [PubMed] [Google Scholar]
- 48.Kataoka K, Suzuki Y, Kitada M, Ohnishi K, Suzuki K, Tanihara M, Ide C, Endo K, Nishimura Y. Alginate, a bioresorbable material derived from brown seaweed, enhances elongation of amputated axons of spinal cord in infant rats. J Biomed Mater Res. 2001;54(3):373–84. doi: 10.1002/1097-4636(20010305)54:3<373::aid-jbm90>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 49.Maysinger D, Krieglstein K, Filipovic-Grcic J, Sendtner M, Unsicker K, Richardson P. Microencapsulated ciliary neurotrophic factor: physical properties and biological activities. Exp Neurol. 1996;138(2):177–88. doi: 10.1006/exnr.1996.0056. [DOI] [PubMed] [Google Scholar]
- 50.Prang P, Muller R, Eljaouhari A, Heckmann K, Kunz W, Weber T, Faber C, Vroemen M, Bogdahn U, Weidner N. The promotion of oriented axonal regrowth in the injured spinal cord by alginate-based anisotropic capillary hydrogels. Biomaterials. 2006;27(19):3560–9. doi: 10.1016/j.biomaterials.2006.01.053. [DOI] [PubMed] [Google Scholar]
- 51.Stabenfeldt SE, Garcia AJ, LaPlaca MC. Thermoreversible laminin-functionalized hydrogel for neural tissue engineering. J Biomed Mater Res A. 2006;77(4):718–25. doi: 10.1002/jbm.a.30638. [DOI] [PubMed] [Google Scholar]
- 52.Tate MC, Shear DA, Hoffman SW, Stein DG, LaPlaca MC. Biocompatibility of methylcellulose-based constructs designed for intracerebral gelation following experimental traumatic brain injury. Biomaterials. 2001;22(10):1113–23. doi: 10.1016/s0142-9612(00)00348-3. [DOI] [PubMed] [Google Scholar]
- 53.Zhong Y, Bellamkonda RV. Controlled release of anti-inflammatory agent alpha-MSH from neural implants. J Control Release. 2005;106(3):309–18. doi: 10.1016/j.jconrel.2005.05.012. [DOI] [PubMed] [Google Scholar]
- 54.Crompton KE, Goud JD, Bellamkonda RV, Gengenbach TR, Finkelstein DI, Horne MK, Forsythe JS. Polylysine-functionalised thermoresponsive chitosan hydrogel for neural tissue engineering. Biomaterials. 2007;28(3):441–9. doi: 10.1016/j.biomaterials.2006.08.044. [DOI] [PubMed] [Google Scholar]
- 55.Freier T, Koh HS, Kazazian K, Shoichet MS. Controlling cell adhesion and degradation of chitosan films by N-acetylation. Biomaterials. 2005;26(29):5872–8. doi: 10.1016/j.biomaterials.2005.02.033. [DOI] [PubMed] [Google Scholar]
- 56.Gamez E, Goto Y, Nagata K, Iwaki T, Sasaki T, Matsuda T. Photofabricated gelatin-based nerve conduits: nerve tissue regeneration potentials. Cell Transplant. 2004;13(5):549–64. doi: 10.3727/000000004783983639. [DOI] [PubMed] [Google Scholar]
- 57.Mahoney MJ, Krewson C, Miller J, Saltzman WM. Impact of Cell Type and Density on Nerve Growth Factor Distribution and Bioactivity in 3-Dimensional Collagen Gel Cultures. Tissue Eng. 2006 doi: 10.1089/ten.2006.12.1915. [DOI] [PubMed] [Google Scholar]
- 58.Wissink MJ, Beernink R, Pieper JS, Poot AA, Engbers GH, Beugeling T, van Aken WG, Feijen J. Binding and release of basic fibroblast growth factor from heparinized collagen matrices. Biomaterials. 2001;22(16):2291–9. doi: 10.1016/s0142-9612(00)00418-x. [DOI] [PubMed] [Google Scholar]
- 59.Wissink MJ, Beernink R, Pieper JS, Poot AA, Engbers GH, Beugeling T, van Aken WG, Feijen J. Immobilization of heparin to EDC/NHS-crosslinked collagen. Characterization and in vitro evaluation. Biomaterials. 2001;22(2):151–63. doi: 10.1016/s0142-9612(00)00164-2. [DOI] [PubMed] [Google Scholar]
- 60.Massia SP, Holecko MM, Ehteshami GR. In vitro assessment of bioactive coatings for neural implant applications. J Biomed Mater Res A. 2004;68(1):177–86. doi: 10.1002/jbm.a.20009. [DOI] [PubMed] [Google Scholar]
- 61.Klaver CL, Caplan MC. Bioactive surface for neural electrodes: decreasing astrocyte proliferation via transforming growth factor beta-1. J Biomed Mater Res A. 2006 doi: 10.1002/jbm.a.31153. [DOI] [PubMed] [Google Scholar]
- 62.Levesque SG, Shoichet MS. Synthesis of cell-adhesive dextran hydrogels and macroporous scaffolds. Biomaterials. 2006;27(30):5277–85. doi: 10.1016/j.biomaterials.2006.06.004. [DOI] [PubMed] [Google Scholar]
- 63.Herbert CB, Bittner GD, Hubbell JA. Effects of fibinolysis on neurite growth from dorsal root ganglia cultured in two- and three-dimensional fibrin gels. J Comp Neurol. 1996;365(3):380–91. doi: 10.1002/(SICI)1096-9861(19960212)365:3<380::AID-CNE4>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 64.Sakiyama SE, Schense JC, Hubbell JA. Incorporation of heparin-binding peptides into fibrin gels enhances neurite extension: an example of designer matrices in tissue engineering. Faseb J. 1999;13(15):2214–24. doi: 10.1096/fasebj.13.15.2214. [DOI] [PubMed] [Google Scholar]
- 65.Sakiyama-Elbert SE, Hubbell JA. Controlled release of nerve growth factor from a heparin-containing fibrin-based cell ingrowth matrix. J Control Release. 2000;69(1):149–58. doi: 10.1016/s0168-3659(00)00296-0. • This paper demonstrated that neurotrophins could be delivered from a heparin based delivery system incorporated into a fibrin scaffold. [DOI] [PubMed] [Google Scholar]
- 66.Sakiyama-Elbert SE, Hubbell JA. Development of fibrin derivatives for controlled release of heparin-binding growth factors. J Control Release. 2000;65(3):389–402. doi: 10.1016/s0168-3659(99)00221-7. [DOI] [PubMed] [Google Scholar]
- 67.Sakiyama-Elbert SE, Panitch A, Hubbell JA. Development of growth factor fusion proteins for cell-triggered drug delivery. Faseb J. 2001;15(7):1300–2. doi: 10.1096/fj.00-0564fje. [DOI] [PubMed] [Google Scholar]
- 68.Schense JC, Hubbell JA. Cross-linking exogenous bifunctional peptides into fibrin gels with factor XIIIa. Bioconjug Chem. 1999;10(1):75–81. doi: 10.1021/bc9800769. [DOI] [PubMed] [Google Scholar]
- 69.Ahmed Z, Underwood S, Brown RA. Nerve guide material made from fibronectin: assessment of in vitro properties. Tissue Eng. 2003;9(2):219–31. doi: 10.1089/107632703764664693. [DOI] [PubMed] [Google Scholar]
- 70.Brown RA, Blunn GW, Ejim OS. Preparation of orientated fibrous mats from fibronectin: composition and stability. Biomaterials. 1994;15(6):457–64. doi: 10.1016/0142-9612(94)90225-9. [DOI] [PubMed] [Google Scholar]
- 71.Ejim OS, Blunn GW, Brown RA. Production of artificial-orientated mats and strands from plasma fibronectin: a morphological study. Biomaterials. 1993;14(10):743–8. doi: 10.1016/0142-9612(93)90038-4. [DOI] [PubMed] [Google Scholar]
- 72.King VR, Henseler M, Brown RA, Priestley JV. Mats made from fibronectin support oriented growth of axons in the damaged spinal cord of the adult rat. Exp Neurol. 2003;182(2):383–98. doi: 10.1016/s0014-4886(03)00033-5. [DOI] [PubMed] [Google Scholar]
- 73.King VR, Phillips JB, Hunt-Grubbe H, Brown R, Priestley JV. Characterization of non-neuronal elements within fibronectin mats implanted into the damaged adult rat spinal cord. Biomaterials. 2006;27(3):485–96. doi: 10.1016/j.biomaterials.2005.06.033. [DOI] [PubMed] [Google Scholar]
- 74.Phillips JB, King VR, Ward Z, Porter RA, Priestley JV, Brown RA. Fluid shear in viscous fibronectin gels allows aggregation of fibrous materials for CNS tissue engineering. Biomaterials. 2004;25(14):2769–79. doi: 10.1016/j.biomaterials.2003.09.052. [DOI] [PubMed] [Google Scholar]
- 75.Gupta D, Tator CH, Shoichet MS. Fast-gelling injectable blend of hyaluronan and methylcellulose for intrathecal, localized delivery to the injured spinal cord. Biomaterials. 2006;27(11):2370–9. doi: 10.1016/j.biomaterials.2005.11.015. [DOI] [PubMed] [Google Scholar]
- 76.Wiesmann C, de Vos AM. Nerve growth factor: structure and function. Cell Mol Life Sci. 2001;58(5–6):748–59. doi: 10.1007/PL00000898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Huang EJ, Reichardt LF. Neurotrophins: roles in neuronal development and function. Annu Rev Neurosci. 2001;24:677–736. doi: 10.1146/annurev.neuro.24.1.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Siegel GJ, Chauhan NB. Neurotrophic factors in Alzheimer’s and Parkinson’s disease brain. Brain Res Brain Res Rev. 2000;33(2–3):199–227. doi: 10.1016/s0165-0173(00)00030-8. [DOI] [PubMed] [Google Scholar]
- 79.Romero MI, Rangappa N, Garry MG, Smith GM. Functional regeneration of chronically injured sensory afferents into adult spinal cord after neurotrophin gene therapy. J Neurosci. 2001;21(21):8408–16. doi: 10.1523/JNEUROSCI.21-21-08408.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Grill R, Murai K, Blesch A, Gage FH, Tuszynski MH. Cellular delivery of neurotrophin-3 promotes corticospinal axonal growth and partial functional recovery after spinal cord injury. J Neurosci. 1997;17(14):5560–72. doi: 10.1523/JNEUROSCI.17-14-05560.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Taylor SJ, McDonald JW, 3rd, Sakiyama-Elbert SE. Controlled release of neurotrophin-3 from fibrin gels for spinal cord injury. J Control Release. 2004;98(2):281–94. doi: 10.1016/j.jconrel.2004.05.003. [DOI] [PubMed] [Google Scholar]
- 82.Taylor SJ, Sakiyama-Elbert SE. Effect of controlled delivery of neurotrophin-3 from fibrin on spinal cord injury in a long term model. J Control Release. 2006 doi: 10.1016/j.jconrel.2006.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Taylor SJ, Rosenzweig ES, McDonald JW, 3rd, Sakiyama-Elbert SE. Delivery of neurotrophin-3 from fibrin enhances neuronal fiber sprouting after spinal cord injury. J Control Release. 2006;113(3):226–35. doi: 10.1016/j.jconrel.2006.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Bramham CR, Messaoudi E. BDNF function in adult synaptic plasticity: the synaptic consolidation hypothesis. Prog Neurobiol. 2005;76(2):99–125. doi: 10.1016/j.pneurobio.2005.06.003. [DOI] [PubMed] [Google Scholar]
- 85.Patist CM, Mulder MB, Gautier SE, Maquet V, Jerome R, Oudega M. Freeze-dried poly(D,L-lactic acid) macroporous guidance scaffolds impregnated with brain-derived neurotrophic factor in the transected adult rat thoracic spinal cord. Biomaterials. 2004;25(9):1569–82. doi: 10.1016/s0142-9612(03)00503-9. [DOI] [PubMed] [Google Scholar]
- 86.Vergara C, Ramirez B. CNTF, a pleiotropic cytokine: emphasis on its myotrophic role. Brain Res Brain Res Rev. 2004;47(1–3):161–73. doi: 10.1016/j.brainresrev.2004.07.010. [DOI] [PubMed] [Google Scholar]
- 87.Ishii K, Nakamura M, Dai H, Finn TP, Okano H, Toyama Y, Bregman BS. Neutralization of ciliary neurotrophic factor reduces astrocyte production from transplanted neural stem cells and promotes regeneration of corticospinal tract fibers in spinal cord injury. J Neurosci Res. 2006;84(8):1669–81. doi: 10.1002/jnr.21079. [DOI] [PubMed] [Google Scholar]
- 88.Grothe C, Haastert K, Jungnickel J. Physiological function and putative therapeutic impact of the FGF-2 system in peripheral nerve regeneration--lessons from in vivo studies in mice and rats. Brain Res Brain Res Rev. 2006;51(2):293–9. doi: 10.1016/j.brainresrev.2005.12.001. [DOI] [PubMed] [Google Scholar]
- 89.Kim DH, Martin DC. Sustained release of dexamethasone from hydrophilic matrices using PLGA nanoparticles for neural drug delivery. Biomaterials. 2006;27(15):3031–7. doi: 10.1016/j.biomaterials.2005.12.021. [DOI] [PubMed] [Google Scholar]
- 90.Ichiyama T, Campbell IL, Furukawa S, Catania A, Lipton JM. Autocrine α-melanocyte-stimulating hormone inhibits NF-kappaB activation in human glioma. J Neurosci Res. 1999;58(5):684–9. [PubMed] [Google Scholar]
- 91.Wells MR, Kraus K, Batter DK, Blunt DG, Weremowitz J, Lynch SE, Antoniades HN, Hansson HA. Gel matrix vehicles for growth factor application in nerve gap injuries repaired with tubes: a comparison of biomatrix, collagen, and methylcellulose. Exp Neurol. 1997;146(2):395–402. doi: 10.1006/exnr.1997.6543. [DOI] [PubMed] [Google Scholar]
- 92.Yu X, Bellamkonda RV. Tissue-engineered scaffolds are effective alternatives to autografts for bridging peripheral nerve gaps. Tissue Eng. 2003;9(3):421–30. doi: 10.1089/107632703322066606. [DOI] [PubMed] [Google Scholar]
- 93.Houweling DA, Lankhorst AJ, Gispen WH, Bar PR, Joosten EA. Collagen containing neurotrophin-3 (NT-3) attracts regrowing injured corticospinal axons in the adult rat spinal cord and promotes partial functional recovery. Exp Neurol. 1998;153(1):49–59. doi: 10.1006/exnr.1998.6867. [DOI] [PubMed] [Google Scholar]
- 94.Moore K, MacSween M, Shoichet M. Immobilized concentration gradients of neurotrophic factors guide neurite outgrowth of primary neurons in macroporous scaffolds. Tissue Eng. 2006;12(2):267–78. doi: 10.1089/ten.2006.12.267. [DOI] [PubMed] [Google Scholar]
- 95.Winter JO, Cogan SF, Rizzo JF., 3rd Neurotrophin-eluting hydrogel coatings for neural stimulating electrodes. J Biomed Mater Res B Appl Biomater. 2006 doi: 10.1002/jbm.b.30696. [DOI] [PubMed] [Google Scholar]
- 96.Benoit JP, Faisant N, Venier-Julienne MC, Menei P. Development of microspheres for neurological disorders: from basics to clinical applications. J Control Release. 2000;65(1–2):285–96. doi: 10.1016/s0168-3659(99)00250-3. [DOI] [PubMed] [Google Scholar]
- 97.Mahoney MJ, Saltzman WM. Controlled release of proteins to tissue transplants for the treatment of neurodegenerative disorders. J Pharm Sci. 1996;85(12):1276–81. doi: 10.1021/js9601602. [DOI] [PubMed] [Google Scholar]
- 98.Lam XM, Duenas ET, Cleland JL. Encapsulation and stabilization of nerve growth factor into poly(lactic-co-glycolic) acid microspheres. J Pharm Sci. 2001;90(9):1356–65. doi: 10.1002/jps.1088. [DOI] [PubMed] [Google Scholar]
- 99.Aubert-Pouessel A, Venier-Julienne MC, Clavreul A, Sergent M, Jollivet C, Montero-Menei CN, Garcion E, Bibby DC, Menei P, Benoit JP. In vitro study of GDNF release from biodegradable PLGA microspheres. J Control Release. 2004;95(3):463–75. doi: 10.1016/j.jconrel.2003.12.012. [DOI] [PubMed] [Google Scholar]
- 100.Rosner BI, Siegel RA, Grosberg A, Tranquillo RT. Rational design of contact guiding, neurotrophic matrices for peripheral nerve regeneration. Ann Biomed Eng. 2003;31(11):1383–401. doi: 10.1114/1.1626118. [DOI] [PubMed] [Google Scholar]
- 101.Yang Y, De Laporte L, Rives CB, Jang JH, Lin WC, Shull KR, Shea LD. Neurotrophin releasing single and multiple lumen nerve conduits. J Control Release. 2005;104(3):433–46. doi: 10.1016/j.jconrel.2005.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Goraltchouk A, Scanga V, Morshead CM, Shoichet MS. Incorporation of protein-eluting microspheres into biodegradable nerve guidance channels for controlled release. J Control Release. 2006;110(2):400–7. doi: 10.1016/j.jconrel.2005.10.019. [DOI] [PubMed] [Google Scholar]
- 103.Edelman ER, Mathiowitz E, Langer R, Klagsbrun M. Controlled and modulated release of basic fibroblast growth factor. Biomaterials. 1991;12(7):619–26. doi: 10.1016/0142-9612(91)90107-l. [DOI] [PubMed] [Google Scholar]
- 104.Lee AC, Yu VM, Lowe JB, 3rd, Brenner MJ, Hunter DA, Mackinnon SE, Sakiyama-Elbert SE. Controlled release of nerve growth factor enhances sciatic nerve regeneration. Exp Neurol. 2003;184(1):295–303. doi: 10.1016/s0014-4886(03)00258-9. [DOI] [PubMed] [Google Scholar]
- 105.Maxwell DJ, Hicks BC, Parsons S, Sakiyama-Elbert SE. Development of rationally designed affinity-based drug delivery systems. Acta Biomater. 2005;1(1):101–13. doi: 10.1016/j.actbio.2004.09.002. [DOI] [PubMed] [Google Scholar]
- 106.Willerth SM, Johnson PJ, Maxwell DJ, Parsons SR, Doukas ME, Sakiyama-Elbert SE. Rationally designed peptides for controlled release of nerve growth factor from fibrin matrices. J Biomed Mater Res A. 2006 doi: 10.1002/jbm.a.30844. [DOI] [PubMed] [Google Scholar]
- 107.Kapur TA, Shoichet MS. Chemically-bound nerve growth factor for neural tissue engineering applications. J Biomater Sci Polym Ed. 2003;14(4):383–94. doi: 10.1163/156856203321478883. • This paper describes how to use photochemistry to covalently incorporate NGF into scaffolds for drug delivery. [DOI] [PubMed] [Google Scholar]
- 108.Gomez N, Lu Y, Chen S, Schmidt CE. Immobilized nerve growth factor and microtopography have distinct effects on polarization versus axon elongation in hippocampal cells in culture. Biomaterials. 2007;28(2):271–84. doi: 10.1016/j.biomaterials.2006.07.043. [DOI] [PubMed] [Google Scholar]
- 109.Yim EK, Leong KW. Proliferation and differentiation of human embryonic germ cell derivatives in bioactive polymeric fibrous scaffold. J Biomater Sci Polym Ed. 2005;16(10):1193–217. doi: 10.1163/156856205774269485. [DOI] [PubMed] [Google Scholar]
- 110.Bradbury EJ, Moon LD, Popat RJ, King VR, Bennett GS, Patel PN, Fawcett JW, McMahon SB. Chondroitinase ABC promotes functional recovery after spinal cord injury. Nature. 2002;416(6881):636–40. doi: 10.1038/416636a. [DOI] [PubMed] [Google Scholar]
- 111.Barritt AW, Davies M, Marchand F, Hartley R, Grist J, Yip P, McMahon SB, Bradbury EJ. Chondroitinase ABC promotes sprouting of intact and injured spinal systems after spinal cord injury. J Neurosci. 2006;26(42):10856–67. doi: 10.1523/JNEUROSCI.2980-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Kim JE, Liu BP, Park JH, Strittmatter SM. Nogo-66 receptor prevents raphespinal and rubrospinal axon regeneration and limits functional recovery from spinal cord injury. Neuron. 2004;44(3):439–51. doi: 10.1016/j.neuron.2004.10.015. [DOI] [PubMed] [Google Scholar]
- 113.Li S, Strittmatter SM. Delayed systemic Nogo-66 receptor antagonist promotes recovery from spinal cord injury. J Neurosci. 2003;23(10):4219–27. doi: 10.1523/JNEUROSCI.23-10-04219.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Pearse DD, Pereira FC, Marcillo AE, Bates ML, Berrocal YA, Filbin MT, Bunge MB. cAMP and Schwann cells promote axonal growth and functional recovery after spinal cord injury. Nat Med. 2004;10(6):610–6. doi: 10.1038/nm1056. [DOI] [PubMed] [Google Scholar]
- 115.Lu P, Yang H, Jones LL, Filbin MT, Tuszynski MH. Combinatorial therapy with neurotrophins and cAMP promotes axonal regeneration beyond sites of spinal cord injury. J Neurosci. 2004;24(28):6402–9. doi: 10.1523/JNEUROSCI.1492-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Krause TL, Bittner GD. Rapid morphological fusion of severed myelinated axons by polyethylene glycol. Proc Natl Acad Sci U S A. 1990;87(4):1471–5. doi: 10.1073/pnas.87.4.1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Lietz M, Ullrich A, Schulte-Eversum C, Oberhoffner S, Fricke C, Muller HW, Schlosshauer B. Physical and biological performance of a novel block copolymer nerve guide. Biotechnol Bioeng. 2006;93(1):99–109. doi: 10.1002/bit.20688. [DOI] [PubMed] [Google Scholar]
- 118.Mahoney MJ, Anseth KS. Three-dimensional growth and function of neural tissue in degradable polyethylene glycol hydrogels. Biomaterials. 2006;27(10):2265–74. doi: 10.1016/j.biomaterials.2005.11.007. [DOI] [PubMed] [Google Scholar]
- 119.Bryan DJ, Tang JB, Doherty SA, Hile DD, Trantolo DJ, Wise DL, Summerhayes IC. Enhanced peripheral nerve regeneration through a poled bioresorbable poly(lactic-co-glycolic acid) guidance channel. J Neural Eng. 2004;1(2):91–8. doi: 10.1088/1741-2560/1/2/004. [DOI] [PubMed] [Google Scholar]
- 120.Evans GR, Brandt K, Niederbichler AD, Chauvin P, Herrman S, Bogle M, Otta L, Wang B, Patrick CW., Jr Clinical long-term in vivo evaluation of poly(L-lactic acid) porous conduits for peripheral nerve regeneration. J Biomater Sci Polym Ed. 2000;11(8):869–78. doi: 10.1163/156856200744066. [DOI] [PubMed] [Google Scholar]
- 121.Sundback CA, Shyu JY, Wang Y, Faquin WC, Langer RS, Vacanti JP, Hadlock TA. Biocompatibility analysis of poly(glycerol sebacate) as a nerve guide material. Biomaterials. 2005;26(27):5454–64. doi: 10.1016/j.biomaterials.2005.02.004. [DOI] [PubMed] [Google Scholar]
- 122.Widmer MS, Gupta PK, Lu L, Meszlenyi RK, Evans GR, Brandt K, Savel T, Gurlek A, Patrick CW, Jr, Mikos AG. Manufacture of porous biodegradable polymer conduits by an extrusion process for guided tissue regeneration. Biomaterials. 1998;19(21):1945–55. doi: 10.1016/s0142-9612(98)00099-4. [DOI] [PubMed] [Google Scholar]
- 123.Evans GR, Brandt K, Widmer MS, Lu L, Meszlenyi RK, Gupta PK, Mikos AG, Hodges J, Williams J, Gurlek A, Nabawi A, Lohman R, Patrick CW., Jr In vivo evaluation of poly(L-lactic acid) porous conduits for peripheral nerve regeneration. Biomaterials. 1999;20(12):1109–15. doi: 10.1016/s0142-9612(99)00010-1. [DOI] [PubMed] [Google Scholar]
- 124.Teng YD, Lavik EB, Qu X, Park KI, Ourednik J, Zurakowski D, Langer R, Snyder EY. Functional recovery following traumatic spinal cord injury mediated by a unique polymer scaffold seeded with neural stem cells. Proc Natl Acad Sci U S A. 2002;99(5):3024–9. doi: 10.1073/pnas.052678899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Hadlock T, Elisseeff J, Langer R, Vacanti J, Cheney M. A tissue-engineered conduit for peripheral nerve repair. Arch Otolaryngol Head Neck Surg. 1998;124(10):1081–6. doi: 10.1001/archotol.124.10.1081. [DOI] [PubMed] [Google Scholar]
- 126.Lesny P, De Croos J, Pradny M, Vacik J, Michalek J, Woerly S, Sykova E. Polymer hydrogels usable for nervous tissue repair. J Chem Neuroanat. 2002;23(4):243–7. doi: 10.1016/s0891-0618(02)00011-x. [DOI] [PubMed] [Google Scholar]
- 127.Belkas JS, Munro CA, Shoichet MS, Johnston M, Midha R. Long-term in vivo biomechanical properties and biocompatibility of poly(2-hydroxyethyl methacrylate-co-methyl methacrylate) nerve conduits. Biomaterials. 2005;26(14):1741–9. doi: 10.1016/j.biomaterials.2004.05.031. [DOI] [PubMed] [Google Scholar]
- 128.Schmidt CE, Shastri VR, Vacanti JP, Langer R. Stimulation of neurite outgrowth using an electrically conducting polymer. Proc Natl Acad Sci U S A. 1997;94(17):8948–53. doi: 10.1073/pnas.94.17.8948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Chen SJ, Wang DY, Wang X, Zhang PY, Gu X. Template synthesis of the polypyrrole tube and its bridging for sciatic nerve regneration. Journal of Material Science Letters. 2000;19(23):2157–2159. [Google Scholar]
- 130.Ikegame M, Tajima K, Aida T. Template synthesis of polypyrrole nanofibers insulated within one-dimensional silicate channels: hexagonal versus lamellar for recombination of polarons into bipolarons. Angew Chem Int Ed Engl. 2003;42(19):2154–7. doi: 10.1002/anie.200250800. [DOI] [PubMed] [Google Scholar]
- 131.Freier T, Montenegro R, Shan Koh H, Shoichet MS. Chitin-based tubes for tissue engineering in the nervous system. Biomaterials. 2005;26(22):4624–32. doi: 10.1016/j.biomaterials.2004.11.040. [DOI] [PubMed] [Google Scholar]
- 132.Guenard V, Valentini RF, Aebischer P. Influence of surface texture of polymeric sheets on peripheral nerve regeneration in a two-compartment guidance system. Biomaterials. 1991;12(2):259–63. doi: 10.1016/0142-9612(91)90210-2. [DOI] [PubMed] [Google Scholar]
- 133.Zhong Y, Yu X, Gilbert R, Bellamkonda RV. Stabilizing electrode-host interfaces: a tissue engineering approach. J Rehabil Res Dev. 2001;38(6):627–32. [PubMed] [Google Scholar]
- 134.Williams LR. Exogenous fibrin matrix precursors stimulate the temporal progress of nerve regeneration within a silicone chamber. Neurochem Res. 1987;12(10):851–60. doi: 10.1007/BF00966306. [DOI] [PubMed] [Google Scholar]
- 135.Williams LR, Danielsen N, Muller H, Varon S. Exogenous matrix precursors promote functional nerve regeneration across a 15-mm gap within a silicone chamber in the rat. J Comp Neurol. 1987;264(2):284–90. doi: 10.1002/cne.902640211. [DOI] [PubMed] [Google Scholar]
- 136.Hahn SK, Jelacic S, Maier RV, Stayton PS, Hoffman AS. Anti-inflammatory drug delivery from hyaluronic acid hydrogels. J Biomater Sci Polym Ed. 2004;15(9):1111–9. doi: 10.1163/1568562041753115. [DOI] [PubMed] [Google Scholar]
- 137.Hou S, Tian W, Xu Q, Cui F, Zhang J, Lu Q, Zhao C. The enhancement of cell adherence and inducement of neurite outgrowth of dorsal root ganglia co-cultured with hyaluronic acid hydrogels modified with Nogo-66 receptor antagonist in vitro. Neuroscience. 2006;137(2):519–29. doi: 10.1016/j.neuroscience.2005.09.029. [DOI] [PubMed] [Google Scholar]
- 138.Hou S, Xu Q, Tian W, Cui F, Cai Q, Ma J, Lee IS. The repair of brain lesion by implantation of hyaluronic acid hydrogels modified with laminin. J Neurosci Methods. 2005;148(1):60–70. doi: 10.1016/j.jneumeth.2005.04.016. [DOI] [PubMed] [Google Scholar]
- 139.Seckel BR, Jones D, Hekimian KJ, Wang KK, Chakalis DP, Costas PD. Hyaluronic acid through a new injectable nerve guide delivery system enhances peripheral nerve regeneration in the rat. J Neurosci Res. 1995;40(3):318–24. doi: 10.1002/jnr.490400305. [DOI] [PubMed] [Google Scholar]
- 140.Tian WM, Hou SP, Ma J, Zhang CL, Xu QY, Lee IS, Li HD, Spector M, Cui FZ. Hyaluronic acid-poly-D-lysine-based three-dimensional hydrogel for traumatic brain injury. Tissue Eng. 2005;11(3–4):513–25. doi: 10.1089/ten.2005.11.513. [DOI] [PubMed] [Google Scholar]


