Summary
In C. elegans, loss-of-function (lf) mutations of the stomatin-like protein (SLP) UNC-1 and the innexin UNC-9 inhibit locomotion [1, 2] and modulate sensitivity to volatile anesthetics [3, 4]. It was unknown why unc-1(lf) and unc-9(lf) mutants have similar phenotypes. We tested the hypothesis that UNC-1 is a regulator of gap junctions formed by UNC-9. Analyses of junctional currents between body-wall muscle cells showed that electrical coupling was inhibited to a similar degree in unc-1(lf), unc-9(lf), and unc-1(lf);unc-9(lf) double mutant, suggesting that UNC-1 and UNC-9 function together. Expression of Punc-1::DsRED2 and Punc-9::GFP transcriptional fusions suggests that unc-1 and unc-9 are coexpressed in neurons and body-wall muscle cells. Immunohistochemistry showed that UNC-1 and UNC-9 colocalized at intercellular junctions, and that unc-1(lf) did not alter UNC-9 expression or subcellular localization. Bimolecular fluorescence complementation (BiFC) assays suggest that UNC-1 and UNC-9 are physically very close at intercellular junctions. Targeted rescue experiments suggest that UNC-9 and UNC-1 function predominantly in neurons to control locomotion. Thus, in addition to the recently reported function of regulating mechanosensitive ion channels [5, 6], SLPs may have a novel function of regulating gap junctions.
Results
UNC-1 dysfunction inhibited electrical coupling of body-wall muscle cells
We previously showed that C. elegans body-wall muscle cells are electrically coupled and that the innexin UNC-9 plays a major role in the coupling [7]. To determine whether UNC-1 regulates gap junctions formed by UNC-9, we analyzed junctional currents (Ij) of body-wall muscle cells in wild-type, unc-1 mutant, and unc-9 mutant. Muscle cells in the two ventral quadrants were analyzed in pairs using the dual whole-cell voltage clamp technique. Compared with wild-type preparations, intra-quadrant coupling (between a pair of neighboring R1-R2 or L1-L2 cells, Figure 1A) was inhibited by approximately 70% in both unc-1(e719) and unc-1(fc53) mutants (Figure 1B), which are putative nulls resulting from premature termination [4, 8]. The coupling defect of unc-1(e719) was completely rescued when wild-type UNC-1 was expressed specifically in body-wall muscle cells under the control of the myosin promoter Pmyo-3 [9] (Figure 1B). These results suggest that UNC-1 is required for normal electrical coupling of body-wall muscle cells.
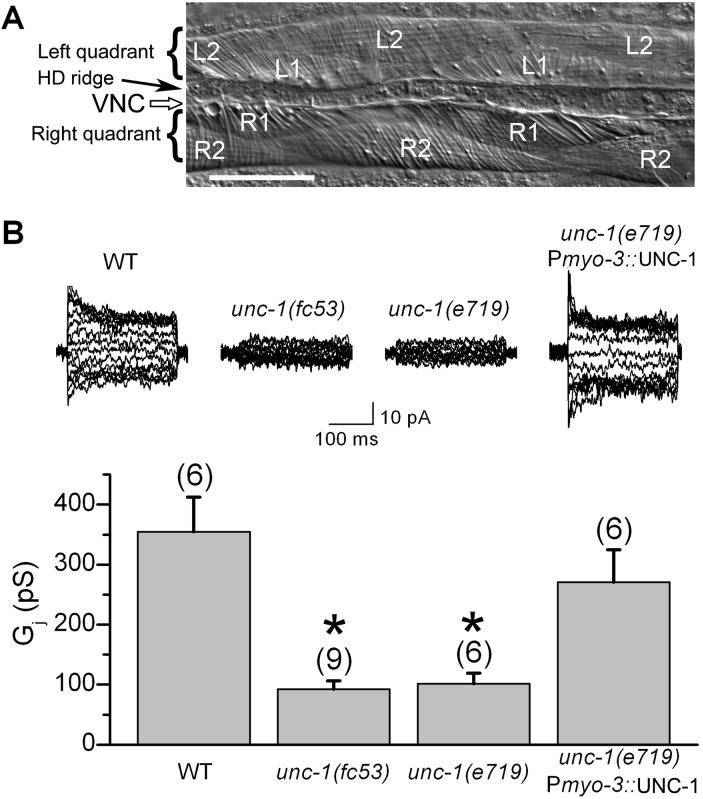
Figure 1.
Electrical coupling of body-wall muscle cells was deficient in unc-1 mutants. A: Photograph of a muscle preparation for electrophysiological recording. The two ventral quadrants, with the ventral nerve cord (VNC) and hypodermal ridge (HD ridge) between them, are shown. Each quadrant consists of a monolayer of two rows of muscle cells. Cells in the right quadrant are designated as R1 or R2, whereas those in the left quadrant as L1 or L2. Scale bar = 50 μm. B: Intra-quadrant coupling (between a pair of neighboring R1-R2 or L1-L2 cells) was significantly inhibited in unc-1(fc53) and unc-1(e719). The coupling defect of unc-1(e719) could be rescued by expressing wild-type UNC-1 specifically in body-wall muscle cells under the control of the myosin promoter Pmyo-3. Representative junctional currents and means of the junctional conductance (Gj) are shown. The asterisk indicates a statistically significant difference compared with the wild-type (one-way ANOVA followed by Bonferroni posthoc tests). The number of samples analyzed is indicated by the value above each column.
Intra-quadrant coupling of body-wall muscle cells is mediated by UNC-9-dependent as well as UNC-9-independent gap junctions, as suggested by the presence of residual coupling in unc-9(fc16) [7], a putative null mutant resulting from a premature stop in the intracellular loop between the second and third membrane spanning domains [10]. To determine if UNC-1 is required specifically for UNC-9-based gap junctions, we compared the degrees of intra-quadrant coupling among wild-type, unc-9(fc16), unc-1(e719), and unc-9(fc16);unc-1(e719) double mutant. Intra-quadrant coupling was inhibited to a similar degree in unc-9(fc16) and unc-1(e719), and was not aggravated in the double mutant (Figure 2A), suggesting that UNC-1 and UNC-9 likely function in the same pathway, and that the residual coupling mediated by other innexin(s) is independent of UNC-1. We previously showed that inter-quadrant coupling (between a pair of neighboring Rl-L1 cells at the same position along the body axis, Figure 1A) was essentially absent in unc-9(fc16) mutant, and the deficiency could be rescued by expressing wild-type UNC-9 [7]. To determine whether UNC-1 is required for the function of UNC-9, we tested whether unc-1(lf) would abolish the rescuing effect of wild-type UNC-9. Indeed, expression of wild-type UNC-9 failed to rescue the coupling defect of unc-9(fc16) in unc-1(e719) genetic background (Figure 2B), suggesting that the function of UNC-9-based gap junctions requires UNC-1. Thus, UNC-1 appears to be required specifically for the function of UNC-9-based gap junctions in body-wall muscle cells.
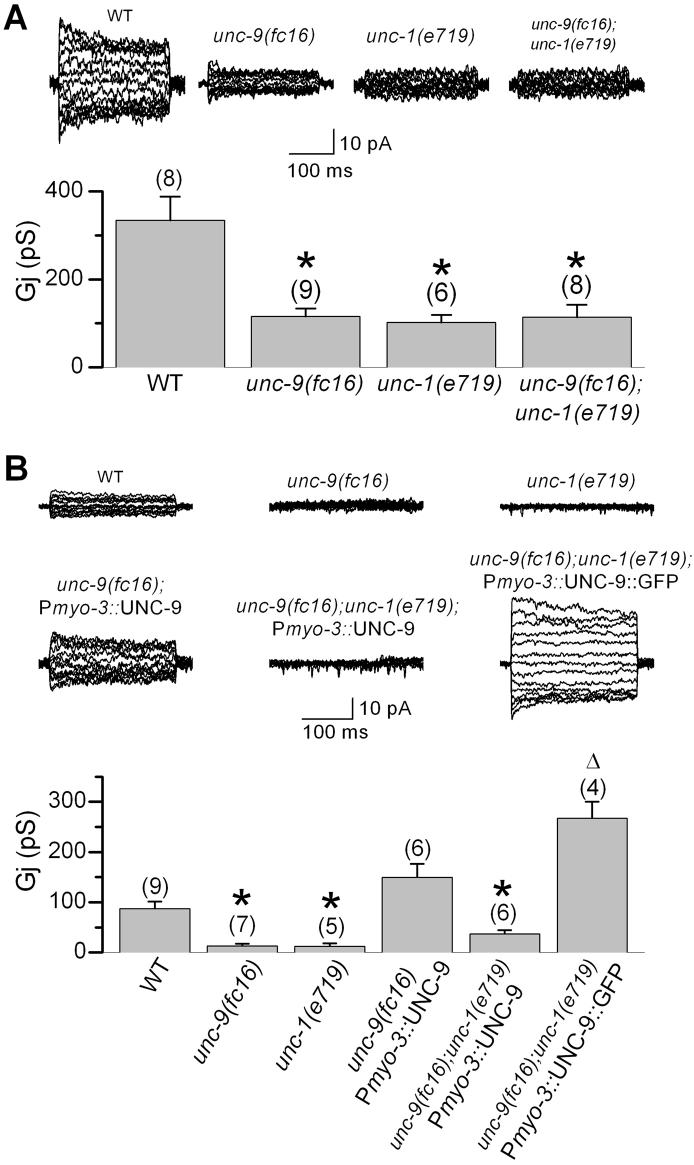
Figure 2.
UNC-1 appeared to be specifically required for the function of UNC-9-based gap junctions in body-wall muscle cells. A: Intra-quadrant coupling (between a pair of neighboring R1-R2 or L1-L2 cells) was indistinguishable between the unc-9(fc16);unc-1(e719) double mutant and either of the single mutant, suggesting that UNC-1 and UNC-9 function in the same pathway. The unc-1(e719) data is the same as that in Figure 1B. B: Wild-type (WT) UNC-9 but not UNC-9::GFP required UNC-1 to rescue electrical coupling of body-wall muscle cells. Inter-quadrant coupling (between a pair of neighboring R1-L1 cells) was nearly absent in unc-9(fc16) or unc-1(e719) mutant. Expression of WT UNC-9 in body-wall muscle cells under the control of Pmyo-3 rescued the coupling defect of unc-9(fc16). In the unc-1(e719) and unc-9(fc16) genetic background, UNC-9::GFP but not WT UNC-9 rescued the coupling defect. The Gj from animals expressing Pmyo-3::UNC-9::GFP might be underestimated because only a small number of progeny expressing the non-integrated transgene survived into adulthood, and these adult animals are conceivably those expressing the transgene at a lower level or in fewer cells. The asterisk indicates a statistically significant difference compared with the WT. The open triangle indicates a statistically significant difference compared with the “unc-9(fc16);unc-1(e719);Pmyo-3::UNC-9” group. One-way ANOVA and Bonferroni posthoc tests were used for the statistical analyses. The number of samples analyzed is indicated by the value above each column. Please note that shown in A and B are intra- and inter-quadrant couplings, respectively, which have different levels of junctional conductance (Gj) and are inhibited to different degrees in unc-9(fc16), as reported previously [7].
During our analyses of UNC-9 subcellular localization by expressing UNC-9::GFP (GFP fused to UNC-9 carboxyl terminus) in muscle cells under the control of Pmyo-3 [7], we observed that the transgenic animal was nearly paralyzed (not shown). Interestingly, this effect of UNC-9::GFP persisted in unc-1(lf) mutants (not shown). One plausible interpretation for these observations is that UNC-9::GFP is a gain-of-function protein that no longer requires UNC-1 to function. Indeed, expression of UNC-9::GFP in unc-9(fc16);unc-1(e719) double mutant led to an unusually high degree of inter-quadrant coupling (Figure 2B), indicating that UNC-9::GFP could form functional gap junctions in the absence of UNC-1. These observations suggest that UNC-1 is unlikely an essential structural component of gap junctions formed by UNC-9. Rather, it might be a regulatory or ancillary protein.
UNC-1 and UNC-9 were coexpressed in muscle cells and neurons
To confirm that UNC-1 is required for the function of UNC-9-based gap junctions, it is important to show that unc-1 and unc-9 are coexpressed in C. elegans. We compared the in vivo expression patterns of unc-1 and unc-9 by expressing Punc-1::DsRED2 and Punc-9::GFP transcriptional fusions in C. elegans. In transgenic animals, both DsRED2 and GFP were expressed in body-wall muscle, vulval muscle, anal depressor muscle, stomatointestinal muscle, most if not all ventral cord motor neurons, and many other neurons (Sup. Figure 1). These observations suggest that UNC-1 and UNC-9 might function together in neurons as well as various muscle cells.
UNC-1 and UNC-9 colocalized at intercellular junctions
We previously showed that UNC-9 is localized to intercellular junctions of body-wall muscle cells when it is expressed under the control of Pmyo-3 [7]. The functional dependence of UNC-9-based gap junctions on UNC-1 suggests that the two proteins might colocalize. To examine this possibility, we coexpressed UNC-1::HA and Myc::UNC-9 either in body-wall muscle cells under the control of Pmyo-3 [9] or in neurons under the control of Punc-47 [11]. In body-wall muscle cells, UNC-1::HA and Myc::UNC-9 colocalized at intercellular junctions between muscle cell bodies, and between muscle arms along the ventral and dorsal nerve cords where muscle cells from the two different quadrants form gap junctions [7, 12] (Figure 3A). UNC-1::HA and Myc::UNC-9 also colocalized in the nervous system (Figure 3B), suggesting that they might also function together in neurons. The subcellular localization patterns of UNC-1 and UNC-9 are consistent with the finding that UNC-1 is required for the electrical coupling mediated by UNC-9-based gap junctions.
Figure 3.
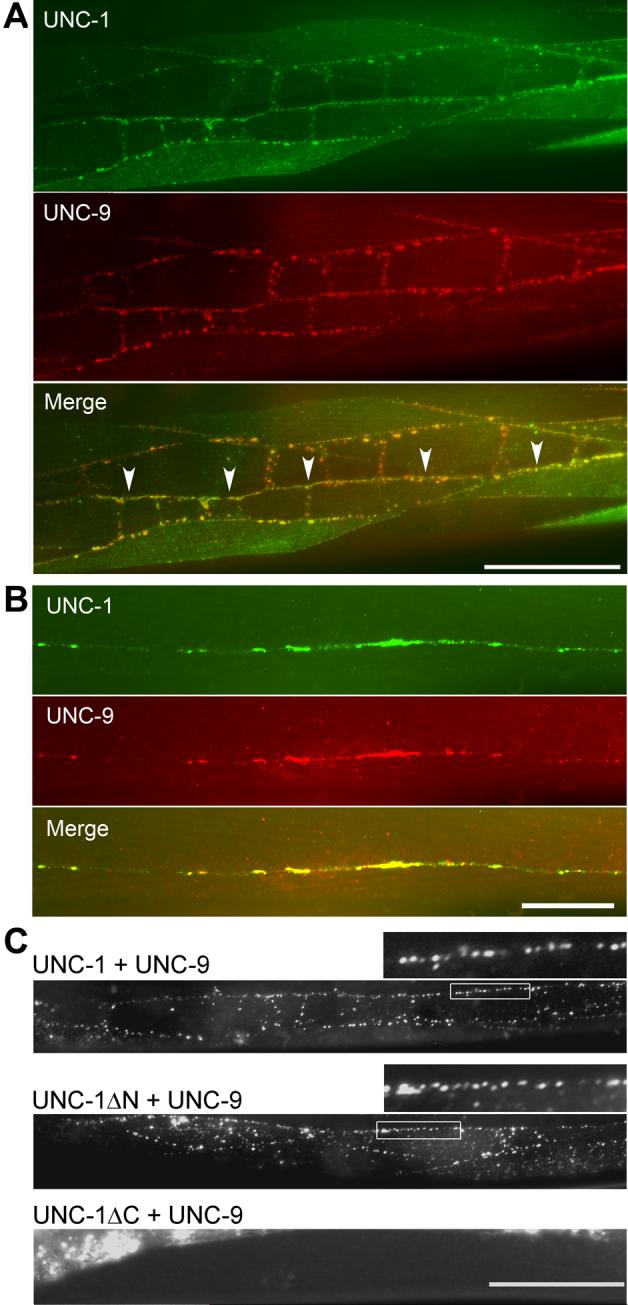
UNC-1 and UNC-9 colocalized in body-wall muscle and the nervous system. A: When UNC-1::HA and Myc::UNC-9 were coexpressed specifically in body-wall muscle cells under the control of Pmyo-3, colocalization was observed at body-wall muscle intercellular junctions, muscle arms, and near the ventral and dorsal nerve cords, where muscle arms from the two ventral or dorsal quadrants interdigitate. Immunoreactivity was absent in some muscle cells due to mosaic expression of the non-integrated transgenes. The nerve cord is indicated by arrow heads in the merged picture. Scale bar = 50 μm. B: When Myc::UNC-9 and UNC-1::HA were coexpressed specifically in neurons under the control of Punc-47, colocalization of the two proteins was observed along the nerve cords. Scale bar = 20 μm. C: Coexpression of UNC-1::YFPa (UNC-1 fused to YFP amino terminal) and UNC-9::YFPc (UNC-9 fused to YFP carboxyl terminal) in body-wall muscle cells under the control of Pmyo-3 reconstituted the fluorophore of YFP in vivo. Top panel: Fluorescent puncta were observed at intercellular junctions between muscle cell bodies and between muscle arms along the nerve cord with full-length UNC-1 and UNC-9. A selected region (marked by a rectangular frame) is enlarged and shown above the image (same for the Middle panel). Middle panel: Fluorescent puncta were still observed with deletion of the amino terminal of UNC-1 (amino acid residues 1−167). Lower panel: Deletion of the carboxyl terminal of UNC-1 (amino acid residues 171−289) prevented the BiFC. The bright fluorescent signal in this image was due to autofluorescence of the gut. Scale bar = 50 μm. A note about functionalities of these fusion proteins may be found in the Supplement.
UNC-1 and UNC-9 appeared to be physically very close at intercellular junctions
The functional interactions and colocalization of UNC-1 and UNC-9 shown above suggest that the two proteins might be physically very close. To examine this possibility, we performed bimolecular fluorescence complementation (BiFC) assays [13] with UNC-1 and UNC-9. The BiFC assay was chosen for our analyses because it shows not only whether protein-protein interactions occur but also where they occur in vivo. In these assays, the non-fluorescent YFP amino and carboxyl terminal fragments were fused to the carboxyl termini of UNC-1 and UNC-9, respectively. The fusion proteins were coexpressed in body-wall muscle cells under the control of Pmyo-3. In transgenic animals, fluorescent puncta were observed between muscle cell bodies, and between muscle arms along the ventral and dorsal nerve cords (Figure 3C, top panel). Similar results were obtained when the amino terminal (amino acids 1−167) of UNC-1 was deleted (Figure 3C, middle panel). However, no fluorescent puncta were observed at intercellular junctions when the carboxyl terminal (amino acids 171−289) of UNC-1 was deleted (Figure 3C, lower panel) despite that the fusion protein was still expressed, as determined by immunohistochemistry (not shown). These observations suggest that UNC-1 is physically very close to UNC-9 at intercellular junctions, and has the potential to physically interact with UNC-9.
UNC-9 expression and subcellular localization were not altered in unc-1 mutant
UNC-1 could potentially be required for UNC-9 function, stability, trafficking, or subcellular localization. To determine how UNC-1 might function to promote electrical coupling, we generated an UNC-9-specific antibody and analyzed UNC-9 expression and subcellular localization in wild-type and unc-1(e719) mutant by immunohistochemistry. In wild-type animals, immunoreactive puncta were observed at body-wall muscle intercellular junctions, along the ventral and dorsal nerve cords, and in the nerve ring (Figure 4, left panels), which is consistent with the UNC-9 expression pattern revealed by the Punc-9::GFP transcriptional fusion (Sup. Figure 1). The immunostaining pattern of UNC-9 in unc-1(e719) genetic background (Figure 4, center panels) was indistinguishable from that in the wild-type, suggesting that the expression and subcellular localization of UNC-9 do not depend on UNC-1. Thus, UNC-1 is most likely required for modulating the function of UNC-9-based gap junctions.
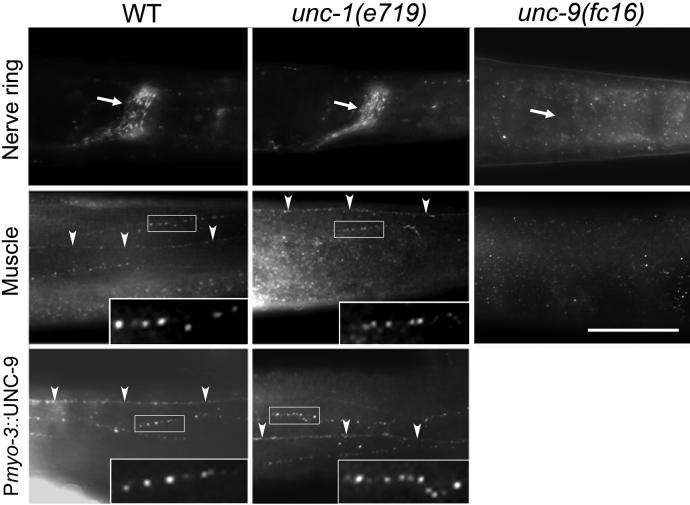
Figure 4.
UNC-9 showed normal expression and subcellular localization in unc-1 mutants. Immunostaining was performed in whole-mount worms with an anti-UNC-9 antibody. In wild-type worms, immunoreactive puncta were observed in the nerve ring (indicated by an arrow), along the ventral or dorsal nerve cord (indicated by arrow heads), and between body-wall muscle cell bodies (not labeled). Overexpression of wild-type UNC-9 in body-wall muscle cells under the control of Pmyo-3 caused an enhancement of the immunoreactive puncta. In unc-1(e719) mutant genetic background, UNC-9 expression and subcellular localization were unaltered. In unc-9(fc16) mutant animals, no immunoreactivity was observed, suggesting that the antibody was specific to UNC-9. The nerve cord is marked with arrow heads. Selected regions of UNC-9-immunoreactive puncta (indicated by rectangular frames) are enlarged and shown as insets. Scale bar = 50 μm.
We also tested whether UNC-1 subcellular localization depends on UNC-9 by comparing the immunostaining patterns of UNC-1::HA in body-wall muscle cells between wild-type and unc-9(fc16) mutant. The localization pattern of UNC-1::HA was indistinguishable between the two transgenic strains (Sup. Figure 2). Thus, UNC-1 subcellular localization is also independent of UNC-9.
Neuronal functions of UNC-1 and UNC-9 predominated in controlling locomotion
The locomotion defects of unc-1(lf) and unc-9(lf) could potentially be caused by deficiencies in both neurons and body-wall muscle cells. To determine whether a neuronal or muscle function of UNC-1 and UNC-9 plays a more important role in locomotion, we analyzed the locomotion behavior of mutant animals in which either a neuronal or muscle deficiency of unc-1 or unc-9 was rescued by expressing the corresponding wild-type gene. We found that expression of wild-type unc-1 or unc-9 in neurons of a corresponding mutant largely rescued the locomotion defect. In contrast, expression of the wild-type gene in body-wall muscle cells of a corresponding mutant showed no obvious effect (Sup. Figure 3). These observations suggest that UNC-1 and UNC-9 function predominantly in neurons to control locomotion. This conclusion is supported by the observation that specific inhibition of UNC-9 function in body-wall muscle cells only causes a moderate locomotion defect [7].
Discussion
The C. elegans genome contains twenty-five innexin genes [14] and ten SLP genes (www.wormbase.org). Despite the existence of so many innexins and SLPs, only two innexins (unc-7 and unc-9) and two SLPs (unc-1 and unc-24) are associated with similar mutant phenotypes [1-4, 15-17], suggesting that specific interactions may occur among them. However, no evidence has been shown that direct interactions exist between the two families of proteins. Our analyses suggest that, in body-wall muscle cells, UNC-1 may be specifically required for the function of UNC-9-based gap junctions. This conclusion may appear somewhat surprising given that unc-7 mutants have grossly similar phenotypes as unc-9 mutant. However, UNC-7 does not contribute to body-wall muscle electrical coupling [7]. Thus, it is probably true that UNC-1 specifically regulates UNC-9-based electrical coupling in body-wall muscle cells.
Gap junction is a head-to-head assembly of two hemichannels, with each hemichannel consisting of six subunits, which are innexins in invertebrates [14, 18] and connexins or pannexins in vertebrates [19-21]. The similar phenotypes of unc-7 and unc-9 mutants could conceivably be due to deficiencies of heteromeric or heterotypic gap junctions formed by UNC-7 and UNC-9. Because UNC-7 does not contribute to body-wall muscle electrical coupling [7] and UNC-1 and UNC-9 appeared to function predominantly in neurons to control locomotion (Sup. Figure 3), UNC-1 may be also required for the function of putative heteromeric or heterotypic gap junctions formed by UNC-7 and UNC-9 in neurons.
How might UNC-1 contribute to the function of gap junctions? The apparently normal expression and subcellular localization of UNC-9 in unc-1(lf) suggest that the main function of UNC-1 is unlikely to be related to UNC-9 synthesis, membrane trafficking, or subcellular localization. UNC-1 also did not appear to be an essential structural component of gap junctions formed by UNC-9 because UNC-9::GFP could form functional intercellular channels in the absence of UNC-1 (Figure 2B). Thus, the primary function of UNC-1 could conceivably be to modulate the gating of gap junctions. Based on our observations and a published model for pH gating of connexin-based gap junctions [22], we propose that UNC-9-based gap junctions are mainly in the closed state in the absence of UNC-1; UNC-1 may interact with a gating domain of UNC-9 to prevent it from closing the gap junction. The carboxyl terminal of UNC-1 may be important for this function because deletion of UNC-1 carboxyl terminal abolished its interaction with UNC-9 in the BiFC assay, which is consistent with the previous observations that all of the temperature-sensitive unc-1 alleles resulted from mutations in the carboxyl terminal, and that the amino terminal was unnecessary for UNC-1 function [4]. Interestingly, the C. elegans SLP MEC-2 also modulates a mechanosensitive ion channel through a gating effect [5]. Both gap junction proteins [23] and SLPs [24, 25] may associate with lipid rafts, which are dynamic assemblies of proteins and lipids in cellular membrane [26]. It remains to be determined whether the modulation of gap junctions by UNC-1 is related to association with lipid rafts.
UNC-24 may be also required for the function of UNC-9-based gap junctions because unc-24 mutants show similar phenotypes as unc-9 mutants [3, 15]. However, UNC-24 might function through a different mechanism compared with UNC-1. It has been suggested that UNC-24 may help to maintain UNC-1 stability because the amount of UNC-1 protein is greatly reduced in unc-24 mutants [15]. Thus, deficiencies of SLPs could potentially affect gap junctions through other mechanisms as well.
Sensitivity to anesthetics in C. elegans is measured according to the effectiveness of an anesthetic to immobilize or paralyze the worm. Although it is well established that lf mutations of unc-7, unc-9, unc-1, or unc-24 suppress the enhanced sensitivity to volatile anesthetics caused by unc-79(lf) or unc-80(lf) [3, 15, 17, 27, 28], molecular mechanisms for these mutant effects are unknown. One possibility is that volatile anesthetics immobilize worms by hyper-activating gap junctions formed by UNC-7 and UNC-9; that these gap junctions are modulated by UNC-1/UNC-24 as well as UNC-79/UNC-80; and that the functions of UNC-79 and UNC-80 are to suppress gap junction activity. Gap junctions are generally inhibited by halothane [29-31], which is a volatile anesthetic. However, there might be a population of gap junctions that are activated by volatile anesthetics.
Previous studies have shown that SLPs could modulate mechanosensitive ion channels of the degenerin/epithelial Na+ channel (DEG/ENaC) family in C. elegans [5] and mice [6]. The present study adds gap junctions as a second type of channels that may be regulated by SLPs. Vertebrate gap junctions are formed by connexins, and possibly, pannexins [19-21]. Pannexins were discovered by database search for invertebrate innexin homologues [32, 33], and belong to the same superfamily of proteins as innexins [34]. Although the three families of proteins are distinct in primary sequence, major structural features are conserved among them [14, 35]. Furthermore, both vertebrate and invertebrate gap junctions may be modulated by similar physiological factors and blocked by a similar spectrum of pharmacological agents [7, 35-39]. Thus, regulation of gap junctions by SLPs is potentially a conserved mechanism of controlling intercellular communication. Gap junctions in the mammalian nervous system are particularly attractive candidates for potential regulation by SLPs because at least ten connexins [19, 21], two pannexins [32], and four SLPs [40-43] are expressed in the central nervous system. The recently discovered erythrocyte pannexin1 hemichannel [44] could potentially be regulated by stomatin, which is enriched in the erythrocyte membrane [45]. The implication of UNC-1 and UNC-9 in anesthetic sensitivity of C. elegans [3, 4] suggests that regulation of gap junctions by SLPs is potentially related to actions of anesthetics. Thus, the biological significance of gap junction regulation by SLPs is likely much broader than what has been revealed by this study.
Experimental Procedures
This information may be found in the supplement.
Acknowledgements
This work was supported by National Science Foundation Grant 0619427 (to Z.W.) and National Institute of Health Grant DA015464 (to B. A. Eipper and R. E. Mains). We thank Matthew Rasband for helping with antibody purification, Lawrence Salkoff for suggestions about the manuscript, Chang-Deng Hu for worm BiFC vectors and technical advice on BiFC assays, Phil Morgan for unc-1(fc53), Caenorhabditis Genetics Center for the other mutant strains, Sanger Centre for cosmids, and Craig Hunter and Andrew Fire for vectors.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Supplementary Material
References
- 1.Brenner S. The genetics of Caenorhabditis elegans. Genetics. 1974;77:71–94. doi: 10.1093/genetics/77.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Park EC, Horvitz HR. Mutations with dominant effects on the behavior and morphology of the nematode Caenorhabditis elegans. Genetics. 1986;113:821–852. doi: 10.1093/genetics/113.4.821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sedensky MM, Meneely PM. Genetic analysis of halothane sensitivity in Caenorhabditis elegans. Science. 1987;236:952–954. doi: 10.1126/science.3576211. [DOI] [PubMed] [Google Scholar]
- 4.Rajaram S, Sedensky MM, Morgan PG. unc-1: a stomatin homologue controls sensitivity to volatile anesthetics in Caenorhabditis elegans. Proc Natl Acad Sci U S A. 1998;95:8761–8766. doi: 10.1073/pnas.95.15.8761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Goodman MB, Ernstrom GG, Chelur DS, O'Hagan R, Yao CA, Chalfie M. MEC-2 regulates C. elegans DEG/ENaC channels needed for mechanosensation. Nature. 2002;415:1039–1042. doi: 10.1038/4151039a. [DOI] [PubMed] [Google Scholar]
- 6.Wetzel C, Hu J, Riethmacher D, Benckendorff A, Harder L, Eilers A, Moshourab R, Kozlenkov A, Labuz D, Caspani O, Erdmann B, Machelska H, Heppenstall PA, Lewin GR. A stomatin-domain protein essential for touch sensation in the mouse. Nature. 2006;445:206–209. doi: 10.1038/nature05394. [DOI] [PubMed] [Google Scholar]
- 7.Liu Q, Chen B, Gaier E, Joshi J, Wang ZW. Low conductance gap junctions mediate specific electrical coupling in body-wall muscle cells of Caenorhabditis elegans. J Biol Chem. 2006;281:7881–7889. doi: 10.1074/jbc.M512382200. [DOI] [PubMed] [Google Scholar]
- 8.Sedensky MM, Pujazon MA, Morgan PG. Tail clamp responses in stomatin knockout mice compared with mobility assays in Caenorhabditis elegans during exposure to diethyl ether, halothane, and isoflurane. Anesthesiology. 2006;105:498–502. doi: 10.1097/00000542-200609000-00013. [DOI] [PubMed] [Google Scholar]
- 9.Okkema PG, Harrison SW, Plunger V, Aryana A, Fire A. Sequence requirements for myosin gene expression and regulation in Caenorhabditis elegans. Genetics. 1993;135:385–404. doi: 10.1093/genetics/135.2.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Barnes TM, Hekimi S. The Caenorhabditis elegans avermectin resistance and anesthetic response gene unc-9 encodes a member of a protein family implicated in electrical coupling of excitable cells. J Neurochem. 1997;69:2251–2260. doi: 10.1046/j.1471-4159.1997.69062251.x. [DOI] [PubMed] [Google Scholar]
- 11.McIntire SL, Reimer RJ, Schuske K, Edwards RH, Jorgensen EM. Identification and characterization of the vesicular GABA transporter. Nature. 1997;389:870–876. doi: 10.1038/39908. [DOI] [PubMed] [Google Scholar]
- 12.White JG, Southgate E, Thomson JN, Brenner S. The structure of the nervous system of the nematode Caenorhabditis elegans. Philos Trans R Soc Lond B Biol Sci. 1986;314:1–340. doi: 10.1098/rstb.1986.0056. [DOI] [PubMed] [Google Scholar]
- 13.Hu CD, Chinenov Y, Kerppola TK. Visualization of interactions among bZIP and Rel family proteins in living cells using bimolecular fluorescence complementation. Mol Cell. 2002;9:789–798. doi: 10.1016/s1097-2765(02)00496-3. [DOI] [PubMed] [Google Scholar]
- 14.Phelan P, Starich TA. Innexins get into the gap. Bioessays. 2001;23:388–396. doi: 10.1002/bies.1057. [DOI] [PubMed] [Google Scholar]
- 15.Sedensky MM, Siefker JM, Morgan PG. Model organisms: new insights into ion channel and transporter function. Stomatin homologues interact in Caenorhabditis elegans. Am J Physiol Cell Physiol. 2001;280:C1340–1348. doi: 10.1152/ajpcell.2001.280.5.C1340. [DOI] [PubMed] [Google Scholar]
- 16.Starich TA, Herman RK, Shaw JE. Molecular and genetic analysis of unc-7, a Caenorhabditis elegans gene required for coordinated locomotion. Genetics. 1993;133:527–541. doi: 10.1093/genetics/133.3.527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Morgan PG, Sedensky M, Meneely PM. Multiple sites of action of volatile anesthetics in Caenorhabditis elegans. Proc Natl Acad Sci U S A. 1990;87:2965–2969. doi: 10.1073/pnas.87.8.2965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bauer R, Loer B, Ostrowski K, Martini J, Weimbs A, Lechner H, Hoch M. Intercellular communication: the Drosophila innexin multiprotein family of gap junction proteins. Chem Biol. 2005;12:515–526. doi: 10.1016/j.chembiol.2005.02.013. [DOI] [PubMed] [Google Scholar]
- 19.Bennett MV, Zukin RS. Electrical coupling and neuronal synchronization in the Mammalian brain. Neuron. 2004;41:495–511. doi: 10.1016/s0896-6273(04)00043-1. [DOI] [PubMed] [Google Scholar]
- 20.Goodenough DA, Goliger JA, Paul DL. Connexins, connexons, and intercellular communication. Annu Rev Biochem. 1996;65:475–502. doi: 10.1146/annurev.bi.65.070196.002355. [DOI] [PubMed] [Google Scholar]
- 21.Connors BW, Long MA. Electrical synapses in the mammalian brain. Annu Rev Neurosci. 2004;27:393–418. doi: 10.1146/annurev.neuro.26.041002.131128. [DOI] [PubMed] [Google Scholar]
- 22.Morley GE, Taffet SM, Delmar M. Intramolecular interactions mediate pH regulation of connexin43 channels. Biophys J. 1996;70:1294–1302. doi: 10.1016/S0006-3495(96)79686-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schubert AL, Schubert W, Spray DC, Lisanti MP. Connexin family members target to lipid raft domains and interact with caveolin-1. Biochemistry. 2002;41:5754–5764. doi: 10.1021/bi0121656. [DOI] [PubMed] [Google Scholar]
- 24.Morrow IC, Parton RG. Flotillins and the PHB domain protein family: rafts, worms and anaesthetics. Traffic. 2005;6:725–740. doi: 10.1111/j.1600-0854.2005.00318.x. [DOI] [PubMed] [Google Scholar]
- 25.Sedensky MM, Siefker JM, Koh JY, Miller DM, 3rd, Morgan PG. A stomatin and a degenerin interact in lipid rafts of the nervous system of Caenorhabditis elegans. Am J Physiol Cell Physiol. 2004;287:C468–474. doi: 10.1152/ajpcell.00182.2003. [DOI] [PubMed] [Google Scholar]
- 26.Simons K, Ehehalt R. Cholesterol, lipid rafts, and disease. J Clin Invest. 2002;110:597–603. doi: 10.1172/JCI16390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Morgan PG, Sedensky MM, Meneely PM. The genetics of response to volatile anesthetics in Caenorhabditis elegans. Ann N Y Acad Sci. 1991;625:524–531. doi: 10.1111/j.1749-6632.1991.tb33883.x. [DOI] [PubMed] [Google Scholar]
- 28.Sedensky MM, Cascorbi HF, Meinwald J, Radford P, Morgan PG. Genetic differences affecting the potency of stereoisomers of halothane. Proc Natl Acad Sci U S A. 1994;91:10054–10058. doi: 10.1073/pnas.91.21.10054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mantz J, Cordier J, Giaume C. Effects of general anesthetics on intercellular communications mediated by gap junctions between astrocytes in primary culture. Anesthesiology. 1993;78:892–901. doi: 10.1097/00000542-199305000-00014. [DOI] [PubMed] [Google Scholar]
- 30.Terrar DA, Victory JG. Influence of halothane on electrical coupling in cell pairs isolated from guinea-pig ventricle. Br J Pharmacol. 1988;94:509–514. doi: 10.1111/j.1476-5381.1988.tb11554.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Burt JM, Spray DC. Volatile anesthetics block intercellular communication between neonatal rat myocardial cells. Circ Res. 1989;65:829–837. doi: 10.1161/01.res.65.3.829. [DOI] [PubMed] [Google Scholar]
- 32.Bruzzone R, Hormuzdi SG, Barbe MT, Herb A, Monyer H. Pannexins, a family of gap junction proteins expressed in brain. Proc Natl Acad Sci U S A. 2003;100:13644–13649. doi: 10.1073/pnas.2233464100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Panchin Y, Kelmanson I, Matz M, Lukyanov K, Usman N, Lukyanov S. A ubiquitous family of putative gap junction molecules. Curr Biol. 2000;10:R473–474. doi: 10.1016/s0960-9822(00)00576-5. [DOI] [PubMed] [Google Scholar]
- 34.Yen MR, Saier MH., Jr. Gap junctional proteins of animals: The innexin/pannexin superfamily. Prog Biophys Mol Biol. 2007;94:5–14. doi: 10.1016/j.pbiomolbio.2007.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Harris AL. Emerging issues of connexin channels: biophysics fills the gap. Q Rev Biophys. 2001;34:325–472. doi: 10.1017/s0033583501003705. [DOI] [PubMed] [Google Scholar]
- 36.Gho M. Voltage-clamp analysis of gap junctions between embryonic muscles in Drosophila. J Physiol. 1994;481(Pt 2):371–383. doi: 10.1113/jphysiol.1994.sp020446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bruzzone R, Barbe MT, Jakob NJ, Monyer H. Pharmacological properties of homomeric and heteromeric pannexin hemichannels expressed in Xenopus oocytes. J Neurochem. 2005;92:1033–1043. doi: 10.1111/j.1471-4159.2004.02947.x. [DOI] [PubMed] [Google Scholar]
- 38.Bohrmann J, Haas-Assenbaum A. Gap junctions in ovarian follicles of Drosophila melanogaster: inhibition and promotion of dye-coupling between oocyte and follicle cells. Cell Tissue Res. 1993;273:163–173. doi: 10.1007/BF00304623. [DOI] [PubMed] [Google Scholar]
- 39.Landesman Y, White TW, Starich TA, Shaw JE, Goodenough DA, Paul DL. Innexin-3 forms connexin-like intercellular channels. J Cell Sci. 1999;112(Pt 14):2391–2396. doi: 10.1242/jcs.112.14.2391. [DOI] [PubMed] [Google Scholar]
- 40.Wang Y, Morrow JS. Identification and characterization of human SLP-2, a novel homologue of stomatin (band 7.2b) present in erythrocytes and other tissues. J Biol Chem. 2000;275:8062–8071. doi: 10.1074/jbc.275.11.8062. [DOI] [PubMed] [Google Scholar]
- 41.Mannsfeldt AG, Carroll P, Stucky CL, Lewin GR. Stomatin, a MEC-2 like protein, is expressed by mammalian sensory neurons. Mol Cell Neurosci. 1999;13:391–404. doi: 10.1006/mcne.1999.0761. [DOI] [PubMed] [Google Scholar]
- 42.Seidel G, Prohaska R. Molecular cloning of hSLP-1, a novel human brain-specific member of the band 7/MEC-2 family similar to Caenorhabditis elegans UNC-24. Gene. 1998;225:23–29. doi: 10.1016/s0378-1119(98)00532-0. [DOI] [PubMed] [Google Scholar]
- 43.Goldstein BJ, Kulaga HM, Reed RR. Cloning and characterization of SLP3: a novel member of the stomatin family expressed by olfactory receptor neurons. J Assoc Res Otolaryngol. 2003;4:74–82. doi: 10.1007/s10162-002-2039-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Locovei S, Bao L, Dahl G. Pannexin 1 in erythrocytes: function without a gap. Proc Natl Acad Sci U S A. 2006;103:7655–7659. doi: 10.1073/pnas.0601037103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Stewart GW. Stomatin. Int J Biochem Cell Biol. 1997;29:271–274. doi: 10.1016/s1357-2725(96)00072-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.