SUMMARY
Opiates addiction is characterized by its long term persistence. In order to study the enduring changes in long-term memory in hippocampus, a pivotal region for this process, we used Suppression Subtractive Hybridization to compare hippocampal gene expression in morphine and saline-treated rats. Animals were subjected to an extended place preference paradigm consisting of four conditioning phases. Sensitization to the reinforcing effects of the drug occurred after 3 conditioning phases. After 26 days of treatment rats were sacrificed and the complementary DNA (cDNA) from the hippocampus of morphine-dependent and saline-treated animals were then screened for differentially expressed cDNAs. The selected 177 clones were then subjected to a microarray procedure and 20 clones were found differentially regulated. The pattern of regulated genes suggest impairments in neurotransmitter release and the activation of neuroprotective pathways.
Keywords: Analysis of Variance; Animals; Conditioning, Operant; drug effects; Drug Administration Schedule; Gene Expression Regulation; drug effects; Hippocampus; drug effects; Male; Microarray Analysis; methods; Morphine; administration & dosage; Narcotics; administration & dosage; Rats; Rats, Sprague-Dawley; Reward
1-INTRODUCTION
The mechanisms underlying opiate dependence, tolerance, and sensitization in response to chronic opiate administration are still poorly understood. Recent studies have shown that morphine alters gene expression in several areas of the brain even after a single injection, these changes in gene expression are hypothesized to be one of the factors underlying the long lasting effects of drugs of abuse (Nestler, 2001).
Recently a large scale gene expression profiling, performed after a single injection of morphine, revealed that two groups of genes encoding for cytoskeleton-related proteins and proteins involved in the mitochondrial respiration are altered in the medial striatum and the spinal cord (Loguinov et al., 2001). Chronic morphine treatment also regulates the expression of several genes involved in receptor regulation and signaling, transcription factors and cytoskeleton associated proteins (Ammon et al., 2003; Fan et al., 2002; Fan et al., 2003; Kaewsuk et al., 2001; Marie-Claire et al., 2004). However these studies were done with massive doses of morphine inducing a physical dependence characterized by a withdrawal syndrome when the treatment is stopped. It is therefore of major interest to study the regulation of gene expression in conditions leading to receptors sensitization and compulsive drug consumption that are the two main characteristics of addiction (Koob and Le Moal, 1997).
With this aim we designed a protocol able to reproduce these conditions in rats through repeated administration of low doses of morphine for 26 days with measurements of drug-dependence at four different times using the conditioned place preference paradigm (CPP). The CPP is a behavioral test, associating drug consumption and memorized environment, extensively used to assess the rewarding properties of drugs of abuse in rodents (review in Bardo and Bevins, 2000). The treatment schedule used in this study is unable to induce obvious physical signs of dependence as previously shown (Contarino et al., 1997). Since hippocampus is the main structure involved in the consolidation of long-term memory (review in Shu et al., 2003), we analyzed gene expression in this structure by “Suppression Subtractive Hybridization” (SSH) (Diatchenko et al., 1996). The candidates cDNAs were then spotted onto glass microarrays and their regulation analyzed by comparing the hybridization pattern of probes from saline- and morphine-treated animals. Results revealed alterations in gene expression patterns of 20 clones representing 13 different genes involved in diverse cellular processes.
2-METHODS
Animals and conditioned place preference procedure
Male Sprague Dawley rats (Charles River Laboratories, France) weighing 180–200g at the beginning of the experiments were used. Experiments were performed according to the European Communities Directive (86/609/EEC). Animals were housed under controlled conditions (12h alternating light/dark cycle and 21±1°C) and had free access to food and water. Conditioned place preference procedure was carried out as described previously (Contarino et al., 1997) and is summarized in Fig. 1A. Each saline and morphine group consisted of 12 animals. Briefly, the protocol consisted of three phases: (1) habituation (pre-conditioning) phase (day 1 and 2), in which drug naive rats had free access to both compartments of the conditioning apparatus for 20 min; and (2) conditioning phase, in which each animal was injected with morphine on alternate days (5 mg/kg, s.c.) and confined in one compartment (days 3, 5, 7, and 9) for 20 min or injected with saline and confined in the other compartment (days 4, 6, 8 and 10) for 20 min. One compartment was randomly chosen to be paired to drug administration and the other to vehicle. Control animals were injected with saline every day and placed alternatively in both compartments. (3) testing phase achieved the day after the last conditioning session: neither drug nor vehicle were administered and the rats underwent a test session (Test 1). The day after the first test session (Test 1), the animals were subjected to further conditioning sessions. The experimental procedures were as in the first conditioning phase except that a test session was given on separate days every four conditioning trials. The experiment lasted totally 26 days and was concluded with a final test session (Test 4) after a total of 10 drug injections. Behavioral results were expressed as mean ± SEM of score values calculated as followed: score = (time spent in the drug paired compartment during the test) − (time spent in the same compartment during the pre-conditioning test). Results were analyzed using two ways ANOVA with repeated measures. Post hoc Newman Keuls tests for multiple comparisons were performed. The accepted value of significance was p<0.05.
Figure 1.
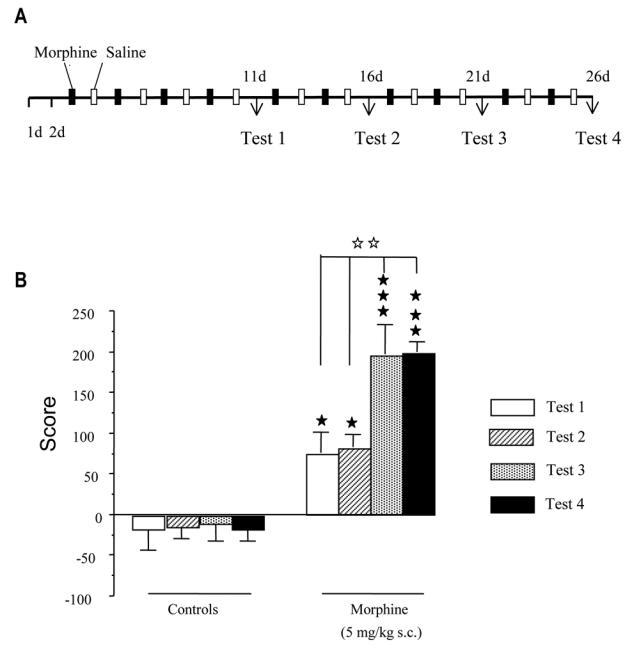
A) Summary of the experimental protocol used in the conditioned place preference procedure B) conditioned place preference induced by morphine (5 mg/kg, s.c.) for test sessions T1 to T4. Data are expressed as scores (means + SEM) calculated as the difference between postconditioning and preconditioning time spent in the compartment associated with the drug. * p<0.05, *** p<0.001 versus the saline group; ## p<0.01 versus test session T2 (Newman Keuls test).
RNA extraction and suppression subtractive hybridization (SSH)
The animals were sacrificed immediately after Test 4 and the brains were rapidly dissected on ice. Hippocampus were then stored at −80°C until RNA isolation. Total RNA was extracted from 5 pooled hippocampi with RNABle reagent (Eurobio, France) following the manufacturer’s protocol. The quality of total RNA was assessed by agarose gel and the quantity measured by spectrophotometry. PCR-based cDNA subtraction method was performed using the SMART PCR cDNA synthesis and the PCR-Select™ cDNA Subtraction Kit (Clontech Laboratories, Inc., Palo Alto, CA) according to the manufacturer protocol. Briefly, total RNA (1 μg) was reverse-transcribed into cDNAs, and then digested with restriction enzyme RsaI. The subtractions were performed by mixing the cDNAs in two rounds of controlled hybridizations. The resulting cDNA molecules were then subjected to two rounds of nested PCR. The amplified cDNAs containing enriched differentially expressed transcripts were cloned into pCR®-Blunt vector using a Zero Blunt® PCR cloning kit (Invitrogen, France).
Differential screening of the subtracted cDNA libraries
Positive clone analysis was carried out by differential screening. Colony PCR was performed on individual colonies from the two subtracted libraries, using the same nested primers used for the libraries amplification. Two identical blots were created by spotting alkaline-denatured PCR products onto Hybond N+ membranes (Amersham Pharmacia Biotech, France). Hybridization was carried out with cDNAs from saline or morphine un-subtracted probes after labeling with the direct nucleic acid labeling system™ (Amersham Pharmacia Biotech, France). Membranes were washed and the labeled nucleic acid were detected using ECL chemioluminescent reagents (Amersham Pharmacia Biotech). Differences were measured by densitometry with the Bio 1D software (Vilber Lourmat, France).
Microarray hybridization
Amplified cDNA clones were arrayed on a poly-L-lysine coated slide with robot Microgrid Pro from Biorobotics (Genomic solutions, United Kingdom). After arraying, slides were rehydrated, dried, UV cross-linked, and blocked as described (Harismendy et al., 2003). 20 μg total RNA from rat hippocampus were reverse transcribed using the Superscript II reverse transcriptase (Invitrogen, Carlsbad, CA) and aminoallyl-dUTP (Sigma). cDNA was labeled by an indirect method using monofunctional NHS-ester Cy3 or Cy5 (Amersham, Buckinghamshire, United Kingdom). Labeled cDNA was then purified by passage through a nucleospin column (Macherey-Nagel, Düren, Germany) and hybridized to the chip harboring the 177 positive clones from the SSH screening. Genes were identified as differently expressed if the fold change was ≥1.2.
cDNA sequencing and homology search
The nucleotide sequence of selected differentially expressed clones was determined using the Big Dye Terminator reagent Mix (Applied Biosystems, France) and automated sequencing (Applied Biosystems model 377). Sequence results were submitted to BLAST searches of various online databases to elucidate the identity of clones. Gene functions were categorized according to GeneOntology (Ashburner et al., 2000).
3-RESULTS
Conditioned place preference
A two- way ANOVA with repeated measures revealed a significant treatment effect (p<0.0001), test session effect (p<0.0035) and a significant interaction between treatment and test session (p=0.0108). As shown in Fig 1B, morphine-treated but not saline-treated rats spent more time in the drug-paired compartment during the four test sessions than during the pre-conditioning test. Post-hoc Newman Keuls test revealed that the significant interaction between treatment and test session resulted from the fact that the score of morphine-treated animals did not differ on test 1 and 2, while an increase was obtained on tests 3 and 4. As expected no difference was observed in control group throughout the protocol.
Subtractive hybridization and differential screening
Two subtractive cDNA libraries were constructed with the total RNAs from the hippocampus of morphine or saline-treated animals. In the first library (up-regulated library), the subtraction enriched for transcripts relatively more abundant in the hippocampus of morphine-treated rats. In the second library (down-regulated library) the subtraction enriched for transcripts relatively more abundant in the hippocampus of saline-treated rats. The resulting libraries were cloned into the pCR®-Blunt vector, generating approximately 1300 independent clones. All the clones were then subjected to differential screening. A representative differential screening is shown in Fig 2. Differential screening on the clones from the up-regulated library resulted in 150 positive clones. The differential screening on the clones from the down-regulated library resulted in 27 positive clones.
Figure 2.
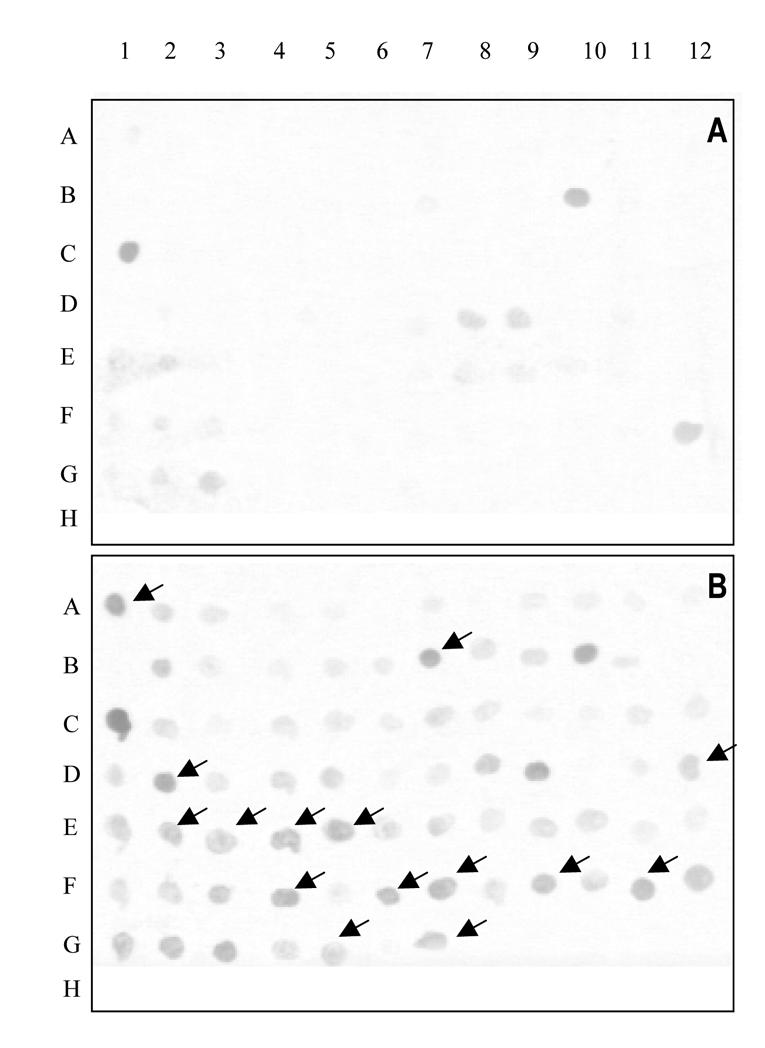
Representative differential screening of clones from the up- and down-regulated libraries. Nylon membranes were spotted with colonies from the down-regulated (A) or the up-regulated (B) libraries and hybridized with labeled probe from subtractive up-regulated cDNAs. Arrows (→) indicate positive colonies in the up-regulated library which predominantly hybridized with the up-regulated cDNAs probe.
Microarray hybridization
In order to confirm the differentially expressed genes microarray hybridizations were performed. Data represent the means of six hybridizations, only spots giving consistent results across all six comparisons, with an average fold change greater than or equal to 1.2, were deemed as modulated. The 20 modulated clones are listed in table I. 13 clones were up-regulated in the hippocampus of morphine-treated animals as compared to saline-treated rats, while the remaining 7 were down-regulated. The 13 up-regulated clones represent 6 genes involved in several molecular functions: vesicular transport (Sip30 and Mss4); heat shock (Hsp90β); steroid synthesis (β HSD type II.2), and oxidoreductase activity (Gapdh). The sequence of one of the up-regulated clones did not match with any known sequence in the databases. The 7 down-regulated clones encode for proteins involved in cellular processes as diverse as cytoskeleton organization (Filamin A), vesicular transport (synphilin), cell adhesion molecule (protocadherin), iron ion transport (sfx4), growth factor receptor binding (Flt1) and transaminase activity (Got1). The sequence of one of the down-regulated clones did not match with any known sequence in the databases.
Table 1.
Genes that are regulated by chronic morphine in rat hippocampus based on microarray analysis. Data are the mean of 6 hybridizations.
| Clone ID | Fold change | Gene | Accession N° | Function |
|---|---|---|---|---|
| M1247
M24 M44 M6 |
1.34
1.30 1.26 1.34 |
Sip30 | NM_147138 | Vesicle mediated transport |
| M482
M15 M81 |
1.27
1.42 1.35 |
3β HSD type II.2 | NM_017265 | Mormon metabolism |
| M19
M38 M41 |
1.38
1.52 1.48 |
Mss4 | X70496 | Intracellular transport |
| M982 | 1.24 | unknown | n.a. | unknown |
| M46 | 1.24 | Hsp90β | XM_217339 | Heat shock protein activity |
| M1532 | 1.27 | Gapdh | NM_017008 | Oxydoreductase activity |
| 73++ | 0.79 | unknown | n.a. | unknown |
| S71 | 0.77 | Flt1 | NM_019306 | growth factor receptor binding |
| S52 | 0.63 | Similar to Sfx4 | XM_342072 | Iron ion transport |
| S66 | 0.70 | Similar to Protocadherin9 | XM_224429 | extracellular matrix constituent |
| S113 | 0.78 | Got1 | NM_012571 | Transaminase activity |
| S82 | 0.79 | Synphilin-1 | XM_225768 | Vesicle mediated transport |
| S94 | 0.8 | Flna | XM_238167 | Cytoskeletal protein binding |
Columns represent the clone ID in the two libraries, fold change values (morphine-treated/controls), gene symbols and GenBank Accession Numbers. Molecular function of the genes according to the Gene Ontology database are also indicated, (n.a. = not applicable).
4-DISCUSSION
It is now well established that chronic morphine treatment induces adaptive processes involving regulation of gene expression (Ammon et al., 2003; Fan et al., 2002; Fan et al., 2003; Kaewsuk et al., 2001; Marie-Claire et al., 2004). A better characterization of these genes is expected to clarify the biological mechanisms of drug dependence and could open new paths to develop treatments of addiction. Few studies have been carried out in this field and they have been essentially devoted to gene modification produced by acute or repeated effects of massive doses (binge) of the alkaloid. In this study we treated rats using an approach leading to a strong sensitization to the reinforcing effects of morphine. The use of repeated conditioning sessions, resulted in an escalated preference for morphine in good agreement with a previous study (Contarino et al., 1997). Due to its implication in long-term memory consolidation (review in Fries et al., 2003; Shu et al., 2003) the rat hippocampus was selected to identify genes that are differentially regulated by morphine in this lengthened CPP procedure. The SSH technique was used to generate custom cDNA microarrays, this combination has been shown to be successful in a variety of systems (review in Dougherty and Geschwind, 2002). Modulated transcripts encode for proteins with functions as diverse as vesicular transport, neurotransmitter release, receptor trafficking, cell metabolism, steroid synthesis, heat shock and angiogenesis.
One of the gene up-regulated in this study is SNAP25 interacting protein 30 (Sip30) which binds directly to synaptosome-associated protein of 25 kDa (Snap25) and facilitates its trafficking towards dendrites. Immunocytochemistry studies show a dense expression of this protein in hippocampal interneurons (Lee et al., 2002). Its long term regulation by chronic morphine may suggest an impairment of the vesicular trafficking by the alkaloid and subsequent neurotransmitter release according to the critical role of Snap25 in exocytosis (Heidelberger and Matthews, 2004). Another gene encoding for a protein involved in exocytosis is also upregulated in the hippocampus of morphine-treated rats. Guanine nucleotide exchange factor Mss4 stimulates GTP-GDP exchange in Sec4 and Rab and binds to a subset of genetically related Rab proteins implicated in Ca2+-dependent exocytosis of neurotransmitters in vivo (Burton et al., 1994). Interestingly, this protein is also up-regulated in several structures of rat brain including hippocampus after chronic treatment with imipramine (Andriamampandry et al., 2002). The gene encoding for synphilin-1, a protein involved in vesicle mediated transport is down-regulated in the CPP procedure used here. Interestingly a slight but not significant down-regulation of this gene was observed in rat striatum after a 5 days chronic morphine treatment (Marie-Claire et al., 2004). Synphilin-1 is expressed at presynaptic nerve terminals where it associates to synaptic vesicles (Ribeiro et al., 2002). Therefore, the down-regulation of synphilin-1 suggests a modulation of the presynaptic vesicular fusion process in the hippocampus after by chronic morphine treatment. Taken together the modulations of these three genes suggest impairments of vesicular transport and neurotransmitter release by this chronic morphine treatment. This is in agreement with the observed increase in release of certain neurotransmitters, like acetylcholine, in the hippocampus induced by repeated administration of opiates (Imperato et al., 1996).
Interestingly, one of the down-regulated gene encodes for Filamin A (Flna) a protein known to couple membrane proteins to actin (van der Flier and Sonnenberg, 2001). Flna is implicated in several receptors regulation and trafficking such as MOR opioid receptors, dopamine D2 and D3 receptors and metabotropic receptor 7 (Enz, 2002; Lin et al., 2001; Onoprishvili et al., 2003). The expression level of this gene is not affected in rat striatum by a 5 days chronic morphine treatment with increasing doses (Marie-Claire et al., 2004). However, a decrease in Flna after a prolonged low dose morphine treatment suggests impairments in receptors endocytosis and recycling in the hippocampus.
A set of genes encoding for proteins involved in neuroprotective pathways were found modulated by this morphine treatment. Heat shock proteins (HSP) are chaperones that guide the folding, assembly, intracellular disposition and proteolytic turnover of many key regulators of cell growth, differentiation and survival (review in Richter-Landsberg and Goldbaum, 2003). HSP are induced in nervous tissue after brain lesions and a role of these proteins in neuroprotection has been proposed (Koroshetz and Bonventre, 1994; Yenari, 2002). Moreover, several heat shock genes are up-regulated by chronic morphine treatment in the frontal cortex of rats (Ammon et al., 2003). Here we found that Hsp90β was up-regulated in rat hippocampus after chronic morphine treatment. Another up-regulated gene is 3 beta-hydroxysteroid dehydrogenase isomerase (3β-HSD type II.2). This enzyme synthesizes progesterone from pregnenolone and is expressed in various structures of the adult rat brain including hippocampus (Guennoun et al., 1995). Progesterone plays an important role in the neurological recovery from traumatic injury of the brain and spinal cord (review in Stein, 2001). The present chronic morphine treatment, like nerve injury, induced an up-regulation of 3β-HSD suggesting an increase in the local production of the neuroprotective and neuroregenerative agent progesterone. Gapdh expression was also slightly increased in the hippocampus after this chronic morphine treatment. Interestingly, Gapdh is also significantly up-regulated in the nucleus accumbens of rats trained to self administer heroin (Jacobs et al., 2002). This gene is involved in various biochemical and cellular activities (review in Tatton et al., 2000) and a role of Gapdh in neuroprotection has also been suggested (review in Sirover, 1999). One of the down-regulated gene, Flt1, encodes for the vascular endothelial growth factor (VEGF) receptor 1 and is one of the key proteins involved in VEGF-induced neuroprotective and neurogenesis effects (review in Yasuhara et al., 2004). Flt1 expression is generally increased after brain injury but here we found a down-regulation after morphine treatment. Interestingly, Flt1 mediates negative effects on neurogenesis and plasticity in hippocampus (Cao et al., 2004), the observed down-regulation of the receptor observed here could thus participate to a neuroprotective and/or increased plasticity mechanism. Taken together the modulations of these four genes suggest modifications of several neuroprotection pathways in the hippocampus of morphine-treated rats.
The results of this study suggest that chronic morphine treatment may lead to some maladaptive changes in hippocampal neuron function. This has also been suggested by the reduced long-term potentiation observed in hippocampus after chronic morphine treatment (Pu et al., 2002). At present no significant neurodegeneration has been observed after long term treatment with opioids including substitutes such as methadone. Several of the genes modulated in this study are involved in neuroprotection, however a protective role of locally released progesterone and/or enhanced intracellular levels of Hsp90β and Gapdh, as suggested by this study, remains to be firmly demonstrated.
Lengthening the CPP procedure induced a sensitization to the reinforcing effects of morphine. Furthermore this treatment altered the expression of genes involved in several cellular processes. The regulation of the 13 genes of this study were validated by successive SSH, differential screening and microarray analyses. Our results suggest profound alterations in vesicular transport, neurotransmitter release and receptor trafficking in this structure resulting from the progressive robust increase in sensitization to morphine emphasized by the enhanced reinforcing effect. Further studies are necessary to elucidate the implications of these genes in the adaptative changes related to long-term memorization of addictive behavior.
Acknowledgments
This work was supported by grant AH020G from the Mission Interministerielle de Lutte contre la Drogue et la Toxicomanie (M.I.L.D.T.).
References
- Ammon S, Mayer P, Riechert U, Tischmeyer H, Hollt V. Microarray analysis of genes expressed in the frontal cortex of rats chronically treated with morphine and after naloxone precipitated withdrawal. Molecular Brain Research. 2003;112:113–125. doi: 10.1016/s0169-328x(03)00057-3. [DOI] [PubMed] [Google Scholar]
- Andriamampandry C, Muller C, Schmidt-Mutter C, Gobaille S, Spedding M, Aunis D, Maitre M. Mss4 gene is up-regulated in rat brain after chronic treatment with antidepressant and down-regulated when rats are anhedonic. Mol Pharmacol. 2002;62:1332–1338. doi: 10.1124/mol.62.6.1332. [DOI] [PubMed] [Google Scholar]
- Ashburner M, Ball CA, Blake JA, Botstein D, Butler H, Cherry JM, Davis AP, Dolinski K, Dwight SS, Eppig JT, Harris MA, Hill DP, Issel-Tarver L, Kasarskis A, Lewis S, Matese JC, Richardson JE, Ringwald M, Rubin GM, Sherlock G. Gene ontology: tool for the unification of biology. The Gene Ontology Consortium. Nat Genet. 2000;25:25–29. doi: 10.1038/75556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bardo MT, Bevins RA. Conditioned place preference: what does it add to our preclinical understanding of drug reward? Psychopharmacology (Berl) 2000;153:31–43. doi: 10.1007/s002130000569. [DOI] [PubMed] [Google Scholar]
- Burton JL, Burns ME, Gatti E, Augustine GJ, De Camilli P. Specific interactions of Mss4 with members of the Rab GTPase subfamily. Embo J. 1994;13:5547–5558. doi: 10.1002/j.1460-2075.1994.tb06892.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao L, Jiao X, Zuzga DS, Liu Y, Fong DM, Young D, During MJ. VEGF links hippocampal activity with neurogenesis, learning and memory. Nat Genet. 2004;36:827–835. doi: 10.1038/ng1395. [DOI] [PubMed] [Google Scholar]
- Contarino A, Zanotti A, Drago F, Natolino F, Lipartiti M, Giusti P. Conditioned place preference: no tolerance to the rewarding properties of morphine. Naunyn Schmiedebergs Arch Pharmacol. 1997;355:589–594. doi: 10.1007/pl00004988. [DOI] [PubMed] [Google Scholar]
- Diatchenko L, Lau YF, Campbell AP, Chenchik A, Moqadam F, Huang B, Lukyanov S, Lukyanov K, Gurskaya N, Sverdlov ED, Siebert PD. Suppression subtractive hybridization: a method for generating differentially regulated or tissue-specific cDNA probes and libraries. Proc Natl Acad Sci U S A. 1996;93:6025–6030. doi: 10.1073/pnas.93.12.6025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dougherty JD, Geschwind DH. Subtraction-coupled Custom Microarray Analysis for Gene Discovery and Gene Expression Studies in the CNS. Chem Senses. 2002;27:293–298. doi: 10.1093/chemse/27.3.293. [DOI] [PubMed] [Google Scholar]
- Enz R. The actin-binding protein Filamin-A interacts with the metabotropic glutamate receptor type 7. FEBS Lett. 2002;514:184–188. doi: 10.1016/s0014-5793(02)02361-x. [DOI] [PubMed] [Google Scholar]
- Fan X, Zhang J, Zhang X, Yue W, Ma L. Acute and chronic morphine treatments and morphine withdrawal differentially regulate GRK2 and GRK5 gene expression in rat brain. Neuropharmacology. 2002;43:809–816. doi: 10.1016/s0028-3908(02)00147-8. [DOI] [PubMed] [Google Scholar]
- Fan XL, Zhang JS, Zhang XQ, Yue W, Ma L. Differential regulation of [beta]-arrestin 1 and [beta]-arrestin 2 gene expression in rat brain by morphine. Neuroscience. 2003;117:383–389. doi: 10.1016/s0306-4522(02)00930-2. [DOI] [PubMed] [Google Scholar]
- Fries P, Fernandez G, Jensen O. When neurons form memories. Trends Neurosci. 2003;26:123–124. doi: 10.1016/S0166-2236(03)00023-7. [DOI] [PubMed] [Google Scholar]
- Guennoun R, Fiddes RJ, Gouezou M, Lombes M, Baulieu EE. A key enzyme in the biosynthesis of neurosteroids, 3[beta]-hydroxysteroid dehydrogenase/[Delta]5-[Delta]4-isomerase (3[beta]-HSD), is expressed in rat brain. Molecular Brain Research. 1995;30:287–300. doi: 10.1016/0169-328x(95)00016-l. [DOI] [PubMed] [Google Scholar]
- Harismendy O, Gendrel CG, Soularue P, Gidrol X, Sentenac A, Werner M, Lefebvre O. Genome-wide location of yeast RNA polymerase III transcription machinery. Embo J. 2003;22:4738–4747. doi: 10.1093/emboj/cdg466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heidelberger R, Matthews G. Vesicle Priming and Depriming: A SNAP Decision. Neuron. 2004;41:311–313. doi: 10.1016/s0896-6273(04)00039-x. [DOI] [PubMed] [Google Scholar]
- Imperato A, Obinu MC, Casu MA, Mascia MS, Carta G, Gessa GL. Chronic morphine increases hippocampal acetylcholine release: possible relevance in drug dependence. Eur J Pharmacol. 1996;302:21–26. doi: 10.1016/0014-2999(96)00047-7. [DOI] [PubMed] [Google Scholar]
- Jacobs EH, Spijker S, Verhoog CW, Kamprath K, de Vries TJ, Smit AB, Schoffelmeer AN. Active heroin administration induces specific genomic responses in the nucleus accumbens shell. Faseb J. 2002;16:1961–1963. doi: 10.1096/fj.02-0272fje. [DOI] [PubMed] [Google Scholar]
- Kaewsuk S, Hutamekalin P, Ketterman AJ, Khotchabhakdi N, Govitrapong P, Casalotti SO. Morphine induces short-lived changes in G-protein gene expression in rat prefrontal cortex. Eur J Pharmacol. 2001;411:11–16. doi: 10.1016/s0014-2999(00)00768-8. [DOI] [PubMed] [Google Scholar]
- Koob GF, Le Moal M. Drug abuse: hedonic homeostatic dysregulation. Science. 1997;278:52–58. doi: 10.1126/science.278.5335.52. [DOI] [PubMed] [Google Scholar]
- Koroshetz WJ, Bonventre JV. Heat shock response in the central nervous system. Experientia. 1994;50:1085–1091. doi: 10.1007/BF01923465. [DOI] [PubMed] [Google Scholar]
- Lee HK, Safieddine S, Petralia RS, Wenthold RJ. Identification of a novel SNAP25 interacting protein (SIP30) J Neurochem. 2002;81:1338–1347. doi: 10.1046/j.1471-4159.2002.00937.x. [DOI] [PubMed] [Google Scholar]
- Lin R, Karpa K, Kabbani N, Goldman-Rakic P, Levenson R. Dopamine D2 and D3 receptors are linked to the actin cytoskeleton via interaction with filamin A. Proc Natl Acad Sci U S A. 2001;98:5258–5263. doi: 10.1073/pnas.011538198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loguinov AV, Anderson LM, Crosby GJ, Yukhananov RY. Gene expression following acute morphine administration. Physiol Genomics. 2001;6:169–181. doi: 10.1152/physiolgenomics.2001.6.3.169. [DOI] [PubMed] [Google Scholar]
- Marie-Claire C, Courtin C, Roques BP, Noble F. Cytoskeletal Genes Regulation by Chronic Morphine Treatment in Rat Striatum. Neuropsychopharmacology. 2004;29:2208–2215. doi: 10.1038/sj.npp.1300513. [DOI] [PubMed] [Google Scholar]
- Nestler EJ. Molecular basis of long-term plasticity underlying addiction. Nat Rev Neurosci. 2001;2:119–128. doi: 10.1038/35053570. [DOI] [PubMed] [Google Scholar]
- Onoprishvili I, Andria ML, Kramer HK, Ancevska-Taneva N, Miller JM, Simon EJ. Interaction Between the {micro} Opioid Receptor and Filamin A Is Involved in Receptor Regulation and Trafficking. Mol Pharmacol. 2003;64:1092–1100. doi: 10.1124/mol.64.5.1092. [DOI] [PubMed] [Google Scholar]
- Pu L, Bao GB, Xu NJ, Ma L, Pei G. Hippocampal long-term potentiation is reduced by chronic opiate treatment and can be restored by re-exposure to opiates. J Neurosci. 2002;22:1914–1921. doi: 10.1523/JNEUROSCI.22-05-01914.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribeiro CS, Carneiro K, Ross CA, Menezes JR, Engelender S. Synphilin-1 is developmentally localized to synaptic terminals, and its association with synaptic vesicles is modulated by alpha-synuclein. J Biol Chem. 2002;277:23927–23933. doi: 10.1074/jbc.M201115200. [DOI] [PubMed] [Google Scholar]
- Richter-Landsberg C, Goldbaum O. Stress proteins in neural cells: functional roles in health and disease. Cell Mol Life Sci. 2003;60:337–349. doi: 10.1007/s000180300028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shu SY, Wu YM, Bao XM, Leonard B. Interactions among memory-related centers in the brain. J Neurosci Res. 2003;71:609–616. doi: 10.1002/jnr.10545. [DOI] [PubMed] [Google Scholar]
- Sirover MA. New insights into an old protein: the functional diversity of mammalian glyceraldehyde-3-phosphate dehydrogenase. Biochim Biophys Acta. 1999;1432:159–184. doi: 10.1016/s0167-4838(99)00119-3. [DOI] [PubMed] [Google Scholar]
- Stein DG. Brain damage, sex hormones and recovery: a new role for progesterone and estrogen? Trends Neurosci. 2001;24:386–391. doi: 10.1016/s0166-2236(00)01821-x. [DOI] [PubMed] [Google Scholar]
- Tatton WG, Chalmers-Redman RM, Elstner M, Leesch W, Jagodzinski FB, Stupak DP, Sugrue MM, Tatton NA. Glyceraldehyde-3-phosphate dehydrogenase in neurodegeneration and apoptosis signaling. J Neural Transm. 2000;(Suppl):77–100. doi: 10.1007/978-3-7091-6301-6_5. [DOI] [PubMed] [Google Scholar]
- van der Flier A, Sonnenberg A. Structural and functional aspects of filamins. Biochim Biophys Acta. 2001;1538:99–117. doi: 10.1016/s0167-4889(01)00072-6. [DOI] [PubMed] [Google Scholar]
- Yasuhara T, Shingo T, Date I. The potential role of vascular endothelial growth factor in the central nervous system. Rev Neurosci. 2004;15:293–307. doi: 10.1515/revneuro.2004.15.4.293. [DOI] [PubMed] [Google Scholar]
- Yenari MA. Heat shock proteins and neuroprotection. Adv Exp Med Biol. 2002;513:281–299. doi: 10.1007/978-1-4615-0123-7_10. [DOI] [PubMed] [Google Scholar]


