Abstract
In this issue of Blood, Chalaris and colleagues show that ADAM17 regulates shedding of interleukin-6 receptor (IL-6R) by apoptotic neutrophils, a process critical for IL-6 trans-signaling in the switch from neutrophil to mononuclear leukocyte recruitment during the resolution of inflammation.
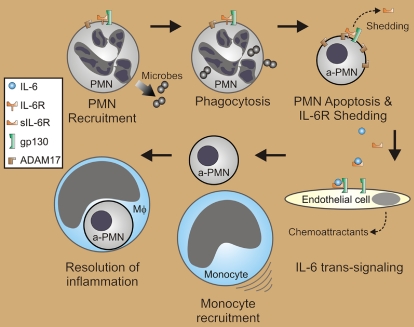
The inflammatory response is characterized largely by influx of neutrophils. These phagocytes possess extraordinary microbicidal capacity and are thus efficient at killing invading microorganisms. On the other hand, cytotoxic molecules of neutrophils have potential to cause significant host tissue damage if released during cell lysis. To avoid this problem, neutrophils undergo apoptosis following phagocytosis as a mechanism to promote resolution of inflammation (see figure). The resolution process also includes switching from neutrophil to mononuclear leukocyte recruitment, and mononuclear phagocytes are responsible for removal of apoptotic neutrophils.1 The switch in leukocyte recruitment involves IL-6 trans-signaling,2 but a trigger for this process has remained elusive.
IL-6 trans-signaling in the resolution of inflammation. The inflammatory response involves influx of polymorphonuclear leukocytes (PMNs or neutrophils). Phagocytosis of microbes and cell activation ultimately lead to PMN apoptosis (a-PMN), during which ADAM17 is activated to cleave IL-6R from the cell surface (shedding). IL-6 and soluble IL-6R derived from neutrophils signal recruitment of mononuclear phagocytes by IL-6 trans-signaling, possibly through endothelial cells. Phagocytosis of apoptotic neutrophils by mononuclear phagocytes, typically macrophages (Mφ), is a key step in the resolution of acute inflammation.
IL-6 is a cytokine that signals through a receptor system consisting of IL-6R, an 80-kDa transmembrane protein that binds IL-6, and a signal transducer molecule known as gp130.2,3 Binding of IL-6 by IL-6R/gp130 ultimately leads to activation of STAT3 and gene transcription in the nucleus.2,3 Expression of IL-6R is restricted to hepatocytes, neutrophils, mononuclear phagocytes, and other leukocytes, whereas gp130 is expressed ubiquitously.3,4 IL-6 can elicit responses directly in cells expressing IL-6R/gp130 or, alternatively, a soluble form of IL-6R (sIL-6R) can bind IL-6 and the complex can be recognized by cells expressing gp130 alone. This process is known as IL-6 trans-signaling.2–4 sIL-6R is generated by shedding (proteolytic cleavage from the cell surface) or from alternatively spliced mRNA.3 There are several known inducers of IL-6R shedding, including bacterial pore-forming toxins, phorbol ester, and metalloproteinases such as ADAM17.
New studies by Chalaris and colleagues reveal that apoptosis is a trigger for IL-6R shedding. Using murine pre-B cells that express human IL-6R, the authors demonstrated that induction of apoptosis by doxorubicin or cytokine withdrawal caused release of IL-6R, thereby forming sIL-6R. Release of IL-6R occurred during early apoptosis and was mediated by the metalloproteinase ADAM17. Next, the authors used HepG2 cells to show that shedding of IL-6R can be mediated by ligation of Fas (CD95), an extrinsic apoptosis pathway stimulus, and that release of IL-6R is caspase-dependent. These results led to a final series of experiments using human neutrophils and a mouse model of acute inflammation. IL-6R was shed from apoptotic human neutrophils in an ADAM17-dependent manner, consistent with recent studies by Walcheck et al.5 Using a mouse model of acute inflammation, the authors discovered that shedding of IL-6R by neutrophils in vivo was essential for subsequent recruitment of mononuclear phagocytes.
These studies extend previous findings by identifying (1) an impetus for the release of IL-6R during inflammation and (2) the signal for recruitment of mononuclear phagocytes and subsequent removal of apoptotic neutrophils during the resolution of acute inflammation (see figure). Given its importance in this process, the IL-6R system is a likely target for new therapeutics designed to treat inflammatory disorders.3
Footnotes
Support for this article was provided by the Intramural Research Program of the NIAID, National Institutes of Health.
Conflict-of-interest disclosure: The author declares no competing financial interests. ■
REFERENCES
- 1.Savill JS, Wyllie AH, Henson JE, et al. Macrophage phagocytosis of aging neutrophils in inflammation. Programmed cell death in the neutrophil leads to its recognition by macrophages. J Clin Invest. 1989;83:865–867. doi: 10.1172/JCI113970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hurst SM, Wilkinson TS, McLoughlin RM, et al. IL-6 and its soluble receptor orchestrate a temporal switch in the pattern of leukocyte recruitment seen during acute inflammation. Immunity. 2001;14:705–714. doi: 10.1016/s1074-7613(01)00151-0. [DOI] [PubMed] [Google Scholar]
- 3.Nishimoto N, Kishimoto T. Interleukin 6: from bench to bedside. Nat Clin Pract Rheumatol. 2007;2:619–626. doi: 10.1038/ncprheum0338. [DOI] [PubMed] [Google Scholar]
- 4.Rose-John S, Waetzig GH, Scheller J, et al. The IL-6/sIL6R complex as a novel target for therapeuitic apparoches. Expert Opin Ther Targets. 2007;11:613–624. doi: 10.1517/14728222.11.5.613. [DOI] [PubMed] [Google Scholar]
- 5.Walcheck B, Herrera AH, Saint Hill C, et al. ADAM17 activity during human neutrophil activation and apoptosis. Eur J Immunol. 2006;36:968–976. doi: 10.1002/eji.200535257. [DOI] [PubMed] [Google Scholar]