Abstract
Sialic acids comprise a large family of derivatives of neuraminic acid containing methyl, acetyl, sulfate and phosphate among other groups, which confer specific physicochemical properties (e.g., hydrophobicity, resistance to hydrolases) to the molecules carrying them. Several years ago a monoclonal antibody, designated H185, was developed, which binds to cell membranes of human corneal, conjunctival, laryngeal, and vaginal epithelia, and whose distribution is altered on the ocular surface of patients with keratinizing disease. Recent findings using immunoprecipitation and immunodepletion techniques have demonstrated that, in human corneal epithelial cells, the H185 antigen is carried by the membrane-associated mucin MUC16. In this study, we show that the H185 epitope on human corneal cells and in tear fluid is an O-acetylated sialic acid epitope that can be selectively hydrolyzed in an enzyme concentration-dependent manner by sialidase from A. ureafaciens, and to a lesser extent by sialidases from Newcastle disease virus, C. perfringens, and S. pneumoniae. Binding of the H185 antibody was impaired by treatment of tear fluid with a recombinant 9-O-acetylesterase from influenza C virus. Two O-acetyl derivatives, Neu5,7Ac2 and Neu5,9Ac2, were identified in human tear fluid by fluorometric HPLC and electrospray mass spectrometry. Immunoprecipitation of the H185 epitope from human corneal epithelial cells revealed that Neu5,9Ac2 was the major derivative on the mucin isolate. These results indicate that exposed wet-surfaced epithelia are decorated with O-acetyl sialic acid derivatives on membrane-associated mucins and suggest that O-acetylation on cell surfaces may protect against pathogen infection by preventing degradation of membrane-associated mucins.
Keywords: cell surface mucin, corneal epithelium, H185 antibody, MUC16, O-acetyl sialic acid
Introduction
Apical membranes on wet-surfaced epithelial cells contain a class of highly O-glycosylated, high molecular weight glycoproteins, termed mucins, which participate in the protection of the underlying epithelium by providing lubrication, maintaining hydration and preventing colonization by pathogenic microorganisms (Hollingsworth and Swanson, 2004). Essential to the functions of mucins are their O-glycans, which constitute 50-80% of their mucin mass. It has been proposed that O-glycans have specialized functions, as evidenced by the fact that different normal mucosal tissues within one individual attach different oligosaccharides to the same mucin protein core, reflecting the distinct requirements of the epithelia (Lan et al., 1990). The composition and sequence of the carbohydrates in the O-glycan chain of mucins are influenced by the specific profile of glycosyltransferases expressed by the cell, their level of activity and their subcellular distribution within the Golgi apparatus (Paulson and Colley, 1989).
Sialic acids occupy a strategic location at the outermost ends of O-glycans, N-glycans, and glycosphingolipids on cell membranes. They are composed of a 9-carbon acidic core, neuraminic acid (Neu)—generally containing N-acetyl (Neu5Ac) or N-glycolyl (Neu5Gc) groups at the 5-carbon position—which can additionally be modified at the 4, 7, 8, and 9-carbon positions (Schauer, 2000). A family of over 40 derivatives of neuraminic acid containing methyl, O-acetyl, sulphate and phosphate, among other groups, have been described in a tissue-specific manner in mammals, where they play important functions in biological recognition phenomena, mediating or preventing binding of cells to each other, and viruses and bacteria to their host cells (Varki, 1997; Schauer, 2000; Schauer et al., 2001). These modifications may also prevent the fast degradation of sialylated glycoconjugates in the glycocalyx by exo-alpha-sialidases (EC 3.2.1.18) produced by certain bacteria, myxoviruses, and animal tissues (Rafelson, 1963). In this instance, the optimal susceptibility to several sialidases requires an intact exocyclic polyhydroxylated side chain (carbons in positions 7, 8 and 9) (Suttajit and Winzler, 1971). O-acetylation of one or more of the hydroxyl groups reduces the action of the sialidases (Corfield et al., 1986).
Monoclonal antibodies that recognize the carbohydrate structure of cell surface glycoconjugates are useful tools for characterizing cell types in physiological and pathophysiological conditions (Kurosaka et al., 1988). Several years ago, a mouse monoclonal antibody, designated H185, was developed, which recognizes a mucin-type glycoprotein in the plasma membrane of suprabasal cells in stratified squamous cells of human corneal, conjunctival, laryngeal, and vaginal epithelium (Watanabe et al., 1995). Binding of the antibody was lost during drying epithelial diseases, such as dry eye and superior limbic keratoconjunctivitis (Watanabe et al., 1997; Danjo et al., 1998). Immunoprecipitation and immunodepletion techniques have shown that the H185 carbohydrate epitope is carried by the membrane-associated mucin MUC16 in human ocular surface epithelial cells (Argueso et al., 2003). This report presents the carbohydrate structure recognized by the H185 antibody as an O-acetylated derivative of sialic acid differentially cleaved by sialidases from different bacterial and viral sources and discusses its biological effects on membrane-associated mucins in stratified wet-surfaced epithelia.
Results
The H185 carbohydrate epitope in human tear fluid is differentially cleaved by sialidases of bacterial and viral origin
The characterization of the H185 carbohydrate was primarily carried out using human tear fluid, which contains high levels of the epitope. By western blot, protein extracts from immortalized human corneal (HCLE) or conjunctival (HCjE) epithelial cell lines contained lesser amounts of the carbohydrate epitope—average levels in the cell lines were 8.8% of those found in tear samples (data not shown). The H185 carbohydrate epitope in tear samples may originate from mucins either secreted or shed from the epithelial cell surface by specific proteases (Thathiah et al., 2003). These proteases cleave the mucin ectodomain in a putative site outside the highly glycosylated, tandem repeat region (Thathiah et al., 2003) and are unlikely to affect terminal carbohydrate antigenicity.
ELISA was initially used to determine the susceptibility of the H185 epitope to hydrolysis by commercial sialidases from different microorganisms (Fig. 1A). Sialidase from A. ureafaciens (α2-3,6,8,9 specific) efficiently abolished H185 antibody binding to tear samples in an enzyme concentration-dependent manner, indicating that a terminal sialic acid residue is involved in H185 antibody recognition. Digestion of tears with increasing concentrations of other bacterial sialidases, S. pneumoniae (α2-3 specific) and C. perfringens (α2-3,6 specific), minimally affected H185 antibody binding—binding was reduced by less than 25%—as compared to that of A. ureafaciens. Treatment with Newcastle disease virus sialidase (α2-3,8 specific) resulted in a 50-85% loss of reactivity. The effect of sialidases on H185 binding was further examined on agarose gels in western blot experiments. A. ureafaciens sialidase totally abolished H185 binding to a high molecular weight band (>250 kDa) on human tears, whereas S. pneumoniae, C. perfringens and Newcastle disease virus did not (Fig. 1B). The membrane-associated mucin MUC16, which has been shown to be a carrier of the H185 carbohydrate epitope in HCLE cells (Argueso et al., 2003), was also detected as shed in tear fluid and migrated on agarose gels to the same position as the H185 epitope. Slight differences in the electrophoretic mobility (particularly after treatment with A. ureafaciens sialidase) were observed in the MUC16 bands, which may have resulted from changes in charge density due to loss of sialic acids, and may have depended on the hydrolysis rate of the enzymes. Additionally, an increase in OC125 antibody binding to MUC16 was observed after desialylation as compared to control (Fig. 1B), which could be explained by the susceptibility of certain mucin antibodies to sialylation (Argueso et al., 2002). The hydrolytic effect of A. ureafaciens sialidase towards the H185 epitope was further confirmed by lack of H185 binding to apical cell membranes on islands of stratified cells in HCLE cultures after enzymatic treatment (Fig. 1C). These results indicate that epithelial mucins carrying the H185 epitope contain sialic acid moieties partially resistant to S. pneumoniae, C. perfringens and Newcastle disease virus sialidases, but labile to digestion with A. ureafaciens sialidase.
Fig. 1. Differential effect of bacterial and viral sialidases on H185 antibody binding.
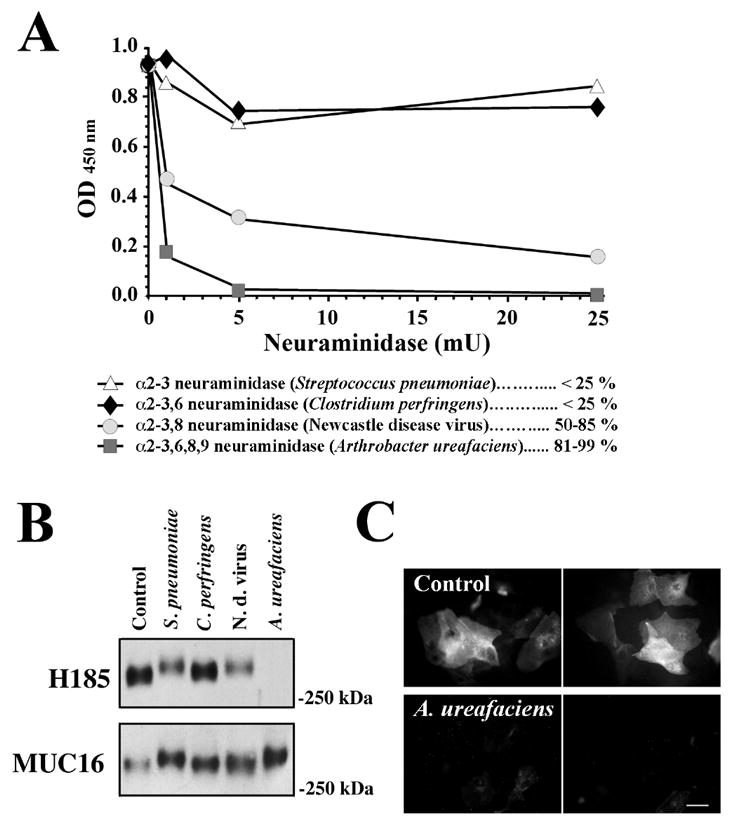
A, In ELISA experiments, 1 μg total protein collected from human tear fluid was enzymatically digested for 1 h at 37°C with 1, 5 and 25 mU of sialidase from S. pneumoniae, C. perfringens, Newcastle disease virus and A. ureafaciens. Reduction in H185 binding (%) was compared to control (0 mU sialidase). B, Effect of sialidases on H185 and MUC16 antibody binding to tear fluid (25 μg of total protein) as demonstrated by western blot. C. Binding of the H185 antibody to apical cell membranes of stratified HCLE cultures (top images) is reduced after digestion with sialidase from A. ureafaciens (bottom images). Bar = 25 μm.
Identification of glycoconjugate-bound O-acetyl sialic acid in human tear fluid
The incomplete hydrolysis of the H185 sialic acid epitope by restricted linkage-specificity sialidases could be explained by the presence of one or several O-acetyl groups on the polyhydroxy side chain of the neuraminic acid molecule (Suttajit and Winzler, 1971; Corfield et al., 1986). Because O-acetyl esters on sialic acids are susceptible to alkaline hydrolysis, we applied mild base treatment to human tears as described in Materials and Methods, and analyzed H185 antibody binding subsequently by ELISA and western blot. By ELISA, there was an average 62% decrease in H185 binding in three tear samples after de-O-acetylation for 30 min (Fig. 2A). H185 binding was not completely abolished after further treatment for up to 120 min. By western blot analysis, there was also a reduction of H185 antibody binding after alkaline hydrolysis (Fig. 2A, inset), suggesting the presence of O-acetyl groups as part of the sialic acid epitope recognized by the H185 antibody. Subsequent treatments of the de-O-acetylated samples with sialidases other than A. ureafaciens did not completely abolish H185 antibody binding, indicating that these sialidases are still unable to hydrolyze the de-O-acetylated H185 epitope under the conditions used in this assay. Treatment of human tears with recombinant 9-O-acetylesterase from influenza C virus resulted in a 90% reduction of H185 binding as determined by ELISA (Fig. 2B), indicating that the H185 carbohydrate epitope is dependent on 9-O-acetyl sialic acid.
Fig. 2. Effect of de-O-acetylation on H185 antibody binding.
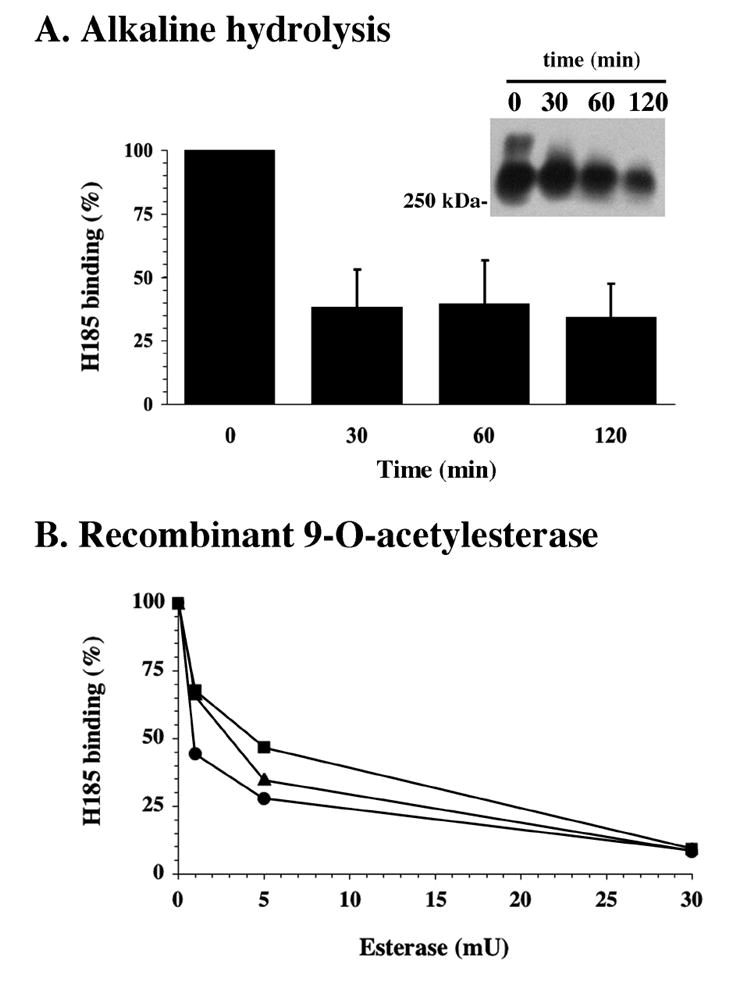
A, O-acetyl esters on sialic acids were removed from tear fluid by alkaline hydrolysis. A reduction of H185 antibody binding was determined by ELISA and western blot (inset). B, Three tear samples containing 1 μg of total protein each were incubated with 9-O-acetylesterase from influenza C virus. A 90 % reduction in H185 binding, as determined by ELISA, was observed after incubation with 30 mU esterase.
The identification of O-acetyl sialic acid derivatives that could potentially constitute the carbohydrate epitope recognized by the H185 antibody was performed by fluorometric HPLC and tandem HPLC-electrospray mass spectrometry (MS) after digestion of tear fluid with A. ureafaciens sialidase. As shown in Fig. 3, crude tear fluid contains a mixture of sialic acids, of which 5-N-acetyl-neuraminic acid (Neu5Ac) is predominant. Two O-acetyl derivatives, Neu5,7Ac2 and Neu5,9Ac2, were also detected, constituting potential determinants of the H185 carbohydrate antigen. Electrospray MS on DMB-derivatized sialic acid peaks further confirmed the presence of Neu5,7Ac2 and Neu5,9Ac2 in three tear samples after digestion with sialidase from A. ureafaciens. The m/z ratio in the mass spectra for Neu5,7Ac2 and Neu5,9Ac2 was 468, and was accompanied by two additional peaks corresponding to the sodium adduct (+22) and the dehydrated form (-18) (data not shown). A minor peak, with a retention time similar to that of the Neu5,7(8),9Ac3 standard, was also detected by fluorometric HPLC but not by electrospray MS. The identity of the O-acetylated residues was confirmed through their susceptibility to alkaline treatment. Hydrolysis of the O-acetyl esters resulted in diminution of the peak area corresponding to the O-acetyl derivatives. This reduction was particularly evident in the case of Neu5,9Ac2, approximately 70%, which correlates to the 62% decrease observed in H185 binding after de-O-acetylation of tears as determined by ELISA (Fig. 2A).
Fig. 3. Identification of glycoconjugate-bound O-acetyl sialic acid derivatives in human tear fluid by fluorometric HPLC.
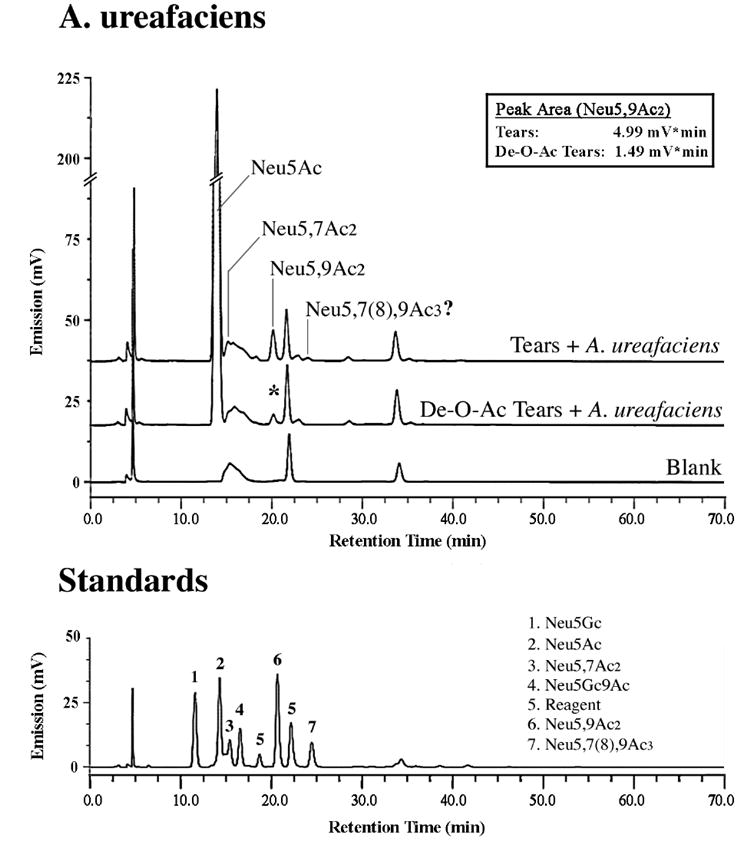
Sialic acids were hydrolyzed using sialidase from A. ureafaciens. In addition to a major peak corresponding to Neu5Ac, two peaks corresponding to Neu5,7Ac2 and Neu5,9Ac2 were identified by fluorometric HPLC and confirmed by tandem HPLC-electrospray MS (see Results). An additional peak, with a retention time similar to that of the Neu5,7(8),9Ac3 standard, was detected by fluorometric HPLC but not by electrospray MS. De-O-acetylation of the sample by alkaline hydrolysis reduced the peak area of Neu5,9Ac2, which correlates with a diminution in H185 binding after alkaline hydrolysis (Fig. 2). The profile of sialic acids is shown relative to standard sialic acids (bottom).
As compared to A. ureafaciens sialidase, digestion of tears with sialidases from S. pneumoniae, C. perfringens and Newcastle disease virus was less efficient (Fig. 4, step 1), correlating with the inability of these enzymes to completely hydrolyze the H185 carbohydrate epitope (Fig. 1A). Further addition of A. ureafaciens sialidase to the samples revealed the presence of remaining O-acetyl derivatives (Fig. 4, step 2), which may account for the H185 antibody binding observed after sialidase treatment in Fig. 1A. Results in Figure 4 also show that the sialidases from S. pneumoniae, C. perfringens and Newcastle disease virus were able to remove non-O-acetylated sialic acid (Neu5Ac) from tear samples. Interestingly, the sialidase from S. pneumoniae seemed to be less efficient than C. perfringens and Newcastle disease virus. Reduced efficiency of the S. pneumoniae sialidase, which only cleaves the α2-3 linkage, suggests that different sialic acid linkages within the mucin are present, α2-3 being a minor component. In this regard, O-acetylation does not seem to take place on α□3-linked sialic acids (Shi et al., 1996; Lewis et al., 2004).
Fig. 4. Differential effect of bacterial and viral sialidases in sialic acid hydrolysis, as determined by fluorometric HPLC.
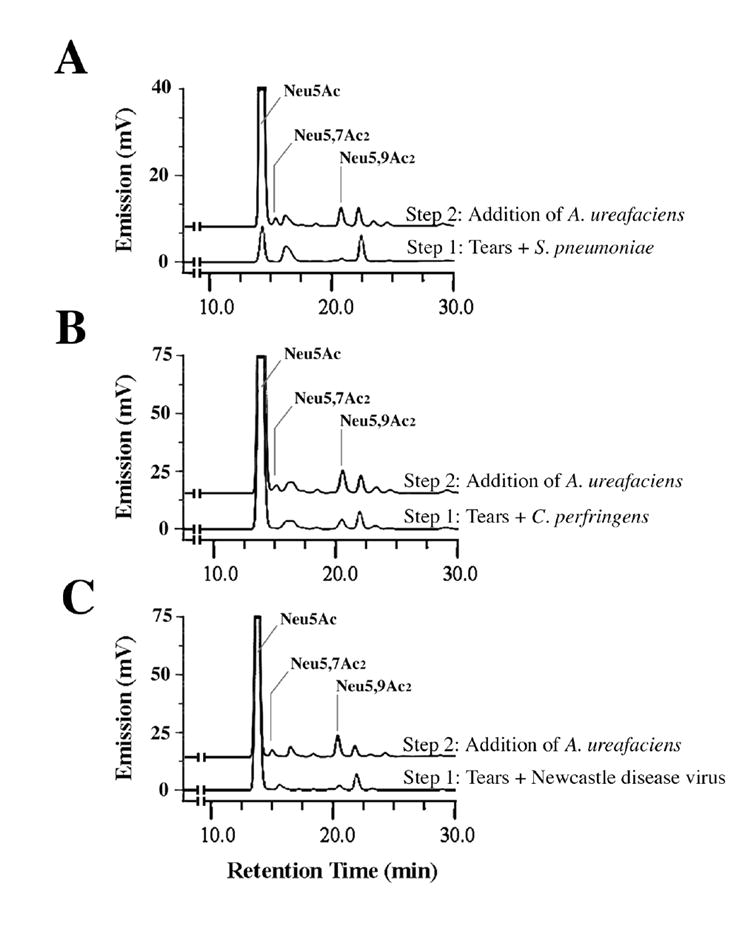
Sialidases from S. pneumoniae (A), C. perfringens (B) and Newcastle disease virus (C), which showed partial or low levels of H185 epitope hydrolysis by ELISA (Fig1A), were less efficient in hydrolyzing Neu5,7Ac2 and Neu5,9Ac2 from human tears than A. ureafaciens sialidase (step 1); further treatment of the digested tears with A. ureafaciens sialidase revealed the presence of remaining Neu5,7Ac2 and Neu5,9Ac2 derivatives in the samples (step 2).
Neu5,7Ac2 and Neu5,9Ac2 were found in all tear samples (n=7) individually analyzed by fluorometric HPLC, and ranged 0.02-6.05% (average 2.4±2.1) of the total sialic acid content for Neu5,7Ac2, and 0.49-4.21% (average 3.5±2.2) for Neu5,9Ac2. The presence of the H185 epitope in each individual sample was confirmed by agarose gel immunoblot, resulting in one or two high molecular weight bands (Fig. 5). A subsequent densitometric analysis did not show a conclusive correlation between the intensity of H185 binding and the relative levels of NeuAc5,7Ac2 and NeuAc5,9Ac2 in these samples (e.g., the sample with the higher amount of NeuAc5,9Ac2 did not have the most intense band by western blot analysis), suggesting that despite the presence of O-acetyl derivatives in the sample, H185 antibody binding intensity may depend on a particular conformation of the epitope and its neighboring sugars.
Fig. 5. Relative abundance of sialic acid derivatives isolated from human tear fluid.
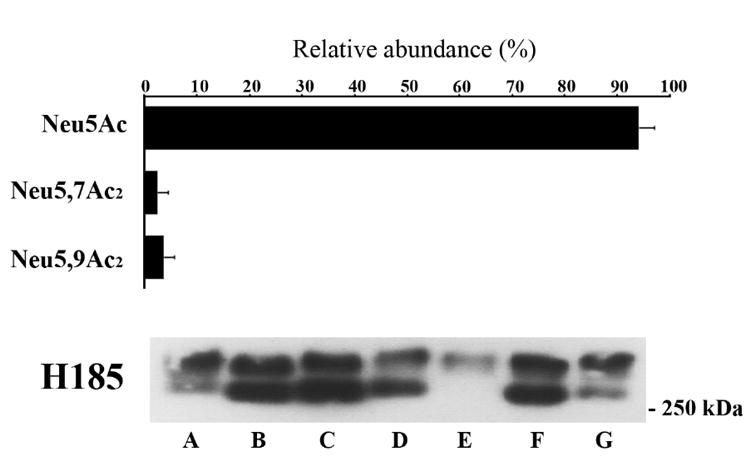
Seven human tear samples were hydrolyzed using sialidase from A. ureafaciens and individually analyzed by fluorometric HPLC. Shown is the relative abundance of individual sialic acids as a percentage of total sialic acid (values are mean ± SD, n=7). The presence of the H185 carbohydrate epitope in each sample (A-G) analyzed by fluorometric HPLC is confirmed by western blot (5-50 μg of total protein). The presence of two molecular weight bands migrating over 250 kDa may result from mucin polymorphism (individual mucin alleles are expressed co-dominantly), or the presence of different mucin gene products or glycoforms containing the H185 carbohydrate epitope in the sample.
All together, these data indicate that O-acetylated species are minor components of the total sialic acid in human tear fluid, but constitute potential antigenic determinants required for H185 antibody binding.
H185/MUC16 cell surface mucin in corneal epithelial cells contains O-acetyl sialic acid
Further analysis of O-acetyl sialic acids on a specific epithelial mucin was performed by immunoprecipitation of the H185 antigen from HCLE cells, previously identified on the membrane-associated mucin MUC16 (Argueso et al., 2003). Analysis of the H185 immunoprecipitate by fluorometric HPLC revealed the presence of Neu5,9Ac2 in the mucin isolate, but not Neu5,7Ac2 (Fig. 6). The relative percentage of Neu5,9Ac2 in the MUC16 molecule was low (6.2%) as compared to that of Neu5Ac (88.4%). Although this data indicates that 9-O-acetyl derivatives are present in MUC16, it should be noted that the 7-O-acetyl ester can migrate to carbon position 9 under physiological conditions or under the conditions of this analysis (Butor et al., 1993; Lewis et al., 2004). A low level of Neu5Gc (5.4%) was additionally found in the immunoprecipitate, most likely metabolically incorporated into the H185 mucin from exogenous glycoproteins present in the calf serum used to grow HCLE cells, as previously described (Tangvoranuntakul et al., 2003; Bardor et al., 2005). In these experiments, immunoprecipitation of the H185 mucin was confirmed by immunoblot (Fig. 6, inset). Taken together, we have used immunoprecipitation followed by fluorometric HPLC to identify a mono-O-acetylated sialic acid derivative, Neu5,9Ac2, on a cell surface-associated mucin in cultures of human corneal epithelial cells.
Fig. 6. Identification of sialic acid derivatives after H185 antigen immunoprecipitation.
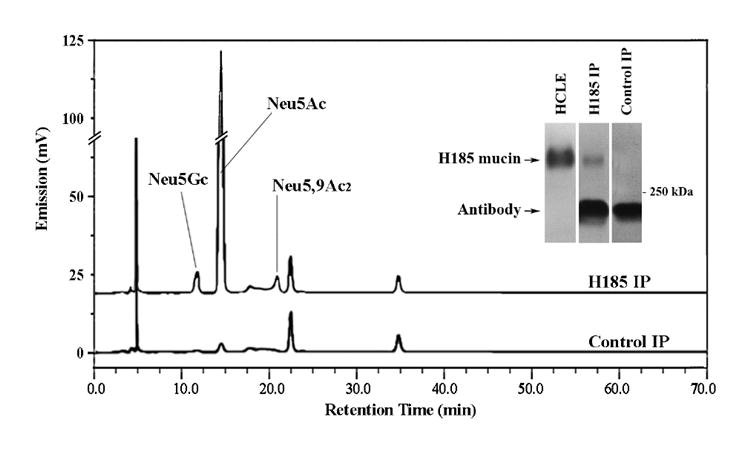
The H185 antigen, which in HCLE cells is carried by the membrane-associated mucin MUC16 (Argueso et al., 2003), was immunoprecipitated from HCLE cultures as described in Materials and Methods. Sialic acids in the H185 mucin immunoprecipitate (H185 IP) were hydrolyzed using A. ureafaciens sialidase, labeled with DMB and analyzed by fluorometric HPLC. A control (no tear sample) was carried out to determine the sialic acid content of the antibodies used for immunoprecipitation. The efficiency of the H185 mucin immunoprecipitation was assessed by immunoblot (inset). The low molecular weight band corresponds to antibodies used in the mucin immunoprecipitation assay.
Discussion
The non-keratinized, human stratified squamous epithelia carry unique carbohydrate antigens on mucin-type glycoproteins, such as the one recognized by the H185 monoclonal antibody, whose distribution has been shown to be altered in the ocular surface of patients with keratinizing dry eye disease (Gipson and Argueso, 2003). This study shows that the H185 antibody recognizes an O-acetylated sialic acid epitope in mucosal secretions and on epithelial surfaces that is differentially cleaved by sialidases of bacterial and viral origin, and identifies membrane-associated mucins as carriers of O-acetyl sialic acid derivatives on exposed epithelial cell surfaces.
Previous analyses have shown that the H185 antibody has a restricted binding pattern, which in humans includes the stratified wet-surfaced epithelia of the cornea, conjunctiva, larynx, and vagina, in addition to the submucosal glands of the esophagus and large intestine, cells of eccrine sweat glands (Watanabe et al., 1995), and the cervical epithelium (data not shown). No binding of the H185 antibody was detected in buccal mucosa, esophagus, ileum, colon, mammary epithelium, and liver, or in non-human species such as monkey, rabbit, rat, cow, and sheep (Watanabe et al., 1995). By comparison, O-acetyl derivatives of Neu5Ac are widely expressed in almost all deuterostomal species in a tissue-specific manner, which in humans include mucosal tissues such as colon and nasal epithelia (Schauer et al., 2001). Lack of H185 antibody binding to these tissues containing O-acetyl sialic acid derivatives suggests that, although the epitope defined by the H185 antibody involves an O-acetylated sialic acid, this structure is a co-determinant and not the entire epitope responsible for antibody recognition. Derivatives of sialic acid that act as co-determinants in antibody recognition have been previously described. Cheresh et al. found that the D1.1 antibody, which reacts with an O-acylated sialic acid epitope on a ganglioside on developing rat embryonic neuroectoderm, did not show any reaction with murine erythroleukemia cells, which are known to have O-acetylated sialic acids on their surface (Cheresh et al., 1984). The unique conformation of the H185 binding domain may be modulated by neighboring sugars, or the presence of other modification(s) on the sialic acid residue not detected under the conditions of our analyses. The presence of carbohydrate moieties in close proximity to the sialic acid antigen site is relevant in defining antibody specificities (Khurana et al., 1997). In mucin-type glycoproteins, the presence of clusters of terminal sialic acid epitopes enhances the reaction of carbohydrate-directed monoclonal antibodies, such as MLS 102, which recognizes a cancer-associated sialic acid antigen on human colonic mucins (Kurosaka et al., 1988). The complete characterization of the molecular structure of the carbohydrate side chains on the H185 mucin may lead to a better understanding of the nature of the H185 antigenic determinant.
Membrane-associated mucins have been proposed as potential carriers of O-acetylated sialic acid derivatives in the glycocalyx of polarized epithelial cells (Maury et al., 1995). In this report, we have identified O-acetyl sialic acid residues in H185 immunoprecipitated material, which in HCLE cells corresponds to the membrane-associated mucin MUC16 (Argueso et al., 2003). Based on immunofluorescence data showing sporadic binding to conjunctival goblet cells (Watanabe et al., 1995), it is possible to speculate that other mucins, such as the secreted gel-forming MUC5AC in goblet cells, may also contain O-acetyl sialic acid. Interestingly, we could not detect H185 antibody binding to the membrane-associated MUC1 immunoprecipitated from HCLE cells (Argueso et al., 2003). Sialic acid O-acetylation on specific membrane-associated mucins may mediate a large number of adhesion events by generating unique patterns of cell surface carbohydrates. In this regard, the success of different microorganisms to colonize an epithelium depend on their capacity to secrete exoproducts, such as sialidases, that modify the host cell surface microenvironment to expose binding sites and facilitate attachment to the epithelial cell surface. This is well illustrated with the Pseudomonas aeruginosa PA01 sialidase, whose activity on primary respiratory epithelial cells is two orders of magnitude greater than that of the C. perfringens enzyme, resulting in an increased adherence of Pseudomonas to respiratory cells treated with the PA01 strain (Cacalano et al., 1992). O-acetylation on mucins can markedly slow or even block the release of sialic acid by sialidases (Varki and Diaz, 1983; Corfield et al., 1986) and may facilitate the protection of the underlying epithelium. Our results show that bacterial and viral sialidases have a differential effect on the hydrolysis of the H185 epitope. The membrane-associated mucin MUC16, which carries the terminal H185 epitope in HCLE cells, contains a long glycosylated extracellular amino terminal domain (O’Brien et al., 2002) that may extend above other membrane-associated mucins and glycoproteins in the glycocalyx, potentially dictating the microbial-host interactions in stratified wet-surfaced epithelia and preventing infection in intact exposed epithelia.
It is now well established, mainly in colorectal cancer, that alterations in the level of O-acetylated species on mucins accompany the development of disease (Schauer et al., 2001). At the ocular surface, Corfield et al. have recently demonstrated a decrease in the level of O-acetylation in tear mucin secretions in a canine model of dry eye (Corfield et al., 2005), which correlates with previous reports in humans showing lack of H185 antibody binding on the cell surface of apical conjunctival cells in patients with dry eye and superior limbic keratoconjunctivitis (Watanabe et al., 1997; Danjo et al., 1998). We hypothesize that lack of O-acetyl groups, known to confer a more hydrophobic character to sialic acid residues (Schauer, 2000), at the ocular surface in dry eye may alter the interaction of the cell surface glycocalyx with other components of the tear film, such as lipids and hydrophobic proteins, leading to tear film instability. Also, the presence of O-acetylated sialic acids on apical cell surface-associated mucins and their alteration may influence the adhesion of circulating leukocytes—which contain sialic acid-binding lectins (Varki, 1997)—present in tears during overnight eye closure (Wilson et al., 1989; Sakata et al., 1997). At the molecular level, the turnover of O-acetyl ester groups on sialic acids is thought to be regulated by the relative activities of two enzymes, sialate-O-acetyltransferases present within the lumen of the Golgi apparatus or Golgi-related organelles, and sialate-O-acetylesterases (Varki, 1999; Shen et al., 2004). Exogenously, O-acetylesterase activity has been reported from bacterial and viral sources (Varki, 1999). Elucidating the relative activities of these enzymes at the ocular surface of patients with keratinizing disease may give insight into the pathophysiology of this disease.
In summary, this study indicates that O-glycans on cell surface mucins from human stratified squamous epithelia contain O-acetyl sialic acids at their non-reducing ends that are recognized by the H185 monoclonal antibody and which can be differentially cleaved by sialidases of bacterial and viral origin. We propose that chemical modifications of neuraminic acid on membrane-associated mucins prevent the fast degradation of the epithelial glycocalyx by microorganisms and mediate the interactions of apical wet surfaces with their extracellular milieu. Alterations in sialic acid O-acetylation, such as that observed at the ocular surface of dry eye patients, may result in cell surface instability and damage to the underlying epithelium.
Materials and Methods
Mucin Collection
Tear fluid and protein extracts from human corneal-limbal epithelial cell cultures were tested for H185 epitope content. The highest level of H185 antigen was found in tear fluid, which contains mucins either secreted or shed by the ocular surface epithelia (Gipson and Argueso, 2003). Therefore, analysis of the H185 carbohydrate epitope was primarily carried out using tear samples. Tear fluid was collected, in compliance with institutional review board regulations, from the inferior fornix of normal subjects after instillation of 60 μl of sterile water onto the cul-de-sac, as described previously (Argueso et al., 2002). Samples were centrifuged for 30 min at 16,000 × g at 4°C. Protein concentration was determined using the MicroBCA protein assay reagent kit (Pierce; Rockford, IL), using a dilution series of bovine serum albumin (BSA) as standard. All samples were promptly frozen at −80°C until the time of analyses.
Cell culture
Telomerase-immortalized human corneal-limbal epithelial (HCLE) cells were plated at a seeding density of 5 × 104 cells/cm2 on culture chamber slides (Lab-Tek, Naperville, IL) or T75 flasks (Corning Costar Corp., Cambridge, MA) and maintained at 37°C in 5% CO2. Characterization of HCLE cell cultures has been previously reported (Gipson et al., 2003). HCLE cultures were grown in a medium optimized for proliferation of keratinocytes (keratinocyte serum-free medium; Gibco-Invitrogen Corporation, Carlsbad, CA) to achieve confluence. After reaching confluence, cells were switched to DMEM/F12 supplemented with 10% calf serum and 10 ng/ml EGF for 7 d, which promotes stratification and differentiation of corneal cells.
Enzymatic treatments and de-O-acetylation
Sample digestions were performed with α2-3-sialidase from Streptococcus pneumoniae (Calbiochem, San Diego, CA), α2-3,6-sialidase from Clostridium perfringens (Calbiochem), α2-3,8-sialidase from Newcastle disease virus (Glyko, Novato, CA) and α2-3,6,8,9-sialidase from Arthrobacter ureafaciens (Glyko). Enzymatic treatments were carried out at 37°C in 50 mM sodium phosphate, pH 6.0 (S. pneumoniae, C. perfringens, A. ureafaciens) or 50 mM sodium acetate, pH 5.5 (Newcastle disease virus). Sialic acids were de-O-acetylated by alkaline hydrolysis as described by Toba et al. (Toba et al., 2000). Briefly, 1-50 μg of total protein from human tears were treated with 0.2 M NaOH at 24°C for up to 120 min. The reaction was neutralized by addition of 3 M acetic acid. De-O-acetylation was also performed enzymatically using a recombinant 9-O-acetylesterase of influenza C virus kindly supplied by Dr. Reinhard Vlasak (Applied Biotechnology GmbH, Salzburg, Austria). Samples were digested with the esterase using conditions previously described (Hellebo et al., 2004).
ELISA
Tear fluid (1 μg of total protein) diluted in PBS was coated on EIA plates (Corning Costar Corp.) overnight at 4°C. After washing with PBS, plates were incubated with 10% normal horse serum (NHS) in PBS for 30 min at room temperature and with the monoclonal mouse anti-human H185 antibody (undiluted hybridoma culture medium) for 1 h. The H185 antibody, which recognizes a human carbohydrate epitope on MUC16 (Argueso et al., 2003), was obtained from culture supernatants of hybridoma cells as described previously (Watanabe et al., 1995). Plates were then incubated with a 1:10,000 dilution of secondary sheep anti-mouse IgG peroxidase-conjugated antibody in PBS containing 10% NHS for 1 h. After washing, plates were incubated with the peroxidase substrate, tetramethylbenzidine (Sigma Co., St. Louis, MO), at room temperature. The reaction was stopped after 30 min by the addition of 0.5 M H2SO4 and the optical density (OD) of each well was read at 450 nm.
Immunoprecipitation
HCLE cells were scraped from T75 flasks after addition of 1 ml of 2% SDS in the presence of Complete Mini protease inhibitor cocktail (Roche Applied Science, Indianapolis, IN), homogenized, and centrifuged at 35,016 × g (RCF) for 45 min. The resulting supernatant was recovered, and the protein concentration determined using the Pierce BCA Protein Assay (Pierce). For H185 mucin immunoprecipitation, the H185 antibody was incubated with anti-mouse IgG agarose particles (Sigma) for 1 h at 4ºC as described previously (Argueso et al., 2003). Five-hundred μg of HCLE protein was diluted in 500 μl of immunoprecipitation buffer (0.1 M NH4HCO3, 2.0 mM PMSF, 0.5 M NaCl, 5 mM ethylenediaminetetraacetic acid (EDTA), 2 mM N-ethylmaleimide, and 0.02% NaN3 and Complete Mini protease inhibitor cocktail), added to the conjugated beads, and incubated for 2 h on a rocker at room temperature. After three washes with a buffer containing 10 mM Tris-HCl, 2 mM EDTA, 0.1% Triton X-100, 0.1% SDS, pH 7.4, the immunoprecipitate was resuspended in 120 μl of 2% SDS, boiled for 5 min, and the beads separated from the soluble H185 mucin and antibody by centrifugation. The supernatant was added to 10K molecular weight cut-off centrifuge tubes (Omega Nanosep 10K, Pall Corp., Ann Arbor, MI), filtered by centrifugation at 16,000 × g for 5 min, and the immunoprecipitated material on the membrane washed with sialidase buffer before enzymatic treatment with 20 mU of sialidase from A. ureafaciens. After sialidase treatment, the sialic acid derivatives were recovered by centrifugation and analyzed by fluorometric HPLC as described below. A control (no tear sample) was carried out to determine the sialic acid content of the antibodies used for immunoprecipitation. The efficiency of the H185 mucin immunoprecipitation was determined by immunoblot.
Agarose gel electrophoresis and immunobloting
Tear samples (1-50 μg of total protein) or immunoprecipitated H185 mucin from HCLE cells were electrophoresed on 1% agarose gels (BMA, Rockland, ME) for 2 h 15 min at 50 V at 4oC under reducing conditions (Thornton et al., 2000). Proteins were transferred for 1.5 h to a nitrocellulose membrane by vacuum blotting in transfer buffer (0.6 M NaCl, 60 mM sodium citrate). Immunolabeling was performed using the H185 or OC125 (DAKO Corporation, Carpinteria, CA) antibodies as previously reported (Argueso et al., 2003). Membranes were incubated with SuperSignal West Pico Chemiluminescent Substrate (Pierce) and protein bands detected after exposure to film (Hyperfilm, Amersham Biosciences, Buckinghamshire, UK).
Immunofluorescence microscopy
HCLE cultures grown for 7 d in cell culture chamber slides were fixed in 4% paraformaldehyde for 24 h. Cultures were then incubated with or without A. ureafaciens sialidase (0.5 units/ml in 50 mM sodium phosphate buffer, pH 6.0) for 2.5 h at 37°C. After blocking with 1% BSA, cultures were maintained overnight at 4°C with a 1:10 dilution of primary mouse monoclonal antibody H185. Cultures were then incubated with a 1:50 dilution of secondary donkey anti-mouse fuorescein-conjugated antibody (Jackson ImmunoResearch, WestGrove, PA) and coverslipped with antifade mounting medium plus propidium iodide (Vectashield; Vector Laboratories; Burlingame, CA) as previously described (Gipson et al., 2003).
Fluorometric HPLC
Tear samples (1-50 μg of total protein) were added to 10K molecular weight cut-off centrifuge tubes and filtered by centrifugation at 16,000 × g for 5 min. Samples on the membrane were treated with 40 mU of sialidase from A. ureafaciens, or 50 mU of sialidases from S. pneumoniae, C. perfringens or Newcastle disease virus for 2 h at 37°C. Tear samples treated with sialidases that showed partial or low levels of H185 epitope digestion in the ELISA assay (S. pneumoniae, C. perfringens, Newcastle disease virus) were further digested on the membranes with A. ureafaciens sialidase for an additional 2 h. The released sialic acids were collected by filtration and lyophilized.
Sialic acids were derivatized with 1,2-diamino-4,5-methylenedioxybenzene (DMB) and analyzed by reverse-phase fluorometric HPLC as described (Manzi and Varki, 1992). Fluorometric HPLC analyses were performed with a Dionex Bio-LC chromatography system (Sunnyvale, CA) using a TSK ODS-120T column (Tosoh Bioscience, Japan) at a flow rate of 0.9 mL/min. Samples were eluted with a linear gradient of acetonitrile (7-11%) in methanol (7%) and H2O over 30 min followed by 10 min at the final conditions. The excitation and emission wavelengths were 373 and 448 nm, respectively. The DMB-derivatized sialic acids were identified by comparing retention times to known standards (sialic acid reference panel, Glyko) that were similarly treated.
Electrospray MS
The identity of DMB-derivatized peaks observed in HPLC analysis was verified using both an on-line UV detector (373 nm) and a Thermo Finnigan LCQ mass spectrometer (San Jose, CA) operated in the positive ion mode using methods previously described (Lewis et al., 2004). Sialic acid hydrolysates were analyzed by electrospray MS after reverse-phase HPLC separation as described above except that the column was eluted isocratically with acetonitrile (7%), methanol (8%), formic acid (0.1%), and H2O.
Acknowledgments
The authors thank Dr. Ilene K. Gipson for providing the mouse monoclonal H185 antibody and for her valuable comments on this work. Dr. Reinhard Vlasak is acknowledged for his generous gift of the recombinant 9-O-acetylesterase from influenza C virus. The authors also thank Dr. Stefano Barabino for collection of human tears. Mass spectrometry analysis of sialic acid derivatives was performed by the UCSD Glycotechnology Core Resource. This work was supported by a grant from the National Institutes of Health/National Eye Institute (R01 EY014847, P.A.).
Abbreviations
- BSA
bovine serum albumin
- DMB
1,2-diamino-4,5-methylenedioxybenzene
- DMEM/F12
Dulbecco’s modified eagle’s medium/ Ham’s nutrient mixture F12
- ELISA
enzyme-linked immunosorbent assay
- HCLE
human corneal-limbal epithelium
- HPLC
high-performance liquid chromatography
- MS
mass spectrometry
- Neu5Ac
5-N-acetyl-neuraminic acid
- Neu5,7(9)Ac2
5-N-acetyl-7(9)-O-acetylneuraminic acid
- Neu5,7(8),9Ac3
5-N-acetyl-7(8),9-di-O-acetylneuraminic acid
- Neu5Gc
5-N-glycolyl-neuraminic acid
- NHS
normal horse serum
- PBS
phosphate buffered saline
References
- 1.Argueso P, Balaram M, Spurr-Michaud S, Keutmann HT, Dana MR, Gipson IK. Decreased levels of the goblet cell mucin MUC5AC in tears of patients with Sjogren syndrome. Invest Ophthalmol Vis Sci. 2002;43:1004–1011. [PubMed] [Google Scholar]
- 2.Argueso P, Spurr-Michaud S, Russo CL, Tisdale A, Gipson IK. MUC16 mucin is expressed by the human ocular surface epithelia and carries the H185 carbohydrate epitope. Invest Ophthalmol Vis Sci. 2003;44:2487–2495. doi: 10.1167/iovs.02-0862. [DOI] [PubMed] [Google Scholar]
- 3.Bardor M, Nguyen DH, Diaz S, Varki A. Mechanism of uptake and incorporation of the non-human sialic acid N-glycolylneuraminic acid into human cells. J Biol Chem. 2005;280:4228–4237. doi: 10.1074/jbc.M412040200. [DOI] [PubMed] [Google Scholar]
- 4.Butor C, Diaz S, Varki A. High level O-acetylation of sialic acids on N-linked oligosaccharides of rat liver membranes. Differential subcellular distribution of 7- and 9-O-acetyl groups and of enzymes involved in their regulation. J Biol Chem. 1993;268:10197–10206. [PubMed] [Google Scholar]
- 5.Cacalano G, Kays M, Saiman L, Prince A. Production of the Pseudomonas aeruginosa neuraminidase is increased under hyperosmolar conditions and is regulated by genes involved in alginate expression. J Clin Invest. 1992;89:1866–1874. doi: 10.1172/JCI115791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cheresh DA, Varki AP, Varki NM, Stallcup WB, Levine J, Reisfeld RA. A monoclonal antibody recognizes an O-acylated sialic acid in a human melanoma-associated ganglioside. J Biol Chem. 1984;259:7453–7459. [PubMed] [Google Scholar]
- 7.Corfield AP, Donapaty SR, Carrington SD, Hicks SJ, Schauer R, Kohla G. Identification of 9-O-acetyl-N-acetylneuraminic acid in normal canine pre-ocular tear film secreted mucins and its depletion in Keratoconjunctivitis sicca. Glycoconj J. 2005;22:409–416. doi: 10.1007/s10719-005-3698-3. [DOI] [PubMed] [Google Scholar]
- 8.Corfield AP, Sander-Wewer M, Veh RW, Wember M, Schauer R. The action of sialidases on substrates containing O-acetylsialic acids. Biol Chem Hoppe Seyler. 1986;367:433–439. doi: 10.1515/bchm3.1986.367.1.433. [DOI] [PubMed] [Google Scholar]
- 9.Danjo Y, Watanabe H, Tisdale AS, George M, Tsumura T, Abelson MB, Gipson IK. Alteration of mucin in human conjunctival epithelia in dry eye. Invest Ophthalmol Vis Sci. 1998;39:2602–2609. [PubMed] [Google Scholar]
- 10.Gipson IK, Argueso P. Role of mucins in the function of the corneal and conjunctival epithelia. Int Rev Cytol. 2003;231:1–49. doi: 10.1016/s0074-7696(03)31001-0. [DOI] [PubMed] [Google Scholar]
- 11.Gipson IK, Spurr-Michaud S, Argueso P, Tisdale A, Ng TF, Russo CL. Mucin gene expression in immortalized human corneal-limbal and conjunctival epithelial cell lines. Invest Ophthalmol Vis Sci. 2003;44:2496–2506. doi: 10.1167/iovs.02-0851. [DOI] [PubMed] [Google Scholar]
- 12.Hellebo A, Vilas U, Falk K, Vlasak R. Infectious salmon anemia virus specifically binds to and hydrolyzes 4-O-acetylated sialic acids. J Virol. 2004;78:3055–3062. doi: 10.1128/JVI.78.6.3055-3062.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hollingsworth MA, Swanson BJ. Mucins in cancer: protection and control of the cell surface. Nat Rev Cancer. 2004;4:45–60. doi: 10.1038/nrc1251. [DOI] [PubMed] [Google Scholar]
- 14.Khurana S, Raghunathan V, Salunke DM. The variable domain glycosylation in a monoclonal antibody specific to GnRH modulates antigen binding. Biochem Biophys Res Commun. 1997;234:465–469. doi: 10.1006/bbrc.1997.5929. [DOI] [PubMed] [Google Scholar]
- 15.Kurosaka A, Kitagawa H, Fukui S, Numata Y, Nakada H, Funakoshi I, Kawasaki T, Ogawa T, Iijima H, Yamashina I. A monoclonal antibody that recognizes a cluster of a disaccharide, NeuAc alpha(2----6)GalNAc, in mucin-type glycoproteins. J Biol Chem. 1988;263:8724–8726. [PubMed] [Google Scholar]
- 16.Lan MS, Batra SK, Qi WN, Metzgar RS, Hollingsworth MA. Cloning and sequencing of a human pancreatic tumor mucin cDNA. J Biol Chem. 1990;265:15294–15299. [PubMed] [Google Scholar]
- 17.Lewis AL, Nizet V, Varki A. Discovery and characterization of sialic acid O-acetylation in group B Streptococcus. Proc Natl Acad Sci U S A. 2004;101:11123–11128. doi: 10.1073/pnas.0403010101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Manzi AE, Varki A. Compositional analysis of glycoproteins. In: Fukuda M, Kobata A, editors. Glycobiology: A Practical Approach. Oxford University Press; Oxford: 1992. pp. 27–77. [Google Scholar]
- 19.Maury J, Bernadac A, Rigal A, Maroux S. Expression and glycosylation of the filamentous brush border glycocalyx (FBBG) during rabbit enterocyte differentiation along the crypt-villus axis. J Cell Sci. 1995;108(Pt 7):2705–2713. doi: 10.1242/jcs.108.7.2705. [DOI] [PubMed] [Google Scholar]
- 20.O’Brien TJ, Beard JB, Underwood LJ, Shigemasa K. The CA 125 gene: a newly discovered extension of the glycosylated N-terminal domain doubles the size of this extracellular superstructure. Tumour Biol. 2002;23:154–169. doi: 10.1159/000064032. [DOI] [PubMed] [Google Scholar]
- 21.Paulson JC, Colley KJ. Glycosyltransferases. Structure, localization, and control of cell type-specific glycosylation. J Biol Chem. 1989;264:17615–17618. [PubMed] [Google Scholar]
- 22.Rafelson ME., Jr The Neuraminidases and Their Action on Glycoproteins and Other Sialic Acid-Containing Compounds. Expos Annu Biochim Med. 1963;24:121–132. [PubMed] [Google Scholar]
- 23.Sakata M, Sack RA, Sathe S, Holden B, Beaton AR. Polymorphonuclear leukocyte cells and elastase in tears. Curr Eye Res. 1997;16:810–819. doi: 10.1076/ceyr.16.8.810.8992. [DOI] [PubMed] [Google Scholar]
- 24.Schauer R. Achievements and challenges of sialic acid research. Glycoconj J. 2000;17:485–499. doi: 10.1023/A:1011062223612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schauer R, Schmid H, Pommerencke J, Iwersen M, Kohla G. Metabolism and role of O-acetylated sialic acids. Adv Exp Med Biol. 2001;491:325–342. doi: 10.1007/978-1-4615-1267-7_21. [DOI] [PubMed] [Google Scholar]
- 26.Shen Y, Kohla G, Lrhorfi AL, Sipos B, Kalthoff H, Gerwig GJ, Kamerling JP, Schauer R, Tiralongo J. O-acetylation and de-O-acetylation of sialic acids in human colorectal carcinoma. Eur J Biochem. 2004;271:281–290. doi: 10.1046/j.1432-1033.2003.03927.x. [DOI] [PubMed] [Google Scholar]
- 27.Shi WX, Chammas R, Varki A. Linkage-specific action of endogenous sialic acid O-acetyltransferase in Chinese hamster ovary cells. J Biol Chem. 1996;271:15130–15138. doi: 10.1074/jbc.271.25.15130. [DOI] [PubMed] [Google Scholar]
- 28.Suttajit M, Winzler RJ. Effect of modification of N-acetylneuraminic acid on the binding of glycoproteins to influenza virus and on susceptibility to cleavage by neuraminidase. J Biol Chem. 1971;246:3398–3404. [PubMed] [Google Scholar]
- 29.Tangvoranuntakul P, Gagneux P, Diaz S, Bardor M, Varki N, Varki A, Muchmore E. Human uptake and incorporation of an immunogenic nonhuman dietary sialic acid. Proc Natl Acad Sci U S A. 2003;100:12045–12050. doi: 10.1073/pnas.2131556100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Thathiah A, Blobel CP, Carson DD. Tumor necrosis factor-alpha converting enzyme/ADAM 17 mediates MUC1 shedding. J Biol Chem. 2003;278:3386–3394. doi: 10.1074/jbc.M208326200. [DOI] [PubMed] [Google Scholar]
- 31.Thornton DJ, Khan N, Sheehan JK. Separation and identification of mucins and their glycoforms. Methods Mol Biol. 2000;125:77–85. doi: 10.1385/1-59259-048-9:077. [DOI] [PubMed] [Google Scholar]
- 32.Toba S, Tenno M, Kurosaka A. An O-acetylated sialyl-Tn is involved in ovarian cancer-associated antigenicity. Biochem Biophys Res Commun. 2000;271:281–286. doi: 10.1006/bbrc.2000.2618. [DOI] [PubMed] [Google Scholar]
- 33.Varki A. Sialic acids as ligands in recognition phenomena. Faseb J. 1997;11:248–255. doi: 10.1096/fasebj.11.4.9068613. [DOI] [PubMed] [Google Scholar]
- 34.Varki A. Essentials of glycobiology. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1999. [PubMed] [Google Scholar]
- 35.Varki A, Diaz S. A neuraminidase from Streptococcus sanguis that can release O-acetylated sialic acids. J Biol Chem. 1983;258:12465–12471. [PubMed] [Google Scholar]
- 36.Watanabe H, Fabricant M, Tisdale AS, Spurr-Michaud SJ, Lindberg K, Gipson IK. Human corneal and conjunctival epithelia produce a mucin-like glycoprotein for the apical surface. Invest Ophthalmol Vis Sci. 1995;36:337–344. [PubMed] [Google Scholar]
- 37.Watanabe H, Maeda N, Kiritoshi A, Hamano T, Shimomura Y, Tano Y. Expression of a mucin-like glycoprotein produced by ocular surface epithelium in normal and keratinized cells. Am J Ophthalmol. 1997;124:751–757. doi: 10.1016/s0002-9394(14)71691-5. [DOI] [PubMed] [Google Scholar]
- 38.Wilson G, O’Leary DJ, Holden BA. Cell content of tears following overnight wear of a contact lens. Curr Eye Res. 1989;8:329–335. doi: 10.3109/02713688908996380. [DOI] [PubMed] [Google Scholar]


