Abstract
Objective
To determine whether regular aerobic exercise improves symptoms of sleep-disordered breathing in overweight children, as has been shown in adults.
Research Methods and Procedures
Healthy but overweight (BMI ≥85th percentile) 7- to 11-year-old children were recruited from public schools for a randomized controlled trial of exercise effects on diabetes risk. One hundred children (53% black, 41% male) were randomly assigned to a control group (n = 27), a low-dose exercise group (n = 36), or a high-dose exercise group (n = 37). Exercise groups underwent a 13 ± 1.5 week after-school program that provided 20 or 40 minutes per day of aerobic exercise (average heart rate = 164 beats per minute). Group changes were compared on BMI z-score and four Pediatric Sleep Questionnaire scales: Snoring, Sleepiness, Behavior, and a summary scale, Sleep-Related Breathing Disorders. Analyses were adjusted for age.
Results
Both the high-dose and low-dose exercise groups improved more than the control group on the Snoring scale. The high-dose exercise group improved more than the low-dose exercise and control groups on the summary scale. No group differences were found for changes on Sleepiness, Behavior, or BMI z-score. At baseline, 25% screened positive for sleep-disordered breathing; half improved to a negative screen after intervention.
Discussion
Regular vigorous exercise can improve snoring, a symptom of sleep-disordered breathing, in overweight children. Aerobic exercise programs may be valuable for prevention and treatment of sleep-disordered breathing in overweight children.
Keywords: sleep-disordered breathing, symptoms, sex, race
Introduction
Currently, 37% of children 6 to 11 years of age in the United States are overweight (≥85th percentile BMI-for-sex and -age) (1). The prevalence of sleep-disordered breathing may be increasing as a result of the childhood obesity epidemic (2). The prevalence of sleep-disordered breathing in children is estimated at 2% (3); however, obese children are more susceptible than lean ones to sleep-disordered breathing (e.g., obstructive sleep apnea) (2,4,5). Sleep disturbances in adolescents have been linked to obesity and inactivity (6).
Among adults, sleep apnea is associated with obesity (7). Exercise training has been shown to ameliorate both obesity and sleep apnea in adults (8,9). The effect of exercise on symptoms of sleep-disordered breathing in overweight children is unknown. We hypothesized that overweight children’s symptoms of sleep-disordered breathing would improve in a dose-response fashion in response to an exercise program.
Research Methods and Procedures
Sample
One hundred children (53% black, the remainder white; 41% male) whose parents completed the Pediatric Sleep Questionnaire (PSQ)1 (10) before and after the intervention were included in this study. One child was excluded because of severe psychiatric illness. One child in the low-dose group underwent a tonsillectomy and adenoidectomy during the intervention period. Three children were taking medications for attention deficit disorder (ADD): one was taking methylphenidate, one dextroamphetamine, and one dextro-amphetamine and clonidine. The children’s average grades were Bs. Table 1 shows the baseline characteristics of the children.
Table 1.
Clinical characteristics of the sample at baseline (N = 100)
| Mean (standard deviation) or Percent | |
|---|---|
| Black | 53% |
| Male | 41% |
| History of tonsillectomy/adenoidectomy | 9% |
| History of premature birth | 19% |
| Attention deficit disorder medications | 3% |
| Positive screen for sleep-disordered breathing* | 25% |
| Take naps | 15% |
| Drink caffeinated beverages | 58% |
| Caffeine intake (if yes, cans or cups/d) | 2.0 (1.3) |
| Age (years) | 9.5 (1.0) |
| Body mass index (kg/m2) | 26.5 (8.2) |
| BMI z-score | 2.08 (0.38) |
| Weekday sleep time (hours) | 9.5 (0.8) |
| Weekend sleep time (hours) | 10.3 (2.6) |
A score >0.33 on the Sleep-Related Breathing Disorder summary scale of the Pediatric Sleep Questionnaire is considered a positive screen.
This study was conducted as part of a larger project examining the effect of exercise on metabolism. The study was advertised by means of presentations and flyers distributed to children attending public elementary schools in the Augusta, GA, area in 2003 and 2004. Healthy black or white children were eligible if they were 7 to 11 years old, were overweight (≥85th percentile BMI) (11), did not participate in a regular physical activity program for more than 1 hour per week, had no medical condition that would affect study results or limit physical activity, and attended a school that was included in the study. Children from whom we were unable to collect blood samples were excluded from the parent study. Three cohorts of children were enrolled in the study, the first from November 2003 to May 2004, the second from June to November 2004, and the third from December 2004 to May 2005.
Procedures
All children and parents completed the informed consent/assent process. The study was reviewed and approved by the Human Assurance Committee (Institutional Review Board) of the Medical College of Georgia. Children were offered incentives of a savings bond worth $50 (face value at maturity $100) at baseline and a savings bond worth $200 (face value at maturity $400) at post-test for completing an 8-hour day of testing procedures including those reported here.
Measures
Parents provided information on the child’s race, sex, birth date, medical history, caffeine intake, bedtime and wake time, whether the child was enrolled in any regular physical activity program, and school grades. Body weight (in shorts and t-shirt) and height (without shoes) were measured with an electronic scale (Detecto, Web City, MO) and stadiometer (Tanita, Arlington Heights, IL) and converted to BMI and a BMI z-score (Epi Info software; Centers for Disease Control and Prevention, Atlanta, GA). BMI z-score reflects the number of standard deviations (SDs) above or below the average value for a child’s age and sex based on the current childhood norms (11). The PSQ, which has been validated against polysomnography in children, was used to assess sleep-disordered breathing symptoms (10). Scores on each scale of the PSQ can range from 0 to 1. Each is an average of several yes or no items. Higher scores indicate more symptoms endorsed. Difference scores (post-test minus baseline) were calculated to evaluate change due to the intervention. Negative change scores indicated improvement. All measures were administered by research staff at our laboratory at baseline and post-test. There were no group differences on PSQ scales at baseline (p > 0.10), whether or not the nine children with a history of tonsillectomy or adenoidectomy were included. Twenty-five children (25%) screened positive for sleep-disordered breathing at baseline [a score of >0.33 on Sleep-Related Breathing Disorders (SRBD), a summary scale of the PSQ].
Exercise Intervention
Children were randomly assigned to one of three experimental conditions: a no-exercise control condition (n = 27), low-dose aerobic exercise (20 minutes per day, n = 36), or high-dose aerobic exercise (40 minutes per day, n = 37). The low- and high-dose groups differed only on time and, therefore, volume (intensity × time) of exercise. Only those children whose parents completed the PSQ before and after the intervention were included in this study. After baseline testing was complete, a statistician assigned the experimental condition by means of a computer-generated randomization sequence balanced by race and sex, which was concealed until the interventions were assigned. The study coordinator enrolled participants and shared their randomization status with them. Children assigned to the control condition were free to do their usual activities after school. The children assigned to intervention were provided free transportation by school bus to our research gymnasium, and then home or to a neighborhood school each weekday after the exercise classes ended. The exercise sessions were held on schoolday afternoons in the research gymnasium for 14 to 15 weeks and instructed by research staff who were trained and supervised daily by a master’s level physical educator. Children so assigned received 13 ± 1.5 weeks of the exercise intervention between baseline and post-test. The post-testing was balanced so that each exercise group received a similar amount of time in the intervention. Children were invited to continue in the intervention after their post-test to ensure an adequate class size for consistency of intervention throughout the testing period. Polar heart rate monitors (610i; Polar Electro, Oy, Finland) were used to monitor intensity and incentivize adherence to the exercise dose. The emphasis was on intensity, enjoyment, and safety, not competition or the enhancement of skills; therefore, activities were selected on the basis of ease of comprehension, fun, and ability to elicit a heart rate of >150 beats per minute. Examples of these activities include running games, tag games, jump rope, basketball, and soccer (12–14). Children remained at our laboratories for approximately 75 minutes each day because of the extra time required for changing clothes, water breaks, etc. The low-dose group engaged in quiet, sedentary activities in another room while the high-dose group completed their exercise session. Both attendance and intensity goals (80% and heart rate >150 beats per minute, respectively) were exceeded by those randomized to the exercise program. The children’s average attendance was 86% of available days between baseline and post-test. Average heart rate during the classes was 164 beats per minute in both groups. There were two dropouts from the exercise program, one of whom completed the post-test. There was one serious adverse event (a fracture).
Analysis
Degree of overweight (BMI z-score) was examined for association with PSQ scores and sleep time at pre-test. Associations of PSQ baseline and change scores with control variables [race, sex, age, history of tonsillectomy or adenoidectomy, use of ADD medications, prematurity status (<36 weeks’ gestation), caffeine intake, hours slept per weekday] were evaluated using ANOVA and correlation coefficients. Any significant associations between PSQ scores and control variables would result in the control variable being included in further analyses. Because cohorts were from different school systems, differed in racial composition, and were intervened on separately, the cohort effect was controlled in analyses comparing dose groups. Relationships with grades were also explored.
Analysis of covariance compared groups’ mean changes (post-test minus baseline) on four scales of the PSQ: Snoring, Sleepiness, Behavior, and a summary scale, SRBD. Additional analyses compared groups on factor analysis-derived scales (10). Tukey’s honestly significant difference was used to follow up any significant group effect to determine which groups differed. We predicted that PSQ scores would improve in a dose-response fashion in response to the exercise intervention. Analyses were repeated excluding children with a history of tonsillectomy or adenoidectomy, or who were taking ADD medications, to assess the stability of the findings. Data were also examined using the screening cut-off point of 0.33 for the SRBD scale to see whether the exercise program caused children to improve from a positive to a negative screen. Data were screened for violation of assumptions. Intent-to-treat analyses were conducted with SPSS software (version 13.0; SPSS, Inc., Chicago, IL) at an α level of 0.05.
Results
No race or sex differences were observed on PSQ scores at baseline or changes on PSQ scores in response to intervention. No differences were detected by prematurity status. BMI z-score was not related to scales from the PSQ or sleep time at baseline. The average change of BMI z-score from baseline to post-test was −0.05, or one-twentieth of an SD on the BMI norms for age and sex. There was no detectable group effect on this change. Age was positively correlated with change from baseline to post-test in Sleepiness, Behavior, and SRBD (r values, 0.25 to 0.29; p values, 0.001 to 0.04), so age was included in models predicting these variables. Caffeine intake (cups per day) was related to improvement on the Sleepiness scale (r = −0.20, p = 0.046). No other relationships of PSQ scales at baseline or PSQ changes from baseline to post-test with tonsillectomy and/or adenoidectomy, caffeine intake, sleep time, or grades were detected.
After adjusting for the effect of age, the high-dose exercise group (mean ± SD = −0.10 ± 0.14) differed from the control group (mean ± SD = 0.01 ± 0.11, p < 0.001) and the low-dose group (mean ± SD = −0.04 ± 0.11, p = 0.03) on improvement on the SRBD scale [F(3,96) = 6.8, p = 0.002]. On the Snoring scale, the control group (mean ± SD = 0.10 ± 0.26) differed from both the low-dose (mean ± SD = −0.08 ± 0.29, p = 0.01) and high-dose groups (mean ± SD = −0.13 ± 0.29) [p = 0.002; F(2,98) = 5.6, p = 0.005]. Adjusted means and standard errors are presented in Figures 1 and 2. These effects remained when children with a history of tonsillectomy or adenoidectomy were excluded and when children on ADD medications were excluded. Similar effects were observed on factor analysis-derived scales. No group differences were found for Sleepiness or Behavior scales, or their factor analytic counterparts. Means by group on PSQ scales at baseline and post-test, along with difference scores, are presented in Table 2.
Figure 1.
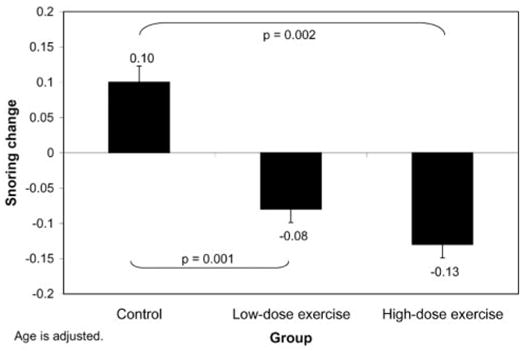
Changes on Snoring scale of PSQ by group assignment. Control group differed from high-dose and low-dose groups. Changes by group (adjusted means) are shown.
Figure 2.
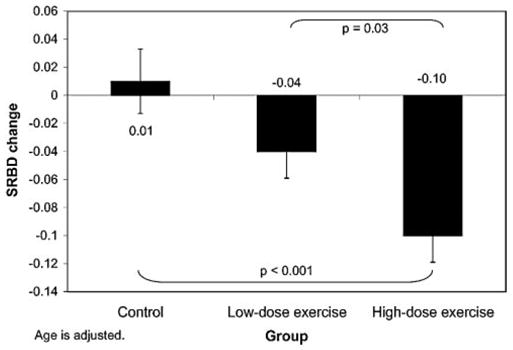
Changes on SRBD scale of PSQ by group assignment. High-dose group differed from controls and low-dose group. Changes by group (adjusted means) are shown.
Table 2.
Means (standard deviation) by group for Pediatric Sleep Questionnaire scores at baseline and post-test
| Baseline
|
Post-test
|
|||||
|---|---|---|---|---|---|---|
| Control (n = 27) | Low-dose (n = 36) | High-dose (n = 37) | Control (n = 27) | Low-dose (n = 36) | High-dose (n = 37) | |
| Snoring* | 0.18 (0.29) | 0.28 (0.35) | 0.32 (0.37) | 0.28 (0.32) | 0.19 (0.29) | 0.20 (0.30) |
| Sleepiness | 0.18 (0.26) | 0.17 (0.27) | 0.16 (0.23) | 0.13 (0.24) | 0.13 (0.25) | 0.08 (0.18) |
| Behavior | 0.32 (0.29) | 0.30 (0.28) | 0.33 (0.33) | 0.30 (0.30) | 0.27 (0.26) | 0.20 (0.26) |
| SRBD† | 0.21 (0.15) | 0.24 (0.15) | 0.25 (0.17) | 0.22 (0.16) | 0.20 (0.14) | 0.15 (0.11) |
Control differs from other groups on changes.
High-dose differs from other groups on changes.
Approximately one-half of the children who screened positive for sleep-disordered breathing improved to a negative screen after the intervention period. Of 13 children whose SRBD screening status improved, 12 were in the exercise groups (Table 3). One child in each of the three groups went from below to above the cut-off point. Statistical tests were not performed because of insufficient numbers in some cells.
Table 3.
Cross-tabulation of status changes on SRBD scale screening criterion by group
| Group
|
||||
|---|---|---|---|---|
| Control | Low-dose | High-dose | Total | |
| Negative to positive | 1 | 1 | 1 | 3 |
| Negative to negative | 21 | 25 | 26 | 72 |
| Positive to positive | 4 | 6 | 2 | 12 |
| Positive to negative | 1 | 4 | 8 | 13 |
Discussion
Overweight children show a moderate level of sleep-disordered breathing symptomatology, particularly snoring. Engaging in 20 to 40 minutes per day of vigorous aerobic exercise, preferably in an organized setting, appears to be helpful in ameliorating symptoms of sleep-disordered breathing in overweight children. This effect was independent of weight change, because the randomized groups did not differ on change in their BMI z-scores. One-quarter of the overweight children in this study screened positive for sleep-disordered breathing; one-half of these children improved to a negative screen after intervention. This randomized trial in a community sample of children at risk for sleep-disordered breathing as a result of their overweight status provides evidence supporting routine clinical prescription of regular aerobic activity in overweight children to ameliorate sleep-disordered breathing symptoms, which may be more common than expected.
A difference between the high- and low-dose groups was found for improvement on the SRBD summary scale, but not the Snoring scale. A difference between the low-dose group and controls was found for the Snoring scale, but not the SRBD scale. These results are consistent with a dose-response effect of exercise on improvement in sleep-disordered breathing symptoms, but they do not conclusively demonstrate this effect, possibly because of limited statistical power.
Although snoring and the SRBD summary scale were impacted by the exercise intervention, no effect was observed on sleepiness. Parents in this sample reported that many of the children regularly drank caffeine, which might suppress sleepiness. On the other hand, these findings are consistent with the literature, in that children with sleep-disordered breathing tend to experience fewer arousals, more preserved sleep architecture, less sleep fragmentation, and less consequent sleepiness than adults with this disorder (15). It is also possible that excessive daytime sleepiness, a subjective complaint, is under-reported and under-diagnosed in children. School-age children with sleep-disordered breathing typically show mild symptoms of inattention and hyperactivity rather than sleepiness (16). Sleep-disordered breathing, sleep fragmentation, and sleep deprivation have been linked with behavior problems and poorer neurobehavioral function in school-age children (17–20).
Snoring in early childhood has been associated with poorer academic performance in middle school and prospectively with hyperactivity (21,22). Five-year-old children with sleep-disordered breathing symptoms such as snoring and noisy breathing have poorer executive function, memory, and general ability than those without symptoms (23). Sleep fragmentation and intermittent nocturnal hypoxia in apneic children may each contribute to difficulty with academic performance (24,25). As suggested by an animal model, there may be irreversible effects of hypoxia on the developing brain (26). Children with symptoms of sleep-disordered breathing tend to show mild symptoms of attention deficit and behavioral disorders, and vice versa (27,28). Thus, the sleep difficulties commonly experienced by overweight children may impair their academic performance. However, no relationship between improved PSQ score and school grades was observed in this study. It may take longer than a few months for improved sleep to affect achievement.
No effect of exercise without dietary intervention was found on overweight, consistent with adult studies showing that exercise without dietary restriction in obese adults improves health without any weight loss (9). No relationship between degree of overweight and sleep-disordered breathing symptoms was detected in this study. The restricted range of BMI z-score in this overweight sample limited our ability to detect any such relationship.
If not weight loss, then what could be the mechanism of this exercise-induced improvement in sleep-disordered breathing? Genes underlying the adenosine system have been implicated in sleep homeostasis by their effects on slow wave activity (29). Increased slow wave activity in adults has been shown to be locally stimulated by a motor task, indicating a neural plasticity basis for this sleep adaptation (30). Orexins present an additional mechanistic link between sleep homeostasis and physical activity, as they have been linked with these behaviors in animal models and have extensive receptors in the brain (31,32). Although not specifically addressed in this study, these areas are lines for future investigations into the possible underlying mechanisms in children.
Black race has been associated with increased risk of sleep-disordered breathing in children, independently of overweight status (3,33). No association between race and sleep-disordered breathing symptoms was detected in this study.
These findings are limited to a sample of overweight children, so they may not be applicable to normal-weight children. However, overweight and inactivity are now epidemic in childhood, which makes this study particularly important (34). This study includes balanced proportions of two ethnic groups and both sexes, drawn from the local school population. Children randomized to different conditions may have interacted at school, potentially allowing contamination; however, the analysis showed distinct group differences on outcomes in the predicted direction. Although the intensive, closely monitored exercise intervention quantified the amount of aerobic activity engaged in by each dose group, children’s activity outside the program was not closely monitored. The unusual amount of resources applied in this study (e.g., transportation, staffing, heart rate monitors, incentives), which enabled it to test the hypothesis that a substantial dose of regular aerobic activity would be efficacious in improving sleep-disordered breathing symptoms, may be difficult to replicate in a field setting. Although not equivalent to polysomnography, a questionnaire that has been validated against polysomnography in children was used in this study to assess symptoms of sleep-disordered breathing (10). A child’s snoring is easily observable by parents and is an important indicator of sleep-disordered breathing (21,22).
Physiological variables such as insulin and adiposity revert to baseline when overweight children cease regular exercise (35). The same may be true for sleep-disordered breathing symptoms. The benefits of behavioral treatments such as regular exercise are often lost when the treatments cease. The long-term consequences of sleep-disordered breathing on children are unknown. There may be lasting benefits of prevention or amelioration of sleep-disordered breathing as a result of protection from neural insult during childhood. Even though proof of long-term benefits is not yet available, it is important to act now, because overweight afflicts many children and leads to numerous adverse health outcomes (2,36-p. 111).
In summary, sleep-disordered breathing may be more common among overweight children than is generally realized. One-quarter of the overweight children in this community sample screened positive for sleep-disordered breathing. Engaging in 40 minutes per day of vigorous aerobic exercise for 10 to 15 weeks seems to improve symptoms of sleep-disordered breathing in overweight children. Achieving this level of physical activity (40 minutes per day, 5 days per week, heart rate >150 beats per minute) among children in ordinary circumstances is likely to require 1 hour per day of physical activity (37). This experimental evidence adds to the knowledge base about the benefits of physical activity on overweight children’s health, even when weight loss does not occur.
Acknowledgments
This study was supported by NIH Grants DK60692 and DK70922. We thank Kashala Carter, Tina Creech, Shena Givens, Sara Groves, Abby Messick, Stephanie Moore, Jennifer Murphy, Ankur Pogula, Laura Power, and Deena Walker for data collection, entry, and management.
Footnotes
This work was presented orally to the North American Association for the Study of Obesity on October 17, 2005, at their meeting in Vancouver, BC, Canada, and was published as an abstract in Obesity Research.
Nonstandard abbreviations: PSQ, Pediatric Sleep Questionnaire; ADD, attention deficit disorder; SD, standard deviation; SRBD, Sleep-Related Breathing Disorders scale of PSQ.
References
- 1.Ogden CL, Carroll MD, Curtin LR, McDowell MA, Tabak CJ, Flegal KM. Prevalence of overweight and obesity in the United States, 1999–2004. JAMA. 2006;295:1549–55. doi: 10.1001/jama.295.13.1549. [DOI] [PubMed] [Google Scholar]
- 2.Daniels SR, Arnett DK, Eckel RH, et al. Overweight in children and adolescents: pathophysiology, consequences, prevention, and treatment. Circulation. 2005;111:1999–2012. doi: 10.1161/01.CIR.0000161369.71722.10. [DOI] [PubMed] [Google Scholar]
- 3.Rosen CL, Larkin EK, Kirchner HL, et al. Prevalence and risk factors for sleep-disordered breathing in 8- to 11-year-old children: association with race and prematurity. J Pediatr. 2003;142:383–9. doi: 10.1067/mpd.2003.28. [DOI] [PubMed] [Google Scholar]
- 4.Mallory GB, Jr, Fiser DH, Jackson R. Sleep-associated breathing disorders in morbidly obese children and adolescents. J Pediatr. 1989;115:892–7. doi: 10.1016/s0022-3476(89)80738-3. [DOI] [PubMed] [Google Scholar]
- 5.Silvestri JM, Weese-Mayer DE, Bass MT, Kenny AS, Hauptman SA, Pearsall SM. Polysomnography in obese children with a history of sleep-associated breathing disorders. Pediatr Pulmonol. 1993;16:124–9. doi: 10.1002/ppul.1950160208. [DOI] [PubMed] [Google Scholar]
- 6.Gupta NK, Mueller WH, Chan W, Meininger JC. Is obesity associated with poor sleep quality in adolescents? Am J Hum Biol. 2002;14:762–8. doi: 10.1002/ajhb.10093. [DOI] [PubMed] [Google Scholar]
- 7.Vgontzas AN, Tan TL, Bixler EO, Martin LF, Shubert D, Kales A. Sleep apnea and sleep disruption in obese patients. Arch Intern Med. 1994;154:1705–11. [PubMed] [Google Scholar]
- 8.Norman JF, Von Essen SG, Fuchs RH, McElligott M. Exercise training effect on obstructive sleep apnea syndrome. Sleep Res Online. 2000;3:121–9. [PubMed] [Google Scholar]
- 9.Lee S, Kuk JL, Davidson LE, et al. Exercise without weight loss is an effective strategy for obesity reduction in obese individuals with and without type 2 diabetes. J Appl Physiol. 2005;99:1220–5. doi: 10.1152/japplphysiol.00053.2005. [DOI] [PubMed] [Google Scholar]
- 10.Chervin RD, Hedger K, Dillon JE, Pituch KJ. Pediatric Sleep Questionnaire (PSQ): validity and reliability of scales for sleep-disordered breathing, snoring, sleepiness, and behavioral problems. Sleep Med. 2000;1:21–32. doi: 10.1016/s1389-9457(99)00009-x. [DOI] [PubMed] [Google Scholar]
- 11.Ogden CL, Kuczmarski RJ, Flegal KM, et al. Centers for Disease Control and Prevention 2000 growth charts for the United States: improvements to the 1977 National Center for Health Statistics version. Pediatrics. 2002;109:45–60. doi: 10.1542/peds.109.1.45. [DOI] [PubMed] [Google Scholar]
- 12.Gutin B, Riggs S, Ferguson M, Owens S. Description and process evaluation of a physical training program for obese children. Res Q Exerc Sport. 1999;70:65–9. doi: 10.1080/02701367.1999.10607731. [DOI] [PubMed] [Google Scholar]
- 13.Hinson C. Fitness for Children. Champaign, IL: Human Kinetics; 1995. [Google Scholar]
- 14.Turner LF, Turner SL. Ready-to-Use Pre-Sport Skills Activities Program. West Nyack, NY: Parker Publishing Co; 2000. [Google Scholar]
- 15.Loughlin GM, Carroll JL, Marcus CL, editors. Lung Biology in Health and Disease Series, No. 147. New York: Marcel Dekker, Inc; 2000. Sleep and Breathing in Children: A Developmental Approach. [Google Scholar]
- 16.Brown WD. The psychosocial aspects of obstructive sleep apnea. Semin Respir Crit Care Med. 2005;26:33–43. doi: 10.1055/s-2005-864199. [DOI] [PubMed] [Google Scholar]
- 17.Beebe DW, Wells CT, Jeffries J, Chini B, Kalra M, Amin R. Neuropsychological effects of pediatric obstructive sleep apnea. J Int Neuropsychol Soc. 2004;10:962–75. doi: 10.1017/s135561770410708x. [DOI] [PubMed] [Google Scholar]
- 18.Chervin RD, Dillon JE, Archbold KH, Ruzicka DL. Conduct problems and symptoms of sleep disorders in children. J Am Acad Child Adolesc Psychiatry. 2003;42:201–8. doi: 10.1097/00004583-200302000-00014. [DOI] [PubMed] [Google Scholar]
- 19.Sadeh A, Gruber R, Raviv A. Sleep, neurobehavioral functioning, and behavior problems in school-age children. Child Dev. 2002;73:405–17. doi: 10.1111/1467-8624.00414. [DOI] [PubMed] [Google Scholar]
- 20.Sadeh A, Gruber R, Raviv A. The effects of sleep restriction and extension on school-age children: what a difference an hour makes. Child Dev. 2003;74:444–55. doi: 10.1111/1467-8624.7402008. [DOI] [PubMed] [Google Scholar]
- 21.Gozal D, Pope DWJ. Snoring during early childhood and academic performance at age thirteen and fourteen. Pediatrics. 2001;107:1394–9. doi: 10.1542/peds.107.6.1394. [DOI] [PubMed] [Google Scholar]
- 22.Chervin RD, Ruzicka DL, Archbold KH, Dillon JE. Snoring predicts hyperactivity four years later. Sleep. 2005;28:885–90. doi: 10.1093/sleep/28.7.885. [DOI] [PubMed] [Google Scholar]
- 23.Gottlieb DJ, Chase C, Vezina RM, et al. Sleep-disordered breathing symptoms are associated with poorer cognitive function in 5-year-old children. J Pediatr. 2004;145:458–64. doi: 10.1016/j.jpeds.2004.05.039. [DOI] [PubMed] [Google Scholar]
- 24.Hunt CE. Neurocognitive outcomes in sleep-disordered breathing. J Pediatr. 2004;145:430–2. doi: 10.1016/j.jpeds.2004.07.026. [DOI] [PubMed] [Google Scholar]
- 25.Urschitz MS, Wolff J, Sokollik C, et al. Nocturnal arterial oxygen saturation and academic performance in a community sample of children. Pediatrics. 2005;115:e204–9. doi: 10.1542/peds.2004-1256. [DOI] [PubMed] [Google Scholar]
- 26.Kheirandish L, Gozal D, Pequignot JM, Pequignot J, Row BW. Intermittent hypoxia during development induces long-term alterations in spatial working memory, monoamines, and dendritic branching in rat frontal cortex. Pediatr Res. 2005;58:594–9. doi: 10.1203/01.pdr.0000176915.19287.e2. [DOI] [PubMed] [Google Scholar]
- 27.Chervin RD, Archbold KH, Dillon JE, et al. Inattention, hyperactivity, and symptoms of sleep-disordered breathing. Pediatrics. 2002;109:449–56. doi: 10.1542/peds.109.3.449. [DOI] [PubMed] [Google Scholar]
- 28.O’Brien LM, Holbrook CR, Mervis CB, et al. Sleep and neurobehavioral characteristics of 5- to 7-year-old children with parentally reported symptoms of attention-deficit/hyper-activity disorder. Pediatrics. 2003;111:554–63. doi: 10.1542/peds.111.3.554. [DOI] [PubMed] [Google Scholar]
- 29.Retey JV, Adam M, Honegger E, et al. A functional genetic variation of adenosine deaminase affects the duration and intensity of deep sleep in humans. Proc Natl Acad Sci U S A. 2005;102:15676–81. doi: 10.1073/pnas.0505414102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Huber R, Ghilardi MF, Massimini M, Tononi G. Local sleep and learning. Nature. 2004;430:78–81. doi: 10.1038/nature02663. [DOI] [PubMed] [Google Scholar]
- 31.Baumann CR, Bassetti CL. Hypocretins (orexins) and sleep-wake disorders. Lancet Neurol. 2005;4:673–82. doi: 10.1016/S1474-4422(05)70196-4. [DOI] [PubMed] [Google Scholar]
- 32.Wayner MJ, Armstrong DL, Phelix CF, Oomura Y. Orexin-A (hypocretin-1) and leptin enhance LTP in the dentate gyrus of rats in vivo. Peptides. 2004;25:991–6. doi: 10.1016/j.peptides.2004.03.018. [DOI] [PubMed] [Google Scholar]
- 33.Redline S, Tishler PV, Schluchter M, Aylor J, Clark K, Graham G. Risk factors for sleep-disordered breathing in children: associations with obesity, race, and respiratory problems. Am J Respir Crit Care Med. 1999;159:1527–32. doi: 10.1164/ajrccm.159.5.9809079. [DOI] [PubMed] [Google Scholar]
- 34.Strauss RS, Pollack HA. Epidemic increase in childhood overweight, 1986–1998. JAMA. 2001;286:2845–8. doi: 10.1001/jama.286.22.2845. [DOI] [PubMed] [Google Scholar]
- 35.Ferguson MA, Gutin B, Le NA, et al. Effects of exercise training and its cessation on components of the insulin resistance syndrome in obese children. Int J Obes Relat Metab Disord. 1999;23:889–95. doi: 10.1038/sj.ijo.0800968. [DOI] [PubMed] [Google Scholar]
- 36.Institute of Medicine Committee on Prevention of Obesity in Children and Youth. Preventing Childhood Obesity: Health in the Balance. Washington DC: National Academies Press; 2005. [PubMed] [Google Scholar]
- 37.Strong WB, Malina RM, Blimkie CJ, et al. Evidence based physical activity for school-age youth. J Pediatr. 2005;146:732–7. doi: 10.1016/j.jpeds.2005.01.055. [DOI] [PubMed] [Google Scholar]


