Abstract
Isolated clefts of the lip and/or palate (ICLP) are developmental craniofacial abnormalities that have consistently been linked to increased social inhibition or shyness. Two explanations have been proposed: 1) psychosocial factors related to differences in facial appearance may lead to low self-concept and subsequent shyness, or 2) abnormal development of brain structures involved in social function, such as the ventral frontal cortex (VFC), may underlie the difference. To investigate these two possibilities this study was designed to evaluate measures of social function in relation to measures of self-concept and VFC morphology. Subjects included 30 boys (age 7-12) with ICLP and a comparison group of 43 boys without cleft in the same age category. Social function and self-concept were assessed using questionnaires with standardized scoring filled out by subjects and one of their parents. The cortical volume and surface area of the VFC, composed of the orbitofrontal cortex (OFC) and straight gyrus (SG), were evaluated using structural magnetic resonance imaging. The ICLP subjects had significantly impaired social function relative to the comparison group. No difference in self-concept was identified. VFC morphology revealed significant differences between groups, particularly decreased volume and surface area in the left SG of the ICLP group. Moreover, abnormal VFC measures were correlated with social dysfunction but measures of self-concept were not. These results are consistent with the possibility that aberrant VFC development may partially underlie social dysfunction in boys with ICLP.
Keywords: social function, neuroimaging, straight gyrus, gyrus rectus, magnetic resonance image, cleft lip and palate
INTRODUCTION
Isolated clefts of the lip and/or palate (ICLP) are craniofacial malformations that occur in approximately 1 in 1000 Caucasians as a congenital anomaly. It is well documented that many children and adults with ICLP have certain cognitive impairments. While often within the normal range, children with ICLP tend to have lower IQs than their peers and the incidence of language problems is substantially higher than in the general population [44,59,60]. Reading disabilities, for example, may affect as many as 30% to 40% of children with ICLP [18,60]. In addition to the cognitive deficits, social dysfunction has been identified repeatedly and in high frequency in individuals with ICLP, as recently reviewed [38]. In general, individuals with ICLP are more likely to be socially inhibited or shy and report fewer friends than non-cleft controls [17,31,41,42,50,58]. They are also less likely to join clubs or societies in school and less likely to marry [19,48]. The etiology of this social dysfunction has been attributed to secondary factors such as low self-concept as a psychological consequence of having abnormal appearance. However, several studies have suggested normal or higher than normal levels of self-concept in subjects with ICLP relative to controls [15,42,45,57].
Alternately, social dysfunction associated with ICLP may not be secondary to psychological factors associated with a facial abnormality, but instead may be a direct consequence of abnormal neurodevelopment. This is plausible from a developmental perspective, considering the intimate relationship of face and brain development in both normal and pathologic conditions [43,64]. Support for this hypothesis is also garnered by recent studies from our laboratory demonstrating abnormal brain structure associated with ICLP in adult males [51-54]. A recent finding of abnormal brain structure in children with ICLP suggests that these differences in brain structure are present early in life and may be related to abnormal development [55].
The neural underpinnings of human social function involve a diverse network of interconnected systems distributed among several regions of the brain. The function of these systems range from those involved in the perception of socially meaningful information, to those linking social stimuli to motivation, reward, emotion, and cognition, as well as the output systems that ultimately produce social behavior [2]. It is well established that a sector of the frontal lobe, the ventral frontal cortex (VFC), is a critical region of convergence for systems involved in the normal processing that underlies adaptive social function, based on evidence from lesion and imaging studies [2,3,10,12,20]. A previous study in our lab evaluated the morphology of the VFC in adult males with ICLP. We reported a direct correlation of abnormal social function and abnormal VFC morphology – the greater the social dysfunction the greater the structural abnormality [54].
The present study extends this line of investigation to children with ICLP. In a sample of boys with ICLP, measures of social function, self-concept, and structure of the VFC (using MRI) are obtained and compared to a healthy comparison group. Our hypothesis is that social function and the structure of the VFC will be abnormal in the ICLP group and the groups will not differ in self-concept. Moreover, there will be a significant correlative relationship between social function and VFC morphology.
MATERIALS AND METHODS
Participants
ICLP
Subjects with clefts were recruited from our Cleft Lip/Palate Clinic. The study group was limited to 30 boys (age 7-12) with isolated clefts of the lip and/or palate; a group that was selected from a larger sample of children and adolescents with ICLP. The criteria for age range and gender were selected a priori for this study to minimize the confounding influence of these variables on brain structure and function. Medical records were reviewed to verify and document cleft status, including type of cleft and side of the cleft (right, left, median, or bilateral). All patients had been examined previously by a trained medical geneticist to rule out congenital syndromes so that only subjects with isolated, or non-syndromic, CLP were included. There were a total of 8 subjects with cleft lip only (CLO), 15 subjects with cleft lip and palate (CLP), and 7 subjects with cleft palate only (CPO).
Comparison group
The comparison group was recruited from the community using local advertisements and included 43 healthy normal subjects matched to the ICLP group based on gender and age.
In both groups subjects were excluded if serious (requiring significant medical intervention) medical or neurological disease was present. In addition, comparison subjects were excluded if they had a history of a learning disorder or psychiatric disorder, such as attention deficit-hyperactivity disorder (ADHD). Written informed consent was obtained for all subjects prior to participation. The study was approved by the University of Iowa Human Subjects Institutional Review Board.
Demographics
Demographic data included age, parental social class (SES), handedness, and race. SES was determined using a modified Hollingshead scale of 1 to 5, with a lower number corresponding to higher social class [37]. A quantitative scale was used to determine handedness [30]. Independent sample t-tests assuming equal variances were used to compare age and SES between groups (see Table 1). Differences in these measures prompted their use as covariates in later analyses. Dominant handedness data in the ICLP group was as follows: 28 right, 1 left, and 1 ambidextrous. The comparison group had 39 right-handed dominant and 4 left. Reflective of the population in Iowa, the race distribution in the study was as follows: 1 African, 1 Asian, 3 Asian American, 65 Caucasian, 1 Hispanic, and 2 mixed.
Table 1.
Characteristics of ICLP and Comparison Group
| ICLP (n=30) | Comparison (n=43) | pa | |
|---|---|---|---|
| Age (years): mean (S.D.) | 9.98 (1.64) | 10.68 (1.45) | .06 |
| Parental Social Class: mean (S.D.) | 2.80 (.55) | 2.26 (.64) | .000 |
independent samples t-test, two-tailed, α = 0.05
Functional Measures
A self-administered Self Description Questionnaire-1 (SDQ-1) [47] provided a scaled score of peer relations and self-concept. For each scale, subjects were asked to rate 8 statements on a 5-point scale, with 5 representing true, 4 mostly true, 3 sometimes false/sometimes true, 2 mostly false and 1 false. The peer relations scale includes statements such as “I have lots of friends” or “I get along with kids easily.”
Measurement of self-concept was obtained from the SDQ-1 general-self scale, which includes statements such as “in general, I like the way I am” and “overall I have a lot to be proud of.” Because the ratings of peer relations and self-concept on the SDQ-1 were self-reported measures, these scales are most appropriately described as a measure of the subject’s self-perception of peer relations and self-concept. Reliability data for the SDQ-1 measures were estimated using internal consistency of responses at .85 for peer relations and .81 for general-self (self-concept) [47]. Construct validity was established by correlating scores to those of instruments measuring similar constructs [47]. For example, SDQ-1 peer relations and general-self scales substantially correlated with the social and general scales of the Perceived Competence Scale [36], at r = .74 and .57 respectively.
In addition to the SDQ-1 peer relations score, another measure of social function was obtained using the Social Adjustment Scale (SAS), a component of the Comprehensive Assessment of Symptoms and History (CASH) [8]. The SAS is a parent-reported assessment tool used to assess a child’s social function. For adults, the parents retrospectively assess their child’s social function. For children, the parents assess the current social function of their child. This includes an overall social adjustment score based on dimensions of withdrawal, peer relations, and interests. The SAS is typically administered as a structured interview, but in the current study the SAS was given as a paper and pencil test. The content remained identical to the original CASH. Extensive inter-rater and test-retest reliability studies and validity studies have demonstrated the effectiveness of the structured interview CASH [8].
Structural Imaging
MRI Acquisition
MRI scans were obtained using a 1.5 Tesla General Electric SIGNA System (GE Medical Systems, Milwaukee, WI). Three-dimensional (3D) T1-weighted images, using a spoiled grass sequence (SPGR), were acquired with the following parameters: 1.5 mm coronal slices, 40 degree flip angle, 24 msec Repetition Time (TR), 5 ms Echo Time (TE), 2 Number of Excitions (NEX), 26 cm Field of View (FOV) and a 256X192 matrix. The Proton Density (PD) and T2 weighted images were acquired with the following parameters: 3.0 mm coronal slices, 36 msec TE (for PD) or 96 msec TE (for T2), 3000 msec TR, 1 NEX, 26 cm FOV, 256X192 matrix and an echo train length=1.
Image Processing
MRI data were processed using BRAINS2 (Brain Research: Analysis of Images, Networks, and Systems), our locally developed software, described elsewhere [6,7,9,22,46]. T1-weighted images were spatially normalized and resampled to 1.015625 mm3 voxels so that the anterior-posterior axis of the brain was realigned parallel to the anterior commissure– posterior commissure (AC-PC) line and the interhemispheric fissure was aligned on the other two axes. T2 and PD weighted images were aligned to the spatially normalized T1-weighted image [68]. The data sets were then segmented using the multi-spectral data and a discriminant analysis method based on automated training class selection [35]. The tissue-classified image was then used to generate a triangle-based iso-surface using a threshold of 130, representing prototypical gray matter that corresponds to the parametric center of the cortex [21]. An initial polygonalization of cortical surface was done using the method described by Wyvill [69]. A retiling algorithm [67] was used to reduce the image to a more manageable size, to approximately 100,000 triangles per hemisphere from the initial 300,000 to 500,000. This triangulated surface was used as the basis for our calculations of cortical areas and volumes.
Surface Anatomy Measures
Hand-traced regions of interest (ROI) were used to surround contiguous areas of the gray-matter triangle iso-surface. On each 2D slice, the cortical surface was visualized as a continuous contour that represents the intersection between the 2D plane and the 3D triangulated surface. Using this surface contour as a guide, VFC structures were defined on each 2D slice. The corresponding gray-matter volume of the cortical plane was found by using the thickness measurement for the selected surface region. The surface area is simply calculated by summing the triangles of the surface in the ROI. Gray-matter volume is represented in cm3 and surface area in mm2.
ROI Definition and Reliability
For the present study the VFC was divided into two subregions: the orbitofrontal cortex (OFC) and the straight gyrus (SG). A detailed description of the anatomical boundaries and the method used to define these subregions is presented elsewhere [23,24]. A visual representation of this region is displayed in figure 1. Two raters (JW and VM) hand-traced these regions after “practicing” on a set of brains entirely different from the set on which the reliability study was done. Each rater was compared to a gold standard set of traces [24]. Inter-rater reliability between the rater and the gold standard traces was calculated with intraclass R coefficients for cortical gray-matter volume. The mean cortical gray-matter volume reliability scores for the left and right OFC and SG were ≥.90 for both tracers.
Figure 1.
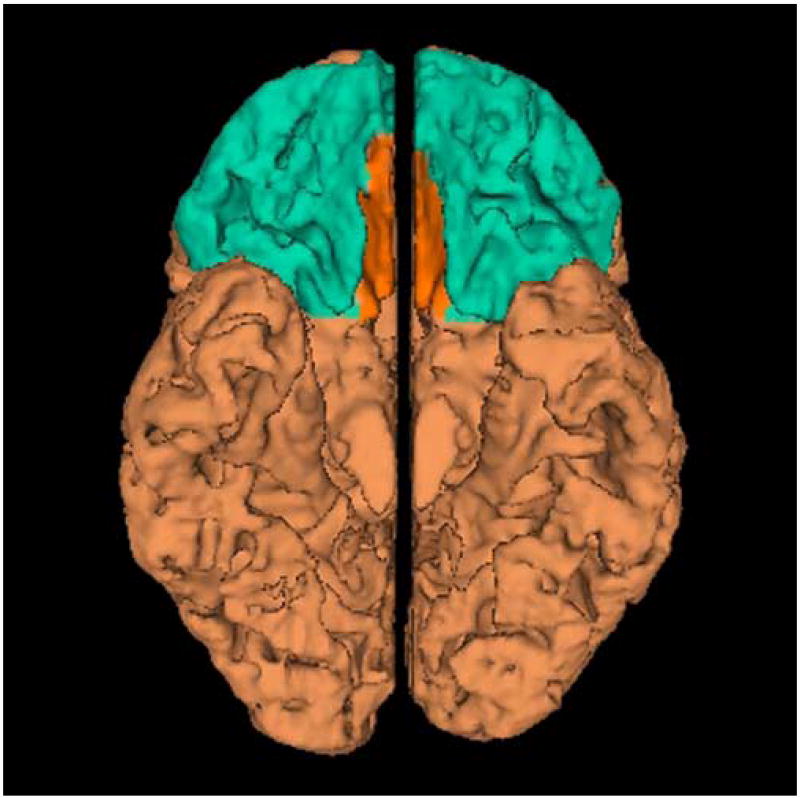
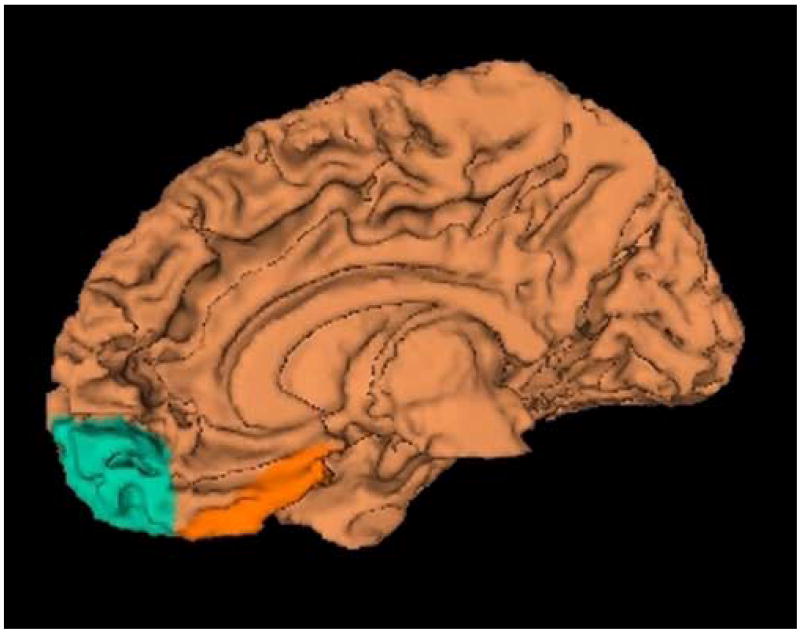
Ventral frontal cortex displayed in three-dimensionally rendered brain with straight gyrus (SG) in orange and the orbitofrontal cortex (OFC) in blue. The medial (left) and inferior view (right) are shown.
Statistical Methods
All analyses were performed using SPSS 13.0 for Windows. Functional measures were compared between groups using MANCOVA (General Linear Models Multivariate Analysis of Variance) with age and SES as covariates. ANCOVA was used to analyze measures of cortical gray matter volume (cm3) and surface area (mm2) for the total frontal lobe (covariates: age, SES, and intracranial volume) and VFC (covariates: age, SES). To correct for any differences in overall frontal lobe size among groups the VFC volumetric measurements were analyzed as a percentage of the total, right, or left frontal lobe gray matter volume for their respective regional measures. Likewise, VFC surface area measures were taken as a percentage of the total, right, or left frontal lobe surface measures. Spearman’s correlation coefficients were calculated to investigate the relationship between social function, self-concept, and VFC morphology. A nonparametric method was used to eliminate the effect of outliers in the data. To help reduce Type I error inflation, only those morphologic measures that showed significant differences between groups were correlated with functional measures.
RESULTS
Functional Measures
Measures of social function and self-concept are presented in Table 2. All SDQ-1 mean scores were within a standard deviation of a larger normative sample of 3,562 children [47]. Normative data for SAS measures were not available. MANCOVA comparison of functional measures revealed that ICLP boys had significantly poorer peer relations according to the self-reported SDQ-1 score. The parent-reported SAS scores also revealed significantly lower overall social function in the ICLP group. Measures of self-concept among the two groups did not reveal statistically significant differences.
Table 2.
Results of Functional Measures
| ICLP
Mean (95% C.I.) |
Comparison
Mean (95% C.I.) |
Significance | |
|---|---|---|---|
| MANCOVAb | |||
| SDQ-1 Peer Relations* | 43.942 (40.426 - 47.459) | 51.782 (48.70 - 54.87) |
F= 10.072
Sig= .002 |
| SAS Score | 1.709 (1.136 - 2.282) | .809 (.306 - 1.311) |
F= 4.996
Sig= .029 |
| SDQ-1 Self Concept* | 33.713 (32.043 - 35.384) | 35.016 (33.552 - 36.480) | F= 1.232
Sig= .271 |
For measures of social function (does not include self concept): Wilks’ lambda = .831 p = .003, F = 6.389 Hypothesis df = 2, Error df = 63
SDQ-1 peer relations are reported as T scores and self-concept scores are reported as raw scores. Conversion of self-concept raw scores to T scores was not possible due to lack of age-matched normative data.
MANCOVA, General Linear Models Multivariate Analysis of Variance. Covariates include age and SES,
Grouped tests included measures of social function. Self-concept was analyzed using ANCOVA.
Structural Measures
Table 3 shows the results of the between-group ANCOVA comparison of frontal lobe and VFC morphology measures. The ICLP sample had lower frontal lobe volume and surface area, but these differences did not reach statistical significance. This is in contrast to the findings of a larger study (of which this group is a subset) that reports smaller frontal lobe size in ICLP subjects [55]. The lack of significance reported here may be due to lack of power, as the larger sample had a total of 74 subjects with ICLP (more than double the sample here) compared to 74 age- and gender-matched controls.
Table 3.
Results of Structural Measures
| Measure | ANCOVA | |||
|---|---|---|---|---|
| ICLP
Adjusted Mean (C.I.) |
Comparison
Adjusted Mean (C.I.) |
F | Sig. | |
| Total Frontal Gray Volume (cm3) | 315.923
(304.556 - 327.290) |
320.879
(311.052 - 330.705) |
.391 | .534 |
| Total Frontal surface area (mm2) | 78294 (75390 - 81198) | 80568 (78057 - 83078) | .715 | .547 |
| Total SG volume | 1.872 (1.725 - 2.020) | 2.106 (1.978 - 2.233) | 5.137 | .027 |
| Right | 2.097 (1.937 - 2.256) | 2.201 (2.063 - 2.339) | .878 | .352 |
| Left | 1.634 (1.459 - 1.809) | 2.006 (1.855 - 2.158) | 9.318 | .003 |
| Total SG surface area | 1.907 (1.755 - 2.058) | 2.160 (2.029 - 2.291) | 5.778 | .019 |
| Right | 2.139 (1.974 - 2.305) | 2.287 (2.144 - 2.430) | 1.630 | .206 |
| Left | 1.660 (1.485 - 1.835) | 2.027 (1.876 - 2.179) | 9.105 | .004 |
| Total OFC volume | 13.566 (13.060 - 14.072) | 13.381 (12.944 - 13.819) | .273 | .603 |
| Right | 13.296 (12.743 - 13.849) | 13.287 (12.809 - 13.765) | .001 | .982 |
| Left | 13.857 (13.285 - 14.428) | 13.485 (12.991 - 13.979) | .870 | .354 |
| Total OFC surface area | 13.962 (13.475 - 14.450) | 13.697 (13.276 - 14.119) | .607 | .439 |
| Right | 13.350 (12.808 - 13.891) | 13.342 (12.873 - 13.810) | .000 | .983 |
| Left | 14.617 (14.068 - 15.166) | 14.078 (13.603 - 14.552) | 1.987 | .163 |
independent samples t-test
General Linear Models Univariate Analysis of Variance. Covariates include age and SES
Table 3 also reports measures of ventral frontal cortex morphology. ANCOVA analysis revealed significantly lower volume and surface area for total SG and left SG measures. Differences in left SG morphology were particularly robust, with statistically significant differences noted regardless of comparing raw data, left SG to left frontal lobe ratio, or ratio comparison with age and SES as covariates. Measures of the right SG were also lower in the ICLP group compared to controls, but these did not reach statistical significance. No significant differences among groups were noted in the OFC.
Structure and Function Relationship
Table 4 displays the Spearman correlations of abnormal VFC measures and functional measures among all subjects. There was a significant correlation between SG measures and self-reported SDQ-1 peer relation scores, suggesting poor peer relation scores are associated with less brain tissue in the straight gyrus. All SAS correlations were in the negative direction, indicating an inverse relationship- the higher the SAS measure (indicating greater social dysfunction)—the lower the volume/surface area of the brain region. The only morphologic measures correlating significantly with SAS score was left straight gyrus volume.
Table 4.
Spearman Correlation of Abnormal Ventral Frontal Cortex Measures and Functional Measures
| Region | Social Function Measures | Self-Concept Measure | ||
|---|---|---|---|---|
| SDQ1 Peer Relations | SAS score | SDQ-1 General Self | ||
| Total SG
volume |
.245 (.038) | -.187 (.113) | .098 (.414) | |
| Left SG
volume |
.286 (.015) | -.239 (.042) | .145 (.223) | |
| Total SG
SA |
.237 (.045) | -.127 (.285) | .064 (.592) | |
| Left SG
SA |
.282 (.016) | -.159 (.179) | .122 (.306) | |
To evaluate the anatomic specificity of these findings, Spearman correlations were then calculated between measures of the entire frontal lobe and social function scores. There were no significant correlations, supporting the concept of anatomic specificity within the frontal cortex for processing social function.
DISCUSSION
It has been widely reported that children with ICLP have abnormal social function in that they are particularly shy and have fewer friends than age-matched peers. In the current study we support this finding of abnormal social function in boys with ICLP using behavioral questionnaires assessing social function. We also report for the first time that VFC morphology is abnormal in boys with ICLP. Specifically, we report decreased SG volume and surface area relative to the comparison group, with morphologic abnormality primarily localized to the left SG. These morphologic changes appear to be specific for the straight gyrus considering no significant differences in overall frontal lobe or adjacent orbitofrontal cortex measures were noted. Finally, the morphologic abnormalities in the SG were significantly, though weakly, correlated to certain abnormal measures of social function, particularly the self-reported SDQ-1 peer relation scores. The parent-reported SAS score was significantly correlated to left SG volume only.
Measures of self-concept were not abnormal in the ICLP group, suggesting that these factors do not underlie the social dysfunction associated with ICLP. Moreover, measures of self-concept had a non-significant correlative relationship with brain morphology. These results leave open the possibility that aberrant development of the VFC may play a role in social dysfunction in boys with ICLP, rather than poor self-concept giving rise to these deficits.
The Role of the Ventral Frontal Cortex in Social Function
A large body of literature has implicated the VFC as a critical region in the guidance of social behavior in humans. Much of the evidence for this has come from a long history of lesion studies spanning over a century [5,13,14,32,34,39,63,65]. More recently functional imaging and non-human primate studies have supported these findings. The role of the VFC in guiding social behavior is multifarious and only partially understood. Functional networks that initiate emotional responses to social stimuli, modulate emotional responses with regard to contextual information, and more general functions like decision making and anticipating the consequences of actions may all play a key role. The anatomy of the orbital and medial prefrontal cortices is well suited to perform these diverse functions. The ventral surface receives multimodal sensory and limbic inputs and is believed to integrate viscerosensory and affective signals, whereas the medial surface provides a major cortical influence on visceromotor output of the autonomic and endocrine systems [4,56]. This anatomical arrangement provides a pathway for information flow whereby sensory input may influence affective processes and ultimately guide behavior. Exactly how this occurs is still an open question, though several plausible theories have been proposed [25,49,61].
Laterality of the Prefrontal Cortex (PFC)
A growing body of literature supports the notion of hemispheric specialization among the prefrontal cortices in the related domains of social behavior and affective style. Richard Davidson has contributed much to the modern literature on this topic, both in original research and in several insightful reviews [26-28]. With respect to the prefrontal cortex, these reviews provide evidence from diverse methodologies that converge on the idea that prefrontal modulation of amygdala activity is a critical determinant of an individual’s affective style. One important theme of this literature focuses on the role of the right prefrontal cortex as a critical cortical substrate in generating physiologic stress responses and negative affect, which favors withdrawal-related behaviors. In contrast, the left PFC may inhibit these stress / negative affect responses and/or generate positive affective states, which facilitate approach-related behaviors. Inhibitory projections from the left medial PFC to the amygdala are hypothesized to be an important mediator of this function [4,26]. This is consistent with a PET study reporting that decreased left prefrontal cortex activity is associated with increased amygdala activity [1]. Also, increased activation of the right prefrontal cortex relative to the left can predispose to behavioral inhibition and social anxiety along a continuum from normal to pathologic, a finding that holds true in children [28].
The current study found morphologic abnormality in the straight gyrus that was most prominent on the left side. In light of the studies on PFC asymmetry discussed above, it is possible that a structural abnormality of the left straight gyrus corresponds to a decrease in inhibitory projections to the amygdala. This may result in deficient top-down modulation of the amygdala and subsequent overactivity in systems influenced by the amygdala (neuroencdocrine, autonomic, and certain behavioral responses), with the overall manifestation being a tendency toward physiologic stress and behavioral inhibition in social settings. This speculation seems congruent with the finding that shy children have increased sympathetic output at rest and in response to social stressors [40]. Amygdala activity has also been shown to be high in a more severe form of social dysfunction, generalized social anxiety. Anxiety is about twice as prevalent in ICLP individuals [58] and social anxiety in particular is seen at greater rates in ICLP individuals than in siblings and controls in a study of Chinese adults [11]. However, we do not know if the 30 ICLP boys in the current study had greater amygdala activity, physiologic stress, or anxiety.
Abnormal VFC morphology does not eliminate the possibility of psychosocial factors playing a critical role in ICLP shyness. Another possible explanation for our results would be that chronic stress related to abnormal appearance or expressive language deficits may result in abnormal brain morphology. Major life stressors in childhood have been shown to have a negative impact on brain development in a pattern that preferentially affects the left hemisphere [29,66]. One mechanism that may mediate such structural changes is elevated corticosteroid levels, which have been shown to cause neuronal atrophy and decreased neurogenesis [16,33,62]. Much of the research in this field has focused on the effects of stress on hippocampal neurogenesis, but the capacity for neurogenesis has also been demonstrated in the prefrontal cortex [33].
A third possibility is that social function and VFC morphology have no direct relationship, but correlate as a result of a third independent variable.
Internalizing versus Externalizing Behavior in ICLP
Social function measures used in the current study did not allow the categorization of internalizing versus externalizing behaviors. As the above discussion would suggest, we are of the assumption that, in general, ICLP boys fall closer to the spectrum of internalizing behaviors. This assumption is based on prior studies demonstrating shyness and increased incidence of depression and anxiety in association with ICLP. However, we can not rule out the possibility that low peer relations scores in the current study may be related to externalizing behavior and disinhibition, which is also linked to the VFC. Future studies hope to clarify this important issue.
Relationship to Previous Study
In 2005 our laboratory published similar results to the current study; adult males with ICLP had significantly abnormal social function, abnormal VFC morphology, and the two measures correlated. Interestingly, the primary morphologic difference among the two groups was the OFC surface area, while the combined right and left SG volume was not significantly different (p=.075). In light of the hemispheric asymmetry noted in the current study, a post-hoc analysis of the data from the right and left straight gyrus of adult males was performed separately. This analysis revealed a statistically significant decrease in the volume of the left straight gyrus relative to the comparison group, while the right was within the normal range. However, this left straight gyrus structural abnormality did not significantly correlate to social function scores. The main structural difference in these two studies of VFC morphology in boys (age 7-12) and adult males with ICLP appears to be the abnormal OFC structure in ICLP adult males but not ICLP boys. One possible explanation for the discrepancy is that the structural difference is not apparent in boys because the OFC is a relatively late developing structure and may be similarly immature in both the ICLP and comparison group. It is possible that a longitudinal follow-up of the two groups in the current study may reveal structural OFC differences at a later developmental stage.
Closing
In closing, we encourage caution when interpreting our findings based on the complex neurobiology of social behavior and the still uncertain role of the VFC in normal and abnormal social behavior. While our understanding of ICLP cognitive and social impairments and their relation to brain structure is still rudimentary, we are optimistic about the prospects of continued investigation in this field for two reasons. First, and most importantly, we believe that insights into the neural underpinnings of these functional deficits of ICLP will help to inform more effective strategies for the remediation of these impairments. Secondly, ICLP may prove to be an important tool for the investigation of the neurobiology of social behavior. Van Der Woude syndrome, for example, is an autosomal dominant clefting disorder with a comparable phenotype to ICLP and a single gene has been shown to be the major etiologic determinant of the disorder [70]. Studies investigating how a single gene influences brain structure and social function may provide an accessible starting point in the effort to study more severe disorders of social function that involve several genes, such as autism.
Acknowledgments
This research was supported in part by the following grant: NIDCR Brain Structure and Function in Children with Oral Clefts 1 RO1 DE01 14399 01 A1.
We thank Amy Conrad and Eric Axelson for their help in collecting and processing the data. The technical assistance of Greg Harris was also appreciated.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Abercrombie H, S SM, Larson CL, Ward RT, Holden JF, Turski PA. Medial prefrontal and amygdala glucose metabolism in depressed and control subjects: an FDG-PET study. Psychophysiology. 1996;33:S17. [Google Scholar]
- 2.Adolphs R. Cognitive neuroscience of human social behaviour. Nature Reviews Neuroscience. 2003;4:165–178. doi: 10.1038/nrn1056. [DOI] [PubMed] [Google Scholar]
- 3.Adolphs R. The neurobiology of social cognition. Curr Opin Neurobiol. 2001;11:231–239. doi: 10.1016/s0959-4388(00)00202-6. [DOI] [PubMed] [Google Scholar]
- 4.Amaral D, Price J, Pitkanen A, Carmichael S. Anatomical organization of the primate amygdaloid complex. New York: Wiley-Liss; 1992. [Google Scholar]
- 5.Anderson SW, Bechara A, Damasio H, Tranel D, Damasio AR. Impairment of social and moral behavior related to early damage in human prefrontal cortex. Nat Neurosci. 1999;2:1032–1037. doi: 10.1038/14833. [DOI] [PubMed] [Google Scholar]
- 6.Andreasen NC, Cizadlo T, Harris G, Swayze V, 2nd, O’Leary DS, Cohen G, Ehrhardt J, Yuh WT. Voxel processing techniques for the antemortem study of neuroanatomy and neuropathology using magnetic resonance imaging. J Neuropsychiatry Clin Neurosci. 1993;5:121–130. doi: 10.1176/jnp.5.2.121. [DOI] [PubMed] [Google Scholar]
- 7.Andreasen NC, Cohen G, Harris G, Cizadlo T, Parkkinen J, Rezai K, Swayze VW., 2nd Image processing for the study of brain structure and function: problems and programs. J Neuropsychiatry Clin Neurosci. 1992;4:125–133. doi: 10.1176/jnp.4.2.125. [DOI] [PubMed] [Google Scholar]
- 8.Andreasen NC, Flaum M, Arndt S. The Comprehensive Assessment of Symptoms and History (CASH). An instrument for assessing diagnosis and psychopathology. Arch Gen Psychiatry. 1992;49:615–623. doi: 10.1001/archpsyc.1992.01820080023004. [DOI] [PubMed] [Google Scholar]
- 9.Andreasen NC, Harris G, Cizadlo T, Arndt S, O’Leary DS, Swayze V, Flaum M. Techniques for measuring sulcal/gyral patterns in the brain as visualized through magnetic resonance scanning: BRAINPLOT and BRAINMAP. Proc Natl Acad Sci U S A. 1994;91:93–97. doi: 10.1073/pnas.91.1.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bechara A, Damasio H, Damasio AR. Emotion, decision making and the orbitofrontal cortex. Cereb Cortex. 2000;10:295–307. doi: 10.1093/cercor/10.3.295. [DOI] [PubMed] [Google Scholar]
- 11.Berk NW, Cooper ME, Liu YE, Marazita ML. Social anxiety in Chinese adults with oral-facial clefts. Cleft Palate Craniofac J. 2001;38:126–133. doi: 10.1597/1545-1569_2001_038_0126_saicaw_2.0.co_2. [DOI] [PubMed] [Google Scholar]
- 12.Blair RJ. The roles of orbital frontal cortex in the modulation of antisocial behavior. Brain Cogn. 2004;55:198–208. doi: 10.1016/S0278-2626(03)00276-8. [DOI] [PubMed] [Google Scholar]
- 13.Blair RJR. The roles of orbital frontal cortex in the modulation of antisocial behavior. Brain Cogn. 2004;55:198–208. doi: 10.1016/S0278-2626(03)00276-8. [DOI] [PubMed] [Google Scholar]
- 14.Blumer D, B DF. Personality changes with frontal and temporal lobe lesions. In: Blumer D, B DF, editors. Psychiatric Aspects of Neurologic Disease. New York: Grune & Stratton; 1975. pp. 151–169. [Google Scholar]
- 15.Brantley HT, Clifford E. Cognitive, self-concept, and body image measures of normal, cleft palate, and obese adolescents. Cleft Palate J. 1979;16:177–182. [PubMed] [Google Scholar]
- 16.Bremmer J. Does stress damage the brain. Biol Psychiatry. 1999;45:797–805. doi: 10.1016/s0006-3223(99)00009-8. [DOI] [PubMed] [Google Scholar]
- 17.Bressmann T, Sader R, Ravens-Sieberer U, Zeilhofer HF, Horch HH. Quality of life research in patients with cleft lip and palate: preliminary results. Mund Kiefer Gesichtschir. 1999;3:134–139. doi: 10.1007/s100060050116. [DOI] [PubMed] [Google Scholar]
- 18.Broder HL, Richman LC, Matheson PB. Learning disability, school achievement, and grade retention among children with cleft: a two-center study. Cleft Palate Craniofac J. 1998;35:127–131. doi: 10.1597/1545-1569_1998_035_0127_ldsaag_2.3.co_2. [DOI] [PubMed] [Google Scholar]
- 19.Broder HL, Smith FB, Strauss RP. Effects of visible and invisible orofacial defects on self-perception and adjustment across developmental eras and gender. Cleft Palate Craniofac J. 1994;31:429–436. doi: 10.1597/1545-1569_1994_031_0429_eovaio_2.3.co_2. [DOI] [PubMed] [Google Scholar]
- 20.Chemerinski E, Nopoulos PC, Crespo-Facorro B, Andreasen NC, Magnotta V. Morphology of the ventral frontal cortex in schizophrenia: relationship with social dysfunction. Biol Psychiatry. 2002;52:1–8. doi: 10.1016/s0006-3223(01)01363-4. [DOI] [PubMed] [Google Scholar]
- 21.Chemerinski E, Nopoulos PC, Crespo-Facorro B, Andreasen NC, Magnotta V. Morphology of the ventral frontal cortex in schizophrenia: relationship with social dysfunction. Biol Psychiatry. 2002;52:1–8. doi: 10.1016/s0006-3223(01)01363-4. [DOI] [PubMed] [Google Scholar]
- 22.Cohen G, Andreasen NC, Alliger R, Arndt S, Kuan J, Yuh WT, Ehrhardt J. Segmentation techniques for the classification of brain tissue using magnetic resonance imaging. Psychiatry Res. 1992;45:33–51. doi: 10.1016/0925-4927(92)90012-s. [DOI] [PubMed] [Google Scholar]
- 23.Crespo-Facorro B, Kim J, Andreasen NC, Spinks R, O’Leary DS, Bockholt HJ, Harris G, Magnotta VA. Cerebral cortex: a topographic segmentation method using magnetic resonance imaging. Psychiatry Res. 2000;100:97–126. doi: 10.1016/s0925-4927(00)00072-x. [DOI] [PubMed] [Google Scholar]
- 24.Crespo-Facorro B, Kim JJ, Andreasen NC, O’Leary DS, Wiser AK, Bailey JM, Harris G, Magnotta VA. Human frontal cortex: an MRI-based parcellation method. Neuroimage. 1999;10:500–519. doi: 10.1006/nimg.1999.0489. [DOI] [PubMed] [Google Scholar]
- 25.Damasio AR. Decartes’ Error: Emotion, Rationality, and the Human Brain. New York: Putnam; 1994. [Google Scholar]
- 26.Davidson RJ. Affective style and affective disorders: Perspectives from affective neuroscience. Cogn Emotion. 1998;12:307–330. [Google Scholar]
- 27.Davidson RJ. Anxiety and affective style: role of prefrontal cortex and amygdala. Biol Psychiatry. 2002;51:68–80. doi: 10.1016/s0006-3223(01)01328-2. [DOI] [PubMed] [Google Scholar]
- 28.Davidson RJ, Rickman M. Behavioral inhibition and the emotional circuitry of the brain: Stability and plasticity during the early childhood years. New York: Oxford University Press; 1999. [Google Scholar]
- 29.De Bellis MD, Baum AS, Birmaher B, Keshavan MS, Eccard CH, Boring AM, Jenkins FJ, Ryan ND. A.E.Bennett Research Award. Developmental traumatology. Part I: Biological stress systems. Biol Psychiatry. 1999;45:1259–1270. doi: 10.1016/s0006-3223(99)00044-x. see comment. [DOI] [PubMed] [Google Scholar]
- 30.Denckla M. Revised Neurological Examination for Subtle Signs. Psychopharmacol Bull. 1985;21:773–800. [PubMed] [Google Scholar]
- 31.Endriga MC, Kapp-Simon KA. Psychological issues in craniofacial care: state of the art. Cleft Palate Craniofac J. 1999;36:3–11. doi: 10.1597/1545-1569_1999_036_0001_piiccs2.3.co_2. [DOI] [PubMed] [Google Scholar]
- 32.Eslinger P, D AR. Behavioral disturbances associated with rupture of anterior communicating artery aneurysms. Semin Neurol. 1984;4:385–389. [Google Scholar]
- 33.Gould E, R AJ, Graziano MS, Gross CG. Neurogenesis in the neocortex of adult primates. Science. 1999;286:548–552. doi: 10.1126/science.286.5439.548. [DOI] [PubMed] [Google Scholar]
- 34.Harlow JM. Recovery after severe injury to the head. Pulication of the Massachusetts Medical Society. 1868;2:327–346. [Google Scholar]
- 35.Harris G, A NC, Cizadlo T, Bailey JM, Bockholt HJ, Magnotta VA. Improving tissue segmentation in MRI: a three-dimensional multispectral discriminant analysis method with automated training class selection. J Comput Assist Tomogr. 1999;23:144–154. doi: 10.1097/00004728-199901000-00030. [DOI] [PubMed] [Google Scholar]
- 36.Harter S. The Perceived Competence Scale for Children. Child Dev. 1982;53:87–97. [Google Scholar]
- 37.Hollingshead AB. Four factor index of social skills. New Haven, CT: Yale; 1975. [Google Scholar]
- 38.Hunt O, Burden D, Hepper P, Johnston C. The psychosocial effects of cleft lip and palate: a systematic review. Eur J Orthod. 2005;27:274–285. doi: 10.1093/ejo/cji004. [DOI] [PubMed] [Google Scholar]
- 39.Hunter R, Blackwood W, Bull J. Three cases of frontal meningiomas presenting psychiatrically. Br Med J. 1968;3:9–16. doi: 10.1136/bmj.3.5609.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kagan J, R S, Snidman N. Biological Bases of Childhood Shyness. Science. 1988;240:167–171. doi: 10.1126/science.3353713. [DOI] [PubMed] [Google Scholar]
- 41.Kapp-Simon KA, Simon DJ, Kristovich S. Self-perception, social skills, adjustment, and inhibition in young adolescents with craniofacial anomalies. Cleft Palate Craniofac J. 1992;29:352–356. doi: 10.1597/1545-1569_1992_029_0352_spssaa_2.3.co_2. [DOI] [PubMed] [Google Scholar]
- 42.Kapp K. Self concept of the cleft lip and or palate child. Cleft Palate J. 1979;16:171–176. [PubMed] [Google Scholar]
- 43.Kjaer I. Human prenatal craniofacial development related to brain development under normal and pathologic conditions. Acta Odontol Scand. 1995;53:135–143. doi: 10.3109/00016359509005963. [DOI] [PubMed] [Google Scholar]
- 44.Kommers MS, Sullivan MD. Written language skills of children with cleft palate. Cleft Palate J. 1979;16:81–85. [PubMed] [Google Scholar]
- 45.Leonard BJ, Brust JD, Abrahams G, Sielaff B. Self-concept of children and adolescents with cleft lip and/or palate. Cleft Palate Craniofac J. 1991;28:347–353. doi: 10.1597/1545-1569_1991_028_0347_scocaa_2.3.co_2. [DOI] [PubMed] [Google Scholar]
- 46.Magnotta VA, Harris G, Andreasen NC, O’Leary DS, Yuh WT, Heckel D. Structural MR image processing using the BRAINS2 toolbox. Comput Med Imaging Graph. 2002;26:251–264. doi: 10.1016/s0895-6111(02)00011-3. [DOI] [PubMed] [Google Scholar]
- 47.Marsh H. Self Description Questionnaire - I. Campbelltown, Australia: University of Western Sydney; 1990. [Google Scholar]
- 48.McWilliams BJ, Paradise LP. Educational, occupational, and marital status of cleft palate adults. Cleft Palate J. 1973;10:223–229. [PubMed] [Google Scholar]
- 49.Nauta WJ. The problem of the frontal lobe: a reinterpretation. J Psychiatr Res. 1971;8:167–187. doi: 10.1016/0022-3956(71)90017-3. [DOI] [PubMed] [Google Scholar]
- 50.Noar JH. Questionnaire survey of attitudes and concerns of patients with cleft lip and palate and their parents. Cleft Palate Craniofac J. 1991;28:279–284. doi: 10.1597/1545-1569_1991_028_0279_qsoaac_2.3.co_2. [DOI] [PubMed] [Google Scholar]
- 51.Nopoulos P, Berg S, Canady J, Richman L, Van Demark D, Andreasen NC. Abnormal brain morphology in patients with isolated cleft lip, cleft palate, or both: a preliminary analysis. Cleft Palate Craniofac J. 2000;37:441–446. doi: 10.1597/1545-1569_2000_037_0441_abmipw_2.0.co_2. [DOI] [PubMed] [Google Scholar]
- 52.Nopoulos P, Berg S, Canady J, Richman L, Van Demark D, Andreasen NC. Structural brain abnormalities in adult males with clefts of the lip and/or palate. Genet Med. 2002;4:1–9. doi: 10.1097/00125817-200201000-00001. [DOI] [PubMed] [Google Scholar]
- 53.Nopoulos P, Berg S, VanDemark D, Richman L, Canady J, Andreasen NC. Increased incidence of a midline brain anomaly in patients with nonsyndromic clefts of the lip and/or palate. J Neuroimaging. 2001;11:418–424. doi: 10.1111/j.1552-6569.2001.tb00072.x. [DOI] [PubMed] [Google Scholar]
- 54.Nopoulos P, Choe I, Berg S, Van Demark D, Canady J, Richman L. Ventral frontal cortex morphology in adult males with isolated orofacial clefts: relationship to abnormalities in social function. Cleft Palate Craniofac J. 2005;42:138–144. doi: 10.1597/03-112.1. [DOI] [PubMed] [Google Scholar]
- 55.Nopoulos P, Murray J, Langbehn D, Canady J, Magnotta V, Richman L. Abnormal Brain Structure in Children with Isolated Clefts of the Lip and/or Palate. Archives of Pediatric and Adolescent Medicine. doi: 10.1001/archpedi.161.8.753. In press. [DOI] [PubMed] [Google Scholar]
- 56.Ongur D, Price JL. The organization of networks within the orbital and medial prefrontal cortex of rats, monkeys and humans. Cerebral Cortex. 2000;10:206–219. doi: 10.1093/cercor/10.3.206. [DOI] [PubMed] [Google Scholar]
- 57.Persson M, Aniansson G, Becker M, Svensson H. Self-concept and introversion in adolescents with cleft lip and palate. Scand J Plast Reconstr Surg Hand Surg. 2002;36:24–27. doi: 10.1080/028443102753478336. [DOI] [PubMed] [Google Scholar]
- 58.Ramstad T, Ottem E, Shaw WC. Psychosocial adjustment in Norwegian adults who had undergone standardised treatment of complete cleft lip and palate. I. Education, employment and marriage. Scand J Plast Reconstr Surg Hand Surg. 1995;29:251–257. doi: 10.3109/02844319509050135. [DOI] [PubMed] [Google Scholar]
- 59.Richman LC. Cognitive patterns and learning disabilities of cleft palate children with verbal deficits. J Speech Hear Res. 1980;23:447–456. doi: 10.1044/jshr.2302.447. [DOI] [PubMed] [Google Scholar]
- 60.Richman LC, Eliason MJ, Lindgren SD. Reading disability in children with clefts. Cleft Palate J. 1988;25:21–25. [PubMed] [Google Scholar]
- 61.Rolls E. The brain and emotion. New York: Oxford University Press; 1999. [Google Scholar]
- 62.Sapolsky R. Why stress is bad for your brain. Science. 1996;273:749–750. doi: 10.1126/science.273.5276.749. [DOI] [PubMed] [Google Scholar]
- 63.Sarazin M, Pillon B, Giannakopoulos P, Rancurel G, Samson Y, Dubois B. Clinicometabolic dissociation of cognitive functions and social behavior in frontal lobe lesions. Neurology. 1998;51:142–148. doi: 10.1212/wnl.51.1.142. [DOI] [PubMed] [Google Scholar]
- 64.Sperber GH. First year of life: prenatal craniofacial development. Cleft Palate Craniofac J. 1992;29:109–111. doi: 10.1597/1545-1569_1992_029_0109_fyolpc_2.3.co_2. [DOI] [PubMed] [Google Scholar]
- 65.Steinman DR, B ED. Neuropsychological sequelae of ruptured anterior communicating artery aneurysm. International Journal of Clinical Neuropsychology. 1986;8:135–140. [Google Scholar]
- 66.Teicher MH. Scars that won’t heal: the neurobiology of child abuse. Sci Am. 2002;286:68–75. doi: 10.1038/scientificamerican0302-68. [DOI] [PubMed] [Google Scholar]
- 67.Turk G. Re-tiling polygonal surfaces. Comput Graphics. 1992;26:620–633. [Google Scholar]
- 68.Woods RP, Cherry SR, Mazziotta JC. Rapid automated algorithm for aligning and reslicing PET images. J Comput Assist Tomogr. 1992;16:620–633. doi: 10.1097/00004728-199207000-00024. [DOI] [PubMed] [Google Scholar]
- 69.Wyvill G, M C. Data structures for soft objects. Vis Comput. 1986;2:227–234. [Google Scholar]
- 70.Zucchero TM, Cooper ME, Maher BS, Daack-Hirsch S, Nepomuceno B, Ribeiro L, Caprau D, Christensen K, Suzuki Y, Machida J, Natsume N, Yoshiura K-I, Vieira AR, Orioli IM, Castilla EE, Moreno L, Arcos-Burgos M, Lidral AC, Field LL, Liu Y-e, Ray A, Goldstein TH, Schultz RE, Shi M, Johnson MK, Kondo S, Schutte BC, Marazita ML, Murray JC. Interferon regulatory factor 6 (IRF6) gene variants and the risk of isolated cleft lip or palate. N Engl J Med. 2004;351:769–780. doi: 10.1056/NEJMoa032909. see comment. [DOI] [PubMed] [Google Scholar]


