Abstract
Choline is known to be involved with numerous physiological functions of the nervous system and also acts as a direct acting agonist of α7 nicotinic acetylcholine receptors (nAChRs). The purpose of this study was to conduct a brain region-specific evaluation of changes in nAChR subtype expression following dietary choline modification. In addition, we assessed changes in body weight, food/water intake, as well as changes spatial learning (Morris Water Maze) in response to dietary choline modification. Male Sprague Dawley rats were exposed to standard, choline supplemented or choline deficient diets for periods of 14 or 28 days. Choline supplemented animals gained significantly less weight over the course of the experiment, in spite of the fact that there were minimal differences in food consumption between the dietary regimens. Spatial memory did not differ between animals maintained on a standard rat diet, and the choline supplemented food. Brains of the animals kept on the diets for 14 and 28 days were used for quantitative autoradiographic analysis of nicotinic receptor subtypes using 125I-Bungarotoxin (α7) and 125I-Epibatidine (non-α7). There were no significant differences in nicotinic receptor binding or physiologic parameters measured between animals fed standard and choline deficient diets. However 2 weeks of dietary choline supplementation caused significant up-regulation of α7 receptors without significant effect on the density of non-α7 nAChRs. Increases in BTX binding predominantly occurred in cortical and hippocampal brain regions and ranged between 14 and 30 percent depending on the brain region. The results of our study suggest that choline acts as a selective agonist at α7 nicotinic cholinergic receptors in the rat central nervous system.
Keywords: ℑ7 nAChR, Cholinergic, Acetylcholine, Morris Water Maze, Up-regulation, Bungarotoxin, Epibatidine
1. Introduction
Choline, a quaternary ammonium compound, was classified by the Food and Nutrition Board of the Institute of Medicine of the National Academy of Sciences as an essential nutrient in 1998. While some de novo synthesis of choline occurs physiologically, dietary sources are far more common. Although found in wide variety of nutritional sources, the highest concentrations of choline are found in beef/chicken liver, pork, eggs, wheat germ, dry soybeans, and peanuts (Blusztajn, 1998; Zeisel, 2004). Since choline is charged at physiological pH, transporters are required for choline movement across biological membranes. At least three distinct choline transporters have been characterized. A low affinity choline transporter (LACT) is located throughout the mammalian body and delivers choline for phospholipid synthesis (e.g. phosphatidylcholine and sphingomyelin), as well as synthesis of other signaling molecules (e.g. diacylglycerol, ceramide). Phosphatidylcholine can also be used to re-synthesize choline de novo. The LACT utilizes a sodium-independent mechanism and is characterized by saturable kinetics, with Km ranging from 80 to 340 μM. In contrast to the LACT, the high affinity choline uptake site (HACU) is sodium-dependent, inhibited by hemicholinium-3 and has a Km in the range of 5μM (Blusztajn and Wurtman, 1983; see Ferguson and Blakely, 2004 or Lockman and Allen, 2002 for a review). The HACU transporter is located presynaptically on acetylcholine (ACh) nerve terminals and the transport of choline into cholinergic neurons is the rate-limiting step in the biosynthesis of ACh (Atweh et al., 1975; Birks and Macintosh, 1957; Kuhar and Murrin, 1978; Simon et al., 1976). Choline enters the brain via a third transporter that is located at the blood-brain barrier (BBB). Since the rat brain BBB transporter is not typically saturated at physiological levels of plasma choline (5–10μM), increases in dietary choline should be paralleled by increases in brain choline concentrations.
Neuronal nicotinic receptors are ligand gated ion channel receptors, coupled to increased flow of Na+ and Ca++. These proteins are pentameric structures consisting of a water-filled pore, surrounded by various combinations of receptor subunits, which assemble to form many distinct receptor isoforms. In the CNS, nAChRs are either heteromeric structures, comprised of an assortment of α subunits (α 2–6) and beta subunits (β 2–4), or homomeric combinations of α subunits (〈7–9). Alpha 7 receptors are the only homomeric nicotinic receptors that have a wide distribution in mammalian CNS and are characterized by high Ca++ permeability (reviewed in Berg and Conroy, 2002; Dani, 2001; Gotti and Clementi, 2004; Pereira et al., 2002). Recent studies have suggested that in addition to the aforementioned physiological functions of choline, this compound may also act as a direct-acting agonist of α7 nAChRs. One of the first observations that suggested this possibility was a demonstration of increased density of α-bungarotoxin binding sites in the CNS of choline supplemented animals, when compared to choline deficient animals (Morley et al., 1977). Increased ACh synthesis occurring secondary to choline supplementation was initially hypothesized to be responsible for this upregulation (Morley et al., 1977; Wecker and Dettbarn, 1979; Wecker and Schmidt, 1979; Wecker and Schmidt, 1980). However, subsequent studies used HACU blockers to deplete cellular levels of ACh, with no alteration in α7 nAChR density (Morley and Garner, 1990). Thus cellular/synaptic levels of ACh do not appear to be the primary determinant of α7 receptor expression. Studies using frog oocytes and heterologously expressed nAChRs demonstrated that choline acts as an agonist at α3/β4, α4/β4, α9 and α9/α10 receptors (Mandelzys et al., 1995; Sgard et al., 2002; Verbitsky et al., 2000; Zwart and Vijverberg, 2000). Additional electrophysiological studies showed conclusively that choline is a full and selective agonist at α7 nAChRs (Alkondon et al., 1997; Albuquerque et al., 1998; Alkondon et al., 1999; Uteshev et al., 2003). Choline-evoked electrophysiological responses from single cells or hippocampal slices are blocked by either methyllycaconitine or α bungarotoxin (BTX), selective α7 receptor antagonists (Albuquerque et al., 1998; Alkondon et al., 1999; Fayuk and Yakel, 2004). The agonistic actions of choline at α7 receptors in the CNS may be useful in brain disorders that involve decreases in the number or function of these proteins. The purpose of this study was to evaluate the effect of dietary choline modification on nicotinic receptor density using quantitative receptor autoradiography. Although previous studies have demonstrated increases in nAChR’s following choline supplementation, a direct comparison of both α7 and non-α7 receptor changes in the same animals has not been reported. If choline were truly a selective α7 agonist, one would expect to see preferential up-regulation of α7 nAChRs following choline administration. Since α7 agonists have been reported to enhance cognitive function (Arendash et al., 1995; Hunter et al., 1994; Levin et al., 1999; Meyer et al., 1997), we also assessed spatial learning in animals that were chronically exposed to dietary choline supplementation.
2. Materials and Methods
2.1 Animals and diets
Male Sprague-Dawley rats were obtained from Harlan Breeding Laboratories (Indianapolis IN). Animals were 31 days old on the first day of choline treatment. Rats were housed two per cage, provided ad libitum food/water, and maintained on a 12:12 light: dark cycle. All rat food was obtained from Harlan Teklad (Madison, WI, USA). Three diets with varying choline content were employed in this study. A standard rat diet containing approximately 0.2% choline (TD 03118) was compared to a choline deficient diet (containing no choline; TD 88052), and a choline supplemented diet (containing approximately 2% choline; TD 03119). All of the diets were nutritionally equivalent, and differed only in choline content. Animals were exposed to the various diets for 14 or 28 days (n = 5–6 per group). Weight gain, food consumption and water intake were measured in all animals subjected to dietary choline manipulation for 4 weeks. Several food pellets were weighed and placed in the animals’ cage each day. On the following day, the food remaining in the cage was re-weighed, and the difference in food weight was calculated as the daily food consumption. Daily water consumption was measured by weighing the water bottles on each day of the experiment. Spillage control water bottles were included for daily corrections due to handling of the water bottles. The body mass of each animal was determined every other day. Results were analyzed using two-way ANOVA (Dietary group × Day of treatment), with the day of treatment as a repeated measure; a Tukey’s procedure was used for post-hoc analysis. All of the experimental procedures described in this manuscript were carried out in accordance with guidelines set forth by the University of Kentucky Institutional Animal Care and Use Committee.
2.2 Cognitive Evaluation
Spatial memory in the Morris Water Maze (MWM) was assessed over 5 consecutive days in animals fed the various diets for 14 or 28 days. The testing room contained a plastic pool (127cm diameter × 56cm in height) with a submerged escape platform (13.5cm in diameter) at the center of one quadrant; visual cues distributed throughout the room helped to aid spatial orientation. All of the cognitive evaluations were videotaped and analyzed using Videomax software (Columbus Instruments). Quadrant entry was randomized for different starting positions, and animals were allowed to swim until they found the platform, where they remained for 15 seconds. Rats that were unable to locate the platform in 60 seconds were manually placed on the platform, and allowed to rest there for 15 seconds. Twenty acquisition trials were administered (4 per day for 5 days). Four hours following the last acquisition trial, the platform was removed and a 30 second retention (probe) trial was performed. Videomax software was used to analyze several aspects of each animals search strategy. Training data were analyzed using the two-way repeated measure analysis of variance (Dietary group × Day of testing), where the day of training was used as a repeated measure. Retention trial data were analyzed using a one-way analysis of variance.
2.3 Receptor Autoradiography
Animals were euthanized, the brains immediately removed and frozen in isopentane that was chilled in dry ice. Brains were sliced using a Lecia CM1850 cryostat (Nussloch, Germany) to make a series of 16-micron thick sections, which were mounted onto gelatin, chromium potassium sulfate, and poly-L-lysine coated slides. Adjacent sets of sections were prepared to directly compare α7 and non-α7 nAChR binding following choline supplementation. Αlpha 7 nAChRs were measured using α-[125I]-Bungarotoxin autoradiography, as previously described (Sparks and Pauly, 1999). A ligand concentration of 2.5nmol [125I] Tyr54-α-BTX (Perkin-Elmer Life Sciences, Inc., Boston, MA; specific activity=102.9Ci/mmol) was used for section incubations. Non-α7 nAChR density was assessed using [125I]-Epibatidine autoradiography (100 nM incubation concentration; Perkin-Elmer Life Sciences, Inc., Boston, MA; specific activity 2200Ci/mmol) (Perry and Kellar, 1995). RayMax Beta High Performance Autoradiography Film (ICN Biomedicals Inc., Aurora, Ohio) was used to visualize the areas of ligand binding. Radioactive rat brain tissue standards were included with each film X-ray cassette in order to determine the response of the film to the increasing amounts of radioactivity. Exposure time was optimized for each ligand: 7 days for [125I]-BTX, and 3 days for [125I]-Epibatidine. All films were processed using Kodak D-19 developer. Binding data were analyzed using NIH image v1.59 on a Power Macintosh connected to a Sony XC-77 CCD camera via a Scion LG-3 frame-grabber. Molar quantities of bound ligand were determined by constructing a standard curve from radioactivity tissue standards fitted to a third degree polynomial. Brain regions for autoradiographic analysis were selected based on differences in nAChR receptor density as well as functional contributions of various regions to MWM performance (D’Hooge and De Deyn, 2001). All binding data were analyzed by one-way ANOVA and Tukey/Kramer post-hoc tests.
3. Results
3.1 Weight gain, food and water consumption
Figure 1 shows the average daily food intake in animals exposed to diets with varying choline content for 28 days. Significant effects of treatment (F=3.42; P=0.049) and time (F=84; P<0.0001) were detected with a repeated measures ANOVA. Post-hoc Tukey analysis demonstrated that choline supplemented animals consumed significantly less food than the standard choline dietary group (P<0.05) on day 3, and significantly less than the choline deficient group (P<0.05) on days 3 and 5. However on all subsequent days there were no differences in food consumption between choline-supplemented animals and the other groups. In addition, there was no difference in food intake in animals consuming the choline deficient and standard choline diets. Figure 2 demonstrates average water intake in animals exposed to diets with varying choline content. Repeated measures ANOVA revealed significant effects of diet (F=12.2; P=0.0002) and time (F=81.8; P<0.0001). Post-hoc Tukey procedure demonstrated that animals consuming the choline supplemented diet drank significantly more water compared to the standard choline diet group (P<0.05) on days 16, 17, 18, 19, 22, 25, 26, and more than the choline deficient group (P<0.05) on days 15, 16, 17, 18, 19, 22 of the experiment. There were no differences in fluid consumption between animals receiving choline deficient and standard choline diets. Figure 3 shows average body weight, assessed every 2–3 days. Repeated measures ANOVA demonstrated significant increase in weight as time went on (F=5162; P<0.0001). In addition, a significant effect of the dietary treatment (F=15.31; P<0.0001) was detected. Post-hoc analysis using Tukey’s procedure showed that choline supplemented animals weighed significantly less then animals consuming the standard choline diet (* P<0.01), as well as the choline deficient animals († P<0.01) starting in Day 5, and continuing throughout the duration of the study. There was no difference in weight gain between the animals exposed to choline deficient diets and the standard choline chow.
Figure 1.
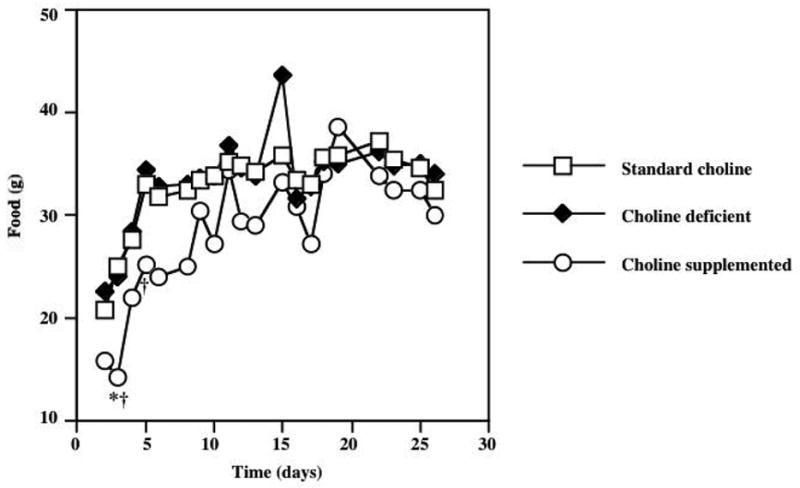
Average daily food intake in animals exposed to diets with varying choline content for 28 days. Repeated measures ANOVA showed significant effects of diet (F=3.42; P=0.049) and time (F=84.0; P<0.0001). Post-hoc Tukey procedure demonstrated a significant difference in food intake between animals consuming a choline-supplemented diet compared to the standard choline diet group on Day 2 of the experiment (* P<0.05). Significant differences in food consumption between choline supplemented and choline deficient groups were detected on days 2 and 4 of the study. Beyond day 4 of the experiment, there were no group differences in food consumption noted. Error bars are omitted from this graph to avoid obstruction of the data points.
Figure 2.
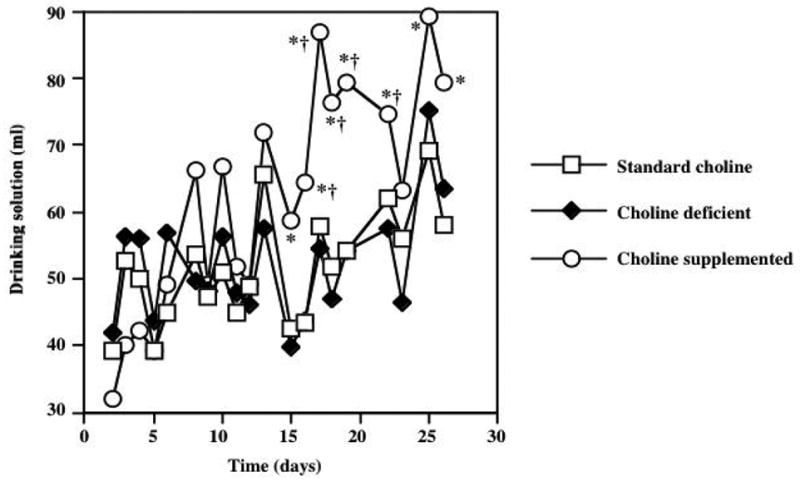
Average water intake in animals exposed to diets with varying choline content for 28 days. ANOVA results showed significant effects of diet (F=12.2; P=0.0002) and time (F=81.8; P<0.0001) on daily water consumption. While there were no group differences in fluid consumption over the first 2 weeks of the study, Post-hoc Tukey procedure demonstrated that choline supplemented animals had significantly increased water consumption on most days in the last 2 weeks of the study (* P<0.05 vs standard choline diet; † P<0.05 vs choline deficient diet). Error bars are omitted from this graph to avoid obstruction of the data points.
Figure 3.
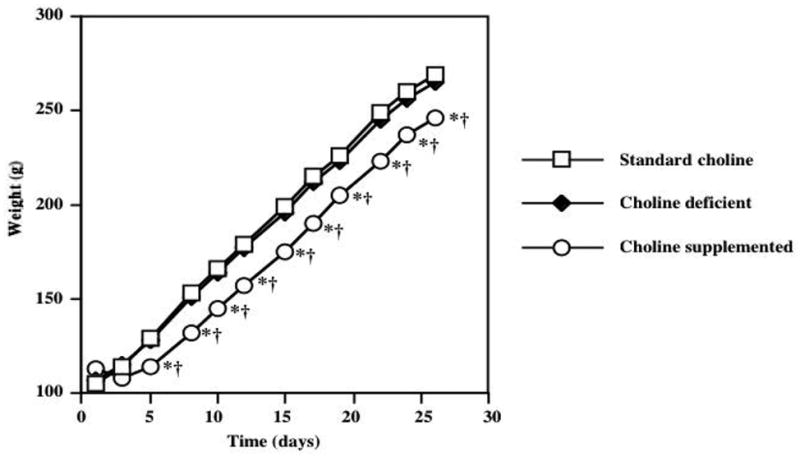
Average body weight gain, assessed approximately every other day in animals exposed to diets with varying choline content for 28 days. ANOVA revealed significant increase in weight as time went on (F=51.62; P<0.0001) as well as a significant effect of dietary treatment (F=15.31; P<0.0001). Post-hoc analysis indicated that choline supplemented animals gained significantly less weight than animals consuming either the standard choline or choline deficient diets. Error bars are omitted to avoid obstruction of the data points.
3.2 Spatial Memory Evaluation
Figure 4 depicts the acquisition of spatial learning in the group of animals tested in the MWM following 2 weeks of dietary choline supplementation. ANOVA results indicated a significant effect of training day (F=21.2, p<0.0001) without significant differences in task acquisition between groups of animals that received the standard and supplemented choline diets. A memory retention test was performed 4 hours following the last acquisition trial, but there were no significant differences between the treatment groups in a number of different parameters involved with search strategy (e.g. relative platform crosses, target quadrant entries, etc; data not shown). Figure 4 shows the acquisition of spatial learning in animals exposed to the dietary choline modification for 4 weeks. ANOVA results demonstrated the expected significant effect of the day of training (F=39.8, p<0.0001) but there were no significant differences in task acquisition between the various dietary groups. Likewise, there were no significant group differences in MWM performance of the memory retention test that was performed in the 28 days animals (data not shown)
Figure 4.
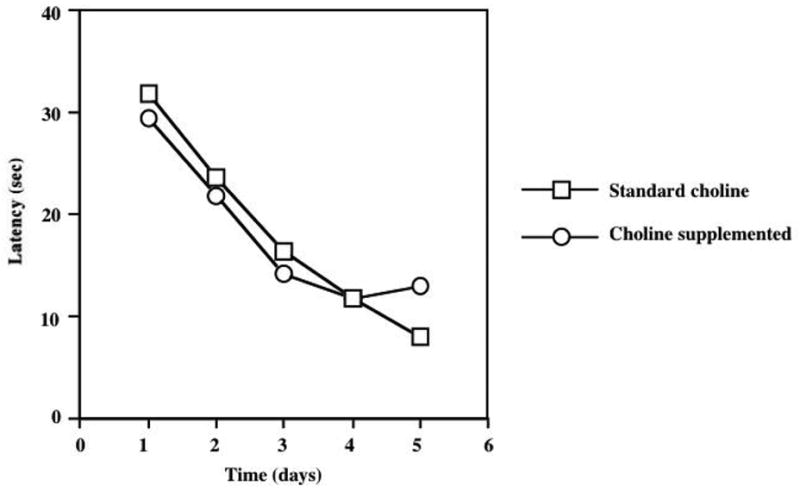
Two weeks of dietary choline supplementation does not alter cognitive performance in the acquisition phase of Morris Water Maze testing. ANOVA demonstrated a significant effect of the training day (F=39.8, P<0.0001), but no significant effect of dietary regimen or diet × day interaction. A retention (probe) test was conducted four hours following the final acquisition trial, but there were no significant effects of dietary treatment (data not shown).
3.3 Receptor Autoradiography
The effects of dietary choline modification on α7 nAChR density were evaluated using [125I]-BTX autoradiography in several cortical, hippocampal and midbrain regions. Alterations in nAChR binding were assessed in groups of animals that were exposed to standard, deficient or choline-supplemented diets for periods of 14 days (Table 1 and Figure 5) or 28 days (Table 2). In animals exposed to the choline-enriched diets for 14 days, there was a significant increase in the density of α7 nAChRs in the cerebral cortex, endopiriform nucleus, hippocampus and superior colliculus. Other brain regions demonstrated a tendency towards increased receptor expression, but the ANOVA results did not reach statistical significance. There were no differences in BTX binding in animals that received the standard and choline deficient diets. In contrast to the 2-week dietary treatment regimen, a more prolonged choline exposure period (4 weeks) did not result in statistically significant increases in BTX binding. Non-α7 receptors evaluated using [125I]-Epibatidine autoradiography were not significantly influenced by dietary choline modification for either 14 days (Table 3) or 28 days (Table 4).
Table 1.
Two weeks of dietary choline modification increases the density of α-7 nAChRs as determined by quantitative [125I]-BTX autoradiography.
| Brain Region | Standard Choline | Choline Deficient | Choline Supplemented |
|---|---|---|---|
| Cortex layers 1–4 | 0.90±0.06 | 0.84±0.1 | 1.29±0.11#, ** |
| Cortex layers 5–6 | 1.63±0.12 | 1.54±0.09 | 2.63±0.33##, ** |
| Endopiriform nucleus | 3.30±0.3 | 2.95±0.18 | 4.87±0.54#, ** |
| CA1 | 2.35±0.03 | 2.31±0.05 | 2.50±0.03 |
| CA2 | 2.55±0.03 | 2.72±0.17 | 2.88±0.05 |
| Hilus of dentate gyrus | 3.32±0.11 | 3.26±0.11 | 4.00±0.11##, ** |
| Dentate gyrus | 2.56±0.04 | 2.51±0.05 | 2.77±0.03 |
| Superior colliculus | 3.81±0.08 | 3.7±0.07 | 4.38±0.11#, ** |
| Inferior colliculus | 4.24±0.15 | 4.34±0.37 | 4.60±0.14 |
| Stratum oriens | 2.67±0.04 | 2.63±0.07 | 2.82±0.05 |
| Ventral lateral geniculate nucleus | 3.58±0.1 | 3.50±0.08 | 4.06±0.15 |
| Posterior hypothalamus | 3.79±0.1 | 3.64±0.37 | 4.06±0.09 |
| Subthalamic nucleus | 4.47±0.12 | 4.10±0.2 | 4.80±0.17 |
| Dorsal tegmental nucleus | 7.08±0.32 | 7.12±0.1 | 7.36±0.09 |
Significantly different from standard choline group (p<0.05);
Significantly different from standard choline group (p<0.01);
Significantly different from choline deficient group (p<0.01).
Figure 5.
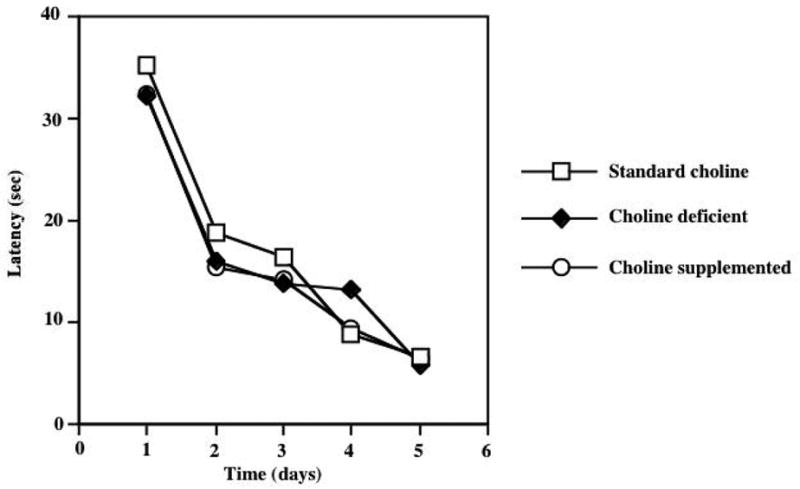
Four weeks of dietary choline supplementation does not alter cognitive performance in the acquisition phase of Morris Water Maze testing. ANOVA demonstrated a significant effect of the training day (F=39.8, P<0.0001), but no significant effect of dietary regimen or diet × day interaction. Animals fed a choline deficient diet and animals consuming a choline-supplemented diet performed similar to animals consuming a standard choline diet on all test days. A retention (probe) test was conducted four hours following the final acquisition trial, but there were no significant effects of dietary treatment (data not shown).
Table 2.
Four weeks of dietary choline modification does not influence the density of α-7 nAChRs as determined by quantitative [125I]-BTX autoradiography.
| Brain Region | Standard Choline | Choline Deficient | Choline Supplemented |
|---|---|---|---|
| Cortex layer 1–4 | 0.87±0.02 | 0.89±0.11 | 1.06±0.08 |
| Cortex layer 5–6 | 1.54±0.08 | 1.52±0.13 | 2.04±0.13 |
| Endopiriform nucleus | 3.16±0.21 | 3.26±0.27 | 3.20±0.29 |
| CA1 | 2.35±0.04 | 2.36±0.02 | 2.42±0.01 |
| CA2 | 2.57±0.05 | 2.59±0.33 | 2.73±0.5 |
| Hilus of dentate gyrus | 3.45±0.1 | 3.49±0.06 | 3.74±0.18 |
| Dentate gyrus | 2.56±0.03 | 2.58±0.02 | 2.67±0.03 |
| Superior colliculus | 3.90±0.14 | 3.92±0.08 | 4.32±0.09 |
| Inferior colliculus | 4.17±0.19 | 4.10±0.09 | 4.16±0.13 |
| Stratum oriens | 2.61±0.06 | 2.66±0.05 | 2.93±0.27 |
| Ventral lateral geniculate nucleus | 3.61±0.12 | 3.77±0.17 | 3.77±0.09 |
| Posterior hypothalamus | 3.6±0.13 | 3.84±0.15 | 3.83±0.09 |
| Subthalamic nucleus | 3.97±0.49 | 4.47±0.12 | 4.33±0.11 |
| Dorsal tegmental nucleus | 6.91±0.81 | 6.84±0.15 | 5.91±1.23 |
Table 3.
Two weeks of dietary choline modification fails to influence the density of non α-7 nAChRs as determined by quantitative [125I]-Epibatidine autoradiography.
| Brain Region | Standard Choline | Choline Deficient | Choline Supplemented |
|---|---|---|---|
| Cortex layer 1 | 3.32±0.12 | 3.44±0.07 | 3.39±0.11 |
| Cortex layers 2–6 | 4.09±0.19 | 4.17±0.13 | 4.00±0.15 |
| Striatum | 4.30±0.18 | 4.32±0.12 | 4.06±0.10 |
| Dentate gyrus (lateral blade) | 2.46±0.08 | 2.71±0.08 | 2.55±0.06 |
| Dentate gyrus (medial blade) | 2.69±0.05 | 2.88±0.03 | 2.75±0.06 |
| Superior colliculus | 5.50±0.28 | 5.23±0.12 | 4.98±0.12 |
| Thalamus | 5.25±0.18 | 5.49±0.23 | 4.93±0.23 |
| Medial habenula | 7.58±0.06 | 8.04±0.14 | 7.51±0.2 |
| Subiculum | 4.63±0.16 | 4.57±0.16 | 4.43±0.13 |
| Medial geniculate nucleus | 4.89±0.17 | 4.74±0.28 | 4.60±0.16 |
| Fasciculus retroflexus | 6.22±0.5 | 6.70±0.27 | 6.12±0.19 |
Table 4.
Four weeks of dietary choline modifications does not change the density of non α-7 nAChRs as determined by quantitative [125I]-Epibatidine autoradiography.
| Brain Region | Standard Choline | Choline Deficient | Choline Supplemented |
|---|---|---|---|
| Cortex layer 1 | 3.32±0.12 | 3.31±0.05 | 3.61±0.23 |
| Cortex layers 2–6 | 4.12±0.24 | 4.05±0.08 | 4.56±0.34 |
| Striatum | 4.23±0.25 | 4.32±0.06 | 4.61±0.30 |
| Dentate gyrus (lateral blade) | 2.67±0.08 | 2.69±0.04 | 2.77±0.09 |
| Dentate gyrus (medial blade) | 2.83±0.07 | 2.84±0.04 | 2.95±0.11 |
| Superior Colliculus | 5.98±0.53 | 5.49±0.13 | 5.98±0.25 |
| Thalamus | 5.38±0.23 | 5.07±0.17 | 5.34±0.29 |
| Medial habenula | 7.61±0.20 | 7.90±0.08 | 7.92±0.23 |
| Subiculum | 4.99±0.35 | 4.62±0.08 | 4.78±0.25 |
| Medial geniculate nucleus | 5.23±0.44 | 4.80±0.16 | 4.94±0.26 |
| Fasciculus retroflexus | 7.11±0.42 | 6.84±0.16 | 6.79±0.28 |
4. Discussion
The results of this study confirm and extend previously investigated actions of dietary choline modification on brain nAChR expression and cognitive performance. The effects of dietary choline supplementation on food/water consumption and average daily weight gain seen in this study were not completely surprising since Morley and Fleck observed that choline supplemented animals ate less and gained weight at lower rate than rats consuming a standard or choline deficient diet (Morley and Fleck, 1987). Our results on food consumption and weight gain were similar; we also determined that choline supplemented animals consume significantly more water than animals on standard or choline deficient diets. It is interesting that weight gain was suppressed in the choline-supplemented group, even though they only had significantly lower food consumption on 2 days of the study. Choline supplemented animals gained less weight even though they ate a similar amount of food and consumed more water than other groups. The effect of choline on body mass regulation could be due to the agonistic actions of choline at α7 nAChRs (Li et al., 2003). Previous studies have demonstrated presence of various nAChR subunit mRNAs (α1–7, α9, α10, β1–4), as well as α7 and β2 receptor proteins in rat adipocytes. Chronic stimulation of rat adipocytes with nicotine has been shown to induce release of adiponectin, a compound that has been suggested to lower body weight in humans (Liu et al., 2004; Matsuzawa, 2005). Alternately it is possible that a stimulant action of choline increased home cage locomotor activity, leading to attenuated weight gain in animals that consumed a choline supplemented diet.
Our data suggest that dietary choline supplementation does not modify spatial learning in otherwise untreated rats. Choline enriched diets did not influence the acquisition or retention phases of MWM testing regardless of whether they were administered diets for 2 weeks or 4 weeks. Animals exposed to a choline-supplemented diet for 2 weeks exhibited a significant increase in the density of α7 nAChRs in several cortical and hippocampal brain regions. Importantly, this up-regulation was not accompanied by a change in MWM performance. Previous studies with chronic nicotine administration have shown that enhanced cognitive performance in the MWM is accompanied by increases in both a7 and non-a7 nAChR expression (Hernandez and Terry, 2005). However up-regulation of a7AChRs alone does not appear to be sufficient for cognitive enhancement. One very surprising finding of our study was that the maximal upregulation of α7 nAChR occurred at 14 days, with no significant up-regulation detected after a longer period of choline supplementation. Importantly, the amount of choline, consumed by the supplemented group remained fairly constant throughout the study (approximately 3mg/kg/day of choline). A study from the Morley lab (Morley and Fleck, 1987) used CNS tissue homogenates to evaluate the effects of choline supplementation on changes in α7 nAChR receptor density in rats of the same strain and age as in our study. This group demonstrated that the effect of choline on α7 nAChR upregulation is dose-dependant, rapid (occurs within 24h), and lasts for 7 days subsequent to the removal of the choline supplementation, after which receptor density declines to control levels. However, significant upregulation of [125I]-BTX binding sites in their study was present after 31 days of choline supplementation. The discrepancies in the results between our study, and the one conducted by Morley’s group could possibly be explained by dissimilarities in the receptor binding methods used, or differences in the method of choline administration. Morley’s group administered choline via drinking solution rather than the dietary supplementation approach used in our study. Pharmacokinetic issues such bioavailability of choline chloride administered in the solid from versus the solution, could potentially be responsible for the discrepancies in the binding results seen following long term choline supplementation. It is also possible that the polydipsia noted in the later portion of out study influenced the clearance of choline administered in solid food. In agreement with the Morley’s findings, the choline deficient diet did not produce any significant difference in α7 nAChR density, as compared to the standard dietary choline group. This is likely explained by continuous replenishment of choline in the brain endogenous sources such as phosphatidylcholine. In fact, Klein et al (Klein et al., 1998) demonstrated that dietary choline deficiency does not result in decreased brain choline, despite a significant reduction in plasma choline levels. The results of present study support the hypothesis that choline is selective for α7 nAChRs since up-regulation was noted for BTX binding, but not Epibatidine binding to non-α7 receptors. With most nicotinic agonists changes in non-α7 binding following chronic treatment are smaller in magnitude than that observed for non-α7 receptors (largely α4/β2). In contrast to our findings, Coutcher et al. reported significant increases in [3H]-nicotine binding in hippocampus and frontal cortex (but not striatum) in rats that were exposed to 30 days of dietary choline supplementation (Coutcher et al., 1992).
Several possible mechanisms could account for the lack of α7 nAChR up-regulation after 28 days of choline supplementation observed in our study. One possibility is that receptor up-regulation that results from short-term (14 day) chronic nicotinic agonist administration is followed by a return to baseline level following longer agonist exposures. If nAChR upregulation is due to receptor desensitization, prolonged treatment with α7 agonists may be associated with changes in the magnitude and/or duration of receptor desensitization, leading to a gradual diminution of receptor upregulation. Previous studies with rats and mice have generally concluded that nicotinic receptor up-regulation increases as the duration of agonist exposure becomes longer (Bhat et al., 1991; Collins et al., 1990; Marks et al., 1983; Marks et al., 1985; Pauly et al., 1991) and results from our own lab showed upregulation of mouse brain BTX binding following nicotine exposure for 30 days via the drinking solution (Sparks and Pauly, 1999). Another possible reason that choline fails to sustain α7 nAChR upregulation beyond 14 days may be a developmental change in the number and/or efficacy of blood-brain barrier choline transporters. Mooradian (1988) studied choline transport across the BBB in rats 2, 18 or 24 months of age and concluded that the BBB choline transporter has reduced capacity and increased affinity in older animals. Furthermore, Conford et al (1982) found that the brain uptake index of choline decreases 3 fold during the first 2 weeks after birth in a rat. Consistent with these findings, choline supplementation-induced increase in α7 nAChRs appears to be age-dependent. Morley and Garner showed a 50% increase in α7 binding in 23-day old rats treated with choline, a 30% increase in 60-day old rats, and no significant changes in rats 6 months of age (Morley and Garner, 1986). Animals in the present study were 45 days old after 14 days on the diets, and 59 days old after 28 days on the diet. If an age-related decrease in choline transporter function occurs, it may be that brain choline levels fall to a level that will not produce significant increase in α7 binding. However, it should be noted not all studies have supported the conclusion that the pharmacology of choline transport changes in development and aging. Allen and Smith compared the kinetics of BBB choline transport in 3, 12, 24 and 28 month old rats and reported that the pharmacokinetics changes minimally in aging (Allen and Smith, 1999).
Choline catabolism could also change with chronic supplementation (or developmentally), leading to alterations in choline concentration at the site of action in the CNS. One study reported that oxidation of choline to betaine increases with age, leading to decreased blood levels of choline (reviewed in Zeisel, 2004). Another possibility is that transport of free choline in to the brain and its clearance out of the brain is increased secondary to chronic choline supplementation. Klein et al has shown, that rats subjected to choline supplementation in drinking solution for 2 weeks demonstrated decreased transport of choline and increased brain efflux of choline (Klein et al., 1991). Wecker and Trommer have reported that serum obtained from choline-supplemented animals contains an unknown factor that inhibits choline transporter function (Wecker and Trommer, 1984). The serum concentration of this factor increased with the duration of choline supplementation. These findings stress the importance of evaluation of brain choline levels following acute and chronic administration. Future studies must pay more careful attention to changes in brain choline levels that could occur over time, influencing the behavioral and neurochemical outcome of chronic treatment.
Dietary choline supplementation has been proposed as a potential method of boosting ACh concentrations in pathologic conditions characterized by deficit in cholinergic signaling, such as Alzheimer’s disease. After early reports of increased brain ACh levels subsequent to choline supplementation (Cohen and Wurtman, 1975; Haubrich et al., 1975) additional studies suggested that under normal physiologic conditions, dietary choline supplementation has no significant effect on brain ACh levels (Trommer et al., 1982; Wecker, 1986; Wecker, 1990; Wecker and Dettbarn, 1979; Wecker and Schmidt, 1979). While this has somewhat diminished enthusiasm regarding choline as a possible therapeutic entity in Alzheimer’s disease, other neurobiological actions of choline may be useful in the clinic. Disorders characterized by a specific deficit in the number and/or function of CNS α7 receptors (such as schizophrenia ( Freedman et al., 2001; for review see Freedman et al., 2000; Leonard et al., 2000; Martin et al., 2004)) could potentially benefit significantly form choline therapy.
Figure 6.
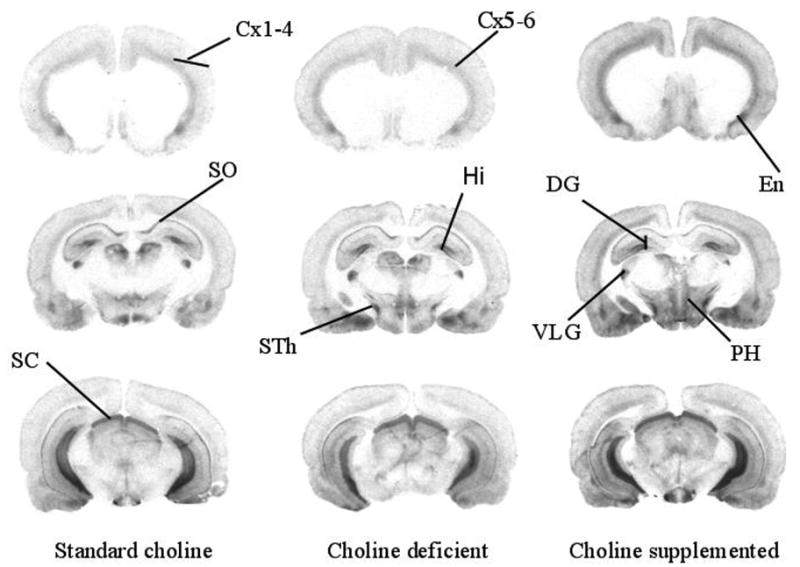
Representative autoradiographs depicting α-[125I]-BTX binding in animals exposed to a standard choline diet (left column), a choline deficient diet (middle column) or a choline supplemented diet (right column) for 14 days. Choline supplemented animals exhibited significantly higher BTX binding in some brain regions compared to animals that consumed the standard choline or choline deficient diets. Cx 1–4, cerebral cortex layers 1–4; Cx 5–6, cerebral cortex layers 5–6; DG, dentate gyrus; En, endopiriform nucleus; Hi, hilus of the dentate gyrus; PH, posterior hypothalamus SC, superior colliculus; SO, stratum oriens; STh, subthalamic nucleus; VLG, ventrolateral geniculate nucleus.
Acknowledgments
Supported by a grant from the National Institute of Health (NS42196 to JRP)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Albuquerque EX, Pereira EF, Braga MF, Alkondon M. Contribution of nicotinic receptors to the function of synapses in the central nervous system: the action of choline as a selective agonist of α7 receptors. J Physiol Paris. 1998;92:309–316. doi: 10.1016/s0928-4257(98)80039-9. [DOI] [PubMed] [Google Scholar]
- Alkondon M, Pereira EF, Cortes WS, Maelicke A, Albuquerque EX. Choline is a selective agonist of α7 nicotinic acetylcholine receptors in the rat brain neurons. Eur J Neurosci. 1997;9:2734–2742. doi: 10.1111/j.1460-9568.1997.tb01702.x. [DOI] [PubMed] [Google Scholar]
- Alkondon M, Pereira EF, Eisenberg HM, Albuquerque EX. Choline and selective antagonists identify two subtypes of nicotinic acetylcholine receptors that modulate GABA release from CA1 interneurons in rat hippocampal slices. J Neurosci. 1999;19:2693–2705. doi: 10.1523/JNEUROSCI.19-07-02693.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen DD, Smith QR. Blood-brain barrier choline transport in the senescent rat. Neurosci Lett. 1999;277:198–202. doi: 10.1016/s0304-3940(99)00869-1. [DOI] [PubMed] [Google Scholar]
- Arendash GW, Sengstock GJ, Sanberg PR, Kem WR. Improved learning and memory in aged rats with chronic administration of the nicotinic receptor agonist GTS-21. Brain Res. 1995;674:252–259. doi: 10.1016/0006-8993(94)01449-r. [DOI] [PubMed] [Google Scholar]
- Atweh S, Simon JR, Kuhar MJ. Utilization of sodium-dependent high affinity choline uptake in vitro as a measure of the activity of cholinergic neurons in vivo. Life Sci. 1975;17:1535–1544. doi: 10.1016/0024-3205(75)90174-5. [DOI] [PubMed] [Google Scholar]
- Berg DK, Conroy WG. Nicotinic α7 receptors: synaptic options and downstream signaling in neurons. J Neurobiol. 2002;53:512–523. doi: 10.1002/neu.10116. [DOI] [PubMed] [Google Scholar]
- Bhat RV, Turner SL, Selvaag SR, Marks MJ, Collins AC. Regulation of brain nicotinic receptors by chronic agonist infusion. J Neurochem. 1991;56:1932–1939. doi: 10.1111/j.1471-4159.1991.tb03450.x. [DOI] [PubMed] [Google Scholar]
- Birks RI, Macintosh FC. Acetylcholine metabolism at nerve-endings. Br Med Bull. 1957;13:157–161. doi: 10.1093/oxfordjournals.bmb.a069605. [DOI] [PubMed] [Google Scholar]
- Blusztajn JK. Choline, a vital amine. Science. 1998;281:794–795. doi: 10.1126/science.281.5378.794. [DOI] [PubMed] [Google Scholar]
- Blusztajn JK, Wurtman RJ. Choline and cholinergic neurons. Science. 1983;221:614–620. doi: 10.1126/science.6867732. [DOI] [PubMed] [Google Scholar]
- Cohen EL, Wurtman RJ. Brain acetylcholine: increase after systemic choline administration. Life Sci. 1975;16:1095–1102. doi: 10.1016/0024-3205(75)90194-0. [DOI] [PubMed] [Google Scholar]
- Collins AC, Romm E, Wehner JM. Dissociation of the apparent relationship between nicotine tolerance and up-regulation of nicotinic receptors. Brain Res Bull. 1990;25:373–379. doi: 10.1016/0361-9230(90)90222-l. [DOI] [PubMed] [Google Scholar]
- Coutcher JB, Cawley G, Wecker L. Dietary choline supplementation increases the density of nicotine binding sites in rat brain. J Pharmacol Exp Ther. 1992;262:1128–1132. [PubMed] [Google Scholar]
- D’Hooge R, De Deyn PP. Applications of the Morris water maze in the study of learning and memory. Brain Res Brain Res Rev. 2001;36:60–90. doi: 10.1016/s0165-0173(01)00067-4. [DOI] [PubMed] [Google Scholar]
- Dani JA. Overview of nicotinic receptors and their roles in the central nervous system. Biol Psychiatry. 2001;49:166–174. doi: 10.1016/s0006-3223(00)01011-8. [DOI] [PubMed] [Google Scholar]
- Fayuk D, Yakel JL. Regulation of nicotinic acetylcholine receptor channel function by acetylcholinesterase inhibitors in rat hippocampal CA1 interneurons. Mol Pharmacol. 2004;66:658–666. doi: 10.1124/mol.104.000042. [DOI] [PubMed] [Google Scholar]
- Ferguson SM, Blakely RD. The choline transporter resurfaces: new roles for synaptic vesicles? Mol Interv. 2004;4:22–37. doi: 10.1124/mi.4.1.22. [DOI] [PubMed] [Google Scholar]
- Freedman R, Adams CE, Leonard S. The α7-nicotinic acetylcholine receptor and the pathology of hippocampal interneurons in schizophrenia. J Chem Neuroanat. 2000;20:299–306. doi: 10.1016/s0891-0618(00)00109-5. [DOI] [PubMed] [Google Scholar]
- Freedman R, Leonard S, Gault JM, Hopkins J, Cloninger CR, Kaufmann CA, Tsuang MT, Farone SV, Malaspina D, Svrakic DM, et al. Linkage disequilibrium for schizophrenia at the chromosome 15q13–14 locus of the α7-nicotinic acetylcholine receptor subunit gene (CHRNA7) Am J Med Genet. 2001;105:20–22. [PubMed] [Google Scholar]
- Gotti C, Clementi F. Neuronal nicotinic receptors: from structure to pathology. Prog Neurobiol. 2004;74:363–396. doi: 10.1016/j.pneurobio.2004.09.006. [DOI] [PubMed] [Google Scholar]
- Haubrich DR, Wang PF, Clody DE, Wedeking PW. Increase in rat brain acetylcholine induced by choline or deanol. Life Sci. 1975;17:975–980. doi: 10.1016/0024-3205(75)90451-8. [DOI] [PubMed] [Google Scholar]
- Hernandez CM, Terry AV. Repeated nicotine exposure in rats: effects on memory function, cholinergic markers and nerve growth factor. Neuroscience. 2005;130:997–1012. doi: 10.1016/j.neuroscience.2004.10.006. [DOI] [PubMed] [Google Scholar]
- Hunter BE, de Fiebre CM, Papke RL, Kem WR, Meyer EM. A novel nicotinic agonist facilitates induction of long-term potentiation in the rat hippocampus. Neurosci Lett. 1994;168:130–134. doi: 10.1016/0304-3940(94)90433-2. [DOI] [PubMed] [Google Scholar]
- Klein J, Koppen A, Loffelholz K. Uptake and storage of choline by rat brain: influence of dietary choline supplementation. J Neurochem. 1991;57:370–375. doi: 10.1111/j.1471-4159.1991.tb03762.x. [DOI] [PubMed] [Google Scholar]
- Klein J, Koppen A, Loffelholz K. Regulation of free choline in rat brain: dietary and pharmacological manipulations. Neurochem Int. 1998;32:479–485. doi: 10.1016/s0197-0186(97)00127-7. [DOI] [PubMed] [Google Scholar]
- Kuhar MJ, Murrin LC. Sodium-dependent, high affinity choline uptake. J Neurochem. 1978;30:15–21. doi: 10.1111/j.1471-4159.1978.tb07029.x. [DOI] [PubMed] [Google Scholar]
- Leonard S, Breese C, Adams C, Benhammou K, Gault J, Stevens K, Lee M, Adler L, Olincy A, Ross R, Freedman R. Smoking and schizophrenia: abnormal nicotinic receptor expression. Eur J Pharmacol. 2000;393:237–242. doi: 10.1016/s0014-2999(00)00035-2. [DOI] [PubMed] [Google Scholar]
- Levin ED. Nicotinic receptor subtypes and cognitive function. J Neurobiol. 2002;53:633–640. doi: 10.1002/neu.10151. [DOI] [PubMed] [Google Scholar]
- Levin ED, Bettegowda C, Blosser J, Gordon J. AR- R17779, and α7 nicotinic agonist, improves learning and memory in rats. Behav Pharmacol. 1999;10:675–680. doi: 10.1097/00008877-199911000-00014. [DOI] [PubMed] [Google Scholar]
- Li MD, Kane JK, Konu O. Nicotine, body weight and potential implications in the treatment of obesity. Curr Top Med Chem. 2003;3:899–919. doi: 10.2174/1568026033452203. [DOI] [PubMed] [Google Scholar]
- Liu RH, Mizuta M, Matsukura S. The expression and functional role of nicotinic acetylcholine receptors in rat adipocytes. J Pharmacol Exp Ther. 2004;310:52–58. doi: 10.1124/jpet.103.065037. [DOI] [PubMed] [Google Scholar]
- Lockman PR, Allen DD. The transport of choline. Drug Dev Ind Pharm. 2002;28:749–771. doi: 10.1081/ddc-120005622. [DOI] [PubMed] [Google Scholar]
- Mandelzys A, De Koninck P, Cooper E. Agonist and toxin sensitivities of ACh-evoked currents on neurons expressing multiple nicotinic ACh receptor subunits. J Neurophysiol. 1995;74:1212–1221. doi: 10.1152/jn.1995.74.3.1212. [DOI] [PubMed] [Google Scholar]
- Marks MJ, Burch JB, Collins AC. Effects of chronic nicotine infusion on tolerance development and nicotinic receptors. J Pharmacol Exp Ther. 1983;226:817–825. [PubMed] [Google Scholar]
- Marks MJ, Stitzel JA, Collins AC. Time course study of the effects of chronic nicotine infusion on drug response and brain receptors. J Pharmacol Exp Ther. 1985;235:619–628. [PubMed] [Google Scholar]
- Martin LF, Kem WR, Freedman R. Α-7 nicotinic receptor agonists: potential new candidates for the treatment of schizophrenia. Psychopharmacology (Berl) 2004;174:54–64. doi: 10.1007/s00213-003-1750-1. [DOI] [PubMed] [Google Scholar]
- Matsuzawa Y. Adipocytokines and metabolic syndrome. Semin Vasc Med. 2005;5:34–39. doi: 10.1055/s-2005-871744. [DOI] [PubMed] [Google Scholar]
- Meyer EM, Tay ET, Papke RL, Meyers C, Huang GL, de Fiebre CM. 3-[2,4-Dimethoxybenzylidene]anabaseine (DMXB) selectively activates rat α7 receptors and improves memory-related behaviors in a mecamylamine-sensitive manner. Brain Res. 1997;768:49–56. doi: 10.1016/s0006-8993(97)00536-2. [DOI] [PubMed] [Google Scholar]
- Morley BJ, Fleck DL. A time course and dose-response study of the regulation of brain nicotinic receptors by dietary choline. Brain Res. 1987;421:21–29. doi: 10.1016/0006-8993(87)91270-4. [DOI] [PubMed] [Google Scholar]
- Morley BJ, Garner LL. Increases in the concentration of brain α-bungarotoxin binding sites induced by dietary choline are age-dependent. Brain Res. 1986;378:315–319. doi: 10.1016/0006-8993(86)90934-0. [DOI] [PubMed] [Google Scholar]
- Morley BJ, Garner LL. AF64A depletes hippocampal high-affinity choline uptake but does not alter the density of α-bungarotoxin binding sites or modify the effect of exogenous choline. Brain Res. 1990;519:1–5. doi: 10.1016/0006-8993(90)90053-e. [DOI] [PubMed] [Google Scholar]
- Morley BJ, Robinson GR, Brown GB, Kemp GE, Bradley RJ. Effects of dietary choline on nicotinic acetylcholine receptors in brain. Nature. 1977;266:848–850. doi: 10.1038/266848a0. [DOI] [PubMed] [Google Scholar]
- Pauly JR, Marks MJ, Gross SD, Collins AC. An autoradiographic analysis of cholinergic receptors in mouse brain after chronic nicotine treatment. J Pharmacol Exp Ther. 1991;258:1127–1136. [PubMed] [Google Scholar]
- Pereira EF, Hilmas C, Santos MD, Alkondon M, Maelicke A, Albuquerque EX. Unconventional ligands and modulators of nicotinic receptors. J Neurobiol. 2002;53:479–500. doi: 10.1002/neu.10146. [DOI] [PubMed] [Google Scholar]
- Perry DC, Kellar KJ. [3H]epibatidine labels nicotinic receptors in rat brain: an autoradiographic study. J Pharmacol Exp Ther. 1995;275:1030–1034. [PubMed] [Google Scholar]
- Sgard F, Charpantier E, Bertrand S, Walker N, Caput D, Graham D, Bertrand D, Besnard F. A novel human nicotinic receptor subunit, α10, that confers functionality to the α9-subunit. Mol Pharmacol. 2002;61:150–159. doi: 10.1124/mol.61.1.150. [DOI] [PubMed] [Google Scholar]
- Simon JR, Atweh S, Kuhar MJ. Sodium-dependent high affinity choline uptake: a regulatory step in the synthesis of acetylcholine. J Neurochem. 1976;26:909–922. doi: 10.1111/j.1471-4159.1976.tb06472.x. [DOI] [PubMed] [Google Scholar]
- Sparks JA, Pauly JR. Effects of continuous oral nicotine administration on brain nicotinic receptors andresponsiveness to nicotine in C57Bl/6 mice. Psychopharmacology (Berl) 1999;141:145–153. doi: 10.1007/s002130050818. [DOI] [PubMed] [Google Scholar]
- Trommer BA, Schmidt DE, Wecker L. Exogenous choline enhances the synthesis of acetylcholine only under conditions of increased cholinergic neuronal activity. J Neurochem. 1982;39:1704–1709. doi: 10.1111/j.1471-4159.1982.tb08006.x. [DOI] [PubMed] [Google Scholar]
- Uteshev VV, Meyer EM, Papke RL. Regulation of neuronal function by choline and 4OH-GTS-21 through α7 nicotinic receptors. J Neurophysiol. 2003;89:1797–1806. doi: 10.1152/jn.00943.2002. [DOI] [PubMed] [Google Scholar]
- Verbitsky M, Rothlin CV, Katz E, Elgoyhen AB. Mixed nicotinic-muscarinic properties of the α9 nicotinic cholinergic receptor. Neuropharmacology. 2000;39:2515–2524. doi: 10.1016/s0028-3908(00)00124-6. [DOI] [PubMed] [Google Scholar]
- Wecker L. Neurochemical effects of choline supplementation. Can J Physiol Pharmacol. 1986;64:329–333. doi: 10.1139/y86-054. [DOI] [PubMed] [Google Scholar]
- Wecker L. Dietary choline: a limiting factor for the synthesis of acetylcholine by the brain. Adv Neurol. 1990;51:139–145. [PubMed] [Google Scholar]
- Wecker L, Dettbarn WD. Relationship between choline availability and acetylcholine synthesis in discrete regions of rat brain. J Neurochem. 1979;32:961–967. doi: 10.1111/j.1471-4159.1979.tb04581.x. [DOI] [PubMed] [Google Scholar]
- Wecker L, Schmidt DE. Central cholinergic function: relationship to choline administration. Life Sci. 1979;25:375–384. doi: 10.1016/0024-3205(79)90269-8. [DOI] [PubMed] [Google Scholar]
- Wecker L, Schmidt DE. Neuropharmacological consequences of choline administration. Brain Res. 1980;184:234–238. doi: 10.1016/0006-8993(80)90605-8. [DOI] [PubMed] [Google Scholar]
- Wecker L, Trommer BA. Effects of chronic (dietary) choline availability on the transport of choline across the blood-brain barrier. J Neurochem. 1984;43:1762–1765. doi: 10.1111/j.1471-4159.1984.tb06107.x. [DOI] [PubMed] [Google Scholar]
- Zeisel SH. Nutritional importance of choline for brain development. J Am Coll Nutr. 2004;23:621S–626S. doi: 10.1080/07315724.2004.10719433. [DOI] [PubMed] [Google Scholar]
- Zwart R, Vijverberg HP. Potentiation and inhibition of neuronal α4beta4 nicotinic acetylcholine receptors by choline. Eur J Pharmacol. 2000;393:209–214. doi: 10.1016/s0014-2999(00)00002-9. [DOI] [PubMed] [Google Scholar]


