Abstract
Divalent cations are important in the folding and stabilization of complex RNA structures. The adenine-sensing riboswitch controls the expression of mRNAs for proteins involved in purine metabolism by directly sensing intracellular adenine levels. Adenine binds with high affinity and specificity to the ligand binding or aptamer domain of the adenine-sensing riboswitch. The X-ray structure of this domain in complex with adenine revealed an intricate RNA-fold consisting of a three-helix junction stabilized by long-range base-pairing interactions and identified five binding sites for hexahydrated Mg2+-ions. Furthermore, a role for Mg2+-ions in the ligand-induced folding of this RNA was suggested. Here, we describe the interaction of divalent cations with the RNA–adenine complex in solution as studied by high-resolution NMR spectroscopy. Paramagnetic line broadening, chemical shift mapping and intermolecular nuclear Overhauser effects (NOEs) indicate the presence of at least three binding sites for divalent cations. Two of them are similar to those in the X-ray structure. The third site, which is important for the folding of this RNA, has not been observed previously. The ligand-free state of the RNA is conformationally heterogeneous and contains base-pairing patterns detrimental to ligand binding in the absence of Mg2+, but becomes partially pre-organized for ligand binding in the presence of Mg2+. Compared to the highly similar guanine-sensing riboswitch, the folding pathway for the adenine-sensing riboswitch aptamer domain is more complex and the influence of Mg2+ is more pronounced.
INTRODUCTION
RNAs are capable of carrying out a multitude of diverse biological functions. Many biologically active RNAs have to adopt intricate 3D structures that rival protein structures in their complexity to be functional in a cellular environment.
Folding of RNA chains into compact globular 3D structures represents a problem. Due to the polyanionic character of the RNA phosphodiester backbone, it inevitably brings negative charges in close proximity to each other. This results in highly unfavorable electrostatic interactions that are anisotropically distributed in the folded form of the RNA. Therefore, divalent metal ions in general and Mg2+ with its favorable charge/size ratio in particular (1) play an important role in RNA folding. Mg2+-ions not only stabilize the final structure through either direct coordination (inner sphere contact) with negatively charged groups of the RNA or in a water-mediated interaction (outer sphere contact) with the hexahydrated ion (Mg(H2O)62+). They also influence the rate of folding, stabilize folding intermediates or destabilize alternative conformations (2).
Riboswitches control the expression of a significant number of bacterial genes by binding with high affinity and specificity to small metabolite molecules. To achieve the binding specificity and affinity required for their function, the ligand binding or aptamer domains of these RNA elements must fold into intricate tertiary structures. This is illustrated in a number of X-ray structures of aptamer domain/ligand complexes of different riboswitches (3). In many cases, the X-ray structures revealed the presence of defined Mg2+-binding sites.
For instance for the thiamine pyrophosphate (TPP)-sensing riboswitch it was shown that Mg2+ binding is absolutely required for TPP binding (4) and cannot be replaced by monovalent ions or Ca2+ (5). The X-ray structure of the thiC TPP-sensing riboswitch from Arabidopsis thaliana bound to TPP reveals that one Mg2+-ion chelates oxygen atoms in the pyrophosphate moiety of TPP and links them to the RNA whereas there are two bridging Mg2+-ions in the X-ray structure of the thiM TPP-sensing riboswitch from Escherichia coli bound to TPP. The presence of bridging Mg2+-ions in the X-ray structures is consistent with the strict Mg2+ requirements for TPP binding to the TPP-sensing riboswitch (6–8). In contrast, the GlmS riboswitch—a ribozyme that undergoes a self-cleavage reaction in the presence of glucosamine-6-phosphate (GlcN6P)—does not strictly require Mg2+ for the self-cleavage reaction. Here, Mg2+ can be substituted by either other divalent metal ions or Co(NH3)63+ and to some extend by high concentrations of monovalent ions (9) indicating that the metal ion is not involved in catalysis but only in electrostatic stabilization of the structure consistent with the X-ray structure of this complex (10).
The purine-sensing riboswitches are among the smallest riboswitches found so far. All purine riboswitches fold into a three-way junction (Figure 1A) where central structural elements such as the ligand-binding core region and the loops L2 and L3 that cap helices II and III, respectively, show a very high degree of sequence conservation. However, despite the sequence conservation and similarity in structure, the adenine-sensing riboswitch binds adenine with high specificity while the guanine-sensing riboswitch binds guanine. This specificity is mediated by a single nucleotide in the ligand-binding core region which is a cytidin in the guanine-sensing riboswitch and a uridine in the adenine-sensing riboswitch (11,12). It was shown that the purine ligand is bound to this specific nucleotide in the core region by forming an intermolecular Watson–Crick base pair (13–15).
Figure 1.
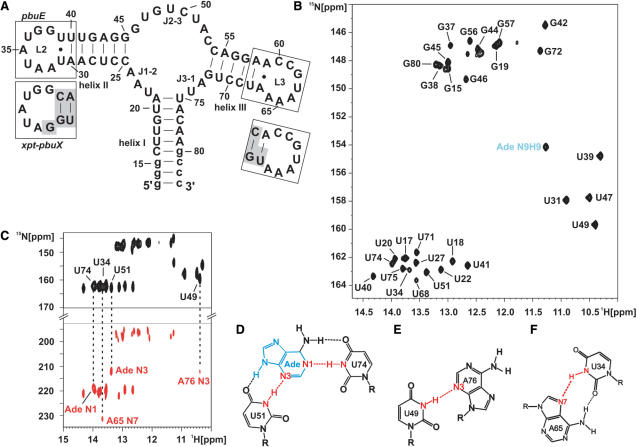
Adenine binding the adenine-sensing riboswitch and complete imino resonance assignment. (A) Secondary structure of the aptamer domain of the pbuE adenine-sensing riboswitch from B. subtilis where loops L2 and L3 are boxed. The sequence of L2 and L3 of the xpt–pbuX guanine-sensing riboswitch from B. subtilis is shown below in separate boxes. Nucleotides that are different in the guanine-sensing riboswitch compared to the adenine-sensing riboswitch are shaded in gray. (B) Imino region of a 1H,15N-HSQC spectrum of the aptamer domain of the adenine-sensing riboswitch RNA in complex with adenine and complete imino resonance assignment in the absence of Mg2+ at 10°C. The signal of the H9N9 imino group of adenine is labeled in blue. (C) HNN-COSY experiment of the RNA–adenine complex in the absence of Mg2+ at 10°C. The correlation from the hydrogen bond donor (N-H, black resonances) to the hydrogen bond acceptor (N, red resonances) is indicated by dashed lines for RNA–adenine intermolecular hydrogen bonds and for selected tertiary interactions. Schematic drawing of the intermolecular base triple (D) involving adenine recognition by U74 and U51 from the adenine-sensing riboswitch, (E) the interaction between U49 and A76, and (F) the long-range reversed-Hoogsteen A65:U34 base pair. All hydrogen bonds are indicated by dashed lines. Hydrogen bonds including the hydrogen bond donor and the hydrogen bond acceptor which are detected and annotated in (C) are shown in red. The adenine ligand is shown in blue.
The importance of Mg2+-ions for ligand binding to these riboswitches is less clear. Thermodynamic studies of ligand binding supported a requirement for the presence of Mg2+ in the case of hypoxanthine binding to the guanine-sensing riboswitch (16) and FRET studies of the pbuE adenine-sensing riboswitch from B. subtilis suggested an important role for Mg2+ in ligand binding (17). Specifically, Mg2+ was required for the formation of the long-range base-pairing interactions between nucleotides in loop 2 and 3. Furthermore, the X-ray structure of the adenine-sensing riboswitch bound to adenine revealed five well-defined Mg2+-binding sites (14) whereas 11 bound Co(NH3)63+-ions were found in the X-ray structure of the guanine-sensing riboswitch in complex with hypoxanthine (13). In contrast, NMR studies found ligand binding to be independent of the presence of Mg2+ for both the adenine and the guanine-sensing riboswitch (14,15). In addition, in the xpt–pbuX guanine-sensing riboswitch from B. subtilis the loop–loop interaction between loops 2 and 3 is preformed in the free form of the riboswitch and becomes strengthened in the presence of Mg2+ but is stable enough to exist even outside the context of the riboswitch (18). Given the high degree of sequence identity between the guanine- and adenine-sensing riboswitch (Figure 1A) the requirement for Mg2+ for the formation of the loop–loop interactions in the adenine-sensing riboswitch as reported in the FRET studies (17) is surprising.
Specifically, with respect to the loop sequences the guanine- and the adenine–sensing riboswitch differ only at the position 32 (Figure 1A) in loop 2. At position 32, the guanine-sensing riboswitch bears a guanosine whereas it is an adenosine in the adenine-sensing riboswitch. The X-ray structures of both riboswitches show that the purine at position 32 stacks between the apical closing base pair of helix II and A33 without making any stabilizing hydrogen bonds and that the residue at position 62 loops out into the solvent (13,14). In addition, there are differences in the closing base pairs of helices II and III in the two riboswitches. The base pair adjacent to loop 2 is an asymmetric U:U base pair in the adenine-sensing riboswitch and a Watson–Crick G:C base pair in the guanine-sensing riboswitch. The closing base pair of helix III is a non-canonical A:A base pair in the adenine-sensing riboswitch but a Watson–Crick A:U base pair in the guanine-sensing riboswitch.
The reported discrepancies in the behavior of the closely related adenine- and guanine-sensing riboswitches prompted us to investigate the binding of divalent metal ions to the pbuE adenine-sensing riboswitch and their influence on ligand-induced RNA folding in solution by high-resolution NMR methods in this study.
MATERIALS AND METHODS
Purine ligand synthesis
15N,13C-labeled adenine was synthesized as described in (15). The ligand concentration was determined by UV absorbance at 261 nm using an extinction coefficient ɛ = 13 400 mol−1 cm−1 (19).
RNA preparation
Labeled RNAs were prepared by in vitro transcription with T7 RNA polymerase from linearized plasmid DNA templates using 15N- and 15N,13C-labeled nucleotides purchased from Silantes (Munich, Germany). The labeled RNA was purified as described (20) and concentrations were determined by UV absorbance at 260 nm. The RNA was folded into a homogenous monomeric conformation through denaturation at 363 K for 5 min and rapid cooling to 273 K by 10-fold dilution with ice-cold H2O. Folding into a homogenous monomeric form was verified by native PAGE (data not shown). The RNA was subsequently exchanged into NMR buffer (25 mM KPO4, pH 6.2, 50 mM KCl) using Centricon-10 microconcentrators.
NMR spectroscopy
All NMR experiments were recorded on Bruker NMR spectrometers operating at 600, 700, 800 and 900 MHz equipped with a 5 mm HCN cryogenic probes and z-axis gradients. The spectra were recorded and processed using Bruker X-WIN NMR3.5 and TopSpin2.0 and were analyzed using XEASY (21). All NMR spectra were recorded at a temperature of 283 K in 90% H2O/10%D2O in NMR buffer (25 mM KPO4, pH 6.2, 50 mM KCl). Water suppression was achieved using the WATERGATE water suppression scheme (22) including water flip back pulses (23). 1H,15N-HSQC, 2JHN-1H,15N-HSQC (24) and 2D- or 15N-edited 3D-NOESY experiments were performed using standard pulse sequences (25). The 2hJNN-HNN-COSY experiments (26) were performed as described previously (15).
Cation titrations and Mg2+-binding affinity studies
Titrations of the RNA with Mg2+, Mn2+ and Co(NH3)63+ were performed directly in the NMR tube by adding aliquots from 125 mM or 500 mM MgCl2, 1 mM MnCl2 or 100 mM Co(NH3)6Cl3 stock solutions. The equilibrium binding constant for Mg2+ to the RNA was measured by the chemical shift perturbation (CSP) of the imino group of G37. CSPs were calculated using the formula 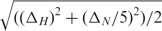 and were correlated with the ratio of [Mg2+]/[RNA]. Non-linear regression using SigmaPlot9.0 was performed using the following fitting function:
and were correlated with the ratio of [Mg2+]/[RNA]. Non-linear regression using SigmaPlot9.0 was performed using the following fitting function:
where f(x) is the CSP at its corresponding ratio x = [Mg2+]/[RNA]. a and b are the fitted parameters where a is the ratio of the dissociation constant (KD) to [RNA] and b is the CSP for infinite Mg2+ concentrations (27).
RESULTS
Adenine binding to the adenine-sensing riboswitch and NMR assignment in solution
The imino region of the 1H,15N-HSQC of the aptamer domain of the adenine-sensing riboswitch in the absence of Mg2+ shows well-resolved and sharp signals in the presence of 1.2 equivalents adenine (Figure 1B). The presence of a signal for the N9H9 imino group of the adenine ligand, which is undetectable in its free form indicates the formation of a high-affinity RNA–ligand complex in the absence of Mg2+. By analyzing 2D-1H,1H-NOESY and 3D-1H,1H,15N-NOESY-HSQC experiments (data not shown) the imino resonances of the RNA–adenine complex could be assigned. The sequence of the aptamer domain of the riboswitch RNA contains 16 guanosine and 19 uridine nucleotides (Figure 1A). For 12 of the guanosine and 17 of the uridine nucleotides imino resonances are detectable in the 1H,15N-HSQC spectrum at 10°C. Imino resonances that could not be assigned correspond to residues G13, G14, U36, G48, G62 and U63. Imino resonances of terminal nucleotides such as G13 and G14 in RNA helices are often not detectable in NMR spectra due to their reduced stability and the resulting fast exchange of the imino proton with the bulk water solvent. The imino groups of residues U36, G48, G62 and U63 are solvent exposed according to the X-ray structure of the adenine–RNA complex (14) which also renders them undetectable due to fast exchange with the bulk water solvent.
The binding mode of adenine to the adenine-sensing riboswitch RNA and tertiary structure characteristics of the adenine–RNA complex were further elucidated by an HNN-COSY experiment (26) which detects hydrogen bonds of the type N-H…N. In the HNN-COSY spectrum of the adenine-sensing riboswitch in complex with adenine (Figure 1C), the imino groups of U74 and of U51 show correlations with the N1 and the N3 nitrogen of the bound adenine, respectively. U74, U51 and adenine form an intermolecular base triple (Figure 1D) as observed previously for a mutant of the adenine-sensing riboswitch aptamer domain (15) and in the X-ray structure of the adenine-sensing riboswitch RNA in complex with adenine crystallized in the presence of 200 mM Mg2+ (14). Furthermore, several non-canonical base pairings and tertiary interactions can be identified in the HNN-COSY or the NOESY spectra. In the HNN-COSY spectrum, the imino group of U49 is correlated to an adenosine N3 as identified by its chemical shift which is consistent with the hydrogen bond between the U49 imino group and the N3 of A76 in the X-ray structure (Figure 1E). The imino group of U34 shows a correlation to the N7 nitrogen of an adenosine as expected for the reversed Hoogsteen A65:U34 base pair (28) (Figure 1F) observed in the X-ray structure. Nuclear Overhauser effect (NOE) data and the chemical shifts of the C2 and C4 carbonyl groups of U31 and U39 indicate that these nucleotides form an asymmetric U:U base pair (data not shown) (29) also in agreement with the X-ray structure.
In summary, the number of observed imino proton signals, the obtained assignments, the observed intra- and intermolecular NOEs, the binding mode of adenine to the RNA as deduced from the HNN-COSY experiment as well as the observed tertiary non-canonical base-pairing interactions strongly indicate that the teriary structure of the adenine-sensing riboswitch aptamer domain bound to adenine in solution in the absence of Mg2+-ions is virtually identical to the corresponding X-ray structure obtained in the presence of high concentrations of Mg2+. Therefore, the presence of Mg2+-ions is not a prerequisite for either ligand binding or for the correct folding of this RNA–ligand complex.
Divalent cation-binding sites in the adenine–RNA complex in solution
We applied three different but complementary techniques to characterize divalent metal binding to the adenine–RNA complex and to analyze possible structural effects in solution by NMR spectroscopy. First, we observed CSPs in Mg2+-titration experiments caused by the interaction of the metal ion with the RNA–ligand complex. Second, we used the Mn2+-ion as a paramagnetic Mg2+-analog to observe paramagnetic line broadening for NMR signals in close proximity to putative divalent metal-binding sites. Third, we utilized the [Co(NH3)6]3+-ion which is considered to be an analog of an hexahydrated Mg2+-ion in CSP and NOE experiments to identify imino groups spatially close to putative divalent metal-binding sites.
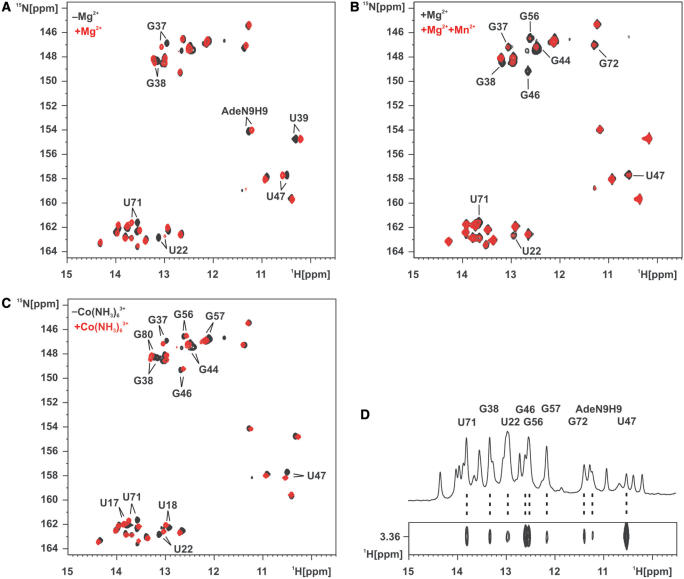
We titrated the adenine-sensing riboswitch RNA–adenine complex with Mg2+ and compared the imino group region of the 1H,15N-HSQC spectrum (Figure 2A). The number of the imino resonances remains unchanged and no large chemical shift changes are observed. This indicates that the overall structure of the complex is not perturbed upon Mg2+ binding and that no new structural elements are formed. However, while some imino resonances show no or only small CSP other resonances showed significant CSP upon addition of 5 mM Mg2+ to the adenine–RNA complex. Such significant CSPs were observed for U22, G37, G38, U39, U47, U71 and the adenine N9H9 imino group. When these imino groups are mapped on the X-ray structure of the complex, four well-defined areas are visible which are affected by Mg2+ binding (Figure 3A). G37 and G38 are located in loop L2 and are involved in the loop–loop interaction forming long-range Watson–Crick base pairs with C61 and C60 in L3, respectively. U39 is located at the apical tip of helix II, U71 is situated in the lower half of helix III pointing to the core region whereas U22, U47 and the adenine N9H9 imino group are all at the interface of the RNA and the bound ligand.
Figure 2.
Divalent metal cation binding to the adenine-sensing riboswitch RNA in complex with adenine. (A) Overlay of the imino region of a 1H,15N-HSQC spectrum of the aptamer domain of the adenine-sensing riboswitch RNA in complex with adenine in the absence of Mg2+ (black) and in the presence of 5 mM Mg2+ (red). Imino resonances that experience chemical shift perturbation >0.05 p.p.m. in 1H or >0.4 p.p.m. in 15N upon addition of 5 mM Mg2+ are labeled. (B) Overlay of the imino region of a 1H,15N-HSQC spectrum of the aptamer domain of the adenine-sensing riboswitch RNA in complex with adenine and 5 mM Mg2+ in the absence of Mn2+ (black) and in the presence of 15 µM Mn2+. Imino resonances that experience line broadening by the paramagnetic Mn2+ are labeled. (C) Overlay of the imino region of a 1H,15N-HSQC spectrum of the aptamer domain of the adenine-sensing riboswitch RNA in complex with adenine in the absence of Co(NH3)63+ (black) and in the presence of 3 mM Co(NH3)63+ (red). Imino resonances that experience chemical shift perturbation >0.05 p.p.m. in 1H or >0.4 p.p.m. in 15N upon addition of 3 mM Co(NH3)63+ are labeled. (D) Imino region of a 1D-1H spectrum of the aptamer domain of the adenine-sensing riboswitch RNA in complex with adenine in the presence of 5 mM Co(NH3)63+ (top) and a section of a 2D-1H,1H-NOESY spectrum showing intermolecular NOEs between the protons of the bulk Co(NH3)63+ and the RNA as well as the adenine ligand imino groups (bottom). RNA and adenine ligand imino groups that show intermolecular contacts to the Co(NH3)63+ protons are labeled.
Figure 3.
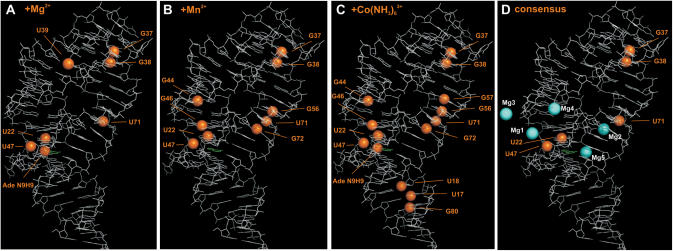
Divalent metal cation-binding sites in solution mapped on the X-ray structure of the closely related add adenine-sensing riboswitch from V. vulnificus in complex with adenine. (A) Imino groups showing chemical shift perturbation upon Mg2+ addition in Figure 2A are represented as orange spheres. There are four regions affected that are located in the ligand-binding core, in the loop–loop interaction, at the apical tip of helix II, and in the center of helix III. (B) Imino groups showing line broadening by paramagnetic Mn2+ in Figure 2B are represented as orange spheres. There are four regions affected that are located in the ligand-binding core, in the loop–loop interaction, and in the center of helix II and helix III. (C) Imino groups showing chemical shift perturbation upon Co(NH3)63+ addition in Figure 2C and imino groups showing intermolecular NOE contacts to Co(NH3)63+ in Figure 2D are represented as orange spheres. There are five regions affected that are located in the ligand-binding core, the loop–loop interaction, and in the center of helix I, helix II and helix III. (D) Consensus of imino groups that exhibit all, chemical shift perturbation upon Mg2+ addition, line broadening by paramagnetic Mn2+, chemical shift perturbation by as well as intermolecular contacts to Co(NH3)63+ are represented as orange spheres. Mg2+-ions found in the X-ray structure are shown as cyan spheres and labeled as in (14). Mg3 is involved in crystal packing. The RNA is drawn in white lines; the adenine ligand is drawn in green lines.
Due to enhanced spin relaxation binding of the paramagnetic Mn2+-ion leads to line broadening of resonances in close proximity to the ion with a distance dependence of 1/r6 (30). Only micromolar concentrations of Mn2+ are required to induce the effect (31–34). In the RNA–adenine complex in the presence of 5 mM Mg2+ imino resonances that show significant line broadening upon titration with 15 µM Mn2+ are those of U22, G37, G38, G44, G46, U47, G56, U71 and G72 (Figure 2B). Except for U39 and the N9H9 group of the bound ligand all the resonances that show significant CSP in the Mg2+ -titrations also show paramagnetic line broadening induced by the presence of Mn2+. Of the four resonances that show line broadening but no CSP G56 and G72 are close in space to U71 that is affected by both ions. Similarly, G46 where only line broadening is observed is adjacent to U47 sensitive to both ions. The residues showing Mn2+-induced line broadening are clustered in four distinct regions when mapped onto the structure (Figure 3B). Three of those regions were previously identified in the Mg2+ -titrations. These regions are close to G37 and G38 involved in the loop–loop interaction, the center of helix III (U71, G56 and G72) and in the ligand-binding core (U22, G46 and U47), respectively. G44 located in the lower half of helix II does not show CSP upon titration with Mg2+ and is not close in space to other residues that are affected by the presence of either Mg2+ or Mn2+.
Co(NH3)63+ has a geometry similar to the hexahydrated Mg(H2O)62+-ion. However, the ligand shell of amino groups is inert to exchange and in contrast to Mg2+ cannot form inner sphere contacts (35). In order to complement our magnesium-binding studies we used the adenine–RNA complex and performed Co(NH3)63+ -titration experiments. As for the Mg2+ -titration of the complex, the overall conformation of the RNA–ligand complex remained unperturbed and no additional structural elements are formed as indicated by the constant number of observable imino resonances. In a 1H,15N-HSQC spectrum the imino resonances of U17, U18, U22, G37, G38, G44, G46, U47, G56, G57, U71 and G80 showed significant CSP upon addition of 3 mM Co(NH3)63+ (Figure 2C).
The binding site of Co(NH3)63+ to the RNA–adenine complex cannot only be studied by CSP but also by intermolecular NOEs between the protons of the NH3-groups of Co(NH3)63+ and RNA protons in spatial proximity. Co(NH3)63+ in contrast to Mg(H2O)62+ is suitable for such NMR experiments since the hydrogens of the ammonia ligands of Co(NH3)63+ exchange slowly with the bulk solvent water and are therefore detectable by NMR. We recorded a 2D-1H,1H-NOESY spectrum for the adenine–RNA complex in the presence of 5 mM Co(NH3)63+ in which we detected intermolecular NOEs from the protons of Co(NH3)63+ to imino group protons of U22, G38, G46, G47, G56, G57, U71, G72 and the N9H9 imino group of the bound adenine (Figure 2D). The residues showing either significant CSP upon the addition of 3 mM Co(NH3)63+ or having an intermolecular NOE in the presence of 5 mM Co(NH3)63+ include all the residues affected by paramagnetic line broadening in the presence of Mn2+-ions. G57 which only shows NOEs and CSP to Co(NH3)63+ but no Mn2+-induced line broadening is located in the center of helix III close to G56, U71 and G72 that are affected by both the presence of Co(NH3)63+ and Mn2+. Similarly, the N9H9 group of the bound adenine which displays an NOE to Co(NH3)63+ and CSP in the presence of Mg2+ but no line broadening in the presence of Mn2+ is in the ligand-binding core in close proximity to U22, G46 and G47. The imino resonances of U17, U18 and G80 in helix I only showed CSP in the presence of Co(NH3)63+ but not in the presence of Mg2+ and no line broadening in the presence of Mn2+-ions.
Mapping of the imino groups experiencing CSP and showing NOEs in the presence Co(NH3)63+ on the X-ray structure reveals five distinct binding sites for Co(NH3)63+ to the adenine-sensing riboswitch RNA in complex with adenine (Figure 3C).
Three of these binding sites have been identified before in the titration experiments with Mg2+ and in the line-broadening experiments with Mn2+. These are located in the vicinity of G37 and G38 in the loop–loop interaction, the center of helix III (U71, G56 and G72) and in the ligand-binding core (U22, G46 and U47), respectively. This is further illustrated by mapping only those imino groups with resonances perturbed by the presence of all three Mg2+-, Mn2+- and Co(NH3)63+-ions on the X-ray structure (Figure 3D). One binding site located in the center of helix II is only identified in the line-broadening experiment with Mn2+ and by CSP in the presence of Co(NH3)63+-ions.
The X-ray structure shows five hexahydrated Mg2+-ions [Mg1 to Mg5, nomenclature of Mg atoms as in (14), Figure 3D] bound to the add adenine-sensing riboswitch in complex with adenine (14). One of these Mg2+-binding sites (Mg3) is involved in a crystal packing interaction (14). Mg1 in the X-ray structure binds to nucleotides in J2-3 and at the lower end of helix II close to U22 and U47. U22 and U47 have been identified in all four experiments as proximal to a divalent metal ion-binding site in solution. Mg2 in the X-ray structure is located close to U71 in helix II that has been identified as a consensus-binding site in solution. Divalent metal ion binding to the lower end of helix II in solution that would correspond to Mg4 in the X-ray structure was only evident in the line-broadening experiments with Mn2+ and in the titrations with Co(NH3)63+ (G44). Therefore, this Mg2+-ion might bind only weakly.
No divalent cation binding in solution could be detected in the vicinity of the positions for Mg3 where the binding site is formed by crystal packing interactions and Mg5 in the X-ray structure. On the other hand, no bound Mg2+ is observed in the X-ray structure in the vicinity of G37 and G38 in the loop–loop interaction where all the solution experiments unambiguously indicate the presence of a divalent cation-binding site. Interestingly, Mg2+ binding at this site appears to be important for the formation of the loop–loop interaction in the pbuE adenine-sensing riboswitch.
The free form of the adenine-sensing riboswitch RNA is conformationally disordered and heterogeneous
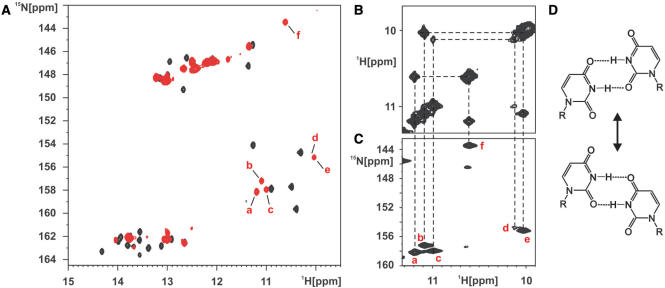
The 1H,15N-HSQC spectrum of the free form of the adenine-sensing riboswitch RNA in the absence of metal ions shows imino resonances that are broad and that vary largely in intensity (Figure 4A). In comparison with the adenine bound form, the number of signals is significantly smaller in the free state of the RNA. The signals for U20, U22, G45, G46, U47, U49, U51, U71, G72, U74, U75 located in the ligand-binding core and at the ends of the helices facing the core region are completely absent. This indicates that there are no stabilizing hydrogen-bond interactions between nucleotides in the core region and the core region apparently is largely disordered. In addition, the signal for the imino group of G38 is absent and those of G37 and U34 are barely detectable. This indicates that the loop–loop interaction between loop 2 and loop 3 is strongly destabilized in the free form of the RNA. Only a minor part of the RNA apparently samples a conformation where the two loops interact in exchange with totally open conformations.
Figure 4.
The conformation of the ligand-free adenine-sensing riboswitch RNA in the absence of Mg2+-ions. (A) Overlay of the imino region of a 1H,15N-HSQC spectrum of the free (red) aptamer domain of the adenine-sensing riboswitch RNA in the absence of Mg2+ and in complex with adenine (black). Resonances of the five uridines (a–e) and one guanosine (f) imino groups involved in non-canonical base pairing in the free form of the RNA are labeled with red letters. (B and C) Section of the 2D-1H,1H-NOESY spectrum (B) of the free aptamer domain of the adenine-sensing riboswitch RNA and the corresponding region of the 1H,15N-HSQC spectrum (C). These spectra show the presence of two different U:U base pairs and one G:U base pair in the free form of the RNA. Imino group correlation in the 2D-1H,1H-NOESY spectrum (B) are indicate by dashed lines and their identity is revealed by dashed lines to the corresponding resonance in the 1H,15N-HSQC spectrum (C). (D) Two alternative conformations for an asymmetric U:U base pair.
However, besides the signals of imino groups belonging to nucleotides in stable Watson–Crick base pairs in the central regions of helices I, II and III, respectively, there are five uridine imino resonances and one guanosine imino resonance with chemical shifts indicating involvement in non-canonical base-pairing interactions. NOEs observed between these imino resonances (Figure 4B) in conjunction with the 1H,15N-HSQC-spectrum (Figure 4C) suggest the presence of two asymmetrical U:U base pairs and of one G:U wobble base pair in the free RNA. However, there is only one asymmetrical U:U base pair (U31:U39) observed in the adenine bound form of the RNA. In addition, there is no evidence for the presence of a stable G:U wobble base pair in the adenine bound RNA. The second U:U base pair and the G:U wobble base pair therefore represent an alternative base-pairing pattern unique to the free form of the RNA and have to dissociate in the process of ligand binding. The location of these alternative base pairs either in the loops 2 and 3 or the ligand-binding core cannot unambiguously be established due to the lack of NOEs to other imino protons. In addition, the presence of the second U:U base pair could be either due to an alternative conformation for the U:U base pair between U31 and U39 (Figure 4D) or due to an additional long-range U:U base-pairing interaction. In the first case, exchange cross peaks should be observable between the uridine imino resonances involved in the U:U base-pairing interactions. Unfortunately, both the proton and the 15N chemical shifts of the uridine imino resonances are very similar and such cross peaks would be obscured by the strong diagonal peaks in either 1H,1H-NOESY spectra and 15N-ZZ-exchange experiments (36). However, Mg2+-titration experiments of the free RNA suggest that the alternative G:U and U:U base pairs are located in the region of the loop–loop interaction and compete with the proper formation of the long-range base pairs between loops 2 and 3.
Mg2+-induced conformational changes in the free adenine-sensing riboswitch RNA
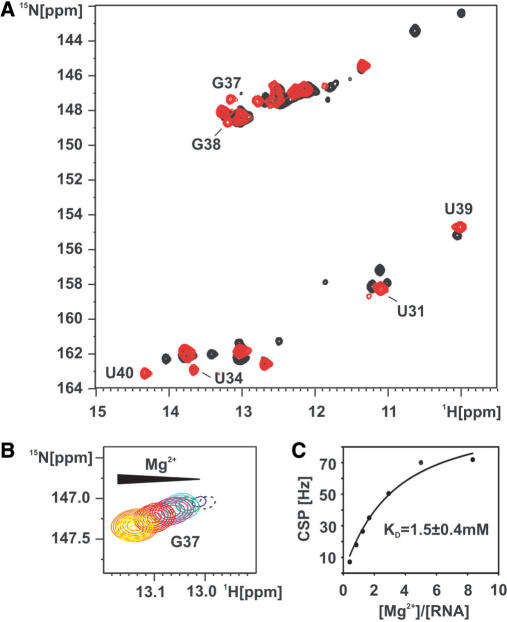
We titrated the free form of the adenine-sensing riboswitch RNA with Mg2+ and observed changes in the signals of the imino groups in a 1H,15N-HSQC spectrum. The overall number of imino residues decreases and the resonances reach equal intensities in the presence of 5 mM Mg2+ (Figure 5A). During the stepwise addition of Mg2+-ions a signal for the imino group of G38 becomes observable and signals for G37 and U34 strongly increase in intensity. This indicates that the presence of Mg2+ promotes the formation of the long-range base-pairing interactions between loops 2 and 3. The signals for G37 and G38 continuously shift upon increasing the Mg2+ concentration. This suggests that Mg2+ shifts the fast equilibrium between open conformations without a loop–loop interaction and closed conformations strongly toward a closed conformation with a stable interaction between loop 2 and loop 3.
Figure 5.
Mg2+ induces folding of the loop–loop interaction in the free form of the adenine-sensing riboswitch RNA. (A) Overlay of the imino region of a 1H,15N-HSQC spectrum of the free (black) and the Mg2+ bound form (red) of the aptamer domain of the adenine-sensing riboswitch RNA. G37 is barely detectable in the free form of the RNA but strongly increases in intensity upon addition of 5 mM Mg2+. (B) 1H,15N-HSQC spectrum of the imino group of G37 upon titration with Mg2+. Mg2+ titration steps are 0 mM (black), 0.25 mM (blue), 0.5 mM (green), 0.75 mM (cyan), 1 mM (purple) 1.75 mM (red), 3 mM (orange) and 5 mM (yellow). The resonance of the G37 imino group at 0mM Mg2+ is shown in dashed contours since it is plotted on a lower level compared to all other titration steps. (C) The chemical shift perturbation (CSP) of the G37 imino resonance is plotted against the ratio [Mg2+]/[RNA] and fitted by non-linear regression (black line). The derived binding constant (KD) of Mg2+ to G37 is 1.5 ± 0.4 mM.
At the same time the stepwise addition of Mg2+ causes the imino proton signals of the non-canonical G:U wobble base pair and one of the U:U base pairs to disappear whereas the imino protons of the other U:U base pair shift strongly towards chemical shifts similar to those of the adenine bound RNA in the presence of Mg2+. Therefore, the formation of the ‘native’ stable loop–loop interaction upon addition of Mg2+ directly leads to the dissociation of the alternative base pairs observed in the free RNA in the absence of Mg2+. Taken together, these observations indicate that the ensemble of rapidly interconverting conformations observed for the free form of the RNA in the absence of Mg2+ converges to a more homogeneous conformation where the loop–loop interaction is present and stable in the presence of Mg2+.
The stepwise CSP of the G37 imino group during the Mg2+ -titration was used to estimate the dissociation constant for Mg2+ binding to the area of the loop–loop interaction (Figure 5B). G37's imino resonance strongly increases in intensity and shifts subsequently by a total of 0.15 p.p.m. in 1H and 0.35 p.p.m. in 15N during the titration in agreement with a rapidly associating and dissociating complex of Mg2+ and RNA. Correlating the CSP for each titration point with the ratio [Mg2+]/[RNA] yielded a saturation curve revealing an equilibrium dissociation constant of 1.5 mM ± 0.4 mM for Mg2+ to the loop L2 region after non-linear regression (Figure 5C).
Interestingly, titrations of the free RNA with Co(NH3)63+-ions lead to virtually identical results with regard to the formation of the native loop–loop interaction and the destabilization of the alternative base-pairing patterns. Therefore, Mg2+ apparently induces the native folding of this RNA purely by using outer-shell coordination.
A mutant stabilizing the loop–loop interaction in the free form
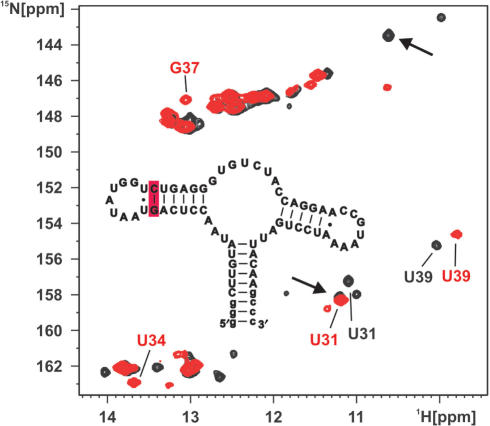
In order to probe the effect of the stability in helix II on the loop–loop formation properties in the free form of the pbuE adenine-sensing riboswitch RNA, we introduced a stabilizing mutation in helix II. In this A30G/U40C double mutant the A30:U40 base pair adjacent to the U31:U39 base pair is replaced by a more stable G:C base pair. In fact, the xpt–pbuX guanine-sensing riboswitch displays a U:A base pair at position 30–40 like the pbuE adenine-sensing riboswitch but in contrast the adjacent base pair facing the loop–loop interaction is a stable canonical G:C base pair in the case of the xpt–pbuX guanine-sensing riboswitch and not a weaker non-canonical U:U base pair as the case in the pbuE adenine-sensing riboswitch (Figure 1A and Figure 6 inset). The A30G/U40C double mutant binds to adenine in a manner identical to the wild-type RNA (15). The imino group 1H,15N-HSQC spectrum of the free form of the mutant shows significant differences compared to the one of the wild-type. In the mutant imino group resonances for G37 and U34 which are located in the loop L2 and form long-range base pairs with nucleotides in loop L3 are readily detectable (Figure 6) with chemical shifts similar to those in the adenine bound RNA. In addition, only one U:U base pair is detected which can be assigned as the U31:U39 base pair and no signals corresponding to a G:U wobble base pair can be found. These data show that the mutant RNA in contrast to the wild-type RNA can already form a stable native loop–loop interaction in the free form in the absence of Mg2+.
Figure 6.
A mutant stabilizing the loop–loop interaction in the free form of the adenine-sensing riboswitch RNA. Overlay of the imino region of a 1H,15N-HSQC spectrum of the free form of the wild-type adenine-sensing riboswitch RNA (black) and a A30G/U40C double mutant of the adenine-sensing riboswitch RNA (red) both in the absence of Mg2+. Signals for the imino groups of G37 and U34 are barely visible in the wild-type RNA but are readily detected in the mutant RNA. Only two signals for the uridine imino groups in an U:U base pair are observable in the mutant. In addition, the imino signals for the wobble G:U base pair (arrows) are absent in the mutant. Inset: Secondary structure of the A30G/U40C double mutant. The mutated base pair at the apical tip of helix II is shaded in red.
DISCUSSION
Divalent cation binding plays a prominent role for RNA folding and stabilization. Mg2+-ions in particular are often required for the proper folding of complex RNA structures giving rise to the notion of an Mg2+-ion-core (37) in highly structured globular RNAs in analogy to the hydrophobic core of protein structures. The X-ray structures of the aptamer domains of the closely related guanine- and adenine-sensing riboswitches are good examples for such intricate globular RNA structures. Not surprisingly, those structures revealed a number of well-defined binding sites for either hexahydrated Mg2+-ions or its structural analog the Co(NH3)63+-ion. Furthermore, an important role for Mg2+-ions for the folding and ligand binding of the adenine-sensing riboswitch was reported. On the other hand, our own NMR investigations of the guanine-sensing riboswitch despite revealing a number of well-defined divalent cation-binding sites in solution showed that Mg2+ binding was not required for ligand binding and that in the free state of the RNA important tertiary interactions were already pre-organized in a Mg2+-independent manner.
Here, we investigated the binding of divalent cations to the aptamer domain of the pbuE adenine-sensing riboswitch in solution and its importance for ligand binding and folding of this RNA. We found that although adenine binding to the aptamer domain is independent of the presence of divalent cations there are a number of well-defined binding sites for divalent cations on the adenine–RNA complex in solution. In contrast to the related guanine-sensing riboswitch, the free state of the adenine-sensing riboswitch is conformationally heterogeneous and displays alternative base-pairing patterns detrimental to ligand binding. The addition of Mg2+-ions induces the formation of a crucial tertiary interaction, the formation of base-pairing interactions between nucleotides in loops 2 and 3, and in turn destabilizes the alternative base pairs thereby pre-organizing the structure of the RNA for ligand binding.
Possible divalent cation-binding sites in solution were identified by using four different techniques: Mg2+-induced CSP, paramagnetic line broadening induced by Mn2+-ions, CSP induced by Co(NH3)63+-ions and intermolecular NOEs between Co(NH3)63+-ions and RNA protons. Despite differences in their inherent sensitivity these experiments yielded consistent results. Three distinct Mg2+-binding sites were identified in all four experiments. Apparently, in all three binding sites the ion binds through ‘outer shell’ coordination since Mg2+ titrations and Co(NH3)63+-binding experiments show similar effects. Two of those binding sites are also seen in the X-ray structure and were also found in solution for the structurally very similar xpt–pbuX guanine-sensing riboswitch. The third binding site identified in all experiments was not seen in the X-ray structure. It is located close to nucleotides involved in tertiary base-pairing interactions between the loops 2 and 3. This is consistent with FRET results showing that Mg2+ binding promotes the formation of this loop–loop interaction already in the absence of ligand (17,38) and with our own results that show Mg2+-dependent formation of the tertiary base-pairing interactions between nucleotides in these two loops. A divalent cation-binding site at this position was also identified in solution for the structurally highly similar guanine-sensing riboswitch (18). A fourth possible binding site was only identified in the line-broadening experiments with Mn2+ and the Co(NH3)63+-binding experiments. Interestingly, this binding site also corresponds to the position of a bound Mg2+-ion in the X-ray structure. Probably the affinity of this site is lower and the magnitude of the Mg2+-induced CSP is too small to identify this site in the Mg2+ experiments. Two Mg2+-binding sites found in the X-ray structure (Mg3 and Mg5) were not found in solution. One of those is created by crystal packing interactions (Mg3). In principle, the failure to detect the Mg2+-binding site corresponding to Mg5 (14) of the X-ray structure might be due to our exclusive use of guanosine and uridine imino groups as probes for metal-induced chemical shift changes. This would prevent the detection of metal ion binding in A, C-rich regions of the structure due to the lack of suitable probes. However, Mg5 is close in space to the imino groups of U74 and U75. Thus, our results suggest that this binding site is apparently not or only rarely occupied by a divalent cation in solution.
On the other hand, Co(NH3)63+-ions in solution seem to bind to a site in helix I in an area with a high density of uridine carbonyl groups where neither the X-ray structure nor the Mg2+- and Mn2+-titration experiments showed a binding site. Due to their higher charge density Co(NH3)63+-ions have an ∼10-fold higher affinity (39) than divalent ions and induce larger CSPs than divalent ions so it seems likely that in this experiment a very weak binding site was detected. The value of the Co(NH3)63+-ion as a structural mimic of hexahydrated Mg2+-ions has been questioned recently (40). Our results indicate that it is a useful probe as long as ion binding occurs through outer shell coordination but also that exclusive reliance on Co(NH3)63+-ions as a probe might lead to an overestimation of the amount of divalent cation binding.
Whereas Mg2+ binding does not influence the structure of the RNA–ligand complex it has a significant influence on the conformation of the free state of the adenine-sensing riboswitch. In contrast to our findings for the aptamer domain of the closely related guanine-sensing riboswitch in the absence of Mg2+ the free aptamer domain of the pbuE adenine-sensing riboswitch studied here does not show a pre-organized stable tertiary interaction between the loops 2 and 3. Instead, an alternative base-pairing pattern is observed that most likely involves loop nucleotides and competes with the formation of this loop–loop interaction and ultimately ligand binding. Mg2+ promotes the formation of this crucial tertiary interaction and directly induces the formation of base-pairing interactions between loop 2 and 3 in agreement with the FRET results reported by Lafontaine and co-workers and Micura and coworkers (17,38). Therefore, the small sequence differences in the closing base pairs of helix II and III and loop 2 and 3, respectively, between the guanine and the adenine-sensing riboswitch lead to significant differences in the conformation of the free state of the RNA and a different role for Mg2+ in the folding pathway (Figure 7). The stabilization of the loop–loop interaction through either Mg2+ binding or more stable base-pairing interactions in helix II as found in the majority of the guanine-sensing riboswitches (12,18) and the resulting pre-organization of the RNA fold might actually have important consequences for the kinetics of ligand binding (kon) which would most likely be faster in the presence of Mg2+ or the stabilizing base-pairing interactions. In turn, the ‘on’ rate of ligand binding is important for the proper function of a ‘kinetically’ controlled riboswitch such as the pbuE riboswitch (41). On the other hand, the base pairing is the same in helix II of the add riboswitch from Vibrio vulnificus which appears to be thermodynamically controlled (38). In fact, all the adenine-sensing riboswitches described so far (42) contain either at least one non-canonical base pair or two A:U base pairs as closing base pairs of loop 2. In contrast, two Watson–Crick base pairs with either one or even both being a G:C base pair are found at these positions in most of the guanine-sensing riboswitches. Therefore, it is tempting to speculate that the Mg2+ concentration might modulate the effectivity of riboswitch-mediated gene regulation in the case of the adenine-sensing riboswitches.
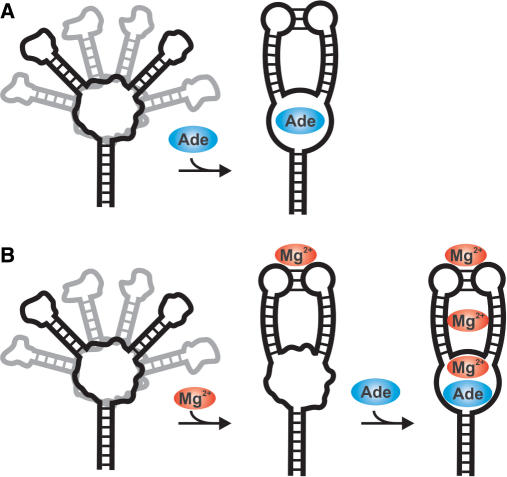
Figure 7.
Cartoon of the folding pathway for the adenine-sensing riboswitch RNA upon adenine and/or Mg2+ binding. (A) In the absence of Mg2+ the free form of the RNA is an ensemble of interconverting structures with different interhelical angles. The core region and the loops L2 and L3 are largely unstructured and partially misfolded. Adenine binding simultaneously induces the folding of the core region and the formation of the long-range base-pairing interactions between loop 2 and 3. (B) The presence of Mg2+ induces the formation of the proper long-range base-pairing interactions between loop II and loop III resulting in a conformation partially pre-organized for adenine binding. The subsequent binding of adenine to the core region yields the fully structured RNA–adenine complex with at least three Mg2+-binding sites.
The detrimental effect for ligand binding of an alternative long-range base-pairing interaction in the core region of the adenine-sensing riboswitch (G48A) has been shown before (42) and the sequence conservation patterns the different variants suggest that they have evolved in a way to minimize this possibility. Here, we demonstrate that the presence of Mg2+-ions apparently helps to avoid an alternative base-pairing pattern in the loop regions. Finally, we demonstrate that small sequence variations can have a profound effect on the folding pathway of this RNA since a slight variation of an apical base pair in helix II is as effective as Mg2+ in stabilizing the loop–loop interaction. Similar effects on RNA-folding pathways have been reported for instance for point mutations in the P5abc domain of the Tetrahymena group I intron (43,44).
The large influence of small sequence variations on the structure of the free state of the purine-binding riboswitches and their folding pathways might have interesting implications for the design of drugs that are targeted against purine-sensing riboswitches (45). The high similarity of the structures of their aptamer domain ligand complexes will render it difficult to find drugs that bind selectively to only a subset of these riboswitches such as those of a given bacterium. However, targeting the free form of these riboswitches where small sequence differences result in different conformations will allow selectivity. For some of these riboswitches it might be promising to develop inhibitors for the formation of the loop–loop interaction which is essential for ligand binding (13) while those with a stable preformed loop–loop interaction will not be affected in their function.
ACKNOWLEDGEMENTS
We are indebted to C. Richter and E. Stirnal for excellent technical support and H. R. Nasiri for the synthesis of 13C,15N-labeled adenine. We are grateful to M. Görlach and K. Abarca Heidemann for critical reading of the manuscript and their helpful comments. This work was supported by the Deutsche Forschungsgemeinschaft (DFG) through the SFB 579 ‘RNA-ligand interactions’, the Center of Biomolecular Magnetic Resonance (State of Hesse), NIH (RR13879) and by start-up funding from the Department of Biochemistry, University of Texas Health Science Center, San Antonio (USA). J. N. (by a fellowship) and H.S. were supported by the Fonds der Chemischen Industrie. Funding to pay the Open Access publication charges for this article was provided by start-up funds to J. W. from the Department of Biochemistry, The University of Texas Health Science Center San Antonio.
Conflict of interest statement. None declared.
REFERENCES
- 1.Woodson SA. Metal ions and RNA folding: a highly charged topic with a dynamic future. Curr. Opin. Chem. Biol. 2005;9:104–109. doi: 10.1016/j.cbpa.2005.02.004. [DOI] [PubMed] [Google Scholar]
- 2.Wu M, Tinoco I., Jr RNA folding causes secondary structure rearrangement. Proc. Natl Acad. Sci. USA. 1998;95:11555–11560. doi: 10.1073/pnas.95.20.11555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schwalbe H, Buck J, Furtig B, Noeske J, Wohnert J. Structures of RNA switches: insight into molecular recognition and tertiary structure. Angewandte Chemie International Ed. 2007;46:1212–1219. doi: 10.1002/anie.200604163. [DOI] [PubMed] [Google Scholar]
- 4.Noeske J, Richter C, Stirnal E, Schwalbe H, Wohnert J. Phosphate-group recognition by the aptamer domain of the thiamine pyrophosphate sensing riboswitch. Chembiochem. 2006;7:1451–1456. doi: 10.1002/cbic.200600151. [DOI] [PubMed] [Google Scholar]
- 5.Yamauchi T, Miyoshi D, Kubodera T, Nishimura A, Nakai S, Sugimoto N. Roles of Mg2+ in TPP-dependent riboswitch. FEBS Lett. 2005;579:2583–2588. doi: 10.1016/j.febslet.2005.03.074. [DOI] [PubMed] [Google Scholar]
- 6.Edwards TE, Ferre-D’Amare AR. Crystal structures of the thi-box riboswitch bound to thiamine pyrophosphate analogs reveal adaptive RNA-small molecule recognition. Structure. 2006;14:1459–1468. doi: 10.1016/j.str.2006.07.008. [DOI] [PubMed] [Google Scholar]
- 7.Serganov A, Polonskaia A, Phan AT, Breaker RR, Patel DJ. Structural basis for gene regulation by a thiamine pyrophosphate-sensing riboswitch. Nature. 2006;441:1167–1171. doi: 10.1038/nature04740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thore S, Leibundgut M, Ban N. Structure of the eukaryotic thiamine pyrophosphate riboswitch with its regulatory ligand. Science. 2006;312:1208–1211. doi: 10.1126/science.1128451. [DOI] [PubMed] [Google Scholar]
- 9.Roth A, Nahvi A, Lee M, Jona I, Breaker RR. Characteristics of the glmS ribozyme suggest only structural roles for divalent metal ions. RNA. 2006;12:607–619. doi: 10.1261/rna.2266506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Klein DJ, Ferre-D’Amare AR. Structural basis of glmS ribozyme activation by glucosamine-6-phosphate. Science. 2006;313:1752–1756. doi: 10.1126/science.1129666. [DOI] [PubMed] [Google Scholar]
- 11.Mandal M, Boese B, Barrick JE, Winkler WC, Breaker RR. Riboswitches control fundamental biochemical pathways in Bacillus subtilis and other bacteria. Cell. 2003;113:577–586. doi: 10.1016/s0092-8674(03)00391-x. [DOI] [PubMed] [Google Scholar]
- 12.Mandal M, Breaker RR. Adenine riboswitches and gene activation by disruption of a transcription terminator. Nat. Struct. Mol. Biol. 2004;11:29–35. doi: 10.1038/nsmb710. [DOI] [PubMed] [Google Scholar]
- 13.Batey RT, Gilbert SD, Montange RK. Structure of a natural guanine responsive riboswitch complexed with the metabolite hypoxanthine. Nature. 2004;432:411–415. doi: 10.1038/nature03037. [DOI] [PubMed] [Google Scholar]
- 14.Serganov A, Yuan YR, Pikovskaya O, Polonskaia A, Malinina L, Phan AT, Hobartner C, Micura R, Breaker RR, et al. Structural basis for discriminative regulation of gene expression by adenine and guanine sensing mRNAs. Chem. Biol. 2004;11:1729–1741. doi: 10.1016/j.chembiol.2004.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Noeske J, Richter C, Grundl MA, Nasiri HR, Schwalbe H, Wohnert J. An intermolecular base triple as the basis of ligand specificity and affinity in the guanine and adenine sensing riboswitch RNAs. Proc. Natl Acad. Sci. USA. 2005;102:1372–1377. doi: 10.1073/pnas.0406347102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gilbert SD, Stoddard CD, Wise SJ, Batey RT. Thermodynamic and kinetic characterization of ligand binding to the purine riboswitch aptamer domain. J. Mol. Biol. 2006;359:754–768. doi: 10.1016/j.jmb.2006.04.003. [DOI] [PubMed] [Google Scholar]
- 17.Lemay JF, Penedo JC, Tremblay R, Lilley DM, Lafontaine DA. Folding of the adenine riboswitch. Chem. Biol. 2006;13:857–868. doi: 10.1016/j.chembiol.2006.06.010. [DOI] [PubMed] [Google Scholar]
- 18.Noeske J, Buck J, Furtig B, Nasiri HR, Schwalbe H, Wohnert J. Interplay of ‘induced fit’ and preorganization in the ligand induced folding of the aptamer domain of the guanine binding riboswitch. Nucleic Acids Res. 2007;35:572–583. doi: 10.1093/nar/gkl1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fasman G. Handbook of Biochemistry and Molecular Biology, Nucleic Acids, I. 3rd. CRC press; 1975. edn. [Google Scholar]
- 20.Stoldt M, Wohnert J, Ohlenschlager O, Gorlach M, Brown LR. The NMR structure of the 5S rRNA E-domain-protein L25 complex shows preformed and induced recognition. EMBO J. 1999;18:6508–6521. doi: 10.1093/emboj/18.22.6508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bartels C, Xia T-H, Billeter M, Güntert P, Wüthrich K. The program XEASY for computer-supported NMR spectral analysis of biological macromolecules. J. Biomol. NMR. 1995;6:1–10. doi: 10.1007/BF00417486. [DOI] [PubMed] [Google Scholar]
- 22.Piotto M, Saudek V, Sklenar V. Gradient-tailored excitation for single-quantum NMR spectroscopy of aqueous solutions. J. Biomol. NMR. 1992;2:661–665. doi: 10.1007/BF02192855. [DOI] [PubMed] [Google Scholar]
- 23.Grzesiek S, Bax A. The importance of not saturating water in protein NMR. Application to sensitivity enhancement and NOE measurements. J. Am. Chem. Soc. 1993;115:12593–12594. [Google Scholar]
- 24.Sklenar V, Peterson RD, Rejante MR, Feigon J. Correlation of nucleotide base and sugar protons in a 15N-labeled HIV-1 RNA oligonucleotide by 1H-15N HSQC experiments. J. Biomol. NMR. 1994;4:117–122. doi: 10.1007/BF00178339. [DOI] [PubMed] [Google Scholar]
- 25.Wijmenga SS, van Buuren BNM. The use of NMR methods for conformational studies of nucleic acids. Prog. Nucl. Magn. Reson. Spectrosc. 1998;32:287–387. [Google Scholar]
- 26.Dingley AJ, Grzesiek S. Direct observation of hydrogen bonds in nucleic acid base pairs by internucleotide 2JNN couplings. J. Am. Chem. Soc. 1998;120:8293–8297. [Google Scholar]
- 27.Kang RS, Daniels CM, Francis SA, Shih SC, Salerno WJ, Hicke L, Radhakrishnan I. Solution structure of a CUE-ubiquitin complex reveals a conserved mode of ubiquitin binding. Cell. 2003;113:621–630. doi: 10.1016/s0092-8674(03)00362-3. [DOI] [PubMed] [Google Scholar]
- 28.Wohnert J, Dingley AJ, Stoldt M, Gorlach M, Grzesiek S, Brown LR. Direct identification of NH … N hydrogen bonds in non-canonical base pairs of RNA by NMR spectroscopy. Nucleic Acids Res. 1999;27:3104–3110. doi: 10.1093/nar/27.15.3104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ohlenschlager O, Wohnert J, Bucci E, Seitz S, Hafner S, Ramachandran R, Zell R, Gorlach M. The structure of the stemloop D subdomain of coxsackievirus B3 cloverleaf RNA and its interaction with the proteinase 3C. Structure. 2004;12:237–248. doi: 10.1016/j.str.2004.01.014. [DOI] [PubMed] [Google Scholar]
- 30.Bertini I, Luchinat C. NMR of Paramagnetic Molecules in Biological Systems. CA, USA: Menlo Park; 1986. [Google Scholar]
- 31.Hurd RE, Azhderian E, Reid BR. Paramagnetic ion effects on the nuclear magnetic resonance spectrum of transfer ribonucleic acid: assignment of the 15-48 tertiary resonance. Biochemistry. 1979;18:4012–4017. doi: 10.1021/bi00585a026. [DOI] [PubMed] [Google Scholar]
- 32.Allain FH, Varani G. Divalent metal ion binding to a conserved wobble pair defining the upstream site of cleavage of group I self-splicing introns. Nucleic Acids Res. 1995;23:341–350. doi: 10.1093/nar/23.3.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Butcher SE, Allain FH, Feigon J. Determination of metal ion binding sites within the hairpin ribozyme domains by NMR. Biochemistry. 2000;39:2174–2182. doi: 10.1021/bi9923454. [DOI] [PubMed] [Google Scholar]
- 34.Feigon J, Butcher SE, Finger LD, Hud NV. Solution nuclear magnetic resonance probing of cation binding sites on nucleic acids. Methods Enzymol. 2001;338:400–420. doi: 10.1016/s0076-6879(02)38230-2. [DOI] [PubMed] [Google Scholar]
- 35.Cowan JA. Metallobiochemistry of RNA. Co(NH3)63+ as a probe for Mg2+(aq) binding sites. J. Inorg. Biochem. 1993;49:171–175. doi: 10.1016/0162-0134(93)80002-q. [DOI] [PubMed] [Google Scholar]
- 36.Hud NV, Schultze P, Sklenar V, Feigon J. Binding sites and dynamics of ammonium ions in a telomere repeat DNA quadruplex. J. Mol. Biol. 1999;285:233–243. doi: 10.1006/jmbi.1998.2327. [DOI] [PubMed] [Google Scholar]
- 37.Cate JH, Hanna RL, Doudna JA. A magnesium ion core at the heart of a ribozyme domain. Nat. Struct. Biol. 1997;4:553–558. doi: 10.1038/nsb0797-553. [DOI] [PubMed] [Google Scholar]
- 38.Rieder R, Lang K, Graber D, Micura R. Ligand-induced folding of the adenosine deaminase A-riboswitch and implications on riboswitch translational control. Chembiochem. 2007;8:896–902. doi: 10.1002/cbic.200700057. [DOI] [PubMed] [Google Scholar]
- 39.Gonzalez RL, Jr, Tinoco I., Jr Solution structure and thermodynamics of a divalent metal ion binding site in an RNA pseudoknot. J. Mol. Biol. 1999;289:1267–1282. doi: 10.1006/jmbi.1999.2841. [DOI] [PubMed] [Google Scholar]
- 40.Fan Y, Gaffney BL, Jones RA. RNA GG x UU motif binds K+ but not Mg2+ J. Am. Chem. Soc. 2005;127:17588–17589. doi: 10.1021/ja0555522. [DOI] [PubMed] [Google Scholar]
- 41.Wickiser JK, Cheah MT, Breaker RR, Crothers DM. The kinetics of ligand binding by an adenine sensing riboswitch. Biochemistry. 2005;44:13404–13414. doi: 10.1021/bi051008u. [DOI] [PubMed] [Google Scholar]
- 42.Lemay JF, Lafontaine DA. Core requirements of the adenine riboswitch aptamer for ligand binding. RNA. 2007;13:339–350. doi: 10.1261/rna.142007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Silverman SK, Zheng M, Wu M, Tinoco I, Jr, Cech TR. Quantifying the energetic interplay of RNA tertiary and secondary structure interactions. RNA. 1999;5:1665–1674. doi: 10.1017/s1355838299991823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zheng M, Wu M, Tinoco I., Jr Formation of a GNRA tetraloop in P5abc can disrupt an interdomain interaction in the Tetrahymena group I ribozyme. Proc. Natl Acad. Sci. USA. 2001;98:3695–3700. doi: 10.1073/pnas.051608598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Blount KF, Breaker RR. Riboswitches as antibacterial drug targets. Nat. Biotechnol. 2006;24:1558–1564. doi: 10.1038/nbt1268. [DOI] [PubMed] [Google Scholar]