Abstract
Rationale: COPD is associated with reduced life expectancy.
Objectives: To determine the association between small airway pathology and long-term survival after lung volume reduction in chronic obstructive pulmonary disease (COPD) and the effect of corticosteroids on this pathology.
Methods: Patients with severe (GOLD-3) and very severe (GOLD-4) COPD (n = 101) were studied after lung volume reduction surgery. Respiratory symptoms, quality of life, pulmonary function, exercise tolerance, chest radiology, and corticosteroid treatment status were assessed preoperatively. The severity of luminal occlusion, wall thickening, and the presence of small airways containing lymphoid follicles were determined in resected lung tissue. Kaplan-Meier survival analysis and Cox proportional hazards models were used to determine the relationship between survival and small airway pathology. The effect of corticosteroids on this pathology was assessed by comparing treated and untreated groups.
Measurements and Main Results: The quartile of subjects with the greatest luminal occlusion, adjusted for covariates, died earlier than subjects who had the least occlusion (hazard ratio, 3.28; 95% confidence interval, 1.55–6.92; P = 0.002). There was a trend toward a reduction in the number of airways containing lymphoid follicles (P = 0.051) in those receiving corticosteroids, with a statistically significant difference between the control and oral ± inhaled corticosteroid–treated groups (P = 0.019). However, corticosteroid treatment had no effect on airway wall thickening or luminal occlusion.
Conclusions: Occlusion of the small airways by inflammatory exudates containing mucus is associated with early death in patients with severe emphysema treated by lung volume reduction surgery. Corticosteroid treatment dampens the host immune response in these airways by reducing lymphoid follicles without changing wall thickening and luminal occlusion.
Keywords: premature death in COPD, airway remodeling, mucosal immune response, corticosteroids
AT A GLANCE COMMENTARY
Scientific Knowledge on the Subject
Premature death is a frequent complication of chronic obstructive pulmonary disease.
What This Study Adds to the Field
Mucoid occlusion of small conducting airways is associated with premature death in advanced chronic obstructive pulmonary disease. We postulate that airway occlusion causes pneumonia that results in early death.
The emphysematous destruction of the lung's gas-exchanging surface and obstruction to flow in the small conducting airways are responsible for the irreversible airflow limitation that defines chronic obstructive pulmonary disease (COPD) (1). We previously reported a negative association between the severity of the pathology in the small conducting airways and FEV1 over the full range of COPD severity (2). The primary objective of the present study was to test the hypothesis that this pathology might influence survival of patients in a 72-month follow-up period after lung volume reduction surgery (LVRS) (3, 4). A secondary objective was to determine if corticosteroid therapy had any influence on small airway pathology that might provide insight into the recent report that long-term steroid therapy reduces exacerbations of COPD but increases the incidence of pneumonia (5). Preliminary reports on the data contained in this article have been presented in abstract form at the Aspen Lung Conference (6) and at annual meetings of the American Thoracic Society (7, 8).
METHODS
Patient Population
Patients with GOLD (Global Initiative for Chronic Obstructive Lung Disease) stages 3 and 4 COPD who were treated by LVRS for advanced emphysema (n = 101) were the subject of this study. Five of these cases from the University of Pittsburgh were treated by LVRS before the start of the National Emphysema Treatment Trial (NETT) and the remaining 96 received this treatment in five sites participating in NETT located at the Universities of Pittsburgh, Michigan, and Colorado, Temple University, and the Baylor College of Medicine. The airway data from these 101 LVRS-treated cases were combined with similar data from 101 cases with milder COPD (GOLD stages 0–2) from the University of British Columbia patient registry and tissue bank to ensure that the cross-sectional regression analysis between airflow limitation and small airway pathology was the same as that observed in our previous report (2). All of the patients who participated in this study provided informed consent under conditions approved by the appropriate committees at the institutions where their surgery was performed. Patient confidentiality was maintained by using unique identifiers to link tissue samples to patient data that could not be traced to any personal identifier.
Preoperative Assessment
Those selected for treatment by LVRS were placed on a preoperative rehabilitation program, and the measurements of body mass index, FEV1, FVC, subdivisions of lung volume, diffusing capacity for carbon monoxide (DlCO), and arterial partial pressure of oxygen (PaO2) used in the analyses were obtained after the rehabilitation period close to the time of surgery. Quality of life was determined using the Physical Component Summary (PCS) and Mental Component Summary (MCS) of the Short Form-36 (SF-36) and St. George's Respiratory Questionnaire (SGRQ), and health utility was assessed by the Quality of Well Being scale (QWB) (9). The presence of cough and sputum production was determined from specific questions about these symptoms in the SGRQ, and the severity of dyspnea was quantified using the University of California, San Diego, Shortness of Breath Questionnaire (UCSD SOBQ) (10).
Histology
Lung tissue samples removed from these 101 patients by LVRS were fixed in formalin immediately after resection and processed for into paraffin blocks at the site where the LVRS took place. The methods of tissue preparation for the 101 non-LVRS cases are published in detail elsewhere (2). Briefly, histologic sections cut from the paraffin blocks were stained using Movat's pentachrome method (11), and all of the small conducting airways in this tissue cut in reasonable cross-section were analyzed (12). Digital images of the airway histology were captured using a Nikon light microscope (Nikon Microphot, Tokyo, Japan) equipped with a JVC3-CCD KY F-70 digital camera (Diagnostic Instruments, Inc., Yokohama, Japan), linked to a computer to record and store the images. None of the clinical, radiologic, lung function, or steroid treatment data were available to the persons making the histologic measurements until this assessment was complete.
The size of the small conducting airways was estimated by measuring the length of the basement membrane and determining lumen area in both the partially collapsed state where the basement membrane was folded and in a simulated fully expanded state calculated by subtracting the area of the epithelium from the area of a circle with a perimeter equal to the airway basement membrane length (2). The severity of the luminal occlusion was expressed by determining the fraction of these lumen areas occluded by inflammatory exudate containing mucus. The thickness of the entire airway wall as well as its epithelial, lamina propria, muscle, and adventitial compartments were determined by dividing their measured areas by the length of the airway basement membrane. The percentage of airways containing a collection of lymphocytes consistent with the formation of a lymphoid follicle was also recorded (2).
Data Analysis
A linear regression analysis (13) was performed on the entire 202 cases to confirm the relationships between FEV1 and airway thickness, and FEV1 and lumen content from our previous report (2). The 101 cases treated by LVRS were then divided into quartiles according to the percentage of occlusion of the fully expanded lumen of their small airways and all of the variables were compared using analysis of variance (ANOVA) followed by Tukey's post hoc test (Table 1) (14). Separate analyses were performed where cases were ranked and divided into quartiles based on either airway wall thickness or the percentage of airways containing lymphoid follicles. In addition, the variables in Table 1 were examined as potential predictors of survival using a Cox proportional hazards model (15). To facilitate valid comparisons, all continuous variables were included as quartiles. A forward stepwise selection procedure was then used to determine which variables best predicted survival, retaining only those variables that, in univariate analysis, provided a P value of 0.25 or less. These variables were included one by one into a multivariate model using a stepwise selection process, retaining only those variables that, in the multivariate analysis, provided a P value of 0.15 or less.
TABLE 1.
THE BASELINE CHARACTERISTICS OF STUDY SUBJECTS ACROSS QUARTILES OF EXPANDED LUMEN RATIO
| Quartile 1 | Quartile 2 | Quartile 3 | Quartile 4 | P Value | |
|---|---|---|---|---|---|
| No. of patients | 25 | 25 | 26 | 25 | — |
| Lumen content of expanded airway, % | 1.1 ± 0.2* | 3.8 ± 0.1 | 7.0 ± 0.3† | 18.4 ± 2.0‡ | <0.001 |
| No. of airways examined per case | 6.8 ± 1.7* | 9.7 ± 1.7 | 13.9 ± 2.5 | 5.3 ± 0.9* | 0.006 |
| No. of male subjects (%) | 13 (52) | 14 (56) | 16 (62) | 16 (64) | 0.823 |
| Age, yr | 64 ± 2 | 66 ± 1 | 68 ± 1 | 66 ± 1 | 0.255 |
| Smoking, pack-years | 55 ± 6 | 58 ± 5 | 68 ± 4 | 68 ± 6 | 0.255 |
| BMI, kg/m2 | 25.1 ± 0.9 | 24.8 ± 0.7 | 25.3 ± 0.8 | 25.8 ± 1.0 | 0.869 |
| FEV1, % predicted | 28.0 ± 1.4 | 24.2 ± 1.3 | 28.0 ± 1.5 | 26.0 ± 1.5 | 0.191 |
| FVC, % predicted | 66.4 ± 4.2 | 62 ± 3.3 | 69.3 ± 3.2 | 67.0 ± 2.7 | 0.501 |
| RV, % predicted | 217 ± 11 | 249 ± 11† | 202 ± 6 | 215 ± 11.6 | 0.012 |
| DlCO, % predicted | 26.8 ± 2.1 | 28.7 ± 1.9 | 28.5 ± 1.9 | 28.9 ± 1.6 | 0.884 |
| PaO2, mm Hg | 63.0 ± 1.7 | 60.9 ± 2.1 | 58.6 ± 2.3 | 59.6 ± 1.6 | 0.468 |
| WBC, 103/mm3 | 7.97 ± 0.52 | 7.63 ± 0.48 | 8.38 ± 0.44 | 8.02 ± 0.50 | 0.732 |
| SF-36 PCS | 30.9 ± 1.6 | 29.2 ± 1.4 | 28.6 ± 2.0 | 28.6 ± 1.8 | 0.789 |
| SF-36 MCS | 60.6 ± 1.1 | 56.6 ± 1.2 | 57.2 ± 1.8 | 55.6 ± 1.8 | 0.189 |
| QWB score | 0.59 ± 0.02 | 0.60 ± 0.02 | 0.54 ± 0.02 | 0.57 ± 0.03 | 0.227 |
| SGRQ overall score | 46.5 ± 2.5 | 55.4 ± 2.7 | 50.5 ± 2.5 | 51.2 ± 3.0 | 0.173 |
| UCSD SOBQ | 53.9 ± 4.0 | 65.9 ± 2.6 | 59.0 ± 4.0 | 63.2 ± 4.3 | 0.152 |
| Six-minute-walk, ft | 1,234 ± 60 | 1,295 ± 52 | 1,274 ± 62 | 1,308 ± 71 | 0.867 |
| Wall thickness, mm | 0.138 ± 0.007 | 0.151 ± 0.007 | 0.142 ± 0.005 | 0.171 ± 0.008*† | 0.004 |
| Epithelial thickness, mm | 0.033 ± 0.002 | 0.034 ± 0.002 | 0.032 ± 0.001 | 0.039 ± 0.002 | 0.103 |
| Lamina propria, mm | 0.037 ± 0.002 | 0.042 ± 0.002 | 0.040 ± 0.002 | 0.040 ± 0.002 | 0.446 |
| Smooth muscle, mm | 0.016 ± 0.001 | 0.018 ± 0.001 | 0.020 ± 0.001 | 0.021 ± 0.001† | 0.027 |
| Adventitia, mm | 0.070 ± 0.005 | 0.073 ± 0.004 | 0.068 ± 0.005 | 0.092 ± 0.006*† | 0.006 |
| Airways with lymphoid follicles, % | 20.8 ± 5.7 | 30.4 ± 3.8 | 20.7 ± 3.0 | 33.9 ± 6.8 | 0.155 |
Definition of abbreviations: BMI = body mass index; DlCO = diffusion capacity of the lung for carbon monoxide; MCS = Mental Component Summary score; PCS = Physical Component Summary score; QWB = Quality of Well-Being scale; SF-36 = Short Form-36; SGRQ = St. George's Respiratory Questionnaire; UCSD SOBQ = University of California, San Diego, Shortness of Breath Questionnaire; WBC = white blood cell count.
Values are mean ± SEM.
Different from quartile 3 at P < 0.05.
Different from quartile 1 at P < 0.05.
Different from all the other quartile groups at P < 0.05.
The effect of corticosteroid treatment on the airway histology was determined in 94 of the 101 cases treated by LVRS where sufficient data on corticosteroid treatment were available, by comparing the histology of those who were known not to receive corticosteroid treatment (n = 16) with those who received inhaled corticosteroids only (n = 45), and those who received oral ± inhaled corticosteroid (n = 33) up until the time of their LVRS. Seven of the 101 cases treated by LVRS were not included in this analysis because there was insufficient information to assign them to one of these three categories. The effect of corticosteroids on survival could not be assessed because we were unable to access data concerning the steroid therapy received by these patients during the follow-up period subsequent to LVRS at the time of this writing.
RESULTS
Relationships between Small Airway Pathology and Clinical Data
The relationships between FEV1 and both small airway wall thickness (P < 0.001) and the percentage of occlusion of the fully expanded airway lumen by inflammatory exudates containing mucus (P < 0.001) in the 202 cases in this study were similar to our previous report that included 159 of these same cases (2). Table 1 shows the results of the analysis performed on the baseline variables after the LVRS cases were divided into quartiles according to a rank ordering of the severity of the lumen obstruction by intraluminal content. These data show weak relationships between the severity of intraluminal airway occlusion and patient demographics (sex, age, smoking history, and body mass index), function (FEV1, FVC residual volume, DlCO, PaO2 white blood cell count), quality of life, and health status scores (SF-36 PCS, SF-36 MCS, QWB, SGRQ overall score, USD SOBQ, and six-minute-walk test). Stronger relationships were observed between quartiles of intraluminal content and the thickness of the total wall (P = 0.004), airway smooth muscle (P = 0.027), and adventitia (P = 0.006).
Small Airway Pathology and Survival Post-LVRS
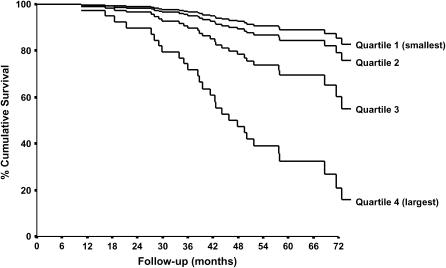
A Kaplan-Meier survival analysis (Figure 1) showed a shortened median survival in the quartile of subjects who had the most severe luminal occlusion. The median time of follow-up was 79 months (95% confidence interval [CI], 68–90 mo). The median survival time was shortest in the quartile with the most severe luminal occlusion (median survival time, 48 mo; 95% CI, 24–71 mo). The median survival time was longest in the group with the least luminal occlusion (> 92 mo). All of the variables listed in Table 1 were evaluated for their relationship with survival. As described in Methods, only those variables that had a univariate P value of 0.25 or less were retained for multivariate analysis. In a stepwise model, only age (P = 0.027) and post-bronchodilator FEV1 (P = 0.031) were significant covariates. The Cox regression analysis revealed that the patients in the quartile with the highest luminal content had a risk of mortality that was over three times higher than the quartile with the lowest luminal content (adjusted hazard ratio, 3.28; 95% CI, 1.55–6.92; P = 0.002) after adjustments for a variety of factors (see Methods).
Figure 1.
An adjusted Kaplan-Meier survival plot of the 101 cases of severe (GOLD [Global Initiative for Chronic Obstructive Lung Disease] stage 3) and very severe (GOLD stage 4) chronic obstructive pulmonary disease, indicating that median survival was shortened in the quartile with the most severe occlusion of the fully expanded lumen (hazard ratio, 3.28; 95% confidence interval, 1.55–6.92; P = 0.002) after adjustments for variety of factors cited in Methods. Note that the smoothing of the curves that occurs with correction for confounding variables to allow accurate comparisons between the quartile groups makes the survival rate in the first 90 days appear higher than it was really was.
The severity of the lumen occlusion measured by histology was not associated with the response to questions in the SGRQ designed to establish a diagnosis of chronic bronchitis, and neither airway wall thickening (P = 0.728) nor the percentage of the airways containing lymphoid follicles (P = 0.531) predicted survival after LVRS.
Impact of Corticosteroid Treatment
Table 2 shows the data concerning patient demographics, physiology, symptoms, and health status, as well as the small airway dimensions for the 94 of the 101 LVRS patients in whom sufficient data were available to analyze the effect of steroid treatment on airway histology. The patients whose treatment included oral ± inhaled steroids were slightly less dyspneic but had a shorter 6-minute-walk, an elevated white blood cell count, and a reduced quality of life compared with the other two groups. Although neither form of corticosteroid therapy had an effect on lumenal content or wall thickness, there was a negative association between steroid therapy and the percentage of airways containing lymphoid follicles (P = 0.051 for ANOVA), with pairwise comparisons showing a significant difference between the group that received oral ± inhaled steroids and the non–steroid-treated subjects (P = 0.019).
TABLE 2.
COMPARISON OF UNTREATED AND CORTICOSTEROID-TREATED GROUPS
| Parameter | Nontreated (n = 16) | Inhaled Steroids (n = 45) | Oral ± Inhaled Steroids (n = 33) | P Value |
|---|---|---|---|---|
| Demographics | ||||
| Age, yr | 65 ± 2 | 68 ± 1 | 64 ± 1* | 0.061 |
| BMI, kg/m2 | 26.3 ± 1.1 | 25.4 ± 0.6 | 24.6 ± 0.8 | 0.402 |
| Smoking, pack-years | 64 ± 8 | 64 ± 4 | 61 ± 5 | 0.882 |
| Physiology | ||||
| FEV1, % predicted | 27.9 ± 2.1 | 26.7 ± 0.9 | 25.2 ± 1.4 | 0.420 |
| TLC, % predicted | 128.3 ± 3.8 | 129.8 ± 2.0 | 128.3 ± 2.9 | 0.837 |
| DlCO, % predicted | 30.4 ± 2.4 | 28.1 ± 1.4 | 27.7 ± 1.5 | 0.602 |
| Six-minute-walk, ft | 1,399 ± 66 | 1,334 ± 45 | 1,146 ± 47*† | 0.004 |
| Reduced maximum load (⩽ 40 W for men; ⩽ 25 W for women) | 13% | 37% | 48% | 0.256 |
| PaO2, mm Hg | 64.3 ± 2.2 | 58.2 ± 1.4 | 61.4 ± 1.7 | 0.139 |
| WBC, 103/mm3 | 7.2 ± 0.3 | 7.3 ± 0.3 | 9.5 ± 0.4*† | <0.001 |
| Symptoms and health status | ||||
| UCSD SOBQ | 63.9 ± 3.0 | 55.0 ± 2.8 | 67.1 ± 3.3* | 0.012 |
| SF-36 PCS | 27.7 ± 1.6 | 31.9 ± 1.3 | 26.2 ± 1.3*† | 0.007 |
| SF-36 MCS | 57.9 ± 2.2 | 58.4 ± 0.8 | 55.4 ± 1.7 | 0.233 |
| QWB | 0.60 ± 0.02 | 0.61 ± 0.02 | 0.51 ± 0.02*† | <0.001 |
| SGRQ overall score | 51.2 ± 2.5 | 47.4 ± 2.0 | 56.0 ± 2.3* | 0.016 |
| Small airway wall dimensions | ||||
| Lumen content of collapsed airway, % | 32.4 ± 3.9 | 30.2 ± 3.0 | 30.4 ± 3.8 | 0.931 |
| Lumen content of expanded airway, % | 8.20 ± 1.73 | 8.02 ± 1.13 | 7.89 ± 1.76 | 0.993 |
| Airways containing lymphoid follicles, % | 40.2 ± 6.4 | 26.1 ± 3.7 | 21.3 ± 4.5† | 0.051 |
| Total wall thickness, mm | 0.16 ± 0.008 | 0.15 ± 0.005 | 0.15 ± 0.006 | 0.913 |
| Epithelial thickness, mm | 0.035 ± 0.003 | 0.035 ± 0.001 | 0.035 ± 0.002 | 0.971 |
| Lamina propria, mm | 0.041 ± 0.002 | 0.040 ± 0.002 | 0.040 ± 0.002 | 0.801 |
| Smooth muscle, mm | 0.020 ± 0.001 | 0.018 ± 0.001 | 0.019 ± 0.001 | 0.320 |
| Adventitia, mm | 0.078 ± 0.006 | 0.077 ± 0.004 | 0.078 ± 0.005 | 0.971 |
For definition of abbreviations, see Table 1.
Values are mean ± SEM.
P < 0.05 versus nontreated group.
P < 0.05 versus inhaled steroids group.
DISCUSSION
The data on from the 202 cases in this study confirm our previous report on 159 cases showing that thickening of the walls of the small conducting airways and occlusion of their lumen by inflammatory exudates containing mucus are negatively associated with FEV1 over the full range of COPD severity (2). They also extend these observations by showing an association between the severity of small airway occlusion by inflammatory exudates containing mucus and early death after LVRS. This association was independent of the LVRS procedure, uninfluenced by correcting for age, level of respiratory symptoms (SGRQ total score, UCSD SOBQ), and level of expiratory flow limitation (FEV1% predicted). The present data also show that treatment with oral ± inhaled corticosteroid therapy had no effect on airway wall thickening or lumen occlusion but was associated with a lowering of the percentage of airways containing lymphoid follicles, which indicates a reduction in the adaptive immune response in the peripheral lung.
Chronic bronchitis is defined by excess cough and sputum production and is associated with decreased clearance of mucus from the lower airways, more frequent pneumonias, and premature death (16, 17). However, the SGRQ questions used to establish the presence of chronic bronchitis failed to associate this diagnosis with occlusion of the small airways by inflammatory mucous exudates in the present dataset. This result is consistent with earlier reports on the pathology of COPD showing that the symptoms of chronic bronchitis are more closely associated with pathology in the central rather than the peripheral airways (18, 19). Therefore, we suspect that it is the inflammatory process in the wall and lumen of the small airways (2, 20) that stimulates the local goblet cells to produce the mucus observed in these exudates. Breuer and associates (21) established that neutrophil elastase induces goblet cells to secrete mucus, and Takeyama and colleagues, Lee and colleagues, and Burgel and associates (22–26) extended these observations by showing that elastase secreted by polymorphonuclear leukocytes (PMNs) cleaves several epithelium-bound ligands for the epidermal growth factor receptor (EGFR) responsible for activating the downstream pathways leading to the secretion of mucus. This same group also established that oxygen free radicals generated by the PMNs can bypass EGFR and have a direct effect on mucus secretion. Collectively, these data suggest that PMNs and possibly other inflammatory cells known to infiltrate the wall and lumen of the small airways are capable of stimulating the generation of the mucus observed in these airways. Alternatively, the accumulation of these exudates might have resulted from defective clearance out of the small airways, with the balance between production and clearance probably determining the level of obstruction at any point in time. Unfortunately, we can only comment on the effect of the level of lumen obstruction of these airways because neither production nor clearance could be addressed in this cross-sectional study.
The most plausible biological link between the level of airway occlusion and early death in COPD is the increased risk for lower respiratory tract infection. Studies of the cause of death in COPD attribute only 11% of deaths to infection, but it is conceivable that undetected infections of the small airways contribute to the 38% of COPD deaths attributed to respiratory failure (27). Many common respiratory pathogens colonize the upper airways first and enter the lower airways by microaspiration (28). The accumulation of mucus containing exudates in the small airway lumen increases the likelihood of developing infection by slowing the clearance of these organisms and allowing them to replicate to levels where they can invade the tissue and produce infection (28). Furthermore, the fact that early deaths occurred at a mean of 24 months with a CI of 24 to 71 months after surgery indicates these deaths occurred far beyond the 1-month time limit for attributing adverse events to complications arising from the surgical procedure.
Sethi and Murphy and their colleagues (29, 30) have shown that the emergence of new strains of organisms that have previously colonized the respiratory tract in COPD are a major source of new infections. These same investigators also demonstrated a sharp increase in the production of sputum IgA and serum IgE antibodies directed against specific microbial proteins from these newly emergent strains (30). We previously attributed the marked increase in the percentage of small airways containing lymphoid follicles to the adaptive immune response to this type of infection (2), and the present results show that steroid therapy is associated with a reduction in the numbers of these follicle-containing airways. Therefore, we postulate that steroid-induced suppression of the host immune response in patients observed in this study might act in conjunction with extensive occlusion of the peripheral airways to account for the increase in pneumonias observed in the recent TORCH trial (5), but further studies will be required to establish this point.
In summary, these results show that severe occlusion of the small conducting airways by inflammatory exudates containing mucus is predictive of early death in patients with advanced COPD. We postulate that this airway occlusion may act in association with steroid-induced immune suppression to increase the probability of infection in the lower respiratory tract.
Supported by grants from the Canadian Institute for Health Research (7246), the National Heart, Lung, and Blood Institute (RO1 HL 63117). The National Emphysema Treatment Trial is supported by the National Heart, Lung, and Blood Institute, the Centers for Medicare and Medicaid and the Agencies for Health Research and Quality, and the George H. Love Research Fund at the University of Pittsburgh.
Originally Published in Press as DOI: 10.1164/rccm.200612-1772OC on June 7, 2007
Conflict of Interest Statement: J.C.H. served as a consultant to Altana Pharmaceuticals in 2003, 2004, and 2005, and also served on the Canadian advisory board for GlaxoSmithKline (GSK) for 1 year (2003). He has participated as a speaker in scientific meetings and courses organized and financed by various pharmaceutical companies, including AstraZeneca (AZ), Altana Pharmaceuticals, and GSK. He serves as the principal investigator on a jointly sponsored Canadian Institute of Health Research (CIHR) industry-sponsored grant which is one-third supported by CIHR and two-thirds supported by GSK and Merck. This grant application was funded after peer review by the regular CIHR mechanism and the funds received from industry are directly related to the operating costs of the study. F.S.F.C. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. W.C.T received grants from AZ, Boehringer Ingelheim, and GSK for the study of prevalence of COPD. D.D.S. has received honoraria for speaking engagements from AZ in 2004 ($3,000) and in 2005 ($11,000), and from GSK in 2004 ($8,000), 2005 ($6,500), and 2006 ($12,000). He has also received unrestricted research funding as either the principal investigator or co-principal investigator from GSK in 2004 for $1.5 million and from AZ in 2007 ($100,000). He also received $1,500 from GSK for consultancy work in 2006. S.A.P. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. P.D.P. is the principal investigator of a project funded by GSK to develop computed tomography (CT)–based algorithms to quantify emphysema and airway disease in COPD. With collaborators, he has received ∼$300,000 to develop and validate these techniques. He is also principal investigator of a Merck Frost–supported research program to investigate gene expression in the lungs of patients who have COPD. He and collaborators have received ∼$200,000 for this project. F.J.M. is a consultant for Altana Pharma and has received compensation greater than $10K. He has been a member of several advisory boards, CME committees, and the speakers' bureau for Boehringer Ingelheim, Pfizer, and GSK. His total compensation per company was greater than $10K. In addition, he is on the advisory board for Novartis and the speakers' bureau for Sepracor and Astra, receiving less than $10K per company. He has been an investigator for industry-sponsored studies for GSK, Boehringer Ingelheim, and Actelion. R.M.R. received honoraria for lectures from Boehringer Ingelheim for $8,000 in 2004, from Dey Pharmaceutical for $10,500 in 2005 and $10,000 in 2006–2007. B.J.M. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. G.J.C. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. R.M.C. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. A.S. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. J.D.L. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. H.O.C. received $11,000 in 2005 for serving on an advisory board for GSK. In addition, he is the coinvestigator on two multicenter studies sponsored by GSK and has received travel expenses to attend meetings related to the project. He has three contract service agreements with GSK to quantify the CT scans in subjects with COPD. A percentage of his salary between 2003 and 2006 ($15,000/yr) derives from contract funds provided to a colleague of P.D.P. by GSK for the development of validated methods to measure emphysema and airway disease using CT. He is the coinvestigator (D.D.S., principal investigator) on a Canadian Institutes of Health–Industry (Wyeth) partnership grant. W.M.E. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. F.C.S. received $63,086 from GSK and $21,518 from AZ in 2005 through 2006 for participation in multicenter clinical trials and has earned less than $10,000 per year serving on advisory boards for GSK and AZ.
References
- 1.Pauwels RA, Buist AS, Calverley PM, Jenkins CR, Hurd SS. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: NHLBI/WHO Global Initiative for Chronic Obstructive Lung Disease (GOLD) workshop summary. Am J Respir Crit Care Med 2001;163:1256–1276. [DOI] [PubMed] [Google Scholar]
- 2.Hogg JC, Chu F, Utokaparch S, Woods R, Elliott WM, Buzatu L, Cherniack RM, Rogers RM, Sciurba FC, Coxson HO, et al. The nature of small-airway obstruction in chronic obstructive pulmonary disease. N Engl J Med 2004;350:2645–2653. [DOI] [PubMed] [Google Scholar]
- 3.National Emphysema Treatment Trial Research Group. Patients at high risk of death after lung-volume-reduction surgery. N Engl J Med 2001;345:1075–1083. [DOI] [PubMed] [Google Scholar]
- 4.National Emphysema Treatment Trial Research Group. A randomized trial comparing lung-volume-reduction surgery with medical therapy for severe emphysema. N Engl J Med 2003;348:2059–2073. [DOI] [PubMed] [Google Scholar]
- 5.Calverly PM, Anderson JA, Celli B, Ferguson GT, Jenkins C, Jones PW, Yates JC, Vestbo J. Salmeterol and fluticasone proprionate and survival in chronic obstructive pulmonary disease. N Engl J Med 2007;356:775–789. [DOI] [PubMed] [Google Scholar]
- 6.Sciurba FC, Martinez FJ, Rogers RM, Make BJ, Criner GJ, Cherniack RM, Patel SA, Chu F, Coxson HO, Sharafkhaneh A, et al. Relationship between pathologic characteristics of peripheral airways and outcome after lung volume reduction surgery in chronic obstructive pulmonary disease. Proc Am Thorac Soc 2006;3:533–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sciurba FC, Martinez FJ, Rogers RM, Make BJ, Criner GJ, Cherniack RM, Patel SA, Chu F, Coxson HO, Sharafkhaneh A, et al. The effect of small airway pathology on survival following lung volume reduction surgery (LVRS) [abstract]. Proc Am Thor Soc 2006;3:A712. [Google Scholar]
- 8.Tan WC, Elliot M, Chu F, Sciurba FC, Martinez F, Rogers RM, Criner GJ, Cherniack RM, Make B, Sharafkhaneh A, et al. Impact of systemic and inhaled corticosteroids on peripheral airways in severe COPD [abstract]. Proc Am Thorac Soc 2005;2:A135. [Google Scholar]
- 9.Jones PW, Quirk FH, Baveystock CM, Littlejohns P. A self-complete measure of health status for chronic airflow limitation: the St George's Respiratory Questionnaire. Am Rev Respir Dis 1992;145:1321–1327. [DOI] [PubMed] [Google Scholar]
- 10.Eakin EG, Resnikoff PM, Prewitt LM, Ries AL, Kaplan RM. Validation of a new dyspnea measure: the UCSD Shortness of Breath Questionnaire. University of California, San Diego. Chest 1998;113:619–624. [DOI] [PubMed] [Google Scholar]
- 11.Movat HZ. Demonstration of all connective tissue elements in a single section: pentachrome stains. AMA Arch Pathol 1995;60:289–295. [PubMed] [Google Scholar]
- 12.James AL, Hogg JC, Dunn LA, Pare PD. The use of the internal perimeter to compare airway size and to calculate smooth muscle shortening. Am Rev Respir Dis 1988;138:136–139. [DOI] [PubMed] [Google Scholar]
- 13.Dobson AJ. An introduction to generalized linear model. 2nd ed. New York: Chapman Hall; 2002.
- 14.Clark TG, Bradburn MJ, Love SB, Altman DG. Survival analysis part I: basic concepts and first analyses. Br J Cancer 2003;89:232–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fisher LD, Van Belle G. Biostatistics: a methodology for the health sciences, New York: Wiley; 1993.
- 16.Pilkington M, Notkola I, Nissinen A, Taukianen H. Thirty year cumulative incidence of chronic bronchitis and COPD in relation to 40 year mortality. Chest 2006;130:1129–1137. [DOI] [PubMed] [Google Scholar]
- 17.Ekberg-Aronsson M, Pehrsson K, Nillsson JA, Nilsson PM, Lofdahl C-G. Mortality in GOLD stages of COPD and its dependence on symptoms of chronic bronchitis. Respir Res 2005;6:98–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mullen JBM, Wright JL, Wiggs B, Pare PD, Hogg JC. Reassessment of inflammation in the airways in chronic bronchitis. BMJ 1985;291:1235–1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Saetta M, Turato G, Facchini FM, Corbino L, Lucchini RE, Casoni G, Maestrelli P, Mapp CE, Ciaccia A, Fabbri LM. Inflammatory cells in the bronchial glands of smokers with chronic bronchitis. Am J Respir Crit Care Med 1997;156:1633–1639. [DOI] [PubMed] [Google Scholar]
- 20.Lo C, Chu F, Elliott WM, Tan WC, Hogg JC. Comparison of the cellular content of the inflammatory exudates in the wall and lumen of the small airways in COPD [abstract]. Proc Am Thorac Soc 2006;3:A459. [Google Scholar]
- 21.Breuer R, Christiansen TG, Lucey EC, Bolbochan G, Stone PJ, Snider GL. Elastase causes secretory discharge in bronchi of hamsters with elastase induced secretory cell metaplasia. Exp Lung Res 1993;19:273–282. [DOI] [PubMed] [Google Scholar]
- 22.Takeyama K, Dabbagh K, Jeong Shim J, Dao-Pick T, Ueki IF, Nadel JA. Oxidative stress causes mucin synthesis via transactivation of epidermal growth factor receptor: role of neutrophils. J Immunol 2000;164:1546–1552. [DOI] [PubMed] [Google Scholar]
- 23.Takeyama K, Jung B, Shim JJ, Burgel PR, Dao-Pick T, Ueki IF, Protin U, Kroschel, Nadel JA. Activation of epidermal growth factor receptors is responsible for mucin synthesis induced by cigarette smoke. Am J Physiol Lung Cell Mol Physiol 2001;280:L165–L172. [DOI] [PubMed] [Google Scholar]
- 24.Takeyama K, Fahy JV, Nadel JA. Relationship of epidermal growth factor receptors to goblet cell production in human bronchi. Am J Respir Crit Care Med 2001;163:511–516. [DOI] [PubMed] [Google Scholar]
- 25.Lee HM, Takeyama K, Dabbagh K, Lausier JA, Ueki IF, Nadel JA. Agarose plug instillation causes goblet cell metaplasia by activating EGF receptors in rat airways. Am J Physiol Lung Cell Mol Physiol 2000;278:L185–L192. [DOI] [PubMed] [Google Scholar]
- 26.Burgel PR, Escudier E, Coste A, Dao-Pick T, Ueki IF, Takeyama K, Shim JJ, Murr AH, Nadel JA. Relation of epidermal growth factor receptor expression to goblet cell hyperplasia in nasal polyps. J Allergy Clin Immunol 2000;106:705–712. [DOI] [PubMed] [Google Scholar]
- 27.Zielinski J, MacNee W, Wedzicha J, Ambrosino N, Braghiroli A, Dolensky J, Howard P, Gorzelak K, Lahdensuo A, Strom K, et al. Causes of death in patients with COPD and chronic respiratory failure. Monaldi Arch Chest Dis 1997;52:43–47. [PubMed] [Google Scholar]
- 28.Tuomanen E, Austrian R, Masure HR. Pathogenesis of pneumococcal infection. N Engl J Med 1995;332:1280–1284. [DOI] [PubMed] [Google Scholar]
- 29.Sethi S, Evans N, Grant BJ, Murphy TF. New strains of bacteria and exacerbations of chronic obstructive pulmonary disease. N Engl J Med 2002;347:465–471. [DOI] [PubMed] [Google Scholar]
- 30.Murphy TF, Brauer AL, Grant BJ, Sethi S. Moraxella catarrhalis in chronic obstructive pulmonary disease: burden of disease and immune response. Am J Respir Crit Care Med 2005;172:195–199. [DOI] [PMC free article] [PubMed] [Google Scholar]