Abstract
Acute lung injury (ALI) is characterized by an early inflammatory response followed by a late fibroproliferative phase, and by an increase in the bronchoalveolar lavage fluid (BALF) concentrations of bioactive soluble FasL (sFasL). Activation of Fas (CD95) has been associated with the development of lung fibrosis in mice. The goal of this study was to determine the mechanisms that link Fas activation with the development of fibrosis in the lungs. We treated mice with three daily intratracheal instillations of a Fas-activating monoclonal antibody (Jo2) or a control IgG, and studied the animals at sequential times. Mice treated with Jo2 had increased caspase-3 activation in alveolar wall cells on Days 2, 4, and 7; an inflammatory response peaking on Day 7, and increased total lung collagen on Day 21. Gene expression profiling performed on Days 2, 4, and 7 showed sequential activation of co-regulated profibrotic genes, including marked up-regulation of matrix metalloproteinase 12 (MMP-12). Targeted deletion of MMP-12 protected mice from Fas-induced pulmonary fibrosis, even though the inflammatory responses in the lungs were similar to those of wild-type mice. Compared with wild-type mice, the mmp12−/− mice showed decreased expression of the profibrotic genes egr1 and cyr61. We conclude that Fas activation in the lungs induces a complex response that includes apoptosis, inflammation, and eventually fibrosis, and that MMP-12 is essential for the fibrotic phenotype. We speculate that MMP-12 activity is required for activation of the profibrotic genes egr1 and cyr61.
Keywords: apoptosis, inflammation, MMP-12, Fas, CYR61/CCN1
CLINICAL RELEVANCE
This study provides novel information suggesting that macrophage activation drives the lung fibrotic response to Fas activation, and that matrix metalloproteinase-12, a metalloelastase previously associated with emphysema, is essential for Fas-induced fibroproliferation.
Acute lung injury (ALI) and its more severe form, the acute respiratory distress syndrome (ARDS), are characterized by an early inflammatory response, which is maximal during the first three days of clinically defined ALI/ARDS, and a subsequent fibroproliferative response (1, 2). The fibroproliferative response is thought to occur late in the course of ALI/ARDS, but autopsy studies have shown histopathologic evidence of fibroproliferation as early as Day 5, and human studies have identified evidence of collagen production in the lungs even on the first day of ALI/ARDS (3, 4). However, the mechanisms linking the early events in acute lung injury with subsequent fibrosis are incompletely understood.
The Fas receptor (CD95) is a membrane surface death receptor that can initiate apoptotic signaling upon binding to its cognate ligand, FasL (CD178). FasL (CD 178) is a 40-kD type II protein that exists as membrane-bound and soluble forms. The soluble form (sFasL) can result from enzymatic cleavage of membrane bound FasL by enzymes such as matrix metalloproteinase-7 (MMP-7, matrilysin), or from rapid release by activated monocytes and monocyte-derived macrophages (5, 6). In the lungs, FasL is present on macrophages, lymphocytes, neutrophils, and epithelial cells, whereas Fas is present on bronchial and alveolar epithelial cells, macrophages, and fibroblasts (7–11). Both the membrane-bound and the soluble forms of FasL can activate the Fas receptor, and in humans with ALI/ARDS, bioactive sFasL present in the BAL fluid is responsible for the majority of the pro-apoptotic effect of ALI/ARDS BAL fluid on normal human lung epithelial cells in vitro (12).
The Fas/FasL system is considered a prototypical apoptosis pathway involved in resolution of inflammation by inducing apoptosis of neutrophils (13). However, activation of the Fas/FasL system can also induce activation of a number of proinflammatory pathways, including those triggered by translocation of NF-κB, and those associated with activation of the IL-1β–converting enzyme (ICE, Caspase-1) (14, 15). In the lungs, the specific effects of activation of the Fas/FasL system depend on the target cell, and include apoptosis in distal lung epithelial cells and alveolar type II cells; cytokine release in alveolar macrophages, and proliferation or apoptosis in fibroblasts (7, 10, 16). In mice, short-term administration of the Fas-activating antibody Jo2 is characterized by an inflammatory response with up-regulation of the cytokines macrophage inflammatory protein (MIP)-2, monocyte chemotactic protein (MCP)-1, TNF-α, and IL-6 (17). Long-term administration of high doses of aerosolized Jo2 mAb every other day for 14 d results in an increase in lung hydroxyproline content, and an increase in TGF-β1 expression (18). Thus, the Fas/FasL system links cell death, cell activation, and fibroproliferation in the lungs.
In a prior study in humans, we found that the concentration of biologically active sFasL is increased in the lungs for the first three days after the onset of ALI (12). To investigate the biological relevance of this finding, in this study we activated Fas in mouse lungs by treating mice with intratracheal Jo2 mAb for three consecutive days, and then studied the pulmonary responses at times that reflect key events in human lung injury. We found that activation of the Fas receptor in the lungs is associated with acute lung inflammation followed by delayed fibrosis. The fibrotic response was mediated by a mechanism involving not only cell death, but also activation of proinflammatory and profibrotic programs of gene expression detectable through microarray analyses. Notably, we identified macrophage MMP-12 as one of the most differentially regulated genes in response to Fas activation. We then confirmed that MMP-12 is a prominent mediator in the fibrotic response following Fas activation by demonstrating that MMP-12–null mice (mmp12−/−) are protected from Fas-induced fibrosis. These studies demonstrate that activation of the Fas/FasL system for a brief period of time induces lung injury that is followed by fibrosis, and that MMP-12 is essential for the development of this fibrotic response.
MATERIALS AND METHODS
Animal Protocols
The animal protocols were approved by the Animal Care Committee of the VA Puget Sound Healthcare System (Seattle, WA). Briefly, male mice weighing 25–30 g were anesthetized with inhaled isoflurane, and then placed on an inclined surface. The larynx was visualized and intubated with a gavage tube attached to a 1.0-ml syringe containing 100 μl of water. Intubation of the trachea was verified by movement of the water bubble with the animal's respiratory efforts. After endotracheal instillation was confirmed, 2.5 ml/g of azide-free, LPS-free Jo2 mAb or an isotype control antibody (both from PharMingen, San Diego, CA) were instilled in three separate aliquots. After instillation, the gavage tube was removed, and the mice were returned to their cages, allowed to recover from anesthesia, and provided with free access to food and water.
The mice were monitored on a daily basis until studied, when they were killed with an intraperitoneal injection of pentobarbital (120 mg/kg) and exsanguinated by closed intracardiac puncture. The thorax was opened and the trachea cannulated and secured. The left hilum was clamped and the left lung was removed and placed in a tube containing 1 ml sterile H2O, for later homogenization. In some mice the left lung was cut into small cubes for RNA extraction.
After removing the left lung, bronchoalveolar lavage (BAL) of the right lung was performed by instilling three separate aliquots of 0.5 ml 0.9% NaCl containing 0.6 mM EDTA. The aliquots were pooled and placed on ice. Immediately after the BAL procedure, the right lung was fixed with 4% paraformaldehyde at 15 cm of water for histologic analysis.
Mice were killed prior to the study endpoint if they showed signed of distress, as determined by the following criteria: loss of > 15% of their body weight at any time during the experiment, dehydration (evaluated by skin tenting test), lethargy, abnormal posture such as hunching and ruffled fur, pale eyes, or loose stools.
Experimental Design
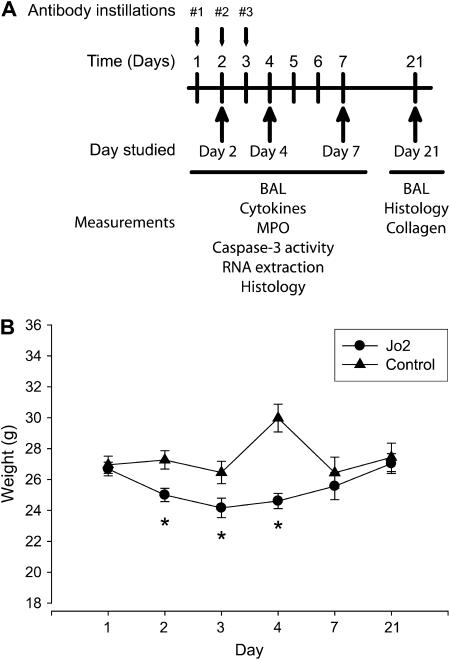
C57BL/6 mice (wild type [WT]) or mice carrying a targeted deletion of the MMP-12 gene (mmp12−/− mice, B6 background) were obtained from the Jackson Laboratories (Bar Harbor, ME). The mice were treated with daily intratracheal instillations of the Fas-activating mAb Jo2 (2.5 ng/g) or an isotype-matched control mAb on two or three consecutive days (Figure 1A). We studied four groups of mice: mice in the “Day 2 group” received instillations on Days 1 and 2, and were killed six hours after the second instillation. Mice in the “Day 4” group received instillations on Days 1, 2, and 3 and were killed on Day 4. Mice in the “Day 7” and “Day 21” groups received instillations on Days 1, 2, and 3 and were killed on Days 7 or 21.
Figure 1.
Experimental design and body weight changes. (A) Mice were treated with up to three consecutive daily intratracheal instillations of Jo2 mAb or an isotype control mAb and then studied on Days 2, 7, 4, or 21 after the first instillation. Both antibodies were given at a dose of 2.5 μg/g. (B) Changes in weight (g) of C57Bl/6 mice after three consecutive daily intratracheal instillations of Jo2 mAb or a control mAb. The mice treated with Jo2 mAb had significantly lower weights from Days 2–4, indicating worse clinical status. *P < 0.05 compared with control mAb.
Sample Processing
The BAL fluid (BALF) aliquots from each mouse were pooled, and an aliquot was processed immediately for total cell counts using a hemacytometer. Differential cell counts were performed on cytospin preparations stained with the Diff-quick method, and a minimum of 200 cells were counted. The remainder of the BALF was spun at 200 × g and the supernatants were stored in individual aliquots at −70°C.
In most animals, the left lung was weighed and then homogenized in 1.0 ml of sterile distilled H2O, using a hand-held homogenizer. The lung homogenate was divided into aliquots for later cytokine, caspase-3 activity, and myeloperoxidase (MPO) measurements. For cytokine and caspase-3 activity measurements, an aliquot of the lung homogenate was vigorously mixed with a buffer containing 0.5% Triton-X-100, 150 mM NaCl, 15 mM Tris, 1 mM CaCl2, and 1mM MgCl2, pH 7.40, incubated for 30 min at 4°C, and then spun at 10,000 × g for 20 min; the supernatants were stored at −70°C. For MPO measurements, the homogenate was vigorously mixed with 50 mM potassium phosphate, pH 6.0, with 5% hexadecyltrimethyl ammonium bromide (Sigma-Aldrich, St. Louis, MO) and 5 mM EDTA. The mixture was sonicated and spun at 12,000 × g for 15 min at 25°C, and the supernatant was stored at −70°C.
In some animals the lungs were cut rapidly into small cubes and placed in 1.0 ml of RNA-later (Ambion, Inc., Austin, TX) and stored overnight at 4°C. Then the lung cubes were homogenized with RNA lysis buffer (Stratagene, La Jolla, CA) and stored at −80°C until the RNA was extracted.
In the animals killed at 21 d the left lung was removed, weighed, and processed for collagen measurements as described below; the right lung was fixed and embedded in paraformaldehyde. No homogenates were generated on the mice studied on Day 21.
Measurements
Myeloperoxidase.
Myeloperoxidase was measured in lung homogenates using the Amplex Red fluorometric assay, following instructions from the manufacturer (Molecular Probes, Eugene, OR).
Cytokine assays.
The concentrations of MIP-2, KC, IL-1β, IL-6, IL-10, IL-12, IFN-γ, TNF-α, and granulocyte macrophage colony-stimulating factor (GM-CSF) were measured simultaneously in lung homogenate supernatants using a cytometric bead-based immunoassay system (Luminex, Austin, TX) according to the manufacturer's protocols (R&D Systems, Minneapolis, MN).
Lung alveolar permeability.
The total protein concentration in BALF was measured using the bicinchoninic acid method (BCA assay, Pierce Co., Rockford, IL). The concentration of IgM in BALF was determined using a specific mouse immunoassay (R&D Systems). The lower limit of the assay was 7.8 pg/ml.
Caspase-3 activity.
Caspase-3 activity in lung homogenates was measured with the Caspase-3/CPP32 Fluorometric Assay kit (BioVision, Mountain View, CA). Fifty-microliter aliquots of lung homogenate were diluted 1:2 with assay reaction buffer containing 10 mM DTT. The caspase-3 substrate DEDV-AFC was added to each substrate at a final concentration of 50 μM. After 2 h incubation at 37°C, fluorescence was read with a fluorometer using 400 nm excitation and 505-nm detection filters. Results are shown as arbitrary fluorescence units.
Total collagen measurements.
The lungs were cut into small cubes and lyophilized for 18 h at −4°C. After lyophilization, distilled H2O was added to each sample to reach a final weight of 15 mg. The samples were homogenized and spun at 10,000 × g for 10 min at 4°C. The pellets were discarded and the supernatants were used for collagen measurements using the Sircol collagen assay, according to the instructions of the manufacturer (Accurate Chemical and Scientific Corporation, Westbury, NY). Briefly, collagen standards were made with type I collagen. Then 1.0 ml of Sircol dye reagent was added to each sample and standard and mixed gently. The tubes containing the samples were then placed in a mechanical shaker for 30 min (Belco Glass Inc., Violyn, NJ). The samples were spun at 10,000 × g for 10 min at room temperature. The supernatants containing the unbound dye were discarded and the tube was blotted dry with tissue paper. One milliliter of Sircol alkaline reagent was added to the pellet, which was then resuspended by vortexing for 10 min to release the bound dye. Afterwards the supernatants were analyzed using a spectrophotometer at a wavelength of 540 nm (Beckman Coulter, Fullerton, CA).
Gene Expression Measurements
Gene expression arrays.
Total RNA isolated from the left lungs of Jo2 or isotype-matched control treated from mice studied on Days 2, 4, and 7 was converted to cDNA, and biotin-labeled cRNA was generated by in vitro transcription (n = 3 for each day and treatment condition). The labeled cRNA was hybridized to 74Av2 mouse oligonucleotide microarrays (Affymetrix, Santa Clara, CA). Each mouse sample was hybridized to a separate microarray (total of 18 separate arrays), and each array yielded a high-quality hybridization. The raw intensity data were transformed (log2) and normalized using the GCRMA program (http://bioconductor.org) (19). The results from Jo2-treated and control animals were compared on each day. Genes that yielded P values < 0.0005 and that were increased or decreased by at least 2-fold from the control animals were selected for unsupervised cluster analysis (∼ 794 genes out of 12,500 tested on the chips). The selected genes were subjected to hierarchical cluster analysis by Euclidean distance and complete linkage using the CLUSTER program (20). The gene expression data clustered by genes and grouped by day of killing and treatment were visualized in a heat map format using the TreeView program (http://rana.lbl.gov/EisenSoftware.htm). The cluster analysis algorithm identified “nodes” (clusters) of genes with similar expression patterns across the 3 d. Known biologic functions were assigned to each gene according to the Gene Ontology (GO) database.
Quantitative PCR.
RNA was isolated using the RT-PCR Miniprep Kit (Stratagene). RNA was reverse-transcribed to cDNA using High Capacity Archive Kit and realtime PCR was performed on each sample in triplicate using Assay-on-demand probe and primer set for mouse MMP-12 (ID: Mm00500554_m1), human MMP-12 (ID: Hs00159178_m1) and TaqMan master mix (ABI, Foster City, CA) Analysis of the amplification was performed using the delta-delta threshold cycle (ddCt) method with18s rRNA (ID: Hs99999901_s1) as the endogenous control, and universal mouse RNA (Strategene) was used as the calibrator.
MMP-12 expression in human alveolar macrophages.
The protocols were approved by the Human Subjects Review Committee of the University of Washington, and informed consent was obtained from the volunteers. BAL was performed in normal, nonsmoking human volunteers, and alveolar macrophages were isolated by plastic adherence to 96-well plates (5 × 104 cells/well) as previously described (21). The cells were incubated for at 37°C, 5% CO2 in RPMI 1640 containing 100 U/ml penicillin, 100 g/ml streptomycin, 5% FCS, and supplemented with either 10 μg/ml of the CH-11 mAb or an isotype control antibody (both from Upstate, Charlottesville, VA). CH-11 specifically activates human Fas (22–24). After 24 h, total RNA was isolated from the cells and MMP-12 mRNA was quantified by real-time PCR as described above, except that the average ddCt of the control group was used as the calibrator.
Histopathology
Caspase-3 immunohistochemistry.
Immunohistochemistry was performed using the Vector “Elite” ABC-HP kit (Vector, Burlingame, CA). Briefly, the lung sections were deparaffinized by heating at 57°C for 60 min, and rehydrated by washing twice in Clear Rite (Richard Allan Scientific, Kalamazoo, MI), twice in 100% ethanol for 3 min, twice in 95% ethanol for 3 min, and once in dH2O for 5 min. The slides were rinsed twice with PBS for 5 min and the samples digested with citric buffer (Vector) in a microwave for 15 min at medium setting. After digestion, the slides were cooled to room temperature for 10 min, rinsed twice with PBS for 5 min, and blocked for 60 min with 3% normal goat serum in nonfat milk, at room temperature. The samples were labeled overnight in a moist chamber at 4°C using rabbit anti-active caspase-3 (PharMingen, San Diego, CA), which labels epitopes on cleaved caspase-3. Next, the slides were rinsed twice with PBS and labeled with goat anti-rabbit biotinylated antibody for 2 h at room temperature. The slides were rinsed twice with PBS, incubated with 0.3% H2O2 in methanol for 60 min to block endogenous peroxidases, and then rinsed twice with PBS. The samples were labeled with ABC-HP and incubated in a moist chamber for 90 min at room temperature, rinsed twice with PBS, and developed in a moist chamber with diaminobenzidine (DAB) substrate (Sigma) for 12 min in the dark at room temperature. The slides were rinsed with running deionized H2O for 5 min and counterstained with 1% methyl green for 6 min, then dehydrated with ethanol, incubated in xylene for 5 min, and mounted with Permount.
Alveolar macrophage immunohistochemistry.
Tissue sections were deparaffinized by heating at 57°C for 60 min, then rehydrated by washing 5 min in 100% xylene, 3 min in 100% ethanol, 3 min in 95% ethanol, and 5 min in dH2O. After washing three times in PBS, the tissues were permeabilized with 0.3% triton-X for 30 min at room temperature, washed three times in PBS, and quenched with Peroxo-block (Zymed, San Francisco, CA) for 90 s at room temperature. The slides were washed three times in PBS, and then blocked with Serum-Free Protein Block (Dako, Carpinteria, CA) for 30 min at room temperature. Immediately after blocking, the tissues were labeled with rat anti-Mac2 antibody at 1:2,000 dilution (Accurate, Westbury NY) in a moist chamber overnight at 4°C. Upon completion of primary antibody staining, tissues were washed three times with PBS, and labeled with biotinylated Mouse anti-Rat IgG2a, 1:100 in PBS (Zymed) for 30 min at room temperature. The samples were labeled with streptavidin–horseradish peroxidase conjugate (Zymed) at room temperature for 10 min, washed two times with PBS, and developed with ice-cold AEC Peroxidase Substrate (Zymed) for 7.5 min. After developing, the slides were rinsed 5 min in dH2O, washed twice in PBS, counterstained with 1% methyl green for 6 min, rinsed 5 min in dH2O, and mounted with aqueous GVA mount (Zymed).
Sirius red collagen stain:
The tissues were deparaffinized by immersion in xylene for 3 min, then rehydrated by submerging twice in 100% ethanol for 3 min, twice in 95% ethyl alcohol, and finally in distilled H2O for 3 min. The nuclei were stained by submerging the slides in Weigert iron hematoxylin for 20 min, then the slides were washed in two changes of tap water. After washing, the slides were submerged for 1 h in a 0.1% wt/vol solution of Sirius red F3B (Roboz Surgical Instrument Company, Rockville, MD) in saturated picric acid (Sigma), then washed twice in acidified water, dehydrated in ethanol, cleared with xylene for 5 min, and mounted with Permount.
The tissue sections (hematoxylin and eosin [H&E] stains) and the immunohistochemistry slides were read by an observer who was unaware of the treatment groups.
Statistical Analysis
Analysis of data from multiple groups was performed by one-way analysis of variance (ANOVA). Fischer's least significant difference post hoc procedure was performed to determine significance between individual groups. In the text and tables, data are shown as means ± SEM. The figures show box plots, in which the box extends from the 25th to 75th percentiles, the middle line depicts the median, and the upper and lower bars show the 90th and the 10th percentiles, respectively. The dots represent individual data points.
RESULTS
Clinical Outcome
A total of 47 mice were treated with the Jo2 mAb (Figure 1A). Of these, 17 (36.2%) died prematurely, usually after one or two instillations of Jo2. A total of 30 mice were treated with the control mAb, and none died. We measured changes in body weight as an indication of the clinical status of the mice. The animals treated with Jo2 mAb lost significant amounts of weight on Days 2, 3, and 4 of the experimental protocol. The surviving animals returned to normal body weight by Days 7 and 21 (Figure 1B).
Tissue and Collagen Responses to Fas Activation in the Lungs
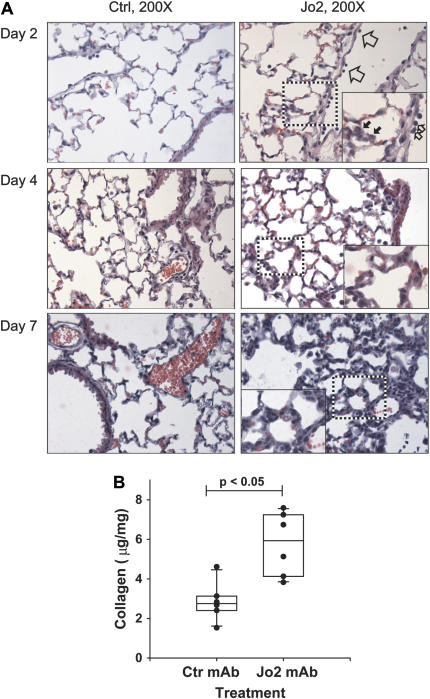
Microscopic analysis of the lungs of the mice showed that on Day 2, mice treated with the Jo2 mAb had neutrophils adhering to the endothelium of venules in the alveolar walls, whereas mice treated with the control antibody had normal lung histology (Figure 2A). On Day 4, the lungs of the mice treated with Jo2 mAb showed thickening of the alveolar walls. By Day 7 the thickening of the alveolar walls had progressed, and there was evidence of cellular proliferation in the interstitium. In contrast, the mice treated with the control antibody had normal lung histology at all times.
Figure 2.
Tissue and collagen response to Fas activation. (A) Tissue response. Mice treated with control mAb showed normal lung histology at all times (left panels). On Day 2, the lungs of mice treated with Jo2 mAb showed increased neutrophil adhesion to the vascular endothelium (open arrows) and increased neutrophils within the alveolar walls (solid arrows). By Day 4, the lungs showed thickening of the alveolar walls, which became more pronounced by Day 7. H&E stain (×400; insets = ×600). (B) Total collagen content in the lungs of mice treated with three consecutive daily intratracheal instillations of either Jo2 mAb, 2.5 μg/g (n = 6), or an isotype control mAb (2.5 μg/g) (n = 6), then killed 21 d after the first instillation. Collagen was measured using the Sircoll Red assay as described in Materials and Methods.
To measure the late fibrotic response in the lungs, we followed some of the animals for up to 21 d after the beginning of the Jo2 treatments. At 21 d, there was a significant increase in the total lung collagen content of the Jo2-treated mice (5.8 ± 0.7 μg/mg versus 2.9 ± 0.4 μg/gm) (Figure 2B). Thus, Fas activation during three consecutive days is sufficient to induce collagen accumulation.
Gene Expression Profiling
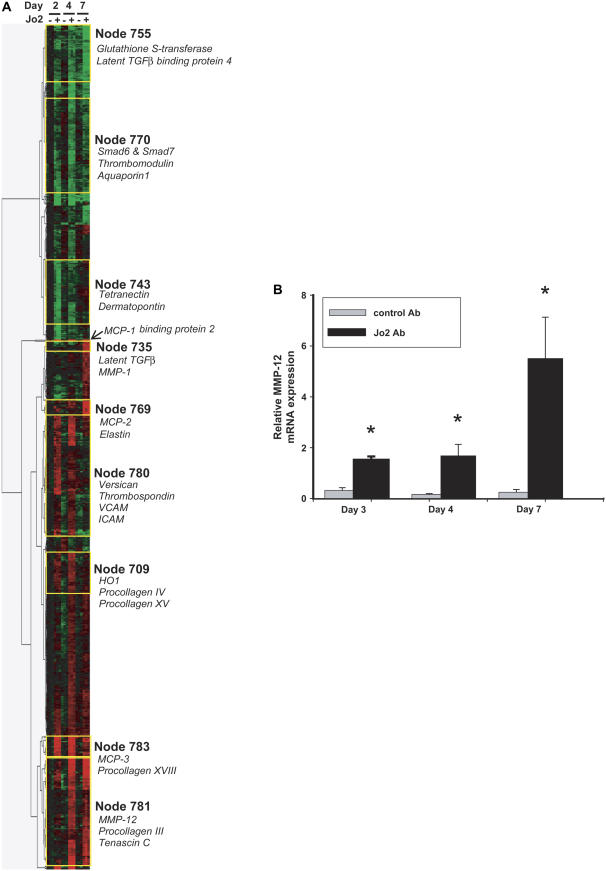
To identify gene expression programs activated by Jo2 administration, we used oligonucleotide microarrays to measure gene expression in whole lung homogenates of mice treated with Jo2 or control mAb and killed on Days 2, 4, and 7 (n = 3/group) after the first instillation. After filtering for genes highly differentially expressed between the Jo2 and isotype control-treated animals (> 2-fold difference for ⩾ 1 group, P < 0.0005) we used hierarchical cluster analysis to identify groups of genes (nodes) with similar expression patterns (Figure 3A). Fas activation by the Jo2 mAb induced four main patterns of gene expression: early up-regulation (e.g., Node 780), late up-regulation (e.g., Nodes 735 and 781), early down-regulation (e.g., Node 743) and late down-regulation (e.g., Node 755). These findings demonstrate that Fas activation not only induces apoptosis in some lung cells, but also affects gene expression in surviving cells, even several days after activation of Fas.
Figure 3.
Gene expression profiling. (A) Hierarchical cluster analysis of gene expression profiles generated from treated with intratracheal instillations of Jo2 mAb (n = 3/d) or an isotype control mAb (Ctrl, n = 3/d), and killed after two instillations (Day 2), 1 d after three daily instillations (Day 4), or 4 d after three daily instillations (Day 7). The analysis was done on 794 genes that were increased (red) or decreased (green) at least 2-fold in the Jo2-treated mice, compared with the control mice, with a P < 0.0005. Selected clusters or “nodes” of genes with similar expression patterns are highlighted. (B) Expression of MMP-12 by real-time PCR in mouse lung RNA. Gene expression was analyzed by the ΔΔ CT method, using 18s rRNA as the reference gene. The vertical axis shows “relative MMP-12 mRNA expression,” which is the n-fold increase in MMP-12 expression over the average delta CT of mouse universal reference RNA (n = 3 mice/group); individual bars show the mean ± SEM. *P < 0.05 compared to control mAb.
Analysis of individual genes of interest showed significant upregulation of matrix-related proteins and proteinases, including MMP-12 (Days 4 and 7), versican (Day 2), and procollagens XV and XVIII (Day 4) (Table 1). Genes that were upregulated early included MCP-1 (Day 2) and several TGF-β–related genes, including HO-1, tropoelastin, and tenascin. In contrast, the TGF-β–negative regulators SMAD6 and SMAD7 were down-regulated. Thus, genome-wide measurements of gene expression suggest an early phase favoring TGF-β signaling, followed by increased expression of certain metalloproteinases and procollagens. Of particular interest, MMP-12, a metalloproteinase associated with the development of emphysema but not of fibrosis, was among the genes showing the strongest up-regulation (25).
TABLE 1.
SELECTED GENES DIFFERENTIALLY EXPRESSED IN C57BL/6 MICE TREATED WITH Jo2 mAb OR A NONSPECIFIC ISOTYPE CONTROL mAb
| Day 2
|
Day 4
|
Day 7
|
||||
|---|---|---|---|---|---|---|
| Class/Gene | Change | P Value | Change | P Value | Change | P Value |
| Matrix Metalloproteinases | ||||||
| MMP-1/3 | 0.03 | 0.9175 | −0.10 | 0.7549 | −1.92 | 0.00001 |
| MMP -2 | 0.05 | 0.5293 | −0.11 | 0.1939 | −0.20 | 0.0225 |
| MMP- 7 | 0.06 | 0.4976 | −0.18 | 0.687 | −0.14 | 0.1416 |
| MMP-9 | 1.35 | 0.0701 | 0.30 | 0.6663 | −1.19 | 0.1052 |
| MMP-12 | 1.34 | 0.0148 | 3.20 | 0.00001 | 3.80 | 0.00001 |
| Tissue Inhibitors of Matrix Metalloproteinases | ||||||
| TIMP-1 | 0.06 | 0.4246 | −0.16 | 0.0629 | −0.23 | 0.0111 |
| TIMP-2 | 0.98 | 0.0292 | −0.21 | 0.5968 | −0.61 | 0.1468 |
| TIMP-3 | 0.52 | 0.0135 | 0.19 | 0.32 | −0.73 | 0.0016 |
| Procollagens | ||||||
| Procollagen, type I, a1 | −0.26 | 0.4873 | −0.21 | 0.5811 | 1.46 | 0.0019 |
| Procollagen, type I, a2 | 0.00 | 0.9964 | −0.41 | 0.1987 | 1.28 | 0.0011 |
| Procollagen, type III, a1 | 0.10 | 0.7316 | 0.01 | 0.9626 | 1.83 | 0.00001 |
| Procollagen, type IV, a2 | 1.55 | 0.00001 | 1.09 | 0.00001 | 0.63 | 0.0062 |
| Procollagen, type IV, a1 | 1.40 | 0.00001 | 1.13 | 0.0003 | 1.04 | 0.0006 |
| Procollagen, type XVIII,a1 | 3.14 | 0.00001 | 3.05 | 0.00001 | 1.42 | 0.0001 |
| Laminin 5 components | ||||||
| Laminin, alpha 3 | −0.05 | 0.8157 | −1.13 | 0.0003 | −0.49 | 0.0513 |
| Laminin, beta 3 | 0.34 | 0.06555 | −0.43 | 0.026 | −0.20 | 0.2673 |
| Laminin, gamma 2 | 0.14 | 0.4599 | −0.58 | 0.01 | −0.02 | 0.922 |
Definition of abbreviations: mAb, monoclonal antibody; MMP, matrix metalloproteinase; TIMP, tissue inhibitor of matrix metalloproteinase.
To confirm the array data suggesting MMP-12 up-regulation, we measured MMP-12 mRNA expression in the lungs using quantitative real-time PCR. As compared with control mice, the Jo2-treated mice showed significant up-regulation of MMP-12 mRNA on Day 2 (4.8-fold increase), Day 4 (10.5-fold increase), and Day 7 (22.4-fold increase) (Figure 3B).
Fas Activation Directly Stimulates MMP-12 Expression in Alveolar Macrophages
Because MMP-12 is a product of activated alveolar macrophages and engagement of membrane Fas is a proinflammatory stimulus in human macrophages (10), we investigated whether Fas activation was capable of directly stimulating MMP-12 expression in alveolar macrophages. Normal human alveolar macrophages incubated for 24 h with the human-specific Fas-activating antibody CH-11 showed a 6.4-fold increase in MMP-12 mRNA, as compared with macrophages incubated with a control antibody (P < 0.05). These data demonstrate that activation of the Fas pathway directly stimulates the expression of MMP-12 in lung alveolar macrophages in vitro.
mmp12−/− Mice Are Protected from Jo2-Induced Lung Fibrosis
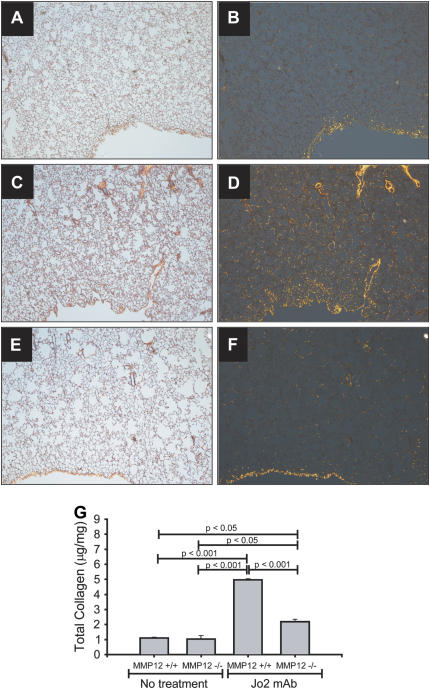
We then used mmp12−/− mice to establish a role for MMP-12 in Fas-induced fibrosis. Wild-type (WT, C57BL/6) and mmp12−/− mice were treated with three doses of Jo2 mAb and and studied according to the protocol shown in Figure 1A. The overall mortality of the mmp12−/− mice was 38.3%; all deaths occurred within 3 d. On Day 21, Sirius red staining showed a strong collagen signal in the WT mice, yet only a minimal signal in mmp12−/− mice (Figure 4A–4F). These histochemical observations were verified by measurement of total lung collagen content, which was 5.0 ± 0.08 μg/mg in the WT mice versus 2.2 ± 0.2 μg/mg in the mmp12−/− mice (P < 0.001) (Figure 4G). The degree of collagen deposition in this model was similar to that observed in mice treated with a standard dose of intratracheal bleomycin (0.0035 U/gm body weight) and studied 21 d (3.7 ± 0.7 μg/mg). These findings strongly suggest that MMP-12 is necessary for the development of Fas-mediated lung fibrosis.
Figure 4.
Collagen response in mmp12−/− mice. Lung sections from mice treated with intratracheal Jo2 mAb for three consecutive days, then killed 21 d after the first instillation (A–F). The tissues were stained with Sirius Red, which stains collagen bright orange under polarized light (right panels). Untreated WT mice have a signal mostly at the pleural surface (A, B). WT mice treated with Jo2 mAb show a marked increase in the orange signal (C, D). In contrast, mmp12−/− mice show markedly less orange signal for collagen (E, F) (×40). Whole collagen content in whole lungs was confirmed using Sircoll collagen assay (G).
The Inflammatory Response in WT and mmp2−/− Mice
Alveolar inflammation, epithelial cell apoptosis and cell proliferation are all thought to be involved in the development of fibrosis after experimental acute lung injury. Given that the lack of MMP-12 could impair macrophage migration into the lungs, resulting in milder inflammation and repair (25) we investigated whether the protection from fibrosis seen in the mmp12−/− mice on Day 21 was associated with decreased inflammatory or apoptotic responses at earlier times.
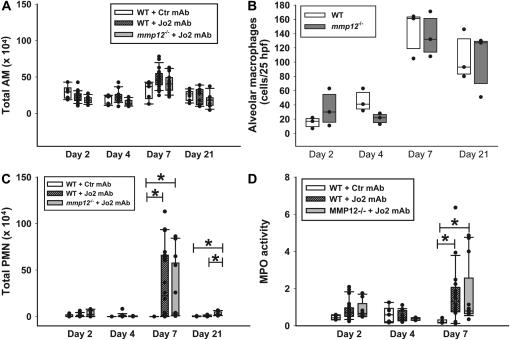
The BALF macrophage counts were similar in WT and mmp12−/− mice at all times tested, regardless of treatment (Figure 5A). Because the BALF macrophages may not represent the total tissue macrophages, we counted macrophages labeled with an antibody against the macrophage-specific antigen mac2 in tissue sections obtained from a subset of the Jo2-treated mice (n = 3/group/d). The macrophage numbers increased after Jo2 treatment; however, there were no significant differences in tissue macrophages between WT and mmp12−/− mice at each of the days tested (Figure 5B). Thus, we could not find evidence that the protection against fibrosis seen in the mmp12−/− mice was due to an impaired macrophage response.
Figure 5.
Cellular responses. (A) Total alveolar macrophages (AM) in the BAL fluid of C57BL/6 mice (WT) treated with Jo2 mAb (hatched bars) or a control mAb (open bars), or mmp12−/− mice treated with Jo2 mAb (shaded bars). Instillations were performed as per the protocol shown in Figure 1. (B) Number of alveolar macrophages in 24 randomly generated high-power fields (×400) from lung tissue sections obtained from C57BL/6 mice (open bars) or mmp12−/− mice (shaded bars) treated with Jo2 mAb (n = 3/group). Macrophages were identified using immunohistochemistry, as described in Materials and Methods. (C) Total PMN in BAL fluids from the same mice shown in A; (D) MPO activity in lung homogenates. MPO is a measurement of the total neutrophil content of the lung. *P < 0.05.
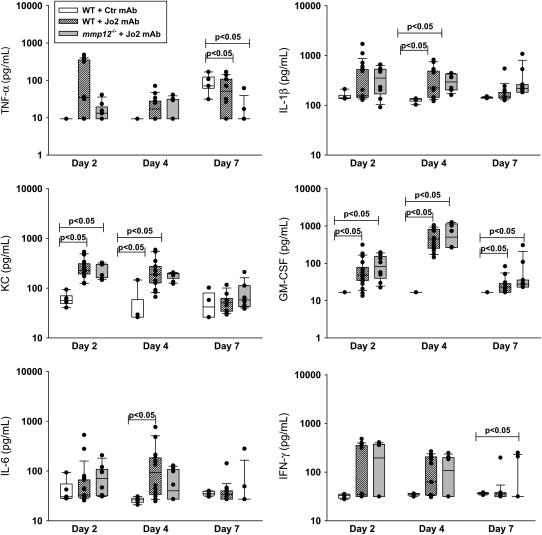
To determine whether the lack of MMP-12 was associated with impaired neutrophilic or cytokine responses to Fas activation, we measured the total BAL PMN, lung homogenate MPO activity, and cytokines in lung tissue. As compared with WT mice treated with control IgG, the administration of Jo2 resulted in an early cytokine response on Days 2 and 4, followed by a neutrophilic response that peaked on Day 7 (Figures 5C, 5D, and 6). However, both the cytokine and the neutrophilic responses were similar in the WT and mmp12−/− mice treated with Jo2.
Figure 6.
Cytokine responses. Concentrations of TNF-α, IL-1β, KC, GM-CSF, IL-6, and IFN-γ in whole lung homogenates from WT mice treated with Jo2 mAb or an isotype control mAb, and mmp12−/− mice treated with intratracheal instillations of Jo2 mAb as per the protocol shown in Figure 1.
The Apoptotic Response in WT and mmp12−/− Mice
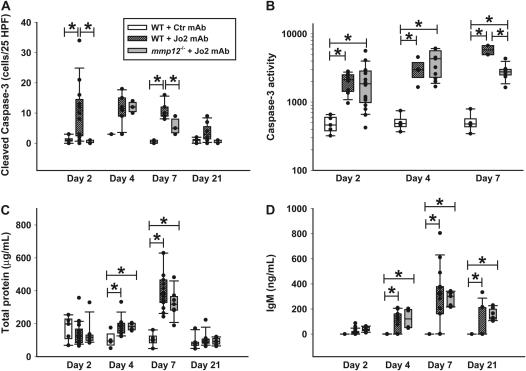
To evaluate the extent of alveolar epithelial apoptosis induced by the Jo2 mAb treatment, we counted the number of cells in the alveolar walls that expressed cleaved (activated) caspase-3, using IHC. The Jo2-treated mmp12−/− mice had significantly less apoptotic cells in the alveolar walls on Days 2 and 7, compared with the WT mice treated with Jo2 mAb (Figure 7A).
Figure 7.
Markers of alveolar epithelial injury. C57BL/6 mice were treated with a Jo2 mAb or with an isotype control mAb, and MMP12-/- mice were treated with Jo2 mAb, according to the protocol shown in Figure 1. (A) Number of alveolar wall cells staining for cleaved caspase-3 in 25 randomly generated high-power fields (×400). (B) Caspase-3 activity in lung homogenates from mice studied on Days 2, 4, and 7. The units are arbitrary fluorescence units reflecting release in the yellow fluorescence by 7-amino-4-trifluoreomethylcoumarin (AFC) cleaved from the peptide DEDV-AFC by caspase-3. (C) Total protein concentration in the BALF. (D) IgM concentration in the BALF. Comparisons were done only within each day. *P < 0.05.
To confirm the IHC data, we measured caspase-3 activity in whole lung homogenates (Figure 7B). Compared with mice treated with control mAb, Jo2 induced a significant increase in whole lung caspase-3 activity on Days 2, 4, and 7. On Day 7, the mmp12−/− mice showed significantly less caspase-3 activity than the WT mice, although it remained elevated compared with mice treated with control mAb.
As a functional measurement of alveolar epithelial disruption, we measured total protein and IgM in BAL fluids. The protein and IgM concentrations increased in all of the animals, peaking on Day 7 after the first Jo2 treatment, and no differences were identified between WT and MMP12-/- animals in either the time course or the magnitude of the responses (Figures 7C –and 7D).
Differential Gene Expression in WT and mmp12−/− Mice
Because the protection seen in the mmp12−/− mice was not explained by differences in inflammation or epithelial permeability, we performed additional expression arrays to investigate whether the WT mice showed a “proliferative” phenotype, compared with the mmp12−/− mice. On Day 7, only 40 genes were differentially expressed between WT and mmp12−/− mice. Of these, 13 genes were down-regulated and 27 genes were up-regulated in the mmp12−/− mice, as compared with the WT mice (Tables 2 and 3). In addition to mmp12, the genes whose expression declined the most in the mmp12−/− mice included egr-1 and cyr61. Early growth response factor-1, the protein coded by egr-1, is a zinc-finger transcription factor that is involved in the fibrotic response to TGF-β (26, 27). The gene cyr61 codes for a cysteine-rich protein that is associated with the extracellular matrix and promotes adhesion, proliferation, and migration of fibroblasts and endothelial cells (28–30). Thus, the gene expression arrays suggest that a key difference between mmp12−/− and WT mice was the expression of genes that participate in the fibrotic response to TGF-β and in fibroblast proliferation.
TABLE 2.
SELECTED GENES DOWN-REGULATED IN THE LUNGS OF mmp12-/- MICE, COMPARED WITH WILD-TYPE MICE (DAY 7 AFTER THREE CONSECUTIVE INSTILLATIONS OF Jo2 mAb)
| Gene | Name | Fold Change | P Value | Biological Process |
|---|---|---|---|---|
| mmp12 | Matrix metalloproteinase 12 | −6.7 | 0.044701 | Proteolysis |
| mmp12 | Matrix metalloproteinase 12 | −5.66 | 0.035201 | Proteolysis |
| egr1 | Early growth response 1 | −2.55 | 0.0383 | Regulation of transcription |
| cyr61 | Cysteine rich protein 61 | −1.71 | 0.017245 | Regulatoin of cell growth |
| ccr2 | Chemokine (C-C motif) receptor 2 | −1.69 | 0.032292 | Chemotaxis |
| plac9 | Placenta specific 9 | −1.63 | 0.014466 | — |
| hnrpdl | Heterogeneous nuclear ribonucleoprotein D-like | −1.6 | 0.04186 | mRNA metabolism |
| st8sia4 | Esterase D/formylglutathione hydrolase | −1.59 | 0.031571 | Protein amino acid glycosylation |
| esd | ST8 alpha-N-acetyl-neuraminide alpha-2,8-sialyltransferase 4 | −1.59 | 0.006283 | — |
| igh-6 | Immunoglobulin heavy chain 6 (heavy chain of IgM) | −1.52 | 0.044387 | Activation of MAPK activity, positive regulation of B cell activity |
| hnrpd | Heterogeneous nuclear ribonucleoprotein D | −1.46 | 0.039531 | Telomere maintenance, regulation of transcription |
| lmo2 | LIM domain only 2 | −1.45 | 0.023731 | — |
| slc6a6 | Solute carrier family 6 member 6 | −1.4 | 0.037234 | Transport (beta-alanine, taurine, neurotransmitters) |
| ewsr1 | Ewing sarcoma breakpoint region 1 | −1.39 | 0.020025 | Regulation of transcription |
Definition of abbreviation: mAb, monoclonal antibody.
TABLE 3.
SELECTED GENES UP-REGULATED IN THE LUNGS OF mmp12-/- MICE, COMPARED WITH WILD-TYPE MICE (DAY 7 AFTER THREE CONSECUTIVE INSTILLATIONS OF Jo2 mAb)
| Gene | Name | Fold Change | P Value | Biological Process |
|---|---|---|---|---|
| hsp105 | Heat shock protein 105 | 5.06 | 0.01991 | Protein folding |
| ctse | Cathepsin E | 4.76 | 0.0474 | Proteolysis |
| mela | Melanoma antigen | 2.17 | 0.02564 | — |
| dnaja1 | DnaJ (Hsp40) homolog, subfamily A, member 1 | 2.13 | 0.04941 | Protein folding-response to heat |
| herpud1 | Homocysteine-inducible, endoplasmic reticulum stress-inducible, ubiquitin-like domain member 1 | 2.07 | 0.01719 | Response to stress |
| cd59a | CD59a antigen | 1.98 | 0.02527 | — |
| stip1 | Stress-induced phosphoprotein 1 | 1.95 | 0.00519 | Response to stress |
| klf9 | Kruppel-like factor 9 | 1.9 | 0.04292 | Embryo implantation |
| xlkd1 | Extra cellular link domain-containing 1 | 1.7 | 0.02760 | Glycosaminoglycan catabolism |
| angptl4 | Angiopoietin-like 4 | 1.69 | 0.03166 | Negative regulation of apoptosis |
| tinagl | Tubulointerstitial nephritis antigen-like | 1.62 | 0.01896 | Proteolysis |
| hspa8 | Heat shock protein 8 | 1.61 | 0.02328 | Regulation of cell cycle |
| fkbp4 | FK506 binding protein 4 | 1.59 | 0.00741 | Protein folding |
| hspa5 | Heat shock 70kD protein 5 | 1.52 | 0.02266 | ER-overload response |
| cryaa | Crystallin, alpha A | 1.51 | 0.03559 | M-phase microtubule process |
| sox7 | SRY-box containing gene 7 | 1.46 | 0.04429 | Regulation of transcription |
| carhsp1 | Calcium regulated heat stable protein 1 | 1.45 | 0.03151 | Regulation of transcription |
| alas1 | Aminolevulinic acid synthase 1 | 1.45 | 0.04172 | Heme biosyntesis |
| timp3 | Tissue inhibitor of metalloproteinase 3 | 1.4 | 0.01070 | — |
| eif4a2 | Eukaryotic translation initiation factor 4A2 | 1.34 | 0.02850 | Protein biosynthesis |
Definition of abbreviation: mAb, monoclonal antibody.
DISCUSSION
The incidence of ALI/ARDS in the United States is 78.9 per 100,000 person-years, with an in-hospital mortality rate of 38.5% (31). Approximately 56–74% of deaths occur more than 72 h after the onset of ARDS, and up to 19% of deaths are entirely due to respiratory failure (32). The development of a fibroproliferative response has been repeatedly identified as an independent poor prognostic factor in ALI/ARDS (2, 33). However, anti-inflammatory strategies aimed at preventing the fibroproliferative response have been unsuccessful, and even deleterious (34, 35). In order to effectively prevent the development of fibroproliferation in ALI/ARDS, it is important to identify specific molecular steps that could be a target for novel therapeutic strategies. The main finding of this study was that activation of Fas in the lungs of mice for 3 d results in a fibroproliferative response in the lungs that is dependent on MMP-12. This is important for two reasons: first, because this experimental model is based on the clinical findings in patients with ALI/ARDS, who have increased concentrations of sFasL in their lungs during the first three days of the disease (12); and second, because the findings suggest that MMP-12 has a mechanistic role in the fibroproliferative response in the lungs.
We used gene expression profiling to identify molecular pathways that are essential for the development of fibroproliferation in mice exposed to a Fas-activating antibody. The gene expression profile revealed several features suggesting activation of the TGF-β1 pathway, including down-regulation of the negative regulators of TGF-β1 signaling, SMAD6 and SMAD7, and up-regulation of TGF-β1–associated genes, such as egr-1, HO-1, elastin, and tenascin. This finding was expected, because Fas activation in the lungs has previously been shown to upregulate TGF-β expression, and several studies have suggested that TGF-β plays an important role in the development of fibrotic responses in mice (18, 27). More surprisingly, we found that activation of the Fas receptor pathway in the lungs is associated with transcriptional activation of metalloproteinase genes, and that MMP-12 was markedly up-regulated. This was unexpected, because MMP-12 has been associated with emphysema, but not fibrosis, and because TGF-β has been reported to down-regulate MMP-12 expression (36, 37).
MMP-12 is a matrix metalloproteinase that was originally identified in human and murine alveolar macrophages (38, 39). MMP-12 is a zinc-containing protein that is released as a 53-kD pro-enzyme, which is readily processed into a 22-kD active form (39). In the lungs, MMP-12 is primarily expressed by alveolar macrophages, although some reports suggest that MMP-12 may also be expressed in human airway bronchial epithelial cells and smooth muscle cells (40, 41). MMP-12 expression is up-regulated by other matrix components such as hyaluronan fragments, cytokines, and growth factors such as TGF-α, IFN-γ and EGF, and serine proteases such as thrombin and plasmin (40, 42, 43). MMP-12 was initially described as an elastase, but like other metalloproteinases, it has been found in vitro to have a wide variety of potential substrates, including type IV collagen, fibronectin, laminin, and gelatin, as well as nonmatrix proteins such as α1-antitrypsin and latent TNF-α (44). Despite the variety of in vitro substrates of MMP-12, no in vivo substrates have been described.
Prior studies have linked MMP-12 with the development of emphysema. The human form of MMP-12 was originally isolated from alveolar macrophages of smokers, and active MMP-12 was later found in the BAL fluid of patients with COPD (38, 45). Furthermore, mmp12−/− mice are protected from cigarette smoke–induced emphysema (25). However, to our knowledge MMP-12 has not been previously associated with ALI or ARDS. The data show that Fas activation directly induces MMP-12 expression in alveolar macrophages. Furthermore, the observation that mice lacking MMP-12 are protected from pulmonary fibrosis after Fas activation supports a central mechanistic role for MMP-12 activation in the pathogenesis of Fas-induced fibrosis. Thus the data suggest a paradigm in which Fas activation causes apoptosis in the alveolar epithelial walls, and MMP-12 expression by activated alveolar macrophages in the airspaces.
An important remaining question is why MMP-12 is essential for the fibrotic response to Jo2. Our findings show that the fibroprotective effect conferred by the lack of MMP-12 is not due to impairment of the macrophage and neutrophilic responses induced by Fas activation in the lungs. Because MMP-12 is a proteinase, it can be speculated that its role in fibrosis is related to its proteolytic activity. One possibility is that Fas-dependent apoptosis of alveolar epithelial cells exposes the alveolar basement membrane and allows MMP-12 and other metalloproteinases to degrade the matrix, triggering a fibroproliferative response. This hypothesis is supported by data suggesting that apoptosis of alveolar epithelial cells is an important step in the development of fibroproliferative responses in the lungs (27). However, the results of the caspase-3 activity assay suggest that the apoptotic response to Jo2 was similar in the WT and mmp12−/− mice. These results raise the possibility that MMP-12 acts in parallel with or distally to apoptosis, by inducing proteolytic activation of a protein required for full expression of the fibrotic response.
The comparison of gene expression profiles in WT and mmp12−/− mice exposed to Jo2 raises an alternative hypothesis. The gene microarray analysis revealed that the in addition to MMP-12, the genes more differentially expressed in mmp12−/− mice compared with WT mice were egr-1 and cyr61. The gene egr-1 encodes for a zinc-finger transcription factor known to be essential for the development of lung fibrosis in response to TGF-β (27). The gene cyr61, encodes for a matrix-associated cysteine-rich secretory protein, CYR61, also known as CCN-1. CYR61/CCN1 is expressed by fibroblasts in response to TGF-β and other growth factors, and is transcriptionally regulated by egr-1 (46). CYR61/CCN1 is associated with fibroblast adhesion, migration, and proliferation; with extracellular matrix remodeling; and with cutaneous wound repair (29, 30, 47–49). CYR61/CCN1 is markedly up-regulated in the lungs of humans and mice with lung disease, including lung tissues from smokers with COPD and lungs from mice with ventilator-induced or hyperoxia-induced lung injury (50–52). The fact that egr1 and cyr61 were markedly down-regulated in the mmp12−/− mice suggests that MMP-12 could be involved in proteolytic processing of a protein that controls expression of egr-1, leading to induction of cyr61 and a repair response with collagen deposition.
In summary, activation of the Fas system in the lungs for 3 d is followed by apoptosis of alveolar wall cells, collagen accumulation by Day 21, and sequential activation of proinflammatory cytokines and the macrophage metalloelastase, MMP-12. MMP-12 provides a molecular mechanism linking Fas activation with late fibrosis, because mice deficient in MMP-12 are protected from the fibrotic response following Fas activation. We conclude that MMP-12 is essential for the development of the Fas-induced fibroproliferative response in the lungs. The data support the rationale for targeting MMP-12 as a potential therapeutic strategy to reduce late fibrosis after Fas activation in the lungs of patients with acute lung injury.
Acknowledgments
The authors thank Venus Wong, Amy Koski, Kate Andrus, Alex Farnand, Reinout Bem, Naoki Hagimoto, and Steve Mongovin for their expert technical assistance.
This work was supported in part by grants HL 70840 and HL083044 (G.M.-B.), HL 65852 and HL073996 (T.R.M.), HL70178 (J.S.L.) from the National Institutes of Health, and the Medical Research Service of the U.S. Department of Veterans Affairs.
Some of the data presented in this manuscript were presented in abstract form at the 2005 ATS International Conference (Proceedings of the American Thoracic Society 2005;2:A903), the 2006 ATS International Conference (Proceedings of the American Thoracic Society 2006;3:A229), and at the 2006 ASCI/AAP Joint meeting.
Originally Published in Press as DOI: 10.1165/rcmb.2006-0471OC on April 19, 2007
Conflict of Interest Statement: None of the authors has a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Park WY, Goodman RB, Steinberg KP, Ruzinski JT, Radella F II, Park DR, Pugin J, Skerrett SJ, Hudson LD, Martin TR. Cytokine balance in the lungs of patients with acute respiratory distress syndrome. Am J Respir Crit Care Med 2001;164:1896–1903. [DOI] [PubMed] [Google Scholar]
- 2.Clark JG, Milberg JA, Steinberg KP, Hudson LD. Type III procollagen peptide in the adult respiratory distress syndrome: association of increased peptide levels in bronchoalveolar lavage fluid with increased risk for death. Ann Intern Med 1995;122:17–23. [DOI] [PubMed] [Google Scholar]
- 3.Olman MA, White KE, Ware LB, Simmons WL, Benveniste EN, Zhu S, Pugin J, Matthay MA. Pulmonary edema fluid from patients with early lung injury stimulates fibroblast proliferation through IL-1β-induced IL-6 expression. J Immunol 2004;172:2668–2677. [DOI] [PubMed] [Google Scholar]
- 4.Fukuda Y, Ishizaki M, Masuda Y, Kimura G, Kawanami O, Masugi Y. The role of intraalveolar fibrosis in the process of pulmonary structural remodeling in patients with diffuse alveolar damage. Am J Pathol 1987;126:171–182. [PMC free article] [PubMed] [Google Scholar]
- 5.Powell WC, Fingleton B, Wilson CL, Boothby M, Matrisian LM. The metalloproteinase matrilysin proteolytically generates active soluble fas ligand and potentiates epithelial cell apoptosis. Curr Biol 1999;9:1441–1447. [DOI] [PubMed] [Google Scholar]
- 6.Kiener PA, Davis PM, Rankin BM, Klebanoff SJ, Ledbetter VA, Starling GC, Liles WC. Human monocytic cells contain high levels of intracellular Fas ligand: Rapid release following cellular activation. J Immunol 1997;159:1594–1598. [PubMed] [Google Scholar]
- 7.Nakamura M, Matute-Bello G, Liles WC, Hayashi S, Kajikawa O, Lin S-M, Frevert CW, Martin TR. Differential response of human lung epithelial cells to Fas-induced apoptosis. Am J Pathol 2004;164:1949–1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hamann KJ, Dorscheid DR, Ko FD, Conforti AE, Sperling AI, Rabe KF, White SR. Expression of Fas (CD95) and FasL (CD95L) in human airway epithelium. Am J Respir Cell Mol Biol 1998;19:537–542. [DOI] [PubMed] [Google Scholar]
- 9.Fine A, Anderson NL, Rothstein TL, Williams MC, Gochuico BR. Fas expression in pulmonary alveolar type II cells. Am J Physiol 1997;273:L64–L71. [DOI] [PubMed] [Google Scholar]
- 10.Park DR, Thomsen AR, Frevert CW, Pham U, Skerrett SJ, Kiener PA, Liles WC. Fas (CD95) induces proinflammatory cytokine responses by human monocytes and monocyte-derived macrophages. J Immunol 2003;170:6209–6216. [DOI] [PubMed] [Google Scholar]
- 11.Matute-Bello G, Liles WC, Frevert CW, Nakamura M, Ballman K, Vathanaprida C, Kiener PA, Martin TR. Recombinant human Fas-ligand induces alveolar epithelial cell apoptosis and lung injury in rabbits. Am J Physiol 2001;281:L328–L335. [DOI] [PubMed] [Google Scholar]
- 12.Matute-Bello G, Liles WC, Steinberg KP, Kiener PA, Mongovin S, Chi EY, Jonas M, Martin TR. Soluble Fas ligand induces epithelial cell apoptosis in humans with acute lung injury (ARDS). J Immunol 1999;163:2217–2225. [PubMed] [Google Scholar]
- 13.Liles WC, Klebanoff SJ. Regulation of apoptosis in neutrophils: Fas track to death? J Immunol 1995;155:3289–3291. [PubMed] [Google Scholar]
- 14.Ponton A, Clement MV, Stamenkovic I. The CD95 (APO-1/Fas) receptor activates NF-κB independently of its cytotoxic function. J Biol Chem 1996;271:8991–8995. [DOI] [PubMed] [Google Scholar]
- 15.Rensing-Ehl A, Hess S, Ziegler-Heitbrock HW, Riethmüller G, Engelmann H. Fas/Apo-1 activates nuclear factor kB and induces interleukin-6 production. J Inflamm 1995;45:161–174. [PubMed] [Google Scholar]
- 16.Freiberg RA, Spencer DM, Choate KA, Duh HJ, Schreiber SL, Crabtree GR, Khavari PA. Fas signal transduction triggers either proliferation or apoptosis in human fibroblasts. J Invest Dermatol 1997;108:215–219. [DOI] [PubMed] [Google Scholar]
- 17.Matute-Bello G, Winn RK, Jonas M, Chi EY, Martin TR, Liles WC. Fas (CD95) induces alveolar epithelial cell apoptosis in vivo: implications for acute pulmonary inflammation. Am J Pathol 2001;158:153–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hagimoto N, Kuwano K, Miyazaki H, Kunitake R, Fujita M, Kawasaki M, Kaneko Y, Hara N. Induction of apoptosis and pulmonary fibrosis in mice in response to ligation of Fas antigen. Am J Respir Cell Mol Biol 1997;17:272–278. [DOI] [PubMed] [Google Scholar]
- 19.Irizarry RA, Hobbs B, Collin F, Beazer-Barclay YD, Antonellis KJ, Scherf U, Speed TP. Exploration, normalization, and summaries of high density oligonucleotide array probe level data. Biostatistics 2003;4:249–264. [DOI] [PubMed] [Google Scholar]
- 20.Eisen MB, Spellman PT, Brown PO, Botstein D. Cluster analysis and display of genome-wide expression patterns. Proc Natl Acad Sci USA 1998;95:14863–14868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Park DR, Skerrett SJ. IL-10 enhances the growth of Legionella pneumophila in human mononuclear phagocytes and reverses the protective effect of IFN-gamma: differential responses of blood monocytes and alveolar macrophages. J Immunol 1996;157:2528–2538. [PubMed] [Google Scholar]
- 22.Itoh N, Yonehara S, Ishii A, Yonehara M, Mizushima S, Sameshima M, Hase A, Seto Y, Nagata S. The polypeptide encoded by the cDNA for human cell surface antigen Fas can mediate apoptosis. Cell 1991;66:233–243. [DOI] [PubMed] [Google Scholar]
- 23.Alderson MR, Tough TW, Braddy S, Davis-Smith T, Roux E, Schooley K, Miller RE, Lynch DH. Regulation of apoptosis and T cell activation by Fas-specific mAb. Int Immunol 1994;6:1799–1806. [DOI] [PubMed] [Google Scholar]
- 24.Liles WC, Kiener PA, Ledbetter JA, Aruffo A, Klebanoff SJ. Differential expression of Fas (CD95) and Fas ligand on normal human phagocytes: implications for the regulation of apoptosis in neutrophils. J Exp Med 1996;184:429–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hautamaki RD, Kobayashi DK, Senior RM, Shapiro SD. Requirement for macrophage elastase for cigarette smoke-induced emphysema in mice. Science 1997;277:2002–2004. [DOI] [PubMed] [Google Scholar]
- 26.Chen S-J, Ning H, Ishida W, Sodin-Semrl S, Takagawa S, Mori Y, Varga J. The early-immediate gene EGR-1 is induced by TGF-beta and mediates stimulation of collagen gene expression. J Biol Chem 2006;281:21183–21197. [DOI] [PubMed] [Google Scholar]
- 27.Lee CG, Cho SJ, Kang MJ, Chapoval SP, Lee PJ, Noble PW, Yehualaeshet T, Lu B, Flavell RA, Milbrandt J, et al. Early growth response gene 1-mediated apoptosis is essential for transforming growth factor β1-induced pulmonary fibrosis. J Exp Med 2004;200:377–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kireeva ML, Latinkic BV, Kolesnikova TV, Chen CC, Yang GP, Abler AS, Lau LF. Cyr61 and Fisp12 are both ECM-associated signaling molecules: activities, metabolism, and localization during development. Exp Cell Res 1997;233:63–77. [DOI] [PubMed] [Google Scholar]
- 29.Kireeva M, Mo F, Yang G, Lau L. Cyr61, a product of a growth factor-inducible immediate-early gene, promotes cell proliferation, migration, and adhesion. Mol Cell Biol 1996;16:1326–1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.O'Brien TP, Yang GP, Sanders L, Lau LF. Expression of cyr61, a growth factor-inducible immediate-early gene. Mol Cell Biol 1990;10:3569–3577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rubenfeld GD, Caldwell E, Peabody E, Weaver J, Martin DP, Neff M, Stern EJ, Hudson LD. Incidence and outcomes of acute lung injury. N Engl J Med 2005;353:1685–1693. [DOI] [PubMed] [Google Scholar]
- 32.Stapleton RD, Wang BM, Hudson LD, Rubenfeld GD, Caldwell ES, Steinberg KP. Causes and timing of death in patients with ARDS. Chest 2005;128:525–532. [DOI] [PubMed] [Google Scholar]
- 33.Ichikado K, Suga M, Muranaka H, Gushima Y, Miyakawa H, Tsubamoto M, Johkoh T, Hirata N, Yoshinaga T, Kinoshita Y, et al. Prediction of prognosis for acute respiratory distress syndrome with thin-section CT: validation in 44 cases. Radiology 2005;238:321–329. [DOI] [PubMed] [Google Scholar]
- 34.The ARDS Network. Ketoconazole for early treatment of acute lung injury and acute respiratory distress syndrome: a randomized controlled trial. JAMA 2000;283:1995–2002. [DOI] [PubMed] [Google Scholar]
- 35.The National Heart, Lung, and Blood Institute Acute Respiratory Distress Syndrome (ARDS) Clinical Trials Network. Efficacy and safety of corticosteroids for persistent acute respiratory distress syndrome. N Engl J Med 2006;354:1671–1684. [DOI] [PubMed] [Google Scholar]
- 36.Morris DG, Huang X, Kaminski N, Wang Y, Shapiro SD, Dolganov G, Glick A, Sheppard D. Loss of integrin alpha(v)beta6-mediated TGF-beta activation causes Mmp12-dependent emphysema. Nature 2003;422:169–173. [DOI] [PubMed] [Google Scholar]
- 37.Bonniaud P, Kolb M, Galt T, Robertson J, Robbins C, Stampfli M, Lavery C, Margetts PJ, Roberts AB, Gauldie J. Smad3 null mice develop airspace enlargement and are resistant to TGF-β-mediated pulmonary fibrosis. J Immunol 2004;173:2099–2108. [DOI] [PubMed] [Google Scholar]
- 38.Shapiro SD, Kobayashi DK, Ley TJ. Cloning and characterization of a unique elastolytic metalloproteinase produced by human alveolar macrophages. J Biol Chem 1993;268:23824–23829. [PubMed] [Google Scholar]
- 39.Shapiro SD, Griffin GL, Gilbert DJ, Jenkins NA, Copeland NG, Welgus HG, Senior RM, Ley TJ. Molecular cloning, chromosomal localization, and bacterial expression of a murine macrophage metalloelastase. J Biol Chem 1992;267:4664–4671. [PubMed] [Google Scholar]
- 40.Lavigne MC, Thakker P, Gunn J, Wong A, Miyashiro JS, Wasserman AM, Wei S-Q, Pelker JW, Kobayashi M, Eppihimer MJ. Human bronchial epithelial cells express and secrete MMP-12. Biochem Biophys Res Commun 2004;324:534–546. [DOI] [PubMed] [Google Scholar]
- 41.Xie S, Issa R, Sukkar MB, Oltmanns U, Bhavsar PK, Papi A, Caramori G, Adcock I, Chung KF. Induction and regulation of matrix metalloproteinase-12 in human airway smooth muscle cells. Respir Res 2005;6:148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Horton MR, Shapiro S, Bao C, Lowenstein CJ, Noble PW. Induction and regulation of macrophage metalloelastase by hyaluronan fragments in mouse macrophages. J Immunol 1999;162:4171–4176. [PubMed] [Google Scholar]
- 43.Raza SL, Nehring LC, Shapiro SD, Cornelius LA. Proteinase-activated receptor-1 regulation of macrophage elastase (MMP-12) secretion by serine proteinases. J Biol Chem 2000;275:41243–41250. [DOI] [PubMed] [Google Scholar]
- 44.Gronski TJ Jr, Martin RL, Kobayashi DK, Walsh BC, Holman MC, Huber M, Van Wart HE, Shapiro SD. Hydrolysis of a broad spectrum of extracellular matrix proteins by human macrophage elastase. J Biol Chem 1997;272:12189–12194. [DOI] [PubMed] [Google Scholar]
- 45.Finlay G, Russell K, McMahon K, D'arcy E, Masterson J, FitzGerald M, O'Connor C. Elevated levels of matrix metalloproteinases in bronchoalveolar lavage fluid of emphysematous patients. Thorax 1997;52:502–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Grote K, Bavendiek U, Grothusen C, Flach I, Hilfiker-Kleiner D, Drexler H, Schieffer B. Stretch-inducible expression of the angiogenic factor CCN1 in vascular smooth muscle cells is mediated by Egr-1. J Biol Chem 2004;279:55675–55681. [DOI] [PubMed] [Google Scholar]
- 47.Brunner A, Chinn J, Neubauer M, Purchio AF. Identification of a gene family regulated by transforming growth factor-beta. DNA Cell Biol 1991;10:293–300. [DOI] [PubMed] [Google Scholar]
- 48.Chen C-C, Mo F-E, Lau LF. The angiogenic factor Cyr61 activates a genetic program for wound healing in human skin fibroblasts. J Biol Chem 2001;276:47329–47337. [DOI] [PubMed] [Google Scholar]
- 49.Leng E, Malcolm T, Tai G, Estable M, Sadowski I. Organization and expression of the Cyr61 gene in normal human fibroblasts. J Biomed Sci 2002;9:59–67. [DOI] [PubMed] [Google Scholar]
- 50.Dolinay T, Kaminski N, Felgendreher M, Kim HP, Reynolds P, Watkins SC, Karp D, Uhlig S, Choi AMK. Gene expression profiling of target genes in ventilator-induced lung injury. Physiol Genomics 2006;26:68–75. [DOI] [PubMed] [Google Scholar]
- 51.Ning W, Li CJ, Kaminski N, Feghali-Bostwick CA, Alber SM, Di YP, Otterbein SL, Song R, Hayashi S, Zhou Z, et al. Comprehensive gene expression profiles reveal pathways related to the pathogenesis of chronic obstructive pulmonary disease. Proc Natl Acad Sci USA 2004;101:14895–14900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Perkowski S, Sun J, Singhal S, Santiago J, Leikauf GD, Albelda SM. Gene expression profiling of the early pulmonary response to hyperoxia in mice. Am J Respir Cell Mol Biol 2003;28:682–696. [DOI] [PubMed] [Google Scholar]