Abstract
Contained within the adult lung are differentiated mesenchymal cell types (cartilage, smooth muscle, and myofibrobasts) that provide structural support for airways and vessels. Alterations in the number and phenotype of these cells figure prominently in the pathogenesis of a variety of lung diseases. While these cells are thought to arise locally, progenitors have yet to be purified. In previous work, we developed a method for isolating progenitors from lung tissue: this technique takes advantage of the unique ability of cell populations enriched for somatic stem and progenitor activity to efflux the vital dye Hoechst 33342, a feature that permits isolation by flow cytometry-based procedures. Using this method, we determined that a rare population of mesenchymal progenitors resides within the CD45− CD31− Hoechst low fraction of the adult murine lung. Similar to other mesenchymal progenitors, these cells express Sca-1, CD106, and CD44; can be serially passaged; and can differentiate to smooth muscle, cartilage, bone, and fat. Overall, these findings demonstrate that a phenotypically distinct mesenchymal progenitor resides within the adult murine lung, and provide a scheme for their isolation and study.
Keywords: mesenchymal, progenitors, lung
CLINICAL RELEVANCE
This article is the first to identify and characterize mesenchymal progenitors in the murine lung. These findings have broad implications for understanding lung development and diseases characterized by the accumulation of mesenchymal elements.
Somatic stem and progenitor cells have been identified in virtually every adult organ functioning to replace damaged and senescent cells during physiologic turnover and tissue injury (1–4). Recent discoveries have provided important clues to the location, phenotype, and signals regulating these cells (2, 5). In the adult lung, progenitor cells of the proximal and distal epithelium have been localized and characterized (4, 6–8). However, lung progenitor cells involved in replacing mesenchymal components (cartilage, smooth muscle, and myofibrobasts) have yet to be purified (9, 10). This is due, in part, to a deficiency of markers, and to slow turnover of these tissues.
In previous work we developed a strategy to isolate candidate stem and progenitor cells from the adult and embryonic lung (11–13). In brief, we took advantage of the fact that rare, somatic cell subsets markedly enriched for progenitor and stem cell activity are characterized by the ability to efflux the vital dye Hoechst 33342. This unique property allows for flow cytometry based purification schemes, since Hoescht-effluxing cells appear as a discrete negatively stained population “off to the side” on density dot plots; hence such cells are termed “side population” or SP cells (14). From organs such as heart, skin, and testes, functional stem cell activity specifically resides within the purified SP cell population (15–17).
We and others found that SP cells in the adult and embryonic lung are a heterogeneous population that uniformly express Sca-1 (12, 13, 18). In fact, at least three distinct subsets have been identified. One subset expresses the pan-hematopoietic marker CD45; phenotypic and functional assays indicate that these cells are hematopoietic progenitors (13). Whether these cells reside within the lung or are trafficking through has yet to be determined. The CD45-negative (CD45−) lung SP population is composed of at least two subsets. These include a CD31-expressing endothelial-like fraction (CD31+), and a population that uniformly expresses α-smooth muscle actin. In the developing lung, this latter population displays several features of a mesenchymal progenitor phenotype (13).
In this study, we investigated whether a discrete mesenchymal progenitor cell population can be identified in the adult mouse lung. Using a flow cytometry–based approach, we isolated a candidate mesenchymal progenitor with the following characteristics: (1) it is contained within the CD45−/CD31− SP cell subset; (2) it can be serially cultured in an undifferentiated state; (3) like other mesenchymal progenitor cell populations, it expresses Sca-1, CD106, and CD44; and (4) these cells can be induced to differentiate into smooth muscle, cartilage, bone, and fat. These features, along with their relative ease of isolation, indicate that these cells are a model for studying adult lung mesenchymal progenitor cell activity and differentiation. Most importantly, these studies demonstrate that a defined mesenchymal progenitor cell population resides in the adult murine lung.
MATERIALS AND METHODS
Animals
Murine lung cells were obtained from adult mice (12–16 wk of age) of background C57BL/6J (Jackson Laboratories, Bar Harbor, ME). Anesthetized mice were killed by cervical dislocation. Animal studies were conducted according to protocols approved by the National Institutes of Health and the Boston University Animal Care and Use Committee.
Isolation and Digestion of Lung Tissue
SP cells were isolated as previously described (12). In brief, animals were bled by transecting the abdominal aorta. Circulating cells were removed from the pulmonary vasculature by perfusing with ice-cold saline. Next, large airways were excised, and remaining lung tissue was dissected from the thoracic cavity. Lung parenchyma from five to seven mice was digested by finely mincing with a razor blade, and by incubating tissue in an enzyme mixture containing 0.05% collagenase, 2.4 U/ml dispase (Roche Diagnostics, Indianapolis, IN), and CaCl2 for 1.5 h at 37°C. During Hoechst staining, the number of cells obtained from five to seven mice was required to generate a readily discrete SP band. Removal of nonspecific debris was performed by filtering single cell suspensions through sterile 100- and 70-μm filters. Cells were re-suspended at a concentration of 1 × 106 nucleated cells/ml in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% fetal bovine serum (FBS), penicillin/streptomycin, and 10 mM HEPES. Hoechst dye (5 μg/ml) was added to the suspension, and cells were stained for 90 min in the dark at 37°C in the presence or absence of verapamil (50 mM). Upon the completion of staining, cells were immediately placed on ice.
Flow Cytometry
Flow cytometry of Hoechst stained cells was performed on a triple laser instrument (MoFlo Cytomation, Fort Collins, CO). An argon multiline ultraviolet (333–363 nm) laser was used to excite Hoechst dye, and fluorescence emission was collected with a 405/30 band-pass filter (Hoechst blue) and a 660 long-pass filter (Hoechst red). Ultraviolet laser was also used to excite allophycocyanin (APC) fluorescent probes. A second 488-nm argon laser was used to excite phycoerythrein and propidium iodide. Flow cytometry analysis of cultured cells was performed using a Becton Dickinson FACScan (Becton Dickinson, San Jose, CA). Data analysis for all flow cytometry experiments was performed using FloJo software (Tree Star Inc., Ashland, OR).
Immunostaining
Immunostaining of Hoechst-stained or cultured cells for flow cytometry experiments was performed using fluorochrome-conjugated monoclonal rat anti-mouse antibodies directed at CD45, CD31, CD44, and CD106 (BD Biosciences, San Jose, CA). Cells were immunostained on ice for 30 min. In parallel, staining was performed using isotype control antibodies. Propidium iodide was used to exclude dead cells from analysis.
Immunohistochemistry was performed with antibodies to smooth muscle α-tropomyosin and type II collagen. Cells grown in monolayer were fixed with 4% paraformaldehyde for 10 min before staining. Autofluorescence was quenched with sodium borohydride for 30 min at room temperature (RT), followed by blocking step with 1% goat serum. Antibody staining with polyclonal rabbit anti-human smooth muscle α-tropomyosin (Sigma Aldrich, St Louis, MO) was performed for 1 h at RT. Secondary labeling used a Texas red–conjugated goat anti-rabbit antibody (1 h at RT). Cells were then washed and counterstained with nuclear 4′6′-diamindino-2-phenylindole dihydrochloride (Sigma-Aldrich). For type II collagen staining, cell pellets were fixed in formalin, and embedded in paraffin wax. Cell blocks were cut into 3-μm sections, and deparaffinized by exposure to graded alcohols. Before antibody staining, sections were treated with hydrogen peroxide to quench endogenous peroxidases, and blocked with 1% goat serum. Primary labeling was performed using a polyclonal rabbit anti-human type II collagen antibody (Chemicon International, Temecula, CA) for 1 h at RT. This was followed by incubation with a secondary biotinylated goat anti-rabbit antibody. Biotin-labeled cells were detected with the use of an ABC kit (Vector Laboratories, Burlingame, CA) and methyl green counter stain.
DNA Labeling
DNA was labeled using propidium iodide as previously described (19). Cells used for these studies were an immortalized mesenchymal cell line and cultured CD45− CD31− SP cells. Before staining, cells were fixed with 35% ethanol for 1 h at 4°C, washed twice with PBS, and resuspended in staining buffer containing RNAse A (25 μg/ml) and propidium iodide (50 μg/ml). Staining was performed for 30 min at 4°C, which was followed by flow cytometry analysis.
Cell Culture
In an attempt to maintain freshly isolated SP cells in an undifferentiated state, cells were cultured in either α-modified minimum essential medium (α-MEM), Roswell Park Memorial Institute medium (RPMI), or DMEM basal media. Media was supplemented with 10–20% FBS, L-glutamine, and penicillin/streptomycin. Fresh media was replaced every 3 d. During culture in differentiation conditions published protocols were followed (20, 21). Briefly, to induce smooth muscle differentiation, cells were transferred to fibronectin-coated chamber slides containing 1 ml of bronchial smooth muscle basal media supplemented with epidermal growth factor (EGF), basic-fibroblast growth factor (FGF2), 5% FBS, insulin, and antimicrobial agents. Cells were observed daily for morphologic changes. After 7 d cells were fixed with alcohol and stained with an antibody against α-tropomyosin. For differentiation of cells to adipocytes, 3 × 104 CD45− CD31− SP cells were maintained in mesenchymal cell maintanence media until reaching 100% confluence. At that time, media was replaced with adipocyte induction media containing h-insulin, l-glutamine, mesenchymal cell growth supplement (MCGS), dexamethasone, indomethacin, 3-isobuty-l-methyl-xanthine, penicillin, and streptomycin). Cells were kept in these conditions for 3 d, after which time adipocyte maintenance media (h-insulin, L-glutamine, MCGS, penicillin, and streptomycin) was added. Cells underwent a total of three induction-maintenance cycles before analysis. Cells were either removed for RNA isolation or stained with Oil Red O dye (10 min RT with 0.5% Oil Red O stock solution 0.5 g/100 ml diluted in distilled water). For differentiation to cartilage, 2.5 × 105 CD45− CD31− SP cells were transferred to 15 ml polypropylene tubes containing chondrogenic media (dexamethasone, ascorbate, insulin, transferrin, selenium, sodium pyruvate, proline, and L-glutamine, TGF-β3 (20 μg/ml), penicillin, and streptomycin). Cells were centrifuged at 150 × g for 5 min at RT and placed in 37°C incubator for 21 d. Fresh media was replaced every 3 d. Upon completion, cell pellets were embedded in paraffin wax, cut into 5-μm tissue sections, and stained with Toluidine blue dye (1% solution for 3 min at RT). To induce bone formation, 3 × 104 cells were plated in one well of a 6-well plate containing osteogenic media (dexamethasone, ascorbate L-glutamine, Beta-glycerophosphate, MCGS, penicillin, and streptomycin). Cells were maintained in these conditions for 21 d followed by fixation and staining with Alizarin Red S dye (2% solution for 5 min at RT).
To obtain primary lung mesenchymal cells CD45− non-SP cells were directly flow sorted into culture vessels and cultured in α-MEM basal media supplemented with 10% FBS, L-glutamine, and penicillin/streptomycin. Epithelial cells from non-SP fractions were removed from culture based on differential adherence. This technique results in a culture containing > 95% mesenchymal cells.
RT-PCR
cDNA was generated from RNA extracts derived from cultured CD45− CD31− lung SP cells using a reverse transcription kit (Promega, Madison, WI). RNA extract from whole lung tissue was used as a positive control. PCR was performed using the following primers: α-smooth muscle actin, 5′-AGCTTTGGGCAGGAATGATTTGG-3′ and 5′-AAGATCATTGCCCCTCCAGAACG-3′; CD31, 5′-GTGCACAGGACTCTCGCAATC-3′ and 5′-GAAGCCAACAGCCATTACGGT-3′; Tie2, 5′-CTTCCATCATCATCCGGTATA-3′ and 5′-CTATAGTTCAGTGATTCGATTGC-3′; keratin 18, 5′-GGCCACTACTTCAAGATCATC-3′ and 5′-GTACTTGTCCAGTTCTCGCG-3′; keratin 19, 5′-CTACAGATTGACAATGCTCGC-3′ and 5′-GGATCTTGGCTAGGTCGACAC-3′; α-smooth muscle tropomyosin, 5′-TGAACT GGACAAATACTCCG-3′ and 5′-CAGTGACTTGAAGTTGTTCG-3′; perilipin, 5′-CAGAAGACCTACAACAGCACCA-3′ and 5′ –TTCCCAGAGCCAGATCAGC-3′.
RESULTS
Isolation and Expansion of CD45− CD31− SP Cells
Based on previous work, we speculated that an adult lung mesenchymal progenitor resides in the CD45− CD31− SP cell population (Figure 1). To further explore this possibility, we set out to identify conditions that promote the expansion of these cells in culture. For these studies, conditions optimized for bone marrow–derived murine mesenchymal progenitor cell populations were employed (α-MEM). Notably, cells cultured in RPMI or DMEM could not be expanded (see Figure E1 in the online supplement).
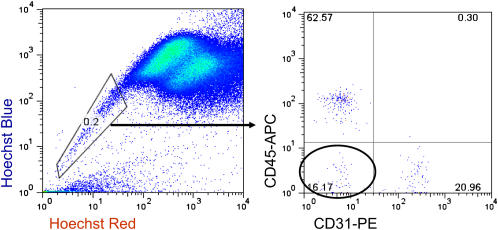
Figure 1.
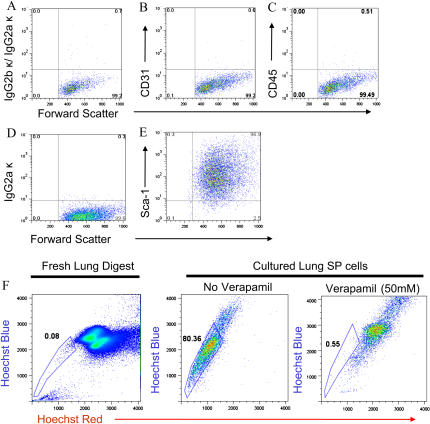
Lung SP cells are a heterogeneous population of cells. Left: Density dot plot demonstrating the presence of SP cells in the adult lung. Right: Gated subset demonstrating that lung SP cells consist of at least three fractions: CD45+, CD45− CD31+, and CD45− CD31−. Circled area contains putative mesenchymal progenitor cell population.
After incubation in α-MEM, CD45− CD31− SP cells were found to readily adhere to culture surfaces, and to display the morphologic features of mesenchymal cells (long, thin, and stellate appearance) (Figures 2A and 2B). Cells with the physical characteristics of hematopoietic (small, round), endothelial (cobblestone), or epithelial (cuboidal) cells were noticeably absent from culture. Consistent with this, cultured cells were found to express by RT-PCR the mesenchymal marker α-smooth muscle actin, which is expressed at early stages of mesenchymal cell differentiation. In contrast, these cells do not express the panepithelial markers keratin 18 and 19 or the panendothelial markers CD31 and Tie-2 (Figure 3). Most notably, other lung SP fractions (CD45+, and CD45− CD31+ subsets) did not grow during culture under these conditions (Figures 2C and 2D).
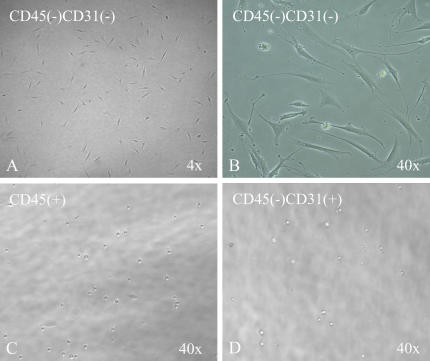
Figure 2.
Lung CD45− CD31− SP cells proliferate in mesenchymal cell maintenance media. (A, B) Low- and high-power view of CD45− CD31− SP cells after culture in mesenchymal cell maintenance media. Cells display the morphologic features of mesenchymal cells (long, thin, and stellate appearance). (C, D) CD45+ and CD45− CD31+ SP cell fractions did not grow or differentiate in mesenchymal cell maintenance conditions.
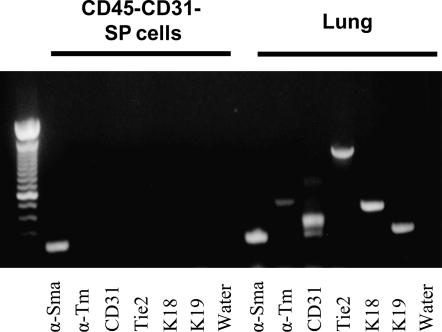
Figure 3.
CD45− CD31− SP cells express genes characteristic of immature mesenchymal cells. RT-PCR was performed on cultured CD45− CD31− SP cells for the mesenchymal genes α-Smooth muscle actin (α-Sma), α-Smooth muscle tropomyosin (α-Tm), the epithelial genes keratin 18 (K18) and keratin 19 (K19), and the endothelial-associated genes CD31 and Tie2. Only expression of the early mesenchymal marker α-smooth muscle actin could be detected in these cells. Markers of epithelial, endothelial, and mature smooth muscle (α-Smooth muscle tropomyosin) were not detected. Whole lung RNA was used as a positive control (Lung).
CD45− CD31− SP cells continued to proliferate even after 50 passages (one passage corresponds to two population doublings). To date, we have maintained two cell lines isolated from pooled lung digests for greater than 50 passages (∼ 100 population doublings), and 10 cell lines for greater than 20 passages (40 population doublings). Kinetic analysis revealed a stable doubling time in culture of ∼ 42 h (Figure 4A).
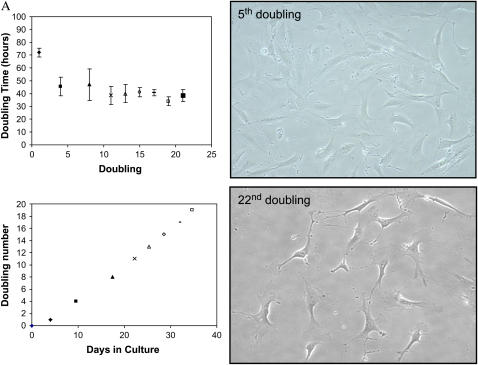
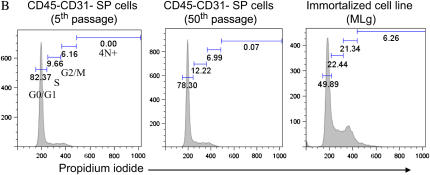
Figure 4.
CD45− CD31− SP cells can be serially passaed in an undifferentiated state. CD45− CD31− SP cells obtained from pooled lung samples continued to proliferate in culture beyond fifty passages. (A) Right: Cells from early and later passages (5th and 22nd doubling) have similar morphologic features. Left: Doubling time for cells after the first passage was estimated to be ∼ 1.75 d (top). Linear growth kinetics was observed over a range of population doublings (bottom). (B) Propidium iodide staining demonstrates that cells at early (5) and late passages (50) have a similar DNA profile. This profile is in contrast to that seen in immortalized cells, which have a greater fraction of cells in S, and G2 phases and with 4N+ DNA.
To determine whether prolonged culturing resulted from the acquisition of widespread chromosomal instability, we examined DNA profiles (propidium iodide staining) after 5 and 50 passages. As depicted in Figure 4B, we found little to no aneuploidy in cells obtained from early or late passages, which suggests that the ability to be maintained in culture is an intrinsic property of CD45− CD31− SP cells. In contrast, profiling of an immortalized mesenchymal cell line (MLg) identified a subpopulation of cells with DNA content greater than 4N.
Phenotype of Cultured CD45− CD31− SP Cells
We compared the phenotype of cultured and freshly isolated CD45− CD31− SP cells. Cultured cells were analyzed after the 5th and 50th passage and found to remain negative for CD45 and CD31 expression (Figures 5A–5C). Moreover, cultured cells, like freshly isolated cells, express the stem cell marker Sca-1 (stem cell antigen-1), and retain the ability to efflux Hoechst dye (Figures 5C and 5D). Together, these latter characteristics are thought to characterize populations enriched for stem/progenitor cell activity, and suggest that serially passaged cells remain in an undifferentiated state (22–24).
Figure 5.
Phenotype of cultured CD45− CD31−SP cells. (A–E) Flow cytometry analysis of cultured cells after 50 passages demonstrates absence of expression of CD31 (B) and CD45 (C), and uniform expression of stem cell antigen-1 (E, Sca-1). (A, D) Isotype staining. (F) Freshly isolated lung digest (left panel) and cultured CD45− CD31− SP cells were stained with Hoechst dye and analyzed by flow cytometry. Cultured SP cells retain the ability to efflux Hoechst dye (middle panel), a process that could be blocked by pre-treatment with verapamil 50 mM (right panel).
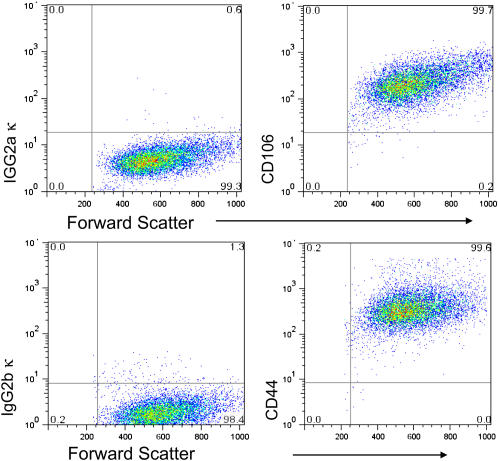
To further evaluate whether the CD45− CD31− SP cell population contains a mesenchymal progenitor, we investigated expression of markers thought to characterize other mesenchymal progenitor cell populations (25, 26). In this regard, bone marrow–derived mesenchymal stem cells uniformly express the adhesion molecule CD106, and the cell surface receptor CD44. To assess expression of these markers, lung CD45− CD31− SP cells were immunostained with fluorescently conjugated antibodies, and analyzed by flow cytometry. As shown in Figure 6, cultured CD45− CD31− SP cells express both markers. Expression of these markers on freshly isolated SP cells could not be determined due to the effects of the enzyme digestion protocol (Figure E2 and data not shown).
Figure 6.
Cultured CD45− CD31− SP cells uniformly express the markers CD106 (upper right) and CD44 (lower right). Isotype staining for CD106 (upper left) and isotype staining for CD44 (lower left).
CD45− CD31− SP Cells Have Mesenchymal Progenitor Activity
To investigate whether CD45− CD31− SP cells possess functional capabilities of progenitor cell populations, cells were expanded in culture before transfer to one of four differentiation conditions: smooth muscle, adipogenic, chondrogenic, and osteogenic media.
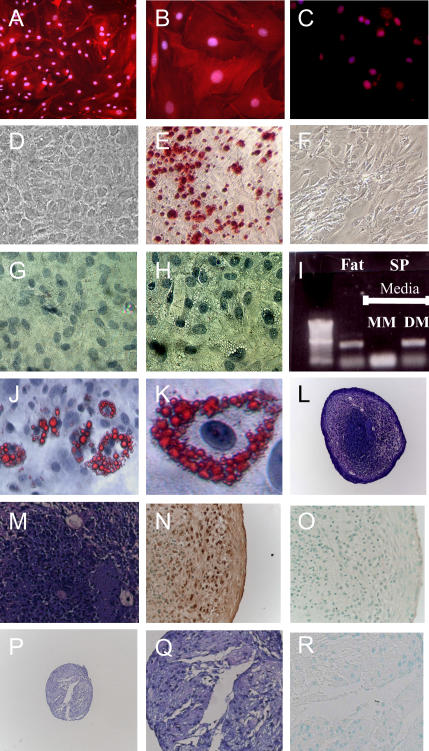
To induce smooth muscle, cells were grown in fibronectin-coated chamber slides containing smooth muscle media. Cells were maintained in culture for 7 d, and observed daily for morphologic changes. After 48 h, cells grown in smooth muscle conditions were easily distinguished based on their large size and copious cytoplasm. Immunostaining with an antibody to α-smooth muscle tropomyosin demonstrated an abundance of this contractile protein. Importantly, cells kept in maintenance media did not express α-smooth muscle tropomyosin protein or message (Figures 3 and 7A–7C).
Figure 7.
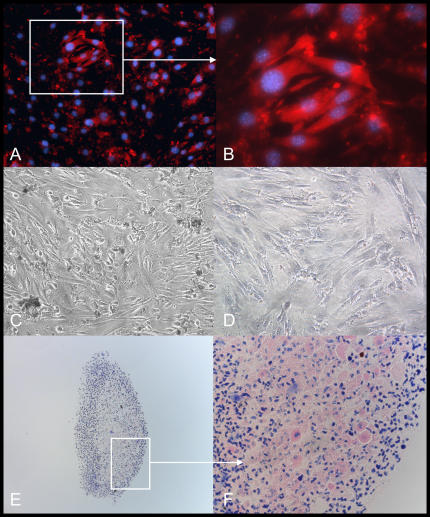
CD45− CD31− SP cells can differentiate to smooth muscle, bone, fat, and cartilage. After exposure to smooth muscle media for 7 d the contractile protein α-smooth muscle tropomyosin was abundantly expressed (low power, A; high power, B). Expression was not detected in cells grown in mesenchymal maintenance conditions (C). In osteogenic conditions, cells readily acquired the features of osteoblasts (rounded shape, D), and demonstrated significant mineralization capacity (orange/red color after Alizarin Red S staining, E). Similar findings were not seen when cells were maintained in mesenchymal cell maintenance media (F). After 21 d in adiopogenic conditions, fat droplets were clearly visible by phase contrast microscopy (H). These features were not observed when cells were grown in mesenchymal cell media for similar periods (G). Consistent with an adipocyte phenotype, droplets stained positive with Oil Red O dye (low and high power view, J and K). RT-PCR demonstrates that cells grown in maintenance media did not express perilipin; however, fresh adipose tissue and cells grown in adipogenic media expressed this adipocyte-specific marker (I). In chondrogenic conditions, CD45− CD31− SP cells formed large cellular structures that contained an abundance of proteoglycans (purple color after Toludine blue stain (L, low power; M, high power). Consistent with a cartilage structure, these cells also stained positive for Type II collagen (N). Negative Isotype staining for Type II collagen (O). Cells grown in mesenchymal cell maintenance media formed smaller, less dense structures (P, low power; Q, high power) that stained weakly to Toludine blue, and negative for Type II collagen (R).
During culture in adipocyte differentiation conditions, CD45− CD31− SP cells readily acquired the morphologic features of adipocytes. Cytoplasmic fat droplets were observed as early as 5 d, and by 21 d fat droplets were visible in the vast majority of cells (Figure 7H). Consistent with a mature fat cell phenotype, cells expressed the adipocyte-specific marker perilipin, and droplets stained positive for Oil Red O (Figures 7I–7K). This is in contrast to cells kept in mesenchymal cell maintanence media, which did not express perilipin or accumulate fat droplets (Figure 7G).
Next, we tested the ability of CD45− CD31− SP cells to form cartilage. For these studies cells were exposed to chrondrogenic media for 21 d. In parallel, cells were cultured in mesenchymal cell maintanence media. As shown in Figure 7, cells grown in chondrogenic growth media formed large, densely packed structures (n = 3). Consistent with cartilage development, these structures were found to be rich in proteoglycans (bright violet color after staining with Toludine Blue dye), and to stain positively for type II collagen. In contrast cells cultured in mesenchymal maintenance media did not form cartilage (Figures 7P and 7Q).
Finally, we examined the osteogenic capacity of CD45− CD31− SP cells. Cells were grown in either osteogenic or mesenchymal cell maintenance media. After 21 d, cells were stained with Alizarin Red S dye. This dye imparts a bright orange-red color in the presence of calcium-rich deposits. As shown in Figure 7E, CD45− CD31− SP cells demonstrated significant mineralization capacity in osteogenic media, but not in maintenance conditions.
Mesenchymal Progenitor Activity Is Restricted to CD45− CD31− SP Cells
While our findings indicate that mesenchymal progenitors exist in lung it is not clear whether activity is restricted to the SP fraction. To explore this further, we tested the differentiation capacity of CD45− CD31− non-SP cells. After flow cytometery–based isolation of this population, contaminating epithelial cells were excluded based on differential adherence (27, 28). After three passages, cells were transferred to each of four differentiation conditions. In smooth muscle conditions, CD45− CD31− non-SP cells demonstrated limited differentiation capacity. As shown in Figure 8, only a small fraction of CD45− CD31− non-SP cells expressed α-tropomyosin at 7 d. Consistent with this limited differentiation capacity, CD45− CD31− non-SP cells lacked adipogenic, chondrogenic, and osteogenic potential. Together, these findings indicate that lung mesenchymal progenitor activity is restricted to the CD45− CD31− SP cell fraction.
Figure 8.
CD45− CD31− non-SP cells lack multi-potent differentiation capacity. CD45− CD31− non-SP has a limited capacity to form smooth muscle in culture. After 7 d in smooth muscle conditions, only a minority of cells expressed α-smooth muscle tropomyosin. (A) Low-power view. (B) High-power view. Culture in other differentiation conditions demonstrated that cells lacked osteogenic, adipogenic, and chondrogenic potential. In all three conditions, cells failed to demonstrate the morphologic characteristic of these cell types. (C) After 21 d in osteogenic media, cells stain negative for Alizarin Red S. (D) Lipid deposits are not seen after culture of CD45− CD31− non-SP cells in adipogenic media. (E) Proteoglycan staining is absent from CD45− CD31− non-SP grown in chondrogenic media.
DISCUSSION
In this work, we provide evidence that a mesenchymal progenitor population exists in the adult murine lung. Our findings indicate that these progenitors reside within the CD45− CD31− SP cell fraction. We found that viable cells can be isolated by relatively simple cell sorting techniques, and that purified cells can be expanded in an undifferentiated state during culture in defined media. Consistent with this, serially passaged cells cultured in this media express markers found in other mesenchymal progenitor populations, lack features of mature cell types, and retain the capacity to efflux Hoechst dye. Further, propidium iodide staining suggests DNA stability during prolonged culturing, though we acknowledge that this method could miss changes in DNA structure and sequence. Most importantly, after transfer to specific induction media these cells assume distinct differentiated cell fates. The ability of CD45− CD31− SP cells to differentiate to smooth muscle, bone, fat, and cartilage suggests that these cells are multi-potent. It is possible, however, that multiple distinct unipotent mesenchymal progenitors (e.g., chondrogenic, osteogenic, adipogenic) reside within the CD45− CD31− SP cell fraction.
In other tissues, mesenchymal progenitor activity also localize to CD45− SP cell fractions (29). Importantly, there are several notable differences between mesenchymal progenitor cell populations that efflux dye and classical mesenchymal stem cell (MSC) populations cultured from the bone marrow. For one thing, classical MSCs are probably not SP cells, in view of the fact that marrow SP cells are uniformly CD45+ (14, 24). Second, classical MSCs probably exist at a far lower frequency in the marrow compared with SP cell mesenchymal progenitors isolated from peripheral tissues (20, 25). One distinctive difference in the mouse is the ease of passaging and expanding of lung-derived mesenchymal progenitors as described here; this is in contrast to the inherent difficulties with isolating and expanding classical mesenchymal stem cells (25). It is also important to note that the relationship of the CD45− CD31− lung SP cells to so-called multipotent adult progenitor cells (MAPCs) remains uncertain (30). One fundamental difference is the fact that MAPCs are pluripotent, whereas the cells described herein have a more limited differentiation repertoire.
Recent work identified a small subset of circulating marrow–derived CD45+ type I collagen+ mononuclear leukocytes (so-called fibrocytes) that are recruited to the lung in response to injury through activation of the chemokine receptor CXCR4 (31–33). At this time we suspect that fibrocytes and lung SP mesenchymal cells represent two distinct unrelated cell types. This is based on several pieces of evidence. Notably, the lung SP mesenchymal progenitor does not express CD11b, CD45, CD34 (11, 12), and CXCR4 (data not shown), whereas fibrocytes express these markers (31, 32). Further, we have not been able to isolate or culture a GFP+ (green fluorescent protein) SP mesenchymal progenitor cell in transplant chimeras (data not shown), suggesting that these cells do not circulate or originate from the bone marrow. Moreover, CD45+ lung SP cells grown in hematopoeitic stem cell conditions do not differentiate into CD45− cells (34).
In conclusion, we identified a defined adult lung cell population that contains cells with a phenotype consistent with mesenchymal progenitor activity. It is important to reiterate that the ability to grow and expand these cells in an undifferentiated state provides a unique tool to evaluate signals controlling mesenchymal differentiation state in lung. This ability may help resolve the relative role of these cells in the pathogenesis of specific lung diseases.
This work was supported by National Institute of Health Grants R21 HL082778 and K08 HL077138.
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1165/rcmb.2006-0386OC on March 29, 2007
Conflict of Interest Statement: None of the authors has a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Theise ND. Liver stem cells: the fall and rise of tissue biology. Hepatology 2003;38:804–806. [DOI] [PubMed] [Google Scholar]
- 2.Blanpain C, Fuchs E. Epidermal stem cells of the skin. Annu Rev Cell Dev Biol 2006;22:339–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bjerknes M, Cheng H. Gastrointestinal stem cells: II. Intestinal stem cells. Am J Physiol Gastrointest Liver Physiol 2005;289:G381–G387. [DOI] [PubMed] [Google Scholar]
- 4.Giangreco A, Reynolds SD, Stripp BR. Terminal bronchioles harbor a unique airway stem cell population that localizes to the bronchoalveolar duct junction. Am J Pathol 2002;161:173–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hong KU, Reynolds SD, Giangreco A, Hurley CM, Stripp BR. Clara cell secretory protein-expressing cells of the airway neuroepithelial body microenvironment include a label-retaining subset and are critical for epithelial renewal after progenitor cell depletion. Am J Respir Cell Mol Biol 2001;24:671–681. [DOI] [PubMed] [Google Scholar]
- 6.Reynolds SD, Hong KU, Giangreco A, Mango GW, Guron C, Morimoto Y, Stripp BR. Conditional clara cell ablation reveals a self-renewing progenitor function of pulmonary neuroendocrine cells. Am J Physiol Lung Cell Mol Physiol 2000;278:L1256–L1263. [DOI] [PubMed] [Google Scholar]
- 7.Boers JE, Ambergen AW, Thunnissen FB. Number and proliferation of basal and parabasal cells in normal human airway epithelium. Am J Respir Crit Care Med 1998;157:2000–2006. [DOI] [PubMed] [Google Scholar]
- 8.Evans MJ, Cabral LJ, Stephens RJ, Freeman G. Transformation of alveolar type 2 cells to type 1 cells following exposure to NO2. Exp Mol Pathol 1975;22:142–150. [DOI] [PubMed] [Google Scholar]
- 9.Sabatini F, Petecchia L, Tavian M, Jodon dVV, Rossi GA, Brouty-Boye D. Human bronchial fibroblasts exhibit a mesenchymal stem cell phenotype and multilineage differentiating potentialities. Lab Invest 2005;85:962–971. [DOI] [PubMed] [Google Scholar]
- 10.da Silva ML, Chagastelles PC, Nardi NB. Mesenchymal stem cells reside in virtually all post-natal organs and tissues. J Cell Sci 2006;119:2204–2213. [DOI] [PubMed] [Google Scholar]
- 11.Summer R, Kotton DN, Sun X, Fitzsimmons K, Fine A. Translational physiology: origin and phenotype of lung side population cells. Am J Physiol Lung Cell Mol Physiol 2004;287:L477–L483. [DOI] [PubMed] [Google Scholar]
- 12.Summer R, Kotton DN, Sun X, Ma B, Fitzsimmons K, Fine A. Side population cells and Bcrp1 expression in lung. Am J Physiol Lung Cell Mol Physiol 2003;285:L97–104. [DOI] [PubMed] [Google Scholar]
- 13.Summer R, Kotton DN, Liang S, Fitzsimmons K, Sun X, Fine A. Embryonic lung side population cells are hematopoietic and vascular precursors. Am J Respir Cell Mol Biol 2005;33:32–40. [DOI] [PubMed] [Google Scholar]
- 14.Goodell MA, Brose K, Paradis G, Conner AS, Mulligan RC. Isolation and functional properties of murine hematopoietic stem cells that are replicating in vivo. J Exp Med 1996;183:1797–1806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Redvers RP, Li A, Kaur P. Side population in adult murine epidermis exhibits phenotypic and functional characteristics of keratinocyte stem cells. Proc Natl Acad Sci USA 2006;103:13168–13173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pfister O, Mouquet F, Summer R, Helmes M, Jain M, Fine A, Colucci WS, Liao R. Cardiac side-population cells exhibit greater cardiomyogenic potential than bone marrow-derived stem cells. Circulation 2004;110:69–70. [Google Scholar]
- 17.Lo KC, Lei Z, Rao C, Beck J, Lamb DJ. De novo testosterone production in luteinizing hormone receptor knockout mice after transplantation of leydig stem cells. Endocrinology 2004;145:4011–4015. [DOI] [PubMed] [Google Scholar]
- 18.Giangreco A, Shen H, Reynolds SD, Stripp BR. Molecular phenotype of airway side population cells. Am J Physiol Lung Cell Mol Physiol 2004;286:L624–L630. [DOI] [PubMed] [Google Scholar]
- 19.Wilson KC, Center DM, Cruikshank WW. The effect of interleukin-16 and its precursor on T lymphocyte activation and growth. Growth Factors 2004;22:97–104. [DOI] [PubMed] [Google Scholar]
- 20.Prockop DJ. Marrow stromal cells as stem cells for nonhematopoietic tissues. Science 1997;276:71–74. [DOI] [PubMed] [Google Scholar]
- 21.Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR. Multilineage potential of adult human mesenchymal stem cells. Science 1999;284:143–147. [DOI] [PubMed] [Google Scholar]
- 22.Zhou S, Schuetz JD, Bunting KD, Colapietro AM, Sampath J, Morris JJ, Lagutina I, Grosveld GC, Osawa M, Nakauchi H, et al. The ABC transporter Bcrp1/ABCG2 is expressed in a wide variety of stem cells and is a molecular determinant of the side-population phenotype. Nat Med 2001;7:1028–1034. [DOI] [PubMed] [Google Scholar]
- 23.Spangrude GJ, Heimfeld S, Weissman IL. Purification and characterization of mouse hematopoietic stem cells. Science 1988;241:58–62. [DOI] [PubMed] [Google Scholar]
- 24.Goodell MA, Rosenzweig M, Kim H, Marks DF, DeMaria M, Paradis G, Grupp SA, Sieff CA, Mulligan RC, Johnson RP. Dye efflux studies suggest that hematopoietic stem cells expressing low or undetectable levels of CD34 antigen exist in multiple species. Nat Med 1997;3:1337–1345. [DOI] [PubMed] [Google Scholar]
- 25.Phinney DG, Kopen G, Isaacson RL, Prockop DJ. Plastic adherent stromal cells from the bone marrow of commonly used strains of inbred mice: variations in yield, growth, and differentiation. J Cell Biochem 1999;72:570–585. [PubMed] [Google Scholar]
- 26.Baddoo M, Hill K, Wilkinson R, Gaupp D, Hughes C, Kopen GC, Phinney DG. Characterization of mesenchymal stem cells isolated from murine bone marrow by negative selection. J Cell Biochem 2003;89:1235–1249. [DOI] [PubMed] [Google Scholar]
- 27.Schuger L, Skubitz AP, de las Morenas A, Gilbride K. Two separate domains of laminin promote lung organogenesis by different mechanisms of action. Dev Biol 1995;169:520–532. [DOI] [PubMed] [Google Scholar]
- 28.Schuger L, Varani J, Mitra R Jr, Gilbride K. Retinoic acid stimulates mouse lung development by a mechanism involving epithelial-mesenchymal interaction and regulation of epidermal growth factor receptors. Dev Biol 1993;159:462–473. [DOI] [PubMed] [Google Scholar]
- 29.Pfister O, Mouquet F, Jain M, Summer R, Helmes M, Fine A, Colucci WS, Liao R. CD31(-) but not CD31(+) cardiac side population cells exhibit functional cardiomyogenic differentiation. Circ Res 2005;97:52–61. [DOI] [PubMed] [Google Scholar]
- 30.Jiang Y, Jahagirdar BN, Reinhardt RL, Schwartz RE, Keene CD, Ortiz-Gonzalez XR, Reyes M, Lenvik T, Lund T, Blackstad M, et al. Pluripotency of mesenchymal stem cells derived from adult marrow. Nature 2002;418:41–49. [DOI] [PubMed] [Google Scholar]
- 31.Gomperts BN, Belperio JA, Rao PN, Randell SH, Fishbein MC, Burdick MD, Strieter RM. Circulating progenitor epithelial cells traffic via CXCR4/CXCL12 in response to airway injury. J Immunol 2006;176:1916–1927. [DOI] [PubMed] [Google Scholar]
- 32.Phillips RJ, Burdick MD, Hong K, Lutz MA, Murray LA, Xue YY, Belperio JA, Keane MP, Strieter RM. Circulating fibrocytes traffic to the lungs in response to CXCL12 and mediate fibrosis. J Clin Invest 2004;114:438–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hashimoto N, Jin H, Liu T, Chensue SW, Phan SH. Bone marrow-derived progenitor cells in pulmonary fibrosis. J Clin Invest 2004;113:243–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liang SX, Summer R, Sun X, Fine A. Gene expression profiling and localization of Hoechst-effluxing. Physiol Genomics 2005;23:172–181. [DOI] [PubMed] [Google Scholar]