Abstract
Background
Bacillus spores are notoriously resistant to unfavorable conditions such as UV radiation, γ-radiation, H2O2, desiccation, chemical disinfection, or starvation. Bacillus pumilus SAFR-032 survives standard decontamination procedures of the Jet Propulsion Lab spacecraft assembly facility, and both spores and vegetative cells of this strain exhibit elevated resistance to UV radiation and H2O2 compared to other Bacillus species.
Principal Findings
The genome of B. pumilus SAFR-032 was sequenced and annotated. Lists of genes relevant to DNA repair and the oxidative stress response were generated and compared to B. subtilis and B. licheniformis. Differences in conservation of genes, gene order, and protein sequences are highlighted because they potentially explain the extreme resistance phenotype of B. pumilus. The B. pumilus genome includes genes not found in B. subtilis or B. licheniformis and conserved genes with sequence divergence, but paradoxically lacks several genes that function in UV or H2O2 resistance in other Bacillus species.
Significance
This study identifies several candidate genes for further research into UV and H2O2 resistance. These findings will help explain the resistance of B. pumilus and are applicable to understanding sterilization survival strategies of microbes.
Introduction
Bacillus pumilus is a Gram-positive, aerobic, rod-shaped, soil-dwelling bacterium [1]. Like other Bacillus species, B. pumilus produces spores that are more resistant than vegetative cells to heat, desiccation, UV radiation, γ-radiation, H2O2, and starvation. B. pumilus has been found in extreme environments such as the interior of Sonoran desert basalt and the Mars Odyssey spacecraft [2], [3]. Spores and vegetative cells of B. pumilus SAFR-032, a strain originally recovered from the Jet Propulsion Lab (Pasadena, CA) spacecraft assembly facility, are endowed with UV radiation and H2O2 resistance capabilities that significantly exceed other Bacillus species and allow survival of standard sterilization practices [3]–[5]. Sterilization is significant not only for prevention of contamination of extraterrestrial environments via spacecraft, but also for fundamental processes in bacteriology, medicine, the pharmaceutical industry, and counter-bioterrorism measures, and hence such resistance is cause for concern.
UV radiation induces the formation of deleterious DNA lesions such as pyrimidine dimers [5], [6]. Bacillus spores are more resistant to UV radiation than vegetative cells because desiccation and the presence of small acid soluble spore proteins (SASP) mitigate DNA damage. A variety of DNA repair mechanisms that become active upon germination also permit survival of UV radiation. H2O2 kills spores by oxidative damage to the inner membrane and it also causes oxidative damage to cellular proteins and DNA [7], [8]. H2O2-induced damage is combated by a variety of reducing agents that react with oxidative agents or oxidized cellular components.
Here we present an analysis of the B. pumilus SAFR-032 genome. In comparing this genome to less UV- and H2O2-resistant Bacillus species (B. subtilis and B. licheniformis) we identify genomic differences that provide important insights into the DNA repair pathways and oxidative stress response pathways of B. pumilus. The genes identified in this study are candidates for further experimental research.
Methods
Bacterial strain growth and DNA isolation
A single B. pumilus SAFR-032 colony exhibiting circular, crateriform morphology and raised ridges on its surface, was used to inoculate trypticase soy yeast (TSY) broth. The culture was grown overnight at 37°C with vigorous shaking. Genomic DNA was purified from CsCl gradients of whole cell lysates [9].
DNA sequencing and genome assembly
DNA sequencing was performed by a combined approach using traditional Sanger dideoxy whole genome shotgun (WGS) sequencing and 454 Life Sciences pyrosequencing strategies [10]. Genomic DNA was nebulized into 5 kb fragments, and cloned into a derivative of pUC18 [11]. The clones were used for WGS DNA sequencing using ABI 3700 sequencers, and reads were assembled using the ATLAS assembler [12]. Read-pair information was used to create higher order scaffolds. WGS reads were sequenced to ten-fold coverage. The WGS plasmid libraries were not random, but had cloning bias of unknown cause. Consequently, the WGS sequence was supplemented with short reads generated on a 454 Life Sciences GS20 sequencer and lacking cloning bias. Here the coverage was thirteen fold.
Gene identification and annotation
Previously described gene prediction and manual annotation protocols were followed [13]. Glimmer [14] and GeneMark [15] were used independently to predict open reading frames (ORFs). Visualization of gene predictions was performed using the Genboree system (www.genboree.org) and the CONAN database [13]. DNA comparisons were performed with BLASTN and BLASTZ. Protein sequences were analyzed by BLASTP vs. the nr database at NCBI [16]. When appropriate, other predictive tools such as InterProScan [17], PFP [18], PSORTb [19], ExPASy ENZYME [20], Helix-Turn-Helix Predictor [21], MEROPs [22], and the Transport Classification Database [23] were used. The B. pumilus SAFR-032 genome is 3.7 Mb and 3848 features (3687 ORFs, 12 frameshifts, 38 pseudogenes, 7 rRNA operons, 69 tRNAs, and 21 ncRNAs) were annotated. The B. pumilus genome has been deposited in GenBank under the accession number CP000813. Locus tags of genes discussed in this paper are listed in Supplementary Table S1.
Comparative Genomic Analysis
The database of annotated genes was searched for genes relevant to DNA repair and H2O2 resistance. B. pumilus genes were considered homologs of B. subtilis and B. licheniformis genes if their translated sequences aligned with ≥50% identity to the homolog of either species. Exceptions were made in deference to conserved gene order and local alignments to functional domains characteristic of specific proteins. We examined the B. subtilis and B. licheniformis genomes and available literature to find DNA repair and H2O2 resistance genes not found in our B. pumilus gene list. Relevant genes absent from the B. pumilus gene list were confirmed as absent using B. subtilis and B. licheniformis sequences as queries for local BLAST against the B. pumilus genome.
Spore survivability to UV radiation and H2O2
Methods of measuring survival of spores exposed to UV radiation and H2O2 have been previously described [3], [4]. Data presented here include but are not limited to measurements previously reported in those studies.
Results and Discussion
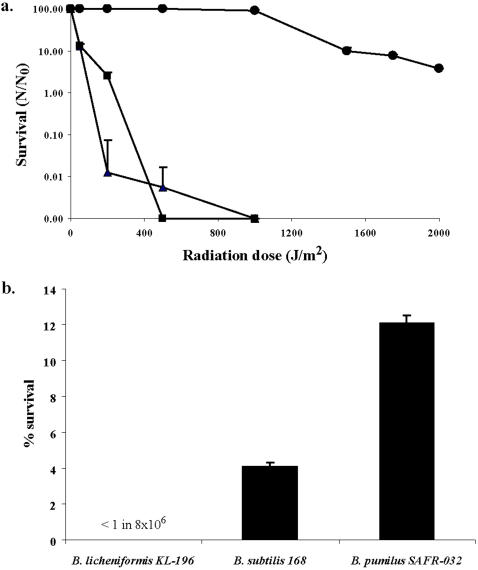
B. pumilus SAFR-032 was selected for genome sequencing and analysis because its spores exhibited unusually high resistance to UV radiation and H2O2 compared to the standard dosimetric strains B. subtilis 168 and B. licheniformis. Whereas >90% lethality of B. subtilis and B. lichenifiormis spores is achieved by exposure to 200 J/m2 UV254, 1500 J/m2 are required to kill 90% of B. pumilus SAFR-032 spores (Figure 1a). Twelve percent of B. pumilus SAFR-032 spores survive 5% liquid H2O2, which is nearly thrice the survival rate of B. subtilis spores (Figure 1b).
Figure 1. Resistance of B. pumilus SAFR-032 spores to UV radiation and H2O2.
a) Survivability of spores exposed to varying doses of UV254 (100 uW sec−1cm−2). Key: B. pumilus SAFR-032, circles; B. subtilis 168, squares; B. licheniformis ME-13-1, triangles. b) Survivability of spores exposed to 5% H2O2 liquid for one hour.
The B. pumilus SAFR-032 genome was annotated and analyzed for features relevant to UV radiation resistance and H2O2 resistance. Mechanisms of DNA repair and the oxidative stress response were compared among B. pumilus, B. subtilis, and B. licheniformis to generate lists of genes common to all three species, genes unique to B. pumilus, and genes absent in B. pumilus (Table 1). The presence or absence of genes is indicative of unique functions that may explain phenotypic differences. Despite gene conservation, the possibility of altered functions of homologous genes due to sequence divergence cannot be excluded. Therefore, the translated sequences of common genes were also compared (Tables 2 and 3). In addition to gene conservation and sequence similarity it is also important to understand gene functions in context of the organism's growth phase. Although the temporal activity of only some proteins discussed here are known, two recent studies describe transcription of many B. subtilis DNA repair and H2O2 resistance genes. Keijser et al. identified transcripts more abundant in spores and germinating cells than in vegetative cells [24]. Moeller et al. identified transcripts induced after exposure of vegetative cells to UVC radiation (200–280 nm) [25]. We cross-referenced our gene lists with these temporal transcription data to augment our genomic comparisons (Table 1). However, it should be understood that because spore survivability assays entail growing surviving spores to countable levels in liquid or solid media, resistance mechanisms at any stage of growth may be important to survivability.
Table 1. List of Bacillus genes involved in DNA repair and oxidative stress resistance.
| Function (No. of genes) | Class | Gene | Missing in Bp [1] | Missing in Bl [2] | Missing in Bs [3] | Missing in Bp & Bl [1], [2] | Missing in Bl & Bs [2], [3] |
| DNA repair (88) | U | ada 2,3; dinG; disA; dnaE; end1; gyrB; hbs; kapD; mfd; mutL,M,S2(yshD); ogt; pcrB; phrB 2,3; polA; polY2(yqjW); priA; recD, J, N,R,Q(recS),X; sbcC,D; scpA,B; sms; uvrX1; xseA,B; yjhB1;ykoW; ylbH; yocI; yobH 1,2; yorK 1,2; yozK 1,2; yqfN; yrrK; yvcI; ywbD | 5 | 5 | 2 | 3 | 2 |
| V | alkA; dinB 1; lexA; mutS1; polY1(yqjH); radC; recF,G,O; ruvA,B; ssb; yjcD; yneB; ywjD 2(uvsE) | 1 | 1 | 0 | 0 | 0 | |
| G&O | addA,B; exoA 1,2; gyrA; mutT 1,Y; nth; pcrA; recU; topA; ung; uvrC; ydiP 2; yhaZ 2 ; ypcP; yprA; ypvA; yrrT; yrvN; ywqA, yxlJ 1 | 3 | 3 | 0 | 1 | 0 | |
| S | nfo; yqhH | 0 | 0 | 0 | 0 | 0 | |
| V+G&O | recA; uvrA,B; ykoU(lig),V(ku) | 0 | 0 | 0 | 0 | 0 | |
| S+G&O | splA,B | 0 | 0 | 0 | 0 | 0 | |
| Oxidative stress resistance (35) | U | bcrC; cotJC 1; katX2 2,3; msrA; ohrA,B,R; sigM; sodF; trxA; ycgT; ygaF; yjqC; ykuU; ylaC 1,D 1;yojM; yqjL | 3 | 1 | 1 | 0 | 1 |
| V | mrgA 1; msrB | 1 | 0 | 0 | 0 | 0 | |
| G&O | ahpC 1,F 1; bsaA; perR; sigB; sodA; spx; tpx; trxB; ydbD 1; yqjM | 3 | 0 | 0 | 0 | 0 | |
| V+G&O | dpsA; katA 1,B 1(katE) | 2 | 0 | 0 | 0 | 0 | |
| S+G&O | katX(yxlI) | 0 | 0 | 0 | 0 | 0 | |
| SASP (18) | S | csgA 1; sspA,B,C 1,2,D,E,F,G 1,2,H 1,I,J,K,L,M,N,O (cotK),P(cotL);tlp | 4 | 2 | 0 | 2 | 0 |
1 = absent in B. pumilus SAFR-032 (Bp). 2 = absent in B. licheniformis (Bl). 3 = absent in B. subtilis 168 (Bs).
U = expression unknown.
V = genes transcribed in B. subtilis vegetative cells [25].
Table 2. Sequence conservation of DNA repair proteins among Bacillus species.
| Pathway | Protein | % identity Bp vs. Bs | % identity Bp vs. Bl | % identity Bs vs. Bl |
| Base excision repair | ||||
| AlkA (YfiP) | 60 | 68 | 78 | |
| Ung | 73 | 73 | 83 | |
| MutM (Fpg) | 64 | 63 | 77 | |
| MutY (YfhQ) | 68 | 66 | 75 | |
| Nth | 88 | 88 | 89 | |
| Nfo (YqfS) | 86 | 87 | 91 | |
| Nucleotide Excision Repair | ||||
| Mfd | 80 | 81 | 85 | |
| PcrA | 83 | 85 | 87 | |
| UvrA | 86 | 87 | 86 | |
| UvrB | 89 | 89 | 92 | |
| UvrC | 82 | 84 | 84 | |
| Mismatch Repair | ||||
| MutS | 79 | 78 | 82 | |
| MutL | 77 | 73 | 78 | |
| XseA (YqiB) | 73 | 71 | 75 | |
| XseB (YqiC) | 74 | 77 | 73 | |
| NHEJ | ||||
| YkoU | 40 | 41 | 58 | |
| YkoV | 49 | 52 | 68 | |
| YkoW | 49 | 34 | 30 | |
| Homologous recombination | ||||
| AddA | 66 | 67 | 72 | |
| AddB | 64 | 65 | 74 | |
| LexA | 87 | 87 | 91 | |
| PriA | 71 | 72 | 79 | |
| RecA | 93 | 95 | 93 | |
| RecD (YrrC) | 79 | 82 | 79 | |
| RecF | 84 | 84 | 89 | |
| RecG (YlpB) | 81 | 80 | 81 | |
| RecJ (YrvE) | 67 | 62 | 68 | |
| RecN | 74 | 75 | 81 | |
| RecO | 75 | 77 | 78 | |
| RecQ (RecS) | 53 | 53 | 58 | |
| RecR | 97 | 98 | 98 | |
| RecU | 70 | 73 | 80 | |
| RecX (YfhG) | 63 | 64 | 70 | |
| RuvA | 81 | 77 | 84 | |
| RuvB | 85 | 85 | 88 | |
| SbcC (YirY) | 51 | 49 | 56 | |
| SbcD | 74 | 74 | 78 | |
| Spore Photoproduct Lyase | ||||
| SplA | 63 | 65 | 73 | |
| SplB | 90 | 86 | 88 | |
| UVDE-dependent excision repair | ||||
| YwjD | 69 | – | – | |
| Y-family polymerase | ||||
| PolY1 (YqjH) | 70 | 71 | 75 | |
| PolY2 (YqjW) | 64 | – | – | |
| Alkyltransferases | ||||
| Ogt | 55 | 56 | 59 | |
| YhaZ | 54 | – | – | |
| Other | ||||
| DinG | 53 | 52 | 59 | |
| End1 (YurI) | 60 | 60 | 61 | |
| KapD | 65 | 66 | 77 | |
| MutS2(YshD) | 77 | 75 | 83 | |
| YpcP | 70 | 69 | 76 | |
| YvcI | 78 | 81 | 77 | |
| YwqA | 76 | 75 | 77 | |
Table 3. Sequence conservation of H2O2 resistance proteins among Bacillus species.
| Function | Protein | % identity Bp vs. Bs | % identity Bp vs. Bl | % identity Bs vs. Bl |
| Catalase | ||||
| KatX1 (YxlI)1 | 82 | 74 | 74 | |
| KatX21 | 47 | 48 | 74 | |
| YjqC | 79 | 64 | 62 | |
| Mn-catalase2 | 25 | 24 | 78 | |
| Redox Proteins | ||||
| BsaA | 69 | 68 | 69 | |
| Tpx | 85 | 84 | 86 | |
| TrxA | 92 | 93 | 97 | |
| TrxB | 90 | 88 | 91 | |
| YcgT | 51 | 46 | 39 | |
| YgaF | 82 | 81 | 85 | |
| YkuU | 96 | 97 | 98 | |
| Organic hydroperoxide | ||||
| OhrA | 73 | 61 | 72 | |
| OhrB | 72 | 78 | 76 | |
| OhrR | 70 | 69 | 65 | |
| Transcription factors | ||||
| PerR | 93 | 92 | 91 | |
| SigM (YhdM) | 91 | 91 | 96 | |
| SigB (RpoF) | 87 | 84 | 86 | |
| Spx (YjbD) | 92 | 93 | 93 | |
| Other | ||||
| DpsA (YktB) | 82 | 82 | 78 | |
| Superoxide resistance | ||||
| BcrC (YwoA) | 50 | 47 | 60 | |
| SodA | 87 | 87 | 89 | |
| SodF | 56 | 60 | 66 | |
| YojM | 42 | 47 | 57 | |
| YqjL | 42 | 56 | 57 | |
blastp vs. KatX.
blastp vs. YdbD.
Previous analyses of the resistance properties of Bacillus spores centered on small acid-soluble spore proteins (SASP) and the spore photoproduct lyase DNA repair system [26], [27]. SASP are spore core proteins that play a crucial role in resistance to UV radiation, heat, desiccation, and oxidative damage by binding DNA and altering its reactivity [5]. When exposed to UV radiation, SASP-bound DNA more readily forms the spore photoproduct (SP), 5-thyminyl-5,6-dihydrothymine, rather than cyclobutane dimers or (6-4)-photoproducts, which are formed in the absence of SASP. Unlike these other DNA lesions, SP is easily repaired by the spore photoproduct lyase (SP lyase), which is encoded by splB gene and is negatively regulated by the splA gene product [6]. B. pumilus has an intact splAB operon. The translated SplB (BPUM_1283) sequence is highly conserved in B. pumilus, but SplA (BPUM_1282) shows much more sequence diversity among B. pumilus, B. subtilis, and B. licheniformis (Table 2), indicating possible differences in SP lyase genetic regulation.
Bacillus subtilis produces 18 SASPs, whose sequences are short (40–100 amino acids) and highly conserved. The α/β-type SspA and SspB predominate, and there are also minor α/β-type SASPs, a γ–type, and novel SASPs [28]. Fifteen SASP genes were annotated in B. pumilus (Table 1); homologs of SspC, SspG, and SspH were not found. SspC is a minor α/β-type SASP that contributes to UV radiation resistance [29], hence its absence from B. pumilus is paradoxical. SspH and SspG are novel type SASPs, that have no effect on B. subtilis UV radiation resistance [28], [30]. The B. pumilus and B. licheniformis homologs of the γ–type SASP appear to be amino-terminal truncations of the 84 amino acid SspE of B. subtilis. The significance of such a truncation is unclear, as the only known function of SspE is as an amino acid source for germinating spores [31]. Although these differences in gene content and sequence conservation may contribute to the enhanced UV and oxidation resistance of B. pumilus, other important factors are likely to be found among DNA repair and oxidative stress response genes
DNA Repair Mechanisms–Single Strand Repair pathways
Base Excision Repair (BER)
Oxidative damage to DNA is repaired by BER, which is performed by DNA glycosylases and AP (apurinic/apyrimidinic) endonucleases [32], [33]. DNA glycosylases remove damaged bases from the DNA backbone to create an AP site. AP endonucleases bind to this site and cleave the DNA 5′ to the abasic site, forming a free 3′-hydroxyl which is repaired by DNA polymerases. Monofunctional DNA glycosylases only have glycosylase activity, whereas bifunctional DNA glycosylases have both glycosylase and lyase activities as well as the ability to cleave the phosphodiester backbone 3′ to the AP site. B. pumilus encodes both monofunctional [AlkA (BPUM_0752), Ung (BPUM_03444)] and bifunctional [MutM (BPUM_2550), Nth (BPUM_1966)] DNA glycosylases in addition to the AP endonuclease IV, Nfo (BPUM_2246). Nth and Nfo are highly conserved among B. pumilus, B. subtilis, and B. licheniformis, but AlkA, Ung, and MutM have greater sequence divergence (Table 2).
B. pumilus lacks a homolog of the AP endonuclease ExoA and the DNA glycosylase YxlJ, both of which are present in B. subtilis and B. licheniformis. The lack of ExoA is not surprising, as B. subtilis exoA mutants do not exhibit enhanced sensitivity to H2O2 [34]. YxlJ functions in the repair of DNA alkylation damage and removal of deaminated purines and cyclic etheno adducts [35], and it is transcribed during spore germination and outgrowth [24]; its absence suggests that another protein compensates for its loss.
Nucleotide Excision Repair (NER)
While BER recognizes and repairs individual bases by specific DNA glycosylases, NER identifies multi-base distortions in the double helix and removes bulky single-stranded lesions, which are repaired by DNA polymerase I [36]. The E. coli NER pathway consists of UvrA and UvrB, which recognize DNA lesions, the UvrC nuclease, and the UvrD helicase. The NER machinery can be recruited to DNA damage by the Mfd protein in a process called transcription-coupled NER. In B. subtilis, NER is associated with UV radiation resistance in vegetative cells [26], and uvrA and uvrB are transcribed in germinating/outgrowing spores [24]. B. subtilis lacks UvrD, but likely uses PcrA to perform the UvrD helicase function [32], [37]. B. pumilus encodes homologs of UvrABC (BPUM_3147, 3148, & 2506), PcrA (BPUM_0625), and Mfd (BPUM_0039), the amino acid sequences of which are conserved with respect to B. subtilis and B. licheniformis (Table 2).
Mismatch Repair (MMR)
MMR recognizes and repairs mismatched bases in newly synthesized DNA daughter strands, and although not associated with DNA repair related to UV radiation or oxidative damage, it is important in maintaining genomic integrity [38]. In E. coli, MMR involves MutS and MutL, which recognize mismatches, and endonuclease MutH. Bacillus species lack MutH and must use another, unidentified mechanism [39]. B. pumilus MutS (BPUM_1608) and MutL (BPUM_1609) homologs are moderately well-conserved compared to those of B. subtilis and B. licheniformis (Table 2). Homologs of XseA (BPUM_2162) and XseB (BPUM_2161), subunits of a MMR exonuclease, were also annotated in B. pumilus.
DNA Repair Mechanisms–Double Strand Repair pathways
Non-Homologous End-Joining (NHEJ)
The NHEJ pathway repairs double-strand DNA (DSB) breaks by directly joining DNA ends without requiring a homologous template to guide the repair [40]. Prokaryotic homologs of the eukaryotic DNA-end-binding protein, Ku, and DNA ligase IV were recently identified in several bacteria [41]. In B. subtilis, the NHEJ proteins are encoded on the ykoUVW operon, and ykoU and ykoV are transcribed both in vegetative cells and germinating/outgrowing spores [42]. B. subtilis YkoV (Ku) specifically recruits YkoU (DNA ligase IV) to DNA ends to stimulate DNA ligation, and loss of these proteins leads to hypersensitivity to UV radiation in B. subtilis [43]. YkoW is hypothesized to interact with dsDNA ends.
There is significant amino acid sequence variation in NHEJ proteins among B. pumilus, B. subtilis, and B. licheniformis (Table 2). The YkoV and YkoU sequences of B. subtilis and B. licheniformis are more closely related to each other than to their B. pumilus homologs (BPUM_1667 & BPUM_1666). Additionally, B. pumilus YkoW (BPUM_1234), at 807 amino acids in length, is much longer than B. subtilis YkoW (749 amino acids) and B. licheniformis (549 amino acids). Beyond these differences in amino acid sequences, which may affect protein function, the regulation of NHEJ genes appears to be different in B. pumilus. The ykoUVW operon structure of B. subtilis and B. licheniformis is not conserved in B. pumilus. In B. pumilus, ykoU and ykoV are adjacent and divergently transcribed, while ykoW is located on a separate locus.
Recombinational Repair
Homologous recombination (HR) is a ubiquitous process that is crucial for DNA repair and maintenance. It is a multi-step pathway involving several proteins that facilitate the invasion of dsDNA by a ssDNA substrate. As DNA is unwound by a helicase, the migrating strand replaces damaged DNA and the intermediate structure is resolved by an endonuclease [32]. HR in Bacillus species can be initiated by the AddAB pathway, which is analogous to E. coli RecBCD [44], or the RecFOR pathway [32]. Strand invasion and exchange is catalyzed by RecA, and recA mutations increase sensitivity to UV radiation [26]. Branch migration is performed by the RuvAB proteins, and resolution is performed by the RecU (RuvC in E. coli), and RecG proteins [32]. B. pumilus encodes homologs of all HR proteins common to B. subtilis and B. licheniformis (Tables 1 & 2).
Control of HR is closely related to the SOS repair system. In B. subtilis, the SOS regulon is similar to that in E. coli but it is also induced in competent cells in the absence of any DNA-damaging treatment [45]. RecA and the SOS transcriptional repressor LexA are the two main proteins involved in this coordinated cellular response to UV-light and DNA-damaging agents [46]. RecA is activated by ssDNA and promotes LexA self-cleavage, causing it to lose affinity to DNA and allowing expression of the SOS-response genes. The B. pumilus LexA (BPUM_1686) sequence is 87% similar to the B. subtilis and B. licheniformis LexA homologs, and their DNA-binding motifs are identical [47], suggesting that their activities are similar in these three species. Several SOS proteins have been identified in E. coli and B. subtilis, but the identification of B. pumilus SOS proteins will require experimental verification of regulation by RecA and LexA [46]. HR is also under the influence of RecX, a repressor of recA [48]. B. pumilus RecX (BPUM_0795) has moderate sequence conservation with its B. subtilis and B. licheniformis homologs.
Other DNA repair systems
UVDE-dependent excision repair
YwjD is a B. subtilis homolog of UVDE, a eukaryotic protein that repairs UV radiation-induced cyclobutane pyrimidine dimers and 6-4 photoproducts [49]. B. pumilus encodes a YwjD (BPUM_3376) homolog that shares only moderate sequence identity with B. subtilis YwjD, which is produced in vegetative cells, and there is no B. licheniformis homolog. Because the sequence conservation is poor, it is possible that B. pumilus YwjD functions in a way that enhances its DNA repair activity.
Y-family polymerases
The Y family polymerases are error-prone, translesional DNA polymerases that are processive through DNA lesions that block the replicative polymerase [50]. Two Y-family polymerases were annotated in B. pumilus and named for their B. subtilus homologs, PolY1 (YqjH; BPUM_2125) and PolY2 (YqjW; BPUM_2102). In B. subtilis PolY2 is an SOS inducible polymerase that functions in UV damage repair and is necessary for UV-induced mutagenesis [50]. PolY2 is missing in B. licheniformis, which may contribute to its relative UV sensitivity. The fact that PolY2 is present in both B. pumilus and B. subtilis means that it alone cannot account for the UV resistance of B. pumilus. However, sequence variation (Table 2) and differences in expression may influence its activity. In contrast, PolY1 is a DinB subfamily polymerase that is constitutively expressed and functions in untargeted mutagenesis rather than UV-induced mutagenesis [50]. PolY1 is common to B. pumilus, B. subtilis, and B. licheniformis, and, therefore, unlikely to be responsible for UV resistance. Two other B. subtilis Y-family polymerases, UvrX and YozK-YobH are encoded on integrated prophages that are not present in B. pumilus [50].
Alkyltransferases
Alkylating chemicals can mutate DNA bases or the phosphodiester backbone by adding an alkyl group to the nitrogen or oxygen atoms. Ogt is a methyltransferase that removes the alkyl group from O6-alkyl guanine or, preferentially, O4-alkyl thymine [51]. Ogt also exhibits suicide inactivation by transferring an alkyl group to a cysteine residue in its own structure. Ogt (BPUM_1248) is found in B. pumilus, B. subtilis and B. licheniformis, although the protein sequence is not well-conserved (Table 2).
The B. pumilus genome encodes a second alkyltransferase, Ada (BPUM_1200), which, like Ogt, removes alkyl moities from DNA by suicide inactivation [32]. Ada also initiates the adaptive response, which activates several DNA repair enzymes [52]. There are notable differences between B. pumilus Ada and the homologs of B. subtilis and B. licheniformis that may be significant in DNA repair. B. pumilus Ada, like E. coli Ada, incorporates a regulatory domain fused to the alkyl glycosylase domain. However, in both B. subtilis and B. licheniformis, these domains are split into two proteins [53], AdaA and AdaB, neither of which align with greater than 50% identity to B. pumilus Ada. The fusion of the two proteins in B. pumilus raises the possibility that the function and transcriptional regulation of this alkyltransferase may be different in B. pumilus compared to B. subtilis and B. licheniformis.
Other proteins
The ATP-dependent DNA helicase DinG can unwind RNA or DNA, and it is a bacterial homolog of a human DNA repair helicase [54], [55]. Homologs of DinG (BPUM_1971) are present in B. pumilus, B. subtilis and B. licheniformis, although their sequences are not well conserved (Table 2).
The Nudix hydrolase superfamily MutT protein hydrolyzes 8-oxo-dGTP (a reactive oxygen species) and prevents its incorporation into DNA [56]. B. subtilis has three MutT superfamily genes, mutT, which is transcribed in germinating/outgrowing spores [24], yjhB, and yvcI. The B. pumilus genome has one yvcI gene (BPUM_3116), but no mutT; or yjhB homologs.
YshD is a MutS2 family protein that maintains genome integrity by inhibiting intergenomic recombination. The YshD (BPUM_2516) sequence is conserved among B. pumilus, B. subtilis, and B. licheniformis (Table 2), but it is unclear if it has an effect on UV or H2O2 resistance.
DNA repair proteins unique to B. pumilus
The B. pumilus genome encodes PhrB (BPUM_1378), a DNA photolyase enzyme that repairs cyclobutane-pyrimidine dimers [57]. Although no homolog exists in B. subtilis and B. licheniformis, there are homologs in other Bacillus species such as B. firmus, B. cereus, B. anthracis, and B. thuringiensis. Nevertheless, none of these species exhibit UV radiation resistance comparable to B. pumilus. The B. subtilis photolyase amino acid sequence is diverse with respect to other photolyases. It shares 32% amino acid identity with E. coli PhrB and only 46% sequence identity with its closest homolog from B. firmus. It is logical that the presence of a photolyase gives B. pumilus UV resistance capabilities that B. subtilis and B. licheniformis lack. However, because less UV-resistant Bacillus species also have photolyase enzymes, the relation of photolyase to enhanced UV resistance is not clear. Although the sequence divergence in the B. pumilus photolyase may indicate altered function, B. pumilus may rely on a combination of other factors for its UV resistance properties.
Genes encoding two DNA repair/modification proteins not found in B. subtilis and B. licheniformis were also annotated in B. pumilus. One sequence (BPUM_0608) is similar to a Superfamily II (SF-2) helicase based upon the presence of a DExD Walker B motif in conserved motif II [58]. SF-2 helicases are known to function in NER and recombinational repair in yeast [59]. Although it cannot be predicted that this helicase functions in DNA repair, if it does have such a function it would be a feature that B. subtilis and B. licheniformis lack, possibly contributing to the enhanced UV radiation resistance. B. pumilus also encodes a C-5 cytosine-specific DNA methyltransferase (BPUM_0656) that has no homolog in either B. subtilis or B. licheniformis. Though unlikely to be directly implicated in DNA repair, it is possible that a unique DNA-modifying protein may contribute to genomic stability in B. pumilus. Additionally, the B. pumilus genome has 517 coding sequences that are not common to B. subtilis and B. licheniformis, including 218 hypothetical proteins that have no sequence similarity to any known sequence in the nr database. It is possible that one more of these coding sequences of unknown function may contribute to UV radiation resistance.
H2O2 resistance
Bacillus species use a variety of proteins to resist the toxic effects of H2O2, including catalases, and various reducing proteins such as alkyl hydroperoxide reductase and peroxiredoxins [60]. Analysis of the B. pumilus genome reveals many striking differences compared to similar proteins in B. subtilis and B. licheniformis.
Catalase
Catalases convert H2O2 into water and oxygen in a highly efficient reaction that requires neither ATP nor an exogenous reducing agent [8]. B. subtilis and B. licheniformis produce two vegetative catalases, KatA and KatB (KatE), and one germination catalase, KatX, which is present in spores and protects germinating cells from H2O2 [61]. All three catalases are transcribed in germinating/outgrowing spores [24]. B. pumilus has no homolog to either vegetative catalase, however, it has two KatX homologs. The sequence conservation of KatX1 (BPUM_3712) is moderate, but KatX2 (BPUM_0892) is more diverse, sharing less than 50% identity with B. subtilis and B. licheniformis KatX. A second germination-specific catalase with substantial sequence diversity is a candidate protein that may explain the enhanced peroxide resistance of B. pumilus spores.
YjqC and YdbD are two additional proteins with catalase domains that are found in B. subtilis and B. licheniformis, although little is known of their functions. B. pumilus YjqC (BPUM_2346) shares moderate sequence identity with its Bacillus homologs (Table 3), but there is no YdbD homolog in B. pumilus. However, a B. pumilus sequence containing a manganese catalase domain does exist (YdbD uses Mn2+ as a cofactor). It is possible that this catalase (BPUM_1305), which differs greatly from YdbD, may have properties that contribute to the H2O2 resistance of B. pumilus.
The spore coat protein CotJC contains a predicted catalase domain in its amino acid sequence. Although CotJC is present in B. subtilis and B. licheniformis, no homolog was identified in B. pumilus, suggesting that it is not necessary for elevated peroxide resistance.
Peroxiredoxins
Bacteria use peroxiredoxins to reduce H2O2 to water [62]. Four peroxiredoxins were annotated in B. pumilus. Three peroxiredoxin protein sequences, YkuU (BPUM_1319), YgaF (BPUM_0826), and Tpx (BPUM_2581), are highly conserved with respect to their B. subtilis and B. licheniformis homologs (Table 3). The fourth peroxiredoxin (BPUM_3690) annotated in B. pumilus has no obvious homolog in B. subtilis or B. licheniformis. Instead, these two species produce an alkyl hydroperoxide reductase that is induced upon H2O2 stress. The enzyme is a heterodimer of AhpC and AhpF, and it uses NADH or NADPH as a reducing agent. In B. subtilis and B. licheniformis the subunits are encoded on the ahpCF operon, and their translated sequences are highly conserved (>90% identity for AhpC and AhpF). The B. pumilus genome does not contain a homologous operon. However, it does it does have a gene encoding an NADH dehydrogenase (BPUM_2106), which, if coupled with the peroxiredoxin (BPUM_3690), could hypothetically function as an alkyl hydroperoxide reductase. Given the lack of sequence and gene order conservation, the function may be distinct from B. subtilis and B. licheniformis AphCF, possibly explaining the abnormal H2O2 resistance of B. pumilus.
Peroxidases
Peroxidases also reduce H2O2 to water using NADH or NADPH as a cofactor [8]. A glutathione peroxidase, BsaA (BPUM_1925), was annotated in B. pumilus. BsaA uses glutathione as a reducing agent to reduce lipid hydroxyperoxides formed by peroxide stress, and bsaA is transcribed during spore germination/outgrowth [24]. There is substantial sequence diversity among the BsaA homologs of B. pumilus, B. subtilis, and B. licheniformis (Table 3), raising the possibility that differences in B. pumilus BsaA may contribute to H2O2 resistance.
Other reducing agents
Thioredoxins and glutaredoxins are instrumental to peroxide stress resistance. They reduce peroxiredoxins and peroxidases, facilitating their functions, and act as hydroxyl radical scavengers [63]. They also maintain oxidation states of cytoplasmic proteins, preventing illegitimate disulfide bond formation [64]. Several redox proteins were annotated in B. pumilus, but only those known to be related to peroxide stress and those unique compared to B. subtilis and B. licheniformis are mentioned in this work.
TrxA is the product of the thioredoxin A gene, which is essential in B. subtilis. The reducing potential of TrxA is recycled by the thioredoxin reductase, TrxB. B. pumilus TrxA (BPUM_2507) and TrxB (BPUM_3117) share approximately 90% identity with their B. subtilis homologs. A second TrxB-like thioredoxin reductase (BPUM_0664) was annotated in B. pumilus (Table 4). This protein has no B. subtilis homolog and a poor alignment to a B. licheniformis reductase, so it may provide peroxide stress resistance capabilities not available to these species. YcgT (BPUM_0777), another thioredoxin-disulfide reductase, is present in B. pumilus, but its sequence is not well-conserved with B. subtilis and B. licheniformis, raising the possibility that differences in YcgT activity may be important to B. pumilus oxidative stress resistance.
Table 4. Unique DNA repair and H2O2 resistance proteins of B. pumilus.
| BPU locus tag2 | Definition |
| DNA repair | |
| BPUM_1378 | photolyase PhrB |
| BPUM_1200 | DNA repair methyltransfrease Ada |
| BPUM_0608 | helicase |
| BPUM_0656 | DNA (cytosine-5-)-methyltransferase |
| H2O2 resistance | |
| BPUM_0664 | TrxB-like thioredoxin-disulfide reductase1 |
| BPUM_0931 | lysine/ornithine N-monooxygenase |
| BPUM_1716 | NADH-dependent flavin oxidoreductase2 |
| BPUM_2106 | NADH dehydrogenase |
| BPUM_3690 | peroxiredoxin |
| BPUM_1153 | possible FAD dependent oxidoreductase |
| BPUM_1731 | flavin reductase |
| BPUM_0802 | possible monooxygenase |
| BPUM_0482 | probable dioxygenase |
| BPUM_3130 | thioredoxin |
homolog in B. licheniformis, but not B. subtilis.
only 42% identity with B. licheniformis and B. subtilis YqjM; B. pumilus YqjM found at BPUM_2112.
The ohr operon
In B. subtilis resistance to organic peroxides is encoded by the ohr locus, which produces the peroxide resistance proteins OhrA and OhrB, and OhrR, a transcriptional regulator of ohrA [65]. The B. pumilus homologs (BPUM_1211-1213) of these proteins share moderate homology with their B. subtilis and B. licheniformis homologs (Table 3), so they may have altered function due to sequence diversity.
Regulation of the oxidative stress response
Several transcriptional regulators of the B. subtilis oxidative stress response are known, including PerR, Spx, and sigma factors SigM and SigB. All four of these proteins are conserved in B. pumilus (Table 3). Dps proteins are DNA- binding proteins that protect bacteria from oxidative stress by sequestering iron and oxidants and storing them as benign ferric oxide minerals [66]. Two Dps proteins, DpsA (YktB) and MrgA are known in B. subtilis and B. licheniformis. B. pumilus does encode a DpsA homolog (Table 3; BPUM_2703), however, it has no MrgA homolog. Although MrgA is important for peroxide resistance proteins in vegetative cells, it has no effect on peroxide resistance in spores [67].
Other oxidative stress proteins
Oxidative stress also occurs in the form of superoxide, O2 −. Although the O2 − and H2O2 stress responses are distinct, the conditions are related, via the chemical conversion of O2 − to H2O2 by superoxide dismutases. B. pumilus has three superoxide dismutases: SodA (BPUM_2230), which uses a manganese cofactor, SodF (BPUM_1859), which uses an iron cofactor, and YojM (BPUM_1865), which uses copper or zinc as a cofactor [8]. B. pumilus SodA is highly conserved with respect to the homologs of B. subtilis and B. licheniformis, but there is much greater sequence diversity in SodF and YojM (Table 3). If these changes in sequence have any effect on protein function, it is difficult to speculate what benefit there would be for H2O2 resistance, as any decrease in superoxide reductase-mediated H2O2 production would mean less efficient removal of O2 −. The hydrolase YqjL (BPUM_2113) and the efflux protein BcrC (BPUM_3294) both contribute to O2 − resistance by unknown mechanisms [68]. The B. pumilus homologs of these proteins are not well-conserved with respect to their B. subtilis and B. licheniformis homologs (Table 3). It is possible that B. pumilus YjqL and/or BcrC may be more adept at O2 − detoxification than their B. subtilis and B. licheniformis homologs, and that their activities may alleviate the production of H2O2 by superoxide dismutases.
Two additional proteins related to oxidative stress resistance in B. subtilis were not annotated in the B. pumilus genome. YlaC is a B. subtilis extracytoplasmic sigma factor that is regulated by the anti-sigma factor YlaD, which contains an oxidative stress-sensing domain [69]. Transcription of ylaCD was also shown to be Spx-dependent, further linking it to the oxidative stress response. Nevertheless, the lack of YlaC and YlaD homologs in B. pumilus indicates that despite the function of these proteins, they are not essential to H2O2 resistance.
The database of annotated B. pumilus coding sequences was examined for oxygenases, oxidoreductases, and redoxins without homologs in B. subtilis or B. licheniformis. The predicted proteins and functions associated with these regions are listed in Table 4. Additionally, it is possible that proteins functioning in peroxide resistance are among the hypothetical proteins and other undefined ORFs that have no B. subtilis or B. licheniformis homolog.
Conclusion
Given the phenotypic differences between B. pumilus and B. subtilis and B. licheniformis in terms of UV radiation resistance and H2O2 resistance, it was expected that a comparison of the genomes of these species would point to unique B. pumilus genes related to these functions. Most genes related to DNA repair and H2O2 resistance are conserved among these species. Paradoxically, B. pumilus lacks several DNA repair and oxidative stress response genes found in B. subtilis and B. licheniformis. Nevetheless, this analysis has identified several B. pumilus genes worthy of further study because of their absence in related organisms, differences in amino acid sequence, or predicted differences in genetic regulation.
Supporting Information
Acknowledgments
We like to especially acknowledge John Rummel, NASA's Planetary Protection Officer for his constant encouragement of our efforts in this area.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: This research was supported in part by grants to GMW (NSF-414410), GEF (NASA-Exobiology #NNG05GN75G) and KV (contract to JPL/Caltech from NASA and funded by NRA ROSS 2005). The sponsors had no role in the conduct of this study.
References
- 1.Priest FG. Systematics and Ecology of Bacillus. In: Sonenshein AL, Hoch JA, Losick R, editors. Bacillus subtilis and Other Gram-Positive Bacteria: Biochemistry, Physiology, and Molecular Genetics. Washington, D.C.: ASM Press; 1993. pp. 3–16. [Google Scholar]
- 2.Benardini JN, Sawyer J, Venkateswaran K, Nicholson WL. Spore UV and acceleration resistance of endolithic Bacillus pumilus and Bacillus subtilis isolates obtained from Sonoran desert basalt: implications for lithopanspermia. Astrobiology. 2003;3:709–717. doi: 10.1089/153110703322736033. [DOI] [PubMed] [Google Scholar]
- 3.Kempf MJ, Chen F, Kern R, Venkateswaran K. Recurrent isolation of hydrogen peroxide-resistant spores of Bacillus pumilus from a spacecraft assembly facility. Astrobiology. 2005;5:391–405. doi: 10.1089/ast.2005.5.391. [DOI] [PubMed] [Google Scholar]
- 4.Newcombe DA, Schuerger AC, Benardini JN, Dickinson D, Tanner R, et al. Survival of spacecraft-associated microorganisms under simulated martian UV irradiation. Applied Environmental Microbiology. 2005;71:8147–8156. doi: 10.1128/AEM.71.12.8147-8156.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Setlow P. Spores of Bacillus subtilis: their resistance to and killing by radiation, heat and chemicals. Journal of Applied Microbiology. 2006;101:514–525. doi: 10.1111/j.1365-2672.2005.02736.x. [DOI] [PubMed] [Google Scholar]
- 6.Setlow P. Resistance of spores of Bacillus species to ultraviolet light. Environ Mol Mutagen. 2001;38:97–104. doi: 10.1002/em.1058. [DOI] [PubMed] [Google Scholar]
- 7.Cortezzo DE, Setlow P. Analysis of factors that influence the sensitivity of spores of Bacillus subtilis to DNA damaging chemicals. Journal of Applied Microbiology. 2005;98:606–617. doi: 10.1111/j.1365-2672.2004.02495.x. [DOI] [PubMed] [Google Scholar]
- 8.Farr SB, Kogoma T. Oxidative stress responses in Escherichia coli and Salmonella typhimurium. Microbiological Reviews. 1991;55:561–585. doi: 10.1128/mr.55.4.561-585.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sambrook J, Russell DW. Molecular cloning: a laboratory manual. Cold Spring Harbor, New York: Cold Spring Harbor Laboratory Press; 2001. [Google Scholar]
- 10.Margulies M, Egholm M, Altman WE, Attiya S, Bader JS, et al. Genome sequencing in microfabricated high-density picolitre reactors. Nature. 2005;437:376–380. doi: 10.1038/nature03959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Andersson B, Wentland MA, Ricafrente JY, Liu W, Gibbs RA. A “double adaptor” method for improved shotgun library construction. Analytical Chemistry. 1996;236:107–113. doi: 10.1006/abio.1996.0138. [DOI] [PubMed] [Google Scholar]
- 12.Havlak P, Chen R, Durbin KJ, Egan A, Ren Y, et al. The Atlas genome assembly system. Genome Research. 2004;14:721–732. doi: 10.1101/gr.2264004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McLeod MP, Qin X, Karpathy SE, Gioia J, Highlander SK, et al. Complete genome sequence of Rickettsia typhi and comparison with sequences of other rickettsiae. Journal of Bacteriology. 2004;186:5842–5855. doi: 10.1128/JB.186.17.5842-5855.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Delcher AL, Harmon D, Kasif S, White O, Salzberg SL. Improved microbial identification with GLIMMER. Nucleic Acids Research. 1999;27:4636–4641. doi: 10.1093/nar/27.23.4636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lukashin A, Borodovsky M. GeneMark.hmm: new solutions for gene finding. Nucleic Acids Research. 1998;26:1107–1115. doi: 10.1093/nar/26.4.1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wheeler DL, Barrett T, Benson DA, Bryant SH, Canese K, et al. Database resources of the National Center for Biotechnology Information. Nucleic Acids Research. 2005;33:D39–45. doi: 10.1093/nar/gki062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zdobnov EM, Apweiler R. InterProScan - an integration platform for the signature-recognition methods in InterPro. Bioinformatics. 2001;17:847–848. doi: 10.1093/bioinformatics/17.9.847. [DOI] [PubMed] [Google Scholar]
- 18.Hawkins T, Luban S, Kihara D. Enhanced automated function prediction using distantly related sequences and contextual association by PFP. Protein Science. 2006;15:1550–1556. doi: 10.1110/ps.062153506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gardy JL, Laird MR, Chen F, Rey S, Walsh CJ, et al. PSORTb v.2.0: Expanded prediction of bacterial protein subcellular localization and insights gained from comparative proteome analysis. Bioinformatics. 2004;21:617–623. doi: 10.1093/bioinformatics/bti057. [DOI] [PubMed] [Google Scholar]
- 20.Gasteiger E, Gattiker A, Hoogland C, Ivanyi I, Appel RD, et al. ExPASy: The proteomics server for in-depth protein knowledge and analysis. Nucleic Acids Research. 2003;31:3784–3788. doi: 10.1093/nar/gkg563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dodd IB, Egan JB. The prediction of helix-turn-helix DNA-binding regions in proteins. Protein Engineering. 1988;2:174–175. doi: 10.1093/protein/2.3.174. [DOI] [PubMed] [Google Scholar]
- 22.Rawlings ND, Morton FR, Barrett AJ. MEROPS: the peptidase database. Nucleic Acids Research. 2006;34:D270–D272. doi: 10.1093/nar/gkj089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Saier MH, Jr., Tran CV, Barabote RD. TCDB: the Transporter Classification Database for membrane transport protein analyses and information. Nucleic Acids Res. 2006;34:D181–186. doi: 10.1093/nar/gkj001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Keijser BJ, Ter Beek A, Rauwerda H, Schuren F, Montijn R, et al. Analysis of temporal gene expression during Bacillus subtilis spore germination and outgrowth. Journal of Bacteriology. 2007;189:3624–3634. doi: 10.1128/JB.01736-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Moeller R, Stackebrandt E, Douki T, Cadet J, Rettberg P, et al. DNA bipyrimidine photoproduct repair and transcriptional response of UV-C irradiated Bacillus subtilis. Archives of Microbiology. 2007 doi: 10.1007/s00203-007-0263-4. [DOI] [PubMed] [Google Scholar]
- 26.Nicholson WL, Munakata N, Horneck G, Melosh HJ, Setlow P. Resistance of Bacillus endospores to extreme terrestrial and extraterrestrial environments. Microbiology and Molecular Biology Reviews. 2000;64:548–572. doi: 10.1128/mmbr.64.3.548-572.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Setlow P. Mechanisms for the prevention of damage to DNA in spores of Bacillus species. Annu Rev Microbiol. 1995;49:29–54. doi: 10.1146/annurev.mi.49.100195.000333. [DOI] [PubMed] [Google Scholar]
- 28.Bagyan I, Setlow B, Setlow P. New small, acid-soluble proteins unique to spores of Bacillus subtilis: identification of the coding genes and regulation and function of two of these genes. Journal of Bacteriology. 1998;180:6704–6712. doi: 10.1128/jb.180.24.6704-6712.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tovar-Rojo F, Setlow P. Effects of mutant small, acid-soluble spore proteins from Bacillus subtilis on DNA in vivo and in vitro. Journal of Bacteriology. 1991;173:4827–4835. doi: 10.1128/jb.173.15.4827-4835.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cabrera-Hernandez A, Sanchez-Salas JL, Paidhungat M, Setlow P. Regulation of four genes encoding small, acid-soluble spore proteins in Bacillus subtilis. Gene. 1999;232:1–10. doi: 10.1016/s0378-1119(99)00124-9. [DOI] [PubMed] [Google Scholar]
- 31.Carrillo-Martinez Y, Setlow P. Properties of Bacillus subtilis small, acid-soluble spore proteins with changes in the sequence recognized by their specific protease. Journal of Bacteriology. 1994;176:5357–5363. doi: 10.1128/jb.176.17.5357-5363.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Eisen JA, Hanawalt PC. A phylogenomic study of DNA repair genes, proteins, and processes. Mutation Research. 1999;435:171–213. doi: 10.1016/s0921-8777(99)00050-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Martins-Pinheiro M, Marques RC, Menck CF. Genome analysis of DNA repair genes in the alpha proteobacterium Caulobacter crescentus. BMC Microbiology. 2007;7:17. doi: 10.1186/1471-2180-7-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Salas-Pacheco JM, Setlow B, Setlow P, Pedraza-Reyes M. Role of the Nfo (YqfS) and ExoA apurinic/apyrimidinic endonucleases in protecting Bacillus subtilis spores from DNA damage. Journal of Bacteriology. 2005;187:7374–7381. doi: 10.1128/JB.187.21.7374-7381.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Aamodt RM, Falnes PO, Johansen RF, Seeberg E, Bjoras M. The Bacillus subtilis counterpart of the mammalian 3-methyladenine DNA glycosylase has hypoxanthine and 1,N6-ethenoadenine as preferred substrates. Journal of Biological Chemistry. 2004;279:13601–13606. doi: 10.1074/jbc.M314277200. [DOI] [PubMed] [Google Scholar]
- 36.Truglio JJ, Croteau DL, Van Houten B, Kisker C. Prokaryotic nucleotide excision repair: the UvrABC system. Chemical Reviews. 2006;106:233–252. doi: 10.1021/cr040471u. [DOI] [PubMed] [Google Scholar]
- 37.Petit MA, Dervyn E, Rose M, Entian KD, McGovern S, et al. PcrA is an essential DNA helicase of Bacillus subtilis fulfilling functions both in repair and rolling-circle replication. Molecular Microbiology. 1998;29:261–273. doi: 10.1046/j.1365-2958.1998.00927.x. [DOI] [PubMed] [Google Scholar]
- 38.Pedraza-Reyes M, Yasbin RE. Contribution of the mismatch DNA repair system to the generation of stationary-phase-induced mutants of Bacillus subtilis. Journal of Bacteriology. 2004;186:6485–6491. doi: 10.1128/JB.186.19.6485-6491.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Smith BT, Grossman AD, Walker GC. Visualization of mismatch repair in bacterial cells. Molecular Cell. 2001;8:1197–1206. doi: 10.1016/s1097-2765(01)00402-6. [DOI] [PubMed] [Google Scholar]
- 40.Moore JK, Haber JE. Cell cycle and genetic requirements of two pathways of nonhomologous end-joining repair of double-strand breaks in Saccharomyces cerevisiae. Molecular and Cellular Biology. 1996;16:2164–2173. doi: 10.1128/mcb.16.5.2164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Weller GR, Kysela B, Roy R, Tonkin LM, Scanlan E, et al. Identification of a DNA nonhomologous end-joining complex in bacteria. Science. 2002;297:1686–1689. doi: 10.1126/science.1074584. [DOI] [PubMed] [Google Scholar]
- 42.Wang ST, Setlow B, Conlon EM, Lyon JL, Imamura D, et al. The forespore line of gene expression in Bacillus subtilis. Journal of Molecular Biology. 2006;358:16–37. doi: 10.1016/j.jmb.2006.01.059. [DOI] [PubMed] [Google Scholar]
- 43.Moeller R, Stackebrandt E, Reitz G, Berger T, Rettberg P, et al. Role of DNA repair by non-homologous end joining (NHEJ) in Bacillus subtilis spore resistance to extreme dryness, mono- and polychromatic UV and ionizing radiation. Journal of Bacteriology. 2007 doi: 10.1128/JB.00018-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kooistra J, Haijema BJ, Venema G. The Bacillus subtilis addAB genes are fully functional in Escherichia coli. Molecular Microbiology. 1993;7:915–923. doi: 10.1111/j.1365-2958.1993.tb01182.x. [DOI] [PubMed] [Google Scholar]
- 45.Yasbin RE, Cheo DL, Bayles KW. Inducible DNA repair and differentiation in Bacillus subtilis: interactions between global regulons. Molecular Microbiology. 1992;6:1263–1270. doi: 10.1111/j.1365-2958.1992.tb00847.x. [DOI] [PubMed] [Google Scholar]
- 46.Au N, Kuester-Schoeck E, Mandava V, Bothwell LE, Canny SP, et al. Genetic composition of the Bacillus subtilis SOS system. Journal of Bacteriology. 2005;187:7655–7666. doi: 10.1128/JB.187.22.7655-7666.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Groban ES, Johnson MB, Banky P, Burnett PG, Calderon GL, et al. Binding of the Bacillus subtilis LexA protein to the SOS operator. Nucleic Acids Research. 2005;33:6287–6295. doi: 10.1093/nar/gki939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lin J, Chen ZZ, Tian B, Hua YJ. Evolutionary pathways of an ancient gene recX. Gene. 2007;387:15–20. doi: 10.1016/j.gene.2006.07.031. [DOI] [PubMed] [Google Scholar]
- 49.Kanno S, Iwai S, Takao M, Yasui A. Repair of apurinic/apyrimidinic sites by UV damage endonuclease; a repair protein for UV and oxidative damage. Nucleic Acids Research. 1999;27:3096–3103. doi: 10.1093/nar/27.15.3096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Duigou S, Ehrlich SD, Noirot P, Noirot-Gros MF. Distinctive genetic features exhibited by the Y-family DNA polymerases in Bacillus subtilis. Molecular Microbiology. 2004;54:439–451. doi: 10.1111/j.1365-2958.2004.04259.x. [DOI] [PubMed] [Google Scholar]
- 51.Sassanfar M, Dosanjh MK, Essigmann JM, Samson L. Relative efficiencies of the bacterial, yeast, and human DNA methyltransferases for the repair of O6-methylguanine and O4-methylthymine. Suggestive evidence for O4-methylthymine repair by eukaryotic methyltransferases. Journal of Biological Chemistry. 1991;266:2767–2771. [PubMed] [Google Scholar]
- 52.Rohankhedkar MS, Mulrooney SB, Wedemeyer WJ, Hausinger RP. The AidB component of the Escherichia coli adaptive response to alkylating agents is a flavin-containing, DNA-binding protein. Journal of Bacteriology. 2006;188:223–230. doi: 10.1128/JB.188.1.223-230.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Morohoshi F, Hayashi K, Munakata N. Bacillus subtilis ada operon encodes two DNA alkyltransferases. Nucleic Acids Research. 1990;18:5473–5480. doi: 10.1093/nar/18.18.5473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Voloshin ON, Vanevski F, Khil PP, Camerini-Otero RD. Characterization of the DNA damage-inducible helicase DinG from Escherichia coli. Journal of Biological Chemistry. 2003;278:28284–28293. doi: 10.1074/jbc.M301188200. [DOI] [PubMed] [Google Scholar]
- 55.Yasuda T, Morimatsu K, Horii T, Nagata T, Ohmori H. Inhibition of Escherichia coli RecA coprotease activities by DinI. The EMBO Journal. 1998;17:3207–3216. doi: 10.1093/emboj/17.11.3207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.McLennan AG. The Nudix hydrolase superfamily. Cellular and Molecular Life Sciences. 2006;63:123–143. doi: 10.1007/s00018-005-5386-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Essen LO, Klar T. Light-driven DNA repair by photolyases. Cellular and Molecular Life Sciences. 2006;63:1266–1277. doi: 10.1007/s00018-005-5447-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Caruthers JM, McKay DB. Helicase structure and mechanism. Current Opinion in Structural Biology. 2002;12:123–133. doi: 10.1016/s0959-440x(02)00298-1. [DOI] [PubMed] [Google Scholar]
- 59.Brosh RM, Jr., Matson SW. A point mutation in Escherichia coli DNA helicase II renders the enzyme nonfunctional in two DNA repair pathways. Evidence for initiation of unwinding from a nick in vivo. Journal of Biological Chemistry. 1997;272:572–579. doi: 10.1074/jbc.272.1.572. [DOI] [PubMed] [Google Scholar]
- 60.Mostertz J, Scharf C, Hecker M, Homuth G. Transcriptome and proteome analysis of Bacillus subtilis gene expression in response to superoxide and peroxide stress. Microbiology. 2004;150:497–512. doi: 10.1099/mic.0.26665-0. [DOI] [PubMed] [Google Scholar]
- 61.Bagyan I, Casillas-Martinez L, Setlow P. The katX gene, which codes for the catalase in spores of Bacillus subtilis, is a forespore-specific gene controlled by sigmaF, and KatX is essential for hydrogen peroxide resistance of the germinating spore. Journal of Bacteriology. 1998;180:2057–2062. doi: 10.1128/jb.180.8.2057-2062.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rhee SG, Chae HZ, Kim K. Peroxiredoxins: a historical overview and speculative preview of novel mechanisms and emerging concepts in cell signaling. Free Radical Biology & Medicine. 2005;38:1543–1552. doi: 10.1016/j.freeradbiomed.2005.02.026. [DOI] [PubMed] [Google Scholar]
- 63.Zeller T, Klug G. Thioredoxins in bacteria: functions in oxidative stress response and regulation of thioredoxin genes. Naturwissenschaften. 2006;93:259–266. doi: 10.1007/s00114-006-0106-1. [DOI] [PubMed] [Google Scholar]
- 64.Smits WK, Dubois JY, Bron S, van Dijl JM, Kuipers OP. Tricksy business: transcriptome analysis reveals the involvement of thioredoxin A in redox homeostasis, oxidative stress, sulfur metabolism, and cellular differentiation in Bacillus subtilis. Journal of Bacteriology. 2005;187:3921–3930. doi: 10.1128/JB.187.12.3921-3930.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Fuangthong M, Atichartpongkul S, Mongkolsuk S, Helmann JD. OhrR is a repressor of ohrA, a key organic hydroperoxide resistance determinant in Bacillus subtilis. Journal of Bacteriology. 2001;183:4134–4141. doi: 10.1128/JB.183.14.4134-4141.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Liu X, Kim K, Leighton T, Theil EC. Paired Bacillus anthracis Dps (mini-ferritin) have different reactivities with peroxide. Journal of Biological Chemistry. 2006;281:27827–27835. doi: 10.1074/jbc.M601398200. [DOI] [PubMed] [Google Scholar]
- 67.Casillas-Martinez L, Setlow P. Alkyl hydroperoxide reductase, catalase, MrgA, and superoxide dismutase are not involved in resistance of Bacillus subtilis spores to heat or oxidizing agents. Journal of Bacteriology. 1997;179:7420–7425. doi: 10.1128/jb.179.23.7420-7425.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Cao M, Moore CM, Helmann JD. Bacillus subtilis paraquat resistance is directed by sigmaM, an extracytoplasmic function sigma factor, and is conferred by YqjL and BcrC. Journal of Bacteriology. 2005;187:2948–2956. doi: 10.1128/JB.187.9.2948-2956.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Matsumoto T, Nakanishi K, Asai K, Sadaie Y. Transcriptional analysis of the ylaABCD operon of Bacillus subtilis encoding a sigma factor of extracytoplasmic function family. Genes & Genetic Systems. 2005;80:385–393. doi: 10.1266/ggs.80.385. [DOI] [PubMed] [Google Scholar]
- 70.Jedrzejas MJ, Huang WJ. Bacillus species proteins involved in spore formation and degradation: from identification in the genome, to sequence analysis, and determination of function and structure. Critical Reviews in Biochemistry and Molecular Biology. 2003;38:173–198. doi: 10.1080/713609234. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.