Abstract
The effects of secondary metabolites produced by waterlogged soils on net K+, H+, and Ca2+ fluxes were studied in the mature zone of roots of two barley (Hordeum vulgare) cultivars contrasting in their waterlogging (WL) tolerance using the noninvasive microelectrode ion flux measuring technique. In WL-sensitive variety ‘Naso Nijo’, all three lower monocarboxylic acids (formic, acetic, and propionic acids) and three phenolic acids (benzoic, 2-hydroxybenzoic, 4-hydroxybenzoic acids) caused a substantial shift toward steady K+ efflux, accompanied by an immediate net influx of H+. Detrimental effects of secondary metabolites on K+ homeostasis in root cells were absent in WL-tolerant ‘TX’ variety. Root treatment with Mn2+ caused only a temporary K+ loss that returned to the initial level 10 min after treatment. Phenolic acids slightly increased Ca2+ influx immediately after treatment, while other metabolites tested resulted in transient Ca2+ efflux from the root. In the long-term (24 h) treatment, all metabolites tested significantly reduced K+ uptake and the adverse effects of phenolic acids were smaller than for monocarboxylic acids and Mn2+. Treatment with monocarboxylic acids for 24 h shifted H+ from net efflux to net influx, while all three phenolic acids did not cause significant effects compared with the control. Based on results of pharmacological experiments and membrane potential measurements, a model explaining the effects of secondary metabolites on membrane transport activity is proposed. We also suggest that plant tolerance to these secondary metabolites could be considered a useful trait in breeding programs.
Owing to the anaerobic metabolism of plants or microbes, significant accumulation of toxic substances occurs in waterlogged soil (Lynch, 1977; Tanaka et al., 1990; Armstrong and Armstrong, 2001). Materials potentially toxic to plants that are accumulated in flooded soils include reduced manganese, iron, hydrogen sulfide, various organic acids, and ethylene (Armstrong and Gaynard, 1976). Surprisingly, to date, all breeding programs aimed at improving waterlogging (WL) tolerance in plants have focused almost exclusively on preventing oxygen loss or improving its transport to, or storage in the root (Jackson and Armstrong, 1999). Plant tolerance to these secondary metabolites has never been considered a useful trait in breeding programs.
The type and amount of organic acids produced depends upon the fermentive character of the microflora, the type and amount of organic materials added, and on the prevailing soil conditions (Rao and Mikkelsen, 1977). Tanaka et al. (1990) found that rice (Oryza sativa) root growth was inhibited by micromolar concentrations of phenolic acids formed in flooded soils amended with wheat (Triticum aestivum) straw both in the laboratory and the field, while Lynch (1978) and Armstrong and Armstrong (1999) reported 15 to 35 mm range values for acetic and propionic acid concentrations under comparable conditions. The accumulation of toxic micronutrients is also increased in waterlogged plants. Ashraf and Rehman (1999) reported that iron and manganese contents increased 80- and 20-fold (to 390 and 148 mg kg−1, respectively) in sandy loam soil as a result of 34 d flooding. The same order of magnitude increase was also reported by Stieger and Feller (1994).
The extent to which the accumulation of toxic metabolites is causally linked to observed deficiencies of macronutrients in waterlogged soils is not clear. Energy deficiency, caused by lack of O2, reduces the availability of many essential nutrients including nitrogen, phosphorus, sulfur, and also most of the trace elements (Drew, 1988). In addition, there are reports suggesting that inorganic nutrient uptake may be impaired by accumulated organic acids (Mitsui et al., 1954; Rao and Mikkelsen, 1977). The mechanisms of such impairment remain to be investigated.
Thus far, most reports have dealt with the analysis of the overall changes in ion content in plant tissues or with monitoring the kinetics of nutrient depletion in a growth solution (Glass, 1973, 1974; Jackson and St. John, 1980). Due to methodological limitations (relatively poor spatial and temporal resolution), these experiments failed to provide answers about the specific ionic mechanisms involved. The above methodological issues may be successfully overcome when using the microelectrode ion flux measuring (MIFE) technique (Shabala, 2003, 2006). The noninvasive MIFE system (University of Tasmania, Hobart, Australia) has very high spatial (a few micrometers) and temporal (several seconds) resolution and has been successfully applied to the measurement of ion flux kinetics under a variety of stress conditions (Shabala and Newman, 1997; Shabala and Lew, 2002; Shabala et al., 2003). In this study, this technique was used to quantify both the immediate responses of ion fluxes and long-term (after 24 h treatment) responses to secondary metabolites associated with anaerobic soils.
RESULTS
The major bulk of experiments were performed on the WL-sensitive variety ‘Naso Nijo’, where much stronger responses were expected. Accordingly, most of results below refer to ‘Naso Nijo’ roots unless specified otherwise.
Transient K+ Fluxes
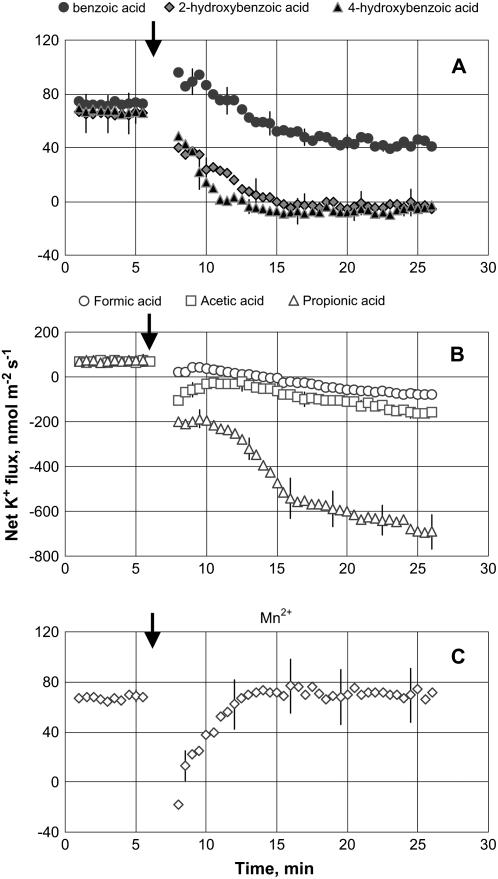
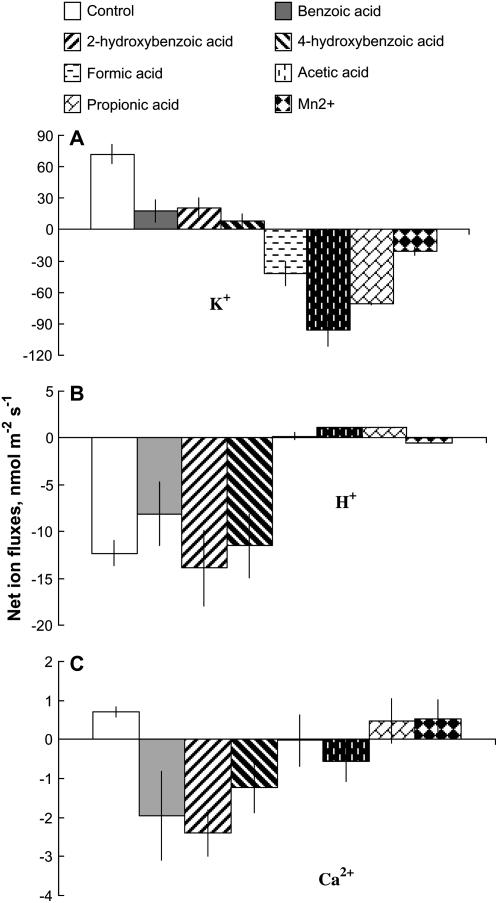
Net K+ uptake of about 60 nmol m−2 s−1 was measured from mature epidermal root cells of 3-d-old seedlings in control (steady-state) conditions. Addition of phenolic compounds (benzoic acid, 2-hydroxybenzoic acid, 4-hydroxybenzoic acid; 200 μm working concentration) and volatile monocarboxylic organic compounds (formic acid, acetic acid, propionic acid; 10 mm working concentration) rapidly decreased net K+ influx (Fig. 1). Among the three different phenolic acids, 2-hydroxybenzoic acid and 4-hydroxybenzoic acid caused much more adverse effects on K+ uptake compared with benzoic acid (Fig. 1A), completely arresting net K+ uptake within 10 min after treatment. All three volatile monocarboxylic organic acids not only arrested K+ influx but also caused significant (P < 0.001) K+ efflux from barley (Hordeum vulgare) roots, with an effect increasing in the following sequence: propionic acid ≫ acetic acid > formic acid (Fig. 1B). This effect was specific and not related to changes in osmolality of the experimental solution, as isotonic treatment with KCl, NaCl, or Na gluconate caused no K+ efflux from barley roots (data not shown). Adding 300 mg L−1 Mn2+ caused an almost instantaneous reduction of K+ uptake, which quickly (within 5 min) returned to its initial value (Fig. 1C). In general, the effect of monocarboxylic organic acids on root K+ fluxes was significantly stronger than one caused by phenolic acids.
Figure 1.
K+ flux kinetics in response to secondary metabolites associated with anaerobic conditions (applied at the time indicated by an arrow). A, 200 μm phenolic acid treatment. B, 10 mm monocarboxylic acid treatment. C, 300 mg L−1 Mn2+ treatment. Measurements were made in the mature zone, 10 to 20 mm from the root tip. Data are means ± se (n = 8).
Transient H+ Fluxes
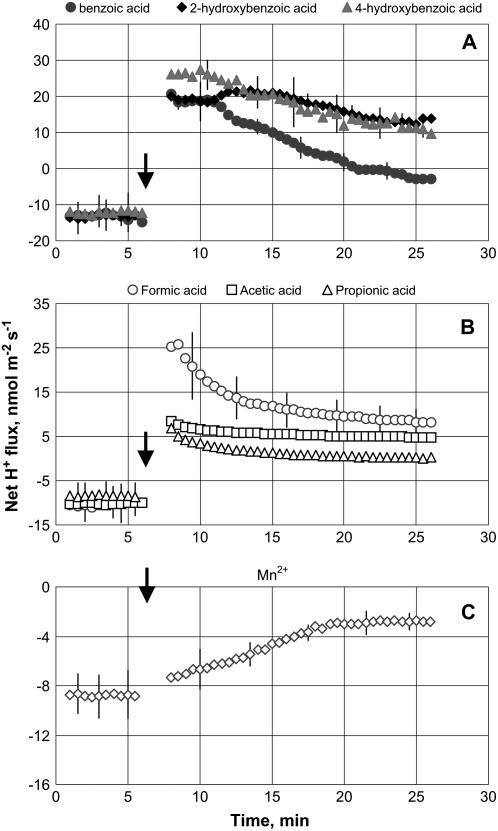
Net H+ efflux of 10 to 15 nmol m−2 s−1 was measured in control (steady-state) conditions from barley roots. Application of all secondary metabolites significantly (P < 0.01) affected H+ fluxes (Fig. 2). An immediate and significant shift toward net H+ uptake was observed in response to all phenolic and monocarboxylic organic acids tested (Fig. 2, A and B), while in the case of Mn2+ treatment, net H+ efflux was significantly (P < 0.01) reduced (Fig. 2C). Among phenolics, the effect followed the ranking: 4-hydroxybenzoic acid ≈ 2-hydroxybenzoic acid > benzoic acid. For monocarboxylic organic acids, responsiveness of H+ flux followed the ranking: formic acid > acetic acid > propionic acid.
Figure 2.
H+ flux kinetics in response to secondary metabolites associated with anaerobic conditions (applied at the time indicated by an arrow). A, 200 μm phenolic acid treatment. B, 10 mm monocarboxylic acid treatment. C, 300 mg L−1 Mn2+ treatment. Measurements were made in the mature zone, 10 to 20 mm from the root tip. Data are means ± se (n = 8).
Transient Ca2+ Fluxes
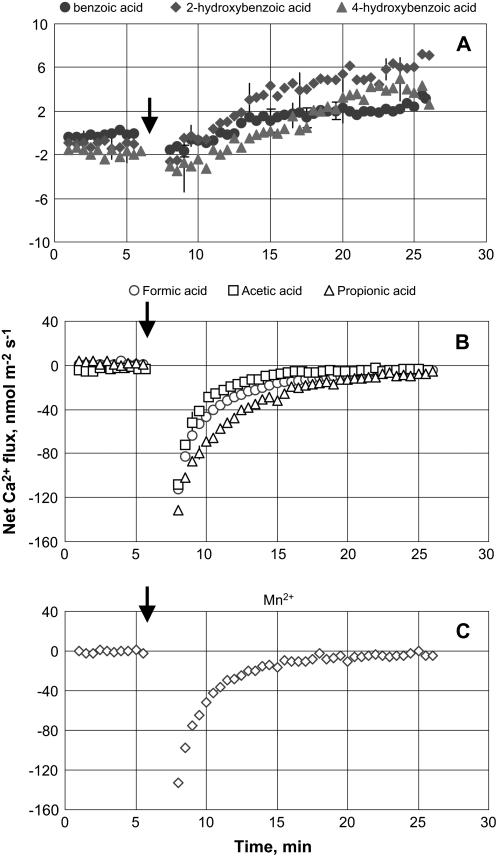
Net zero Ca2+ flux was measured in control (steady-state) conditions. Root treatment with phenolic acids led to a gradual and prolonged increase in net Ca2+ uptake (Fig. 3A). Such a slowness of response may be indicative of a cytosolic mode of action. No significant (P < 0.05) difference between the effects of various phenolic acids was found. Adding 10 mm monocarboxylic organic acid to the bath, however, caused an immediate and substantial Ca2+ efflux from barley roots (Fig. 3B). A similar trend was observed for Mn2+ treatment (Fig. 3C). In both cases, net Ca2+ flux recovered to its original (zero) value within 10 to 15 min (Fig. 3, B and C).
Figure 3.
Ca2+ flux kinetics in response to secondary metabolites associated with anaerobic conditions (applied at the time indicated by an arrow). A, 200 μm phenolic acid treatment. B, 10 mm monocarboxylic acid treatment. C, 300 mg L−1 Mn2+ treatment. Measurements were made in the mature zone, 10 to 20 mm from the root tip. Data are means ± se (n = 8).
Genotypical Difference
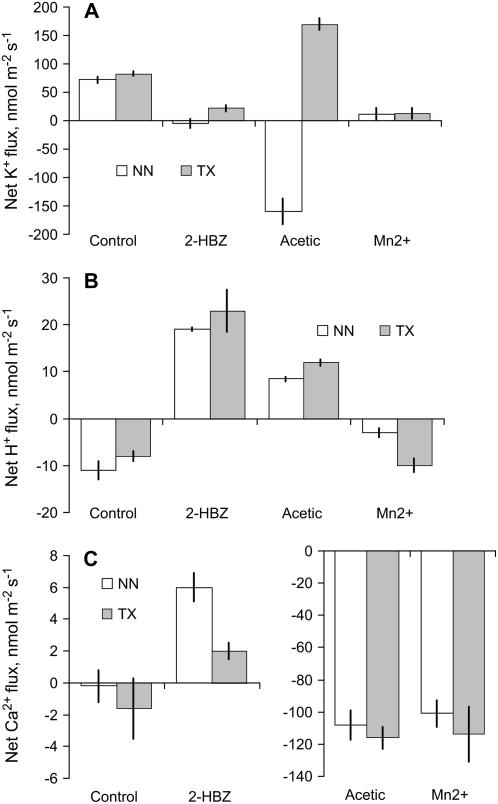
No significant (P < 0.05) difference in initial (steady-state) flux levels was found for any ions measured between two contrasting genotypes (WL-sensitive ‘Naso Nijo’ and WL-tolerant ‘TX’; Fig. 4). However, transient flux kinetics differed substantially between genotypes. The most striking difference was observed for K+ flux (Fig. 4A). Contrary to WL-sensitive ‘Naso Nijo’ genotype, lower monocarboxylic (acetic) acid treatment did not cause any net K+ loss in the WL-tolerant ‘TX’ variety. Moreover, ‘TX’ roots even increased net K+ uptake 20 min after treatment with 10 mm acetic acid (Fig. 4A), while similar treatment caused a very substantial K+ loss from the roots of WL-sensitive ‘Naso Nijo’ variety. Also significantly reduced was ‘TX’ net Ca2+ uptake in response to 2-hydroxybenzoic treatment compared with ‘Naso Nijo’ (Fig. 4C). No clear difference was evident between genotypes in terms of H+ flux responses for either acetic or 2-hydroxybenzoic treatment (Fig. 4B). Treatment with Mn2+, however, has significantly reduced H+ efflux in WL-sensitive ‘Naso Nijo’ variety (compared with control) but was not significant in WL-tolerant ‘TX’ variety (Fig. 4B).
Figure 4.
Magnitude of net K+ (A), H+ (B), and Ca+ (C) responses to various metabolic compounds (as depicted in Figs. 1–3) for two genotypes contrasting in WL tolerance, 20 min after treatment. NN, ‘Naso Nijo’ (WL sensitive); ‘TX’, TX9425 (WL tolerant). Data are mean ± se (n = 8). 2-HBZ, 2-Hydroxybenzoic acid.
Long-Term Ion Flux Responses
K+ uptake was significantly reduced in ‘Naso Nijo’ roots after 24 h treatment with all secondary metabolites tested (Fig. 5A). Root treatment with phenolic compounds (benzoic acid, 2-hydroxybenzoic acid, 4-hydroxybenzoic acid) caused a significant (P < 0.01) decrease in net K+ uptake. No significant (P < 0.05) difference between the effects of various phenolic compounds was found. In monocarboxylic acid treated roots, K+ fluxes were shifted to substantial (−40 to −100 nmol m−2 s−1) net efflux. Among them, acetic acid and propionic acid caused more severe effects than formic acid. Mn2+ treatment also caused net K+ efflux. In general, the adverse effects of phenolic acids were smaller than the other four treatments.
Figure 5.
Fluxes of K+ (A), H+ (B), Ca2+ (C) measured in the mature zone of barley root after being exposed to various secondary metabolites for 24 h. Data are means ± se (n = 12).
The monocarboxylic acid treatments shifted H+ from net efflux to net influx (Fig. 5B). Mn2+ treatment reduced H+ efflux to around zero. Among the phenolic acids, 2-hydroxybenzoic acid and 4-hydroxybenzoic acid did not cause significant (P < 0.05) changes to H+ fluxes, while benzoic acid slightly reduced H+ efflux (Fig. 5B).
Phenolic acids caused significant (P < 0.05) net Ca2+ efflux from roots pretreated for 24 h (Fig. 5C). Formic and acetic acids also slightly reduced net Ca2+ uptake, while propionic acid and Mn2+ did not significantly (P < 0.05) affect Ca2+ fluxes (Fig. 5C).
Pharmacology
Effects of various channel blockers and metabolic inhibitors on ion fluxes kinetics were studied using one chemical from each group (specifically, acetic acid, 2-hydroxybenzoic acids, and Mn2+).
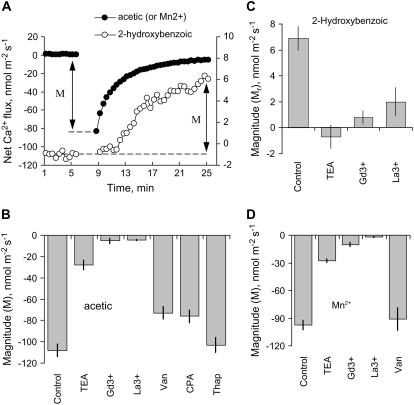
None of the inhibitors used significantly affected the initial Ca2+ flux after 1 h of incubation (data not shown). However, La3+ and Gd3+ (two known nonselective cation channel [NSCC] blockers; Demidchik et al., 2002) almost completely inhibited Ca2+ flux responses to either acetic acid (Fig. 6B), 2-hydroxybenzoic acid (Fig. 6C), or Mn2+ (Fig. 6D) treatment. TEA+ was also efficient (approximately 70% inhibition; Fig. 6). At the same time, cyclopiazonic acid (CPA; a specific Ca2+-ATPase inhibitor) and vanadate (general ATPase inhibitor) caused approximately 25% reduction of the magnitude of acetic acid-induced Ca2+ efflux observed (Fig. 6B), with thapsigargin (another specific Ca2+-ATPase inhibitor) being ineffective.
Figure 6.
Pharmacology of Ca2+ flux responses to acetic acid (B), 2-hydroxybenzoic acid (C), and Mn2+ (D). Peak Ca2+ efflux 2 min after the treatment is shown for acetic and Mn2+, while for 2-hydroxybenzoic acid, increment in net Ca2+ uptake is shown 20 min after the treatment (as depicted in A). Data are means ± se (n = 6–8). Roots were pretreated with various metabolic inhibitors for 1 h before the specific metabolite was added (still in the presence of inhibitor). M, Magnitude of response.
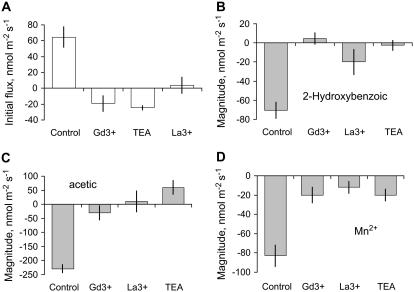
Initial K+ uptake in barley roots was strongly suppressed by TEA+ (Fig. 7A). All these inhibitors were also efficient in reducing the magnitude of K+ flux response to 2-hydroxybenoic (Fig. 7B) and acetic (Fig. 7C) acids and Mn2+ (Fig. 7D; significant at P < 0.05). Both Gd3+ and La3+ (two known NSCC channel blockers; Demidchik et al., 2002) also significantly reduced initial K+ uptake and the magnitude of K+ flux response to all chemicals tested (Fig. 7; significant at P < 0.05).
Figure 7.
Pharmacology of K+ flux responses to acetic acid (B), 2-hydroxybenzoic acid (C), and Mn2+ (D). The magnitude of K+ efflux was determined as the difference between steady-state K+ flux before the treatment and K+ flux value 20 min after adding a particular compound to the root. Effect of channel blockers on initial (steady state) K+ fluxes is shown in A. Data are means ± se (n = 6). Roots were pretreated with various metabolic inhibitors for 1 h before the specific metabolite was added (still in the presence of inhibitor).
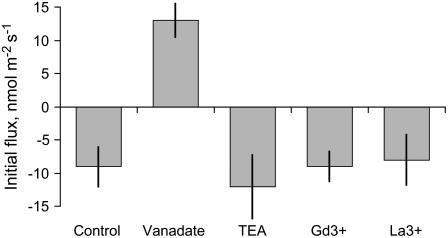
Root pretreatment in 1 mm vanadate (a known inhibitor of the plasma membrane [PM] H+-ATPase) shifted the initial H+ flux from net efflux to net influx (Fig. 8), while no significant effect of TEA+, La3+, or Gd3+ on initial H+ flux was observed. Vanadate treatment also significantly (P < 0.01) reduced the magnitude of H+ flux changes in response to all treatments tested (data not shown).
Figure 8.
Pharmacology of H+ flux responses in barley roots—effect of metabolic inhibitors on steady-state net H+ fluxes in ‘Naso Nijo’ roots. Data are means ± se (n = 6).
Membrane Potential Responses and H+-ATPase Activity
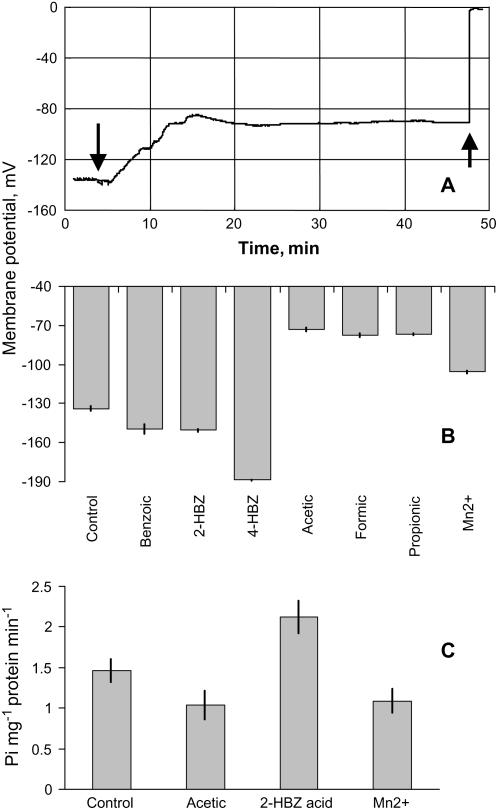
The average membrane potential in the mature zone of barley roots was −133.9 ± 2.0 mV in control. Phenolic compounds caused substantial membrane depolarization (as illustrated in Fig. 9A for the treatment with 200 μm 2-hydroxybenzoic acid), stabilizing at −90 mV level approximately 10 min after the treatment was applied. Membrane potential kinetics in response to other compounds was not measured.
Figure 9.
A, A typical example of transient change of membrane potential upon the addition of 200 μm 2-hydroxybenzoic acid to root mature zone. The first arrow indicates commencing of the treatment, while the second arrow shows the moment when electrode was removed from the cell. B, Cell membrane potentials in root mature zones after 24 h treatment with various secondary metabolites. Data are means ± se (n = 16). C, ATPase hydraulic activity of 7-d-old barley root PM after 30 min treatment of 2-hydroxybenzoic acid, acetic acid, and Mn2+. 2-HBZ, 2-Hydroxybenzoic acid; 4-HBZ, 4-hydroxybenzoic acid.
Contrary to the short-term effects of 2-hydroxybenzoic acid, 24 h treatment with 200 μm phenolics caused significant (P < 0.01) hyperpolarization of the membrane potential (Fig. 6B). The largest hyperpolarization effect was found in roots treated with 4-hydroxybenzoic acid. All three monocarboxylic acids and Mn2+ treatments induced significant (P < 0.001) long-term depolarization of membrane potential (Fig. 6B).
Results of membrane potential measurements were consistent with direct estimation of ATP hydrolytic activity from PM vesicles isolated from the microsomal fraction of barley roots (Fig. 9C). No significant (P < 0.05) difference was found in ATP hydrolytic activity between control samples and samples treated with either acetic acid or Mn2+. At the same time, the PM vesicles from roots treated with 2-hydroxybenzoic acid had about 40% higher ATP hydrolytic activity compared with control roots (significant at P < 0.05; Fig. 9C).
DISCUSSION
Secondary Metabolites Toxicity and WL Tolerance in Barley
All seven secondary metabolites associated with anaerobic soil conditions inhibited root elongation in 24 h treatments (data not shown), highlighting their detrimental effects on root metabolism. They also caused significant alterations in root membrane-transport activity even in the presence of oxygen. The most significant was a pronounced shift toward K+ efflux, caused by both phenolics and monocarboxylic acids (Fig. 1). In the case of monocarboxylic acids, the result was a very substantial K+ loss measured from the roots of WL-sensitive ‘Naso Nijo’ (Fig. 1B). Such K+ loss has been previously reported from barley roots in response to salinity (Chen et al., 2005, 2007) and oxidative (Cuin and Shabala, 2007) stress, with a strong positive correlation (r2 > 0.7) between a root's ability to retain K+ and the level plant stress tolerance reported (Chen et al., 2007). A tight link between net K+ efflux and cytosolic free K+ concentration was also shown (Shabala et al., 2006). All these findings suggested that the magnitude of stress-induced K+ loss may be used as a quantitative characteristic of plant stress tolerance.
In this study, we extend these findings to plant WL tolerance. The WL-tolerant ‘TX’ was capable not only of completely preventing net K+ loss after acetic acid treatment (Fig. 4A) but even slightly enhancing net K+ uptake by roots under stress conditions. Also less affected (compared with WL-sensitive ‘Naso Nijo’) was K+ uptake in response to phenolics (Fig. 4A). These data support the idea that WL tolerance in barley is conferred not only by differences in root anatomy (high percentage of aerenchyma in ‘TX’ genotype; Pang et al., 2004), but may, to a large extent, be determined by the superior ability of tolerant genotypes to reduce detrimental effects of secondary metabolites on membrane-transport processes in roots (specifically, improving K+ retention). With K+ being the most abundant cytosolic cation, and its involvement in numerous enzymatic reactions in plants (Shabala, 2003), the physiological significance of such retention is obvious. Thus far, plant breeding for WL tolerance has never considered responses to these secondary metabolites as a useful trait; we suggest this issue should be given special attention in future work.
Phenolics: Short-Term Effects
Early reports of Glass (1974) showed that various benzoic compounds tested caused a substantial inhibition of potassium absorption (measured as 86Rb uptake from excised barley roots) after 3 h of treatment. However, as 86Rb measures unidirectional K+ uptake, the extent to which K+ efflux systems were affected was unclear. Our data (Fig. 1A) show that net K+ fluxes from roots treated with 2-hydroxybenzoic and 4-hydroxybenzoic acids were even slightly negative (net efflux), suggesting not only a reduction in K+ uptake, but also an increase in K+ loss from cells, pointing toward multiple targets in barley root membranes.
Among the three phenolics, the effects of 2-hydroxybenzoic and 4-hydroxybenzoic acids on K+ flux were larger than effects caused by benzoic acid (Fig. 1). Earlier, Glass (1973) suggested that increasing hydroxylation within a series tends to decrease the inhibitory capacity of phenolics. This was obviously not the case in our experiments.
Under the conditions of this experiment (pH 5.5), most phenolic acids in solution will be in the dissociated form. This undissociated acid concentration can be calculated according to the Henderson-Hasselbalch equation:
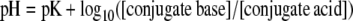 |
As shown in Table I, the amount of undissociatd acids was relatively low and comprised 4.7%, 0.3%, and 8.7% for benzoic, 2-hydroxybenzoic, and 4-hydroxybenzoic acids, respectively. Therefore, no obvious correlation between the magnitude of effect and the amount of dissociated compound was found.
Table I.
Concentrations of undissociated phenolic acids under experimental conditions
| Compound | Dissociation Constant | pK | Concentration of Undissociated Compound |
|---|---|---|---|
| μm | % | ||
| Benzoic acid | 64.6 | 4.19 | 4.7 |
| 2-Hydroxybenzoic acid | 1,071 (K1) | 2.97 (pK1) | 0.3 |
| 4-Hydroxybenzoic acid | 33.1 (K1) | 4.48 (pK1) | 8.7 |
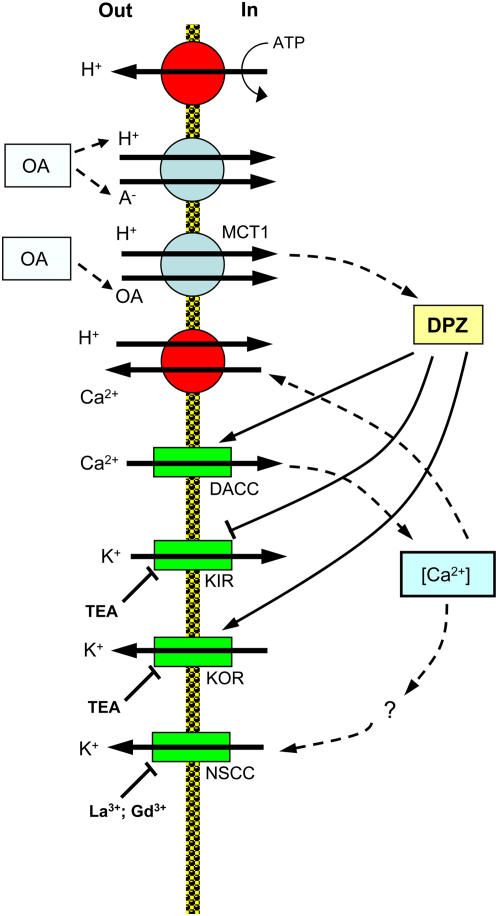
The mechanisms by which phenolic compounds control K+ transport across the PM remain elusive. Based on the fact that removal of phenolics caused a rapid recovery of K+ reabsorption, Glass (1974) suggested a direct effect on cell membranes. No specific details were offered though. Our data reported in this study suggests that major voltage-dependent K+-transporting systems may be key players. This is supported by the observed immediate membrane depolarization (Fig. 9A). Moreover, our data suggest that both increased H+ (Fig. 2A) and Ca2+ (Fig. 3A) uptake could contribute to this depolarization as depicted in Figure 10.
Figure 10.
A suggested model explaining short-term effects of secondary metabolites on membrane transport activity. OA, Organic acid; KIR, potassium inward-rectifying channel; KOR, potassium outward-rectifying channel; DACC, depolarization-activated Ca2+ channel; DPZ, depolarization of the PM. [See online article for color version of this figure.]
It is traditionally believed that most phenolic acids cross the cell membrane in an undissociated form by passive diffusion (Jackson and St. John, 1980). However, recent cloning and functional characterization of the MCT1 family of transporters suggest that uptake of both monocarboxylic acids and benzoic acid occur via the H+-coupled cotransport mechanism, at least in animal systems (Kido et al., 2000). We suggest that a similar scenario is also applicable to plant tissues. As undissociated phenolic acid is electrically neutral, not only will increased net H+ flux be generated as a result of such activity (consistent with our H+ flux data; Fig. 2A), but also a substantial membrane depolarization is expected (Fig. 9). Such a depolarization will affect intracellular K+ homeostasis by reducing K+ uptake via inward-rectifying K+ channels and enhancing K+ efflux via depolarization-activated outward-rectifying K+ channels (Maathuis and Sanders, 1996; Fig. 10), explaining the rapid shift toward net K+ efflux after phenolics application (Fig. 1A). As both of these channels are TEA+ sensitive, inhibition of K+ efflux by TEA+ in our experiments (Fig. 7) is consistent with this model.
Several Ca2+-permeable channels may mediate Ca2+ uptake into root cells; each of these may contribute to the observed Ca2+ influx after phenolics application. Of special interest may be depolarization-activated Ca2+ channels (Thion et al., 1998; Miedema et al., 2001). These play a prominent role in signal perception and transduction in plants (Thion et al., 1998). Regardless of the type of Ca2+-permeable channel, increased Ca2+ uptake will provide a positive feedback to further depolarize the membrane potential, amplifying the effect of phenolics on K+ transport (as depicted in a tentative model in Fig. 10). It also appears that NSCCs contribute partly to the K+ efflux, as both Gd3+ and La3+ (two known NSCC blockers; Demidchik et al., 2002) were efficient in preventing 2-hydroxybenzoic acid-induced K+ loss (Fig. 7B).
Phenolics: Long-Term Effects
Once inside the cell, permeated phenolic acids dissociate and acidify the cytosol (Guern et al., 1986; Ehness et al., 1997). This will activate the PM H+-ATPase and increase H+ extrusion (Frachisse et al., 1988; Felle, 1989; Beffagna and Romani, 1991). As a result of such activation, the net H+ uptake observed in the 1st min after treatment with phenolics would gradually decline, and membrane potential restored. Indeed, after 24 h treatment, roots treated by each of the three phenolic acids had net H+ flux values not significantly (P < 0.05) different from the control (Fig. 5B), while membrane potential values were even more negative (hyperpolarized) compared with control roots (Fig. 9B), most likely the result of higher ATP hydrolytic activity in phenolic-treated roots (Fig. 9C).
Theoretically, membrane hyperpolarization observed after long-term phenolic treatment (Fig. 9B) was expected to reverse the detrimental effects of metabolites on K+ transport. However, this was not the case, and K+ uptake after 24 h of treatment with phenolic acids was significantly (P < 0.05) lower than in the control (Fig. 5A). The answer may lie in the fact that net Ca2+ uptake measured soon after treatment (Fig. 3A) may result in a substantial elevation in cytosolic free Ca2+. Patch-clamp experiments on guard cells suggest that the inward K+ current is greatly reduced by elevating [Ca]cyt to micromolar concentrations (Schroeder and Hagiwara, 1989). At the same time, outward-rectifying K+ channels are much less sensitive to [Ca]cyt (Hosoi et al., 1988; Blatt and Grabov, 1997; Grabov and Blatt, 1997). Such differential sensitivity of inward-rectifying K+ channels and outward-rectifying K+ channels to elevations in cytosolic Ca2+ may shift the balance in net K+ flux toward higher efflux, thus diminishing any beneficial effects of membrane hyperpolarization on K+ nutrition.
It should be also mentioned that some benzoic acid derivatives [e.g. 5-nitro-2-(3-phenylpropylamino) benzoic acid] were found to be potent inhibitors of anion channels (Roberts, 2006). Also, as a result of dissociation, a significant amount of organic anions will be accumulated in the cytosol. This accumulation might block their removal from the cytosol via anion channels (positive feedback), thus exacerbating toxicity effects. At the same time, anion channel blockage will add to the observed membrane hyperpolarization by reducing the amount of negatively charged particles leaving the cytosol.
Effects of Monocarboxylic Acids
Similar to phenolics, lipid-soluble undissociated forms of the volatile monocarboxylic acids are often regarded as the most toxic (Jackson and Taylor, 1970; Jackson and St. John, 1980). Given that the concentration of monocarboxylic acids used in our experiment is much higher than that of the phenolic acids, the concentration of H+ in the cell cytosol would be much higher than in phenolic acid-treated plants. This might explain the difference in membrane potential after 24 h of treatment (Fig. 9). All monocarboxylic compounds resulted in a significant (P < 0.05) net K+ efflux from barley roots (Fig. 1B); of these, propionic acid caused the largest K+ efflux, followed by acetic acid, and formic acid the least, proportional to the amount of undissociated acid in the bath solution (Table II). This is consistent with previous reports on the adverse effects of these acids on the K+ uptake (Jackson and Taylor, 1970; Jackson and St. John, 1980).
Table II.
Concentrations of undissociated monocarboxylic acids under experimental conditions
| Compound | Dissociation Constant | pK | Concentration of Undissociated Compound |
|---|---|---|---|
| μm | % | ||
| Formic acid | 177 | 3.75 | 1.7 |
| Acetic acid | 17.6 | 4.75 | 15.1 |
| Propionic acid | 13.4 | 4.87 | 18.9 |
These authors also suggested that changes in membrane lipid composition might be responsible for the observed leak of K+ and Ca2+ from roots treated with monocarboxylic acids. However, it is highly unlikely that such a non-ion-specific change in general membrane permeability may occur almost immediately (within 1 min) after the treatment, as resolved by the MIFE system for K+ efflux in our experiments (Fig. 1B). Such changes in permeability are usually associated with a change in membrane lipid components (Jackson and Taylor, 1970; Glass, 1974; Jackson and St. John, 1980), the latter process most likely operating on a slower time scale. Importantly, the above changes in membrane permeability are believed to be nonspecific (Glass and Dunlop, 1974), thus, a mirror image kinetics for K+ efflux and Ca2+ uptake (according to electrochemical potential for each ion) should be observed. This was obviously not the case in our study. While the K+ leak gradually increased with time (Fig. 1B), Ca2+ efflux was short lived and returned back to control values within 10 to 15 min after treatment. This suggests that fluxes of these two ions are mediated by different transport systems, and thus cannot be attributed to a general change in membrane permeability.
A plausible alternative explanation may be offered. Similar to our model, phenolics, monocarboxylic acids are transported into the cytosol most likely in an undissociated form (Kido et al., 2000; Fig. 10). Using the H+-coupled cotransport mechanism, such transport through the MCT will cause the significant H+ influx measured in our experiments (Fig. 2B). This might depolarize the membrane (Fig. 10) and cause K+ efflux through depolarization-activated K+ channels (Fig. 2B). In addition, it appears that at least part of the observed K+ efflux may be also mediated by NSCCs, as both Gd3+ and La3+ (two known NSCC blockers; Demidchik et al., 2002) were also efficient in preventing acetic acid-induced K+ loss (Fig. 7C).
Contrary to the effect of phenolics, monocarboxylic acids did not cause any substantial increase in Ca2+ uptake (Fig. 3, A and B, respectively), indicating a specificity of regulation of Ca2+ signaling by these secondary metabolites. The effect of specific blockers of the Ca2+-ATPase (CPA and thapsigargin) on acetic-induced transient Ca2+ efflux was rather small (Fig. 6B), suggesting a relatively minor role for the PM Ca2+ pump in this process and pointing toward a possible mediation of Ca2+ efflux by the PM Ca2+/H+ exchanger. At the same time, Ca2+ flux responses to monocarboxylic acids were completely blocked by either Gd3+ and La3+ (Fig. 6, B and C), as well as strongly inhibited by TEA+. The latter results may suggest that a substantial component of Ca2+ efflux may originate from the K+/Ca2+ Donnan exchange in the cell wall (Shabala and Newman, 2000), as all these inhibitors were also efficient in preventing acetic acid-induced K+ efflux from roots (Fig. 7C).
The above scenario is further supported by the results of long-term experiments (Fig. 5). While a substantial K+ leak was measured 24 h after treatment with monocarboxylic acids (Fig. 5A), no significant (P < 0.05) Ca2+ leak was found (Fig. 5C). Thus, it is highly unlikely that general changes in membrane permeability were involved as was suggested by Jackson and coauthors (Jackson and Taylor, 1970; Jackson and St. John, 1980). Strong membrane depolarization (Fig. 9) also supports the idea of voltage-gated control of activity of K+-permeable channels by monocarboxylic acids.
In summary, this study shows that secondary metabolites associated with waterlogged soil conditions adversely affect root nutrient uptake and that the perturbation to root ionic homeostasis is much stronger in WL-sensitive genotypes. Accordingly, we suggest that tolerance to these stresses should be targeted in any program to breed crops for WL tolerance.
MATERIALS AND METHODS
Plant Material and Growth Conditions
Two barley (Hordeum vulgare) varieties, WL-sensitive ‘Naso Nijo’ and WL-tolerant ‘TX9425’ (Pang et al., 2004, 2007) were grown hydroponically for 3 to 4 d on a floating mesh in plastic containers above 0.5 L of aerated nutrient solution containing 0.1 mm CaCl2 and 0.2 mm KCl (pH 5.5 unbuffered). Seedlings were grown under laboratory conditions (temperature + 24°C; 16 h photoperiod; fluorescent lighting about 150 μmol m−2 s−1) essentially as described by Pang et al. (2006) and used for measurement when their root length was 60 to 80 mm.
Ion Flux Measurements
Net fluxes of H+, Ca2+, and K+ were measured using the noninvasive MIFE technique (University of Tasmania, Hobart, Australia). Details on fabrication and calibration of H+, Ca2+, and K+ ion selective microelectrodes have been described previously (Shabala et al., 1997, 2000). Briefly, pulled and silanized microelectrodes with tip diameters of about 3 μm were back filled with the appropriate solution (0.15 mm NaCl + 0.4 mm KH2PO4 adjusted to pH 6.0 using NaOH for the proton electrode; 0.5 m CaCl2 for calcium; 0.5 m KCl for potassium). The electrode tips were then filled with ionophore cocktails (Fluka; catalog no. 95297 for H+; 21048 for Ca2+; 60031 for K+). Electrodes were mounted on a three-dimensional electrode holder (MMT-5, Narishige), positioned with their tips spaced 2 to 3 μm apart in line. They were calibrated in an appropriate set of standards before and after use (pH from 4.4–7.8; Ca2+ from 0.1–0.5 mm; K+ from 0.2–1 mm).
Experimental Protocol
Two major groups of organic acids, namely monocarboxylic acids and phenolic acids, were chosen for experiments (Table I and II). These are the most widely reported compounds associated with anaerobic soil conditions (Lynch, 1977; Tanaka et al., 1990; Armstrong and Armstrong, 2001). In water, weak acid establishes an equilibrium between the weak acid and the conjugate base. A weaker acid has less dissociation to the conjugate base and the equilibrium favors the undissociated weak acid form.
One hour before measurement, 5 mL basic salt medium (BSM) solution (0.1 mm CaCl2, 0.2 mm KCl, pH 5.5 unbuffered) was added to a plexiglass measuring chamber (100 mm long, 30 mm deep, and 4 mm wide). A seedling was taken from the growth container and placed immediately into the chamber. The root was immobilized in the horizontal position by fine Teflon partitions 5 mm above the floor of the chamber as described in Pang et al. (2006). The chamber was put onto the microscope stage in the Faraday cage and the plant was allowed to adapt to experimental conditions. Ion selective microelectrodes were positioned 50 μm above the root tissue in the mature zone (10 mm from the tip). During measurements electrodes moved vertically in a square-wave manner (10-s cycle; travel range 50 μm) driven by a hydraulic manipulator as described in Shabala et al. (1997).
In transient experiments, steady-state fluxes were measured for 5 min, then 5 mL of BSM solution containing a double concentration of an appropriate chemical was added into the chamber, and the measurement continued for a further 30 min. Solution pH was adjusted to 5.5 in advance using NaOH/HCl, and no substantial changes in Ca2+ or Mn2+ activity was caused by addition of any of organic acids. About 2 min is required for unstirred layer conditions to be reached. This period of time was discarded from the analysis and appears as a gap in the figures.
For measurement of the long-term effects of secondary metabolites on root ion fluxes and membrane potential the components studied were added to the growth plastic container (basic solution) 24 h before measurement. The final concentrations of phenolic acids (benzoic acid, 2-hydroxybenzoic acid, and 4-hydroxybenzoic acid) were 200 μm, volatile monocarboxylic organic acids (formic acid, acetic acid and propionic acid) were 10 mm, Mn2+ (added as MnSO4 salt) was 300 mg L−1; all these concentrations were selected based on previous literature reports showing they are physiologically relevant. Solution pH was adjusted to 5.5 (using HCl/NaOH) in all treatments and monitored continuously by the pH microelectrode. Solutions were aerated continuously during the 24-h treatment period.
Pharmacology
Pretreatment with inhibitors was carried out when the root was transferred to the measuring chamber. Orthovanadate (an inhibitor of P-type ATPase), TEACl (a putative K+ channel blocker), GdCl3 and LaCl3 (NSCCs blockers), and CPA and thapsigargin (specific Ca2+-ATPase inhibitors) were used to modify the activity of selected PM transporters. These inhibitors were mixed with the basic solution (0.2 mm KCl, 0.1 mm CaCl2) to achieve their final concentrations that were as follows: vanadate, 1 mm; TEA+, 10 mm; Gd3+, 50 μm; La3+, 200 μm; CPA, 50 μm; thapsigargin, 5 μm. After 1 h pretreatment in the appropriate inhibitor, transient ion flux responses to one of the secondary metabolites were measured, as described above (still in the presence of inhibitor in the bath solution).
Membrane Potential Measurements
The roots of intact barley plants were mounted in a measuring chamber and the roots were gently secured in a horizontal position with small plastic blocks. Experimental conditions were the same as those for the ion flux measurement. The plant was allowed to stabilize for 60 min. Measurements of the electrical potential difference (Vm) across the root-cell membranes were made in the root mature zone, 1 to 2 cm from the root tip essentially as described by Cuin and Shabala (2005). The borosilicate glass microelectrodes (Clark Electromedical Instruments) were filled with 1 m KCl, connected to an electrometer via a Ag-AgCl half cell, and inserted into the root tissue with a manually operated micromanipulator (Narishige MMT-5).
Assay of ATPase Activity of PM Vesicles
Barley was grown in vermiculite for 7 d in the dark. The vermiculite was watered with basic nutrient solution containing 0.2 mm KCl and 0.1 mm CaCl2 (BS). The roots were washed carefully and rinsed with BSM. Around 10 g roots (fresh weight) were taken into BS, with one of 20 mm acetic acid in BSM (pH 5.5), 200 μm 2-hydroxybenzoic acid in BSM (pH 5.50), or 300 mg/L MnSO4 in BSM (pH 5.5) added for 30 min. Roots were then homogenized in 200 mL ice-cold homogenization buffer (50 mm MOPS, 5 mm EDTA, 330 mm Suc, 0.6% polyvinylpyrrolidone, 5 mm ascorbate, 5 mm dithiothreitol, 0.5 mm phenylmethylsulfonyl fluoride). PM was isolated from the mirosomal fraction (30,000 g) by partitioning at 4°C at an aqueous polymer two-phase system (9 g + 3 g) composed of 6.2% Dextran D1037 (Sigma), 6.2% PEG3350 (Sigma), 330 mm Suc, 5 mm potassium phosphate pH 7.8, 3 mm KCl, 0.1 mm EDTA, and 1 mm dithiothreitol (Larsson et al., 1994). The final PM pellet was suspended in 330 mm Suc, 5 mm potassium phosphate pH 7.8, 50 mm KCl, 5 mm EDTA. Protein concentration was determined according to Bradford colorimetric assay (Bradford, 1976). ATP hydraulic activity was measured as described in Regenberg et al. (1995). The assay medium (20 mm MOPS, 8 mm MgSO4, 50 mm KNO3, 5 mm NaN3, 250 μm NaMo, 0.02% Brij58, pH was adjusted to 7.0 with KOH) included 3 mm ATP. The reaction was initiated by the addition of 10 μL of root PMs to the assay medium and kept for 30 min by leaving at 30°C in heating block.
Acknowledgments
We are grateful to Dr. A. Fuglsang (University of Copenhagen) for her valuable advice on H+-ATPase hydrolytic assay experiments and Mrs. Julie Harris (University of Tasmania) for her kind assistance with using ultracentrifuge.
This work was supported by Grain Research and Development Corporation (M.Z. and N.M.) and Australian Research Council (S.S.) grants.
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantphysiol.org) is: Sergey Shabala (sergey.shabala@utas.edu.au).
Some figures in this article are displayed in color online but in black and white in the print edition.
References
- Armstrong J, Armstrong W (1999) Phragmites die-back: toxic effects of propionic, butyric and caproic acids in relation to pH. New Phytol 142 201–217 [Google Scholar]
- Armstrong J, Armstrong W (2001) Rice and Phragmites: effects of organic acids on growth, root permeability, and radial oxygen loss to the rhizosphere. Am J Bot 88 1359–1370 [PubMed] [Google Scholar]
- Armstrong W, Gaynard TJ (1976) The critical oxygen pressures for respiration in intact plants. Physiol Plant 37 200–206 [DOI] [PubMed] [Google Scholar]
- Ashraf M, Rehman H (1999) Mineral nutrient status of corn in relation to nitrate and long-term waterlogging. J Plant Nutr 22 1253–1268 [Google Scholar]
- Beffagna N, Romani G (1991) Modulation of the plasmalemma proton pump activity by intracellular pH in Elodea densa leaves: correlation between acid load and H+ pumping activity. Plant Physiol Biochem 29 471–480 [Google Scholar]
- Blatt MR, Grabov A (1997) Signalling gates in abscisic acid-mediated control of guard cell ion channels. Physiol Plant 100 481–490 [Google Scholar]
- Bradford M (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72 248–254 [DOI] [PubMed] [Google Scholar]
- Chen Z, Newman I, Zhou M, Mendham N, Zhang G, Shabala S (2005) Screening plants for salt tolerance by measuring K+ flux: a case study for barley. Plant Cell Environ 28 1230–1246 [Google Scholar]
- Chen Z, Zhou M, Newman I, Mendham N, Zhang G, Shabala S (2007) Potassium and sodium relations in salinised barley tissues as a basis of differential salt tolerance. Funct Plant Biol 34 150–162 [DOI] [PubMed] [Google Scholar]
- Cuin T, Shabala S (2005) Exogenously supplied compatible solutes rapidly ameliorate NaCl-induced potassium efflux from barely roots. Plant Cell Physiol 46 1924–1933 [DOI] [PubMed] [Google Scholar]
- Cuin T, Shabala S (2007) Amino acids regulate salinity-induced potassium efflux in barley root epidermis. Planta 225 753–761 [DOI] [PubMed] [Google Scholar]
- Demidchik V, Davenport RJ, Tester M (2002) Nonselective cation channels in plants. Annu Rev Plant Biol 53 67–107 [DOI] [PubMed] [Google Scholar]
- Drew MC (1988) Effects of flooding and oxygen deficiency on plant mineral nutrition. In A Lauchli, PB Tinker, eds, Advances in Plant Nutrition, Vol 3. Praeger, New York, pp 115–159
- Ehness R, Ecker M, Godt DE, Roitsch T (1997) Glucose and stress independently regulate source and sink metabolism and defense mechanisms via signal transduction pathways involving protein phosphorylation. Plant Cell 9 1825–1841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felle H (1989) K+/H+-antiport in Riccia Fluitans: an alternative to the plasma membrane H+ pump for short-term pH regulation? Plant Sci 61 9–15 [Google Scholar]
- Frachisse JM, Johannes E, Felle H (1988) The use of weak acids as physiological tools: a study of the effects of fatty acids on intracellular pH and electrical plasmalemma properties of Riccia fluitans rhizoid cells. Biochim Biophys Acta 938 199–210 [Google Scholar]
- Glass ADM (1973) Influence of phenolic acids on ion uptake. I. Inhibition of phosphate uptake. Plant Physiol 51 1037–1041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glass ADM (1974) Influence of phenolic acids upon ion uptake. III. Inhibition of potassium absorption. J Exp Bot 25 1104–1113 [Google Scholar]
- Glass ADM, Dunlop J (1974) Influence of phenolic acids on ion uptake. IV. Depolarization of membrane potentials. Plant Physiol 54 855–858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grabov A, Blatt MR (1997) Parallel control of the inward-rectifier K+ channel by cytosolic free Ca2+ and pH in Vicia guard cells. Planta 201 84–95 [Google Scholar]
- Guern J, Mathieu Y, Pean M, Pasquier C, Beloeil JC, Lallemand JY (1986) Cytoplasmic pH regulation in Acer pseudoplatanus cells. I. A 31P NMR description of acid-load effects. Plant Physiol 82 840–845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosoi S, Iino M, Shimazaki K (1988) Outward-rectifying K+ channels in stomatal guard-cell protoplasts. Plant Cell Physiol 29 907–911 [Google Scholar]
- Jackson MB, Armstrong W (1999) Formation of aerenchyma and the processes of plant ventilation in relation to soil flooding and submergence. Plant Biol 1 274–287 [Google Scholar]
- Jackson PC, St. John JB (1980) Changes in membrane lipids of roots associated with changes in permeability. Plant Physiol 66 801–804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson PC, Taylor JM (1970) Effects of organic acids on ion uptake and retention in barley roots. Plant Physiol 46 538–542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kido Y, Tamai I, Okamoto M, Suzuki F, Tsuji A (2000) Functional clarification of MCT1-mediated transport of monocarboxylic acids at the blood-brain barrier using in vitro cultured cells and in vivo BUI studies. Pharm Res 17 55–62 [DOI] [PubMed] [Google Scholar]
- Larsson C, Sommarin M, Widell S (1994) Isolation of highly purified plasma membranes and the separation of inside-out and right-side-out vesicles. Methods Enzymol 228 451–469 [Google Scholar]
- Lynch JM (1977) Phytotoxicity of acetic acid produced in the anaerobic decomposition of wheat straw. J Appl Bacteriol 42 81–87 [DOI] [PubMed] [Google Scholar]
- Lynch JM (1978) Production and phytotoxicity of acetic acid in anaerobic soils containing plant residues. Soil Biol Biochem 10 131–135 [Google Scholar]
- Maathuis FJM, Sanders D (1996) Mechanisms of potassium absorption by higher plant roots. Physiol Plant 96 158–168 [Google Scholar]
- Miedema H, Bothwell JHF, Brownlee C, Davies JM (2001) Calcium uptake by plant cells—channels and pumps acting in concert. Trends Plant Sci 6 514–519 [DOI] [PubMed] [Google Scholar]
- Mitsui S, Aso S, Kumazawa K, Ishiwara T (1954) The nutrient uptake of the rice plant as influenced by H2S and butyric acid abundantly evolving under waterlogged soil conditions. Transactions of the International Congress of Soil Science 5 364–368 [Google Scholar]
- Pang JY, Ross J, Zhou MX, Mendham N, Shabala S (2007) Amelioration of detrimental effects of waterlogging by foliar nutrient spray in barley. Funct Plant Biol 34 221–227 [DOI] [PubMed] [Google Scholar]
- Pang JY, Newman I, Mendham N, Zhou MX, Shabala S (2006) Microelectrode ion and O2 flux measurements reveal differential sensitivity of barley root tissues to hypoxia. Plant Cell Environ 29 1107–1121 [DOI] [PubMed] [Google Scholar]
- Pang JY, Zhou MX, Mendham N, Shabala S (2004) Growth and physiological responses of six barley genotypes to waterlogging and subsequent recovery. Aust J Agric Res 55 895–906 [Google Scholar]
- Rao DN, Mikkelsen DS (1977) Effects of acetic, propionic, and butyric acids on rice seedling growth and nutrition. Plant Soil 47 323–334 [Google Scholar]
- Regenberg B, Villalba JM, Lanfermeijer FC, Palmgren MG (1995) C-terminal deletion analysis of plant plasma membrane H+-ATPase: yeast as a model system for solute transport across the plant plasma membrane. Plant Cell 7 1655–1666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts SK (2006) Plasma membrane anion channels in higher plants and their putative functions in roots. New Phytol 169 647–666 [DOI] [PubMed] [Google Scholar]
- Schroeder JI, Hagiwara S (1989) Cytosolic calcium regulates ion chanels in the plasma membrane of Vicia faba guard cells. Nature 338 427–430 [Google Scholar]
- Shabala S (2003) Physiological implications of ultradian oscillations in plant roots. Plant Soil 255 217–226 [Google Scholar]
- Shabala S (2006) Non-invasive microelectrode ion flux measurements in plant stress physiology. In A Volkov, ed, Plant Electrophysiology—Theory and Methods. Springer, Heidelberg, pp 35–72
- Shabala S, Demidchik V, Shabala L, Cuin T, Smith S, Miller A, Davies J, Newman I (2006) Extracellular Ca2+ ameliorates NaCl-induced K+ loss from Arabidopsis root and leaf cells by controlling plasma membrane K+-permeable channels. Plant Physiol 141 1653–1665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shabala S, Newman I (2000) Salinity effects on the activity of plasma membrane H+ and Ca2+ transporters in bean leaf mesophyll: masking role of the cell wall. Ann Bot (Lond) 85 681–686 [Google Scholar]
- Shabala S, Newman I, Wilson S, Clark R (2000) Nutrient uptake patterns over the surface of germinating wheat seeds. Aust J Plant Physiol 27 89–97 [Google Scholar]
- Shabala S, Shabala L, Van Volkenburgh E (2003) Effect of calcium on root development and root ion fluxes in salinised barley seedlings. Funct Plant Biol 30 507–514 [DOI] [PubMed] [Google Scholar]
- Shabala SN, Lew RR (2002) Turgor regulation in osmotically stressed Arabidopsis epidermal root cells: direct support for the role of inorganic ion uptake as revealed by concurrent flux and cell turgor measurements. Plant Physiol 129 290–299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shabala SN, Newman IA (1997) H+ flux kinetics around plant roots after short-term exposure to low temperature: identifying critical temperatures for plant chilling tolerance. Plant Cell Environ 20 1401–1410 [Google Scholar]
- Shabala SN, Newman IA, Morris J (1997) Oscillations in H+ and Ca2+ ion fluxes around the elongation region of corn roots and effects of external pH. Plant Physiol 113 111–118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stieger PA, Feller U (1994) Nutrient accumulation and translocation in maturing wheat plants grown on waterlogged soil. Plant Soil 160 87–95 [Google Scholar]
- Tanaka F, Ono S, Hayasaka T (1990) Identification and evaluation of toxicity of rice elongation inhibitors in flooded soils with added wheat straw. Soil Sci Plant Nutr 36 97–103 [Google Scholar]
- Thion L, Mazars C, Nacry P, Bouchez D, Moreau M, Ranjeva R, Thuleau P (1998) Plasma membrane depolarization-activated calcium channels, stimulated by microtubule-depolymerizing drugs in wild-type Arabidopsis thaliana protoplasts, display constitutively large activities and a longer half-life in ton 2 mutant cells affected in the organization of cortical microtubules. Plant J 13 603–610 [DOI] [PubMed] [Google Scholar]