Abstract
The role of gibberellins (GAs) in tomato (Solanum lycopersicum) fruit development was investigated. Two different inhibitors of GA biosynthesis (LAB 198999 and paclobutrazol) decreased fruit growth and fruit set, an effect reversed by GA3 application. LAB 198999 reduced GA1 and GA8 content, but increased that of their precursors GA53, GA44, GA19, and GA20 in pollinated fruits. This supports the hypothesis that GA1 is the active GA for tomato fruit growth. Unpollinated ovaries developed parthenocarpically in response to GA3 > GA1 = GA4 > GA20, but not to GA19, suggesting that GA 20-oxidase activity was limiting in unpollinated ovaries. This was confirmed by analyzing the effect of pollination on transcript levels of SlCPS, SlGA20ox1, -2, and -3, and SlGA3ox1 and -2, encoding enzymes of GA biosynthesis. Pollination increased transcript content of SlGA20ox1, -2, and -3, and SlCPS, but not of SlGA3ox1 and -2. To investigate whether pollination also altered GA inactivation, full-length cDNA clones of genes encoding enzymes catalyzing GA 2-oxidases (SlGA2ox1, -2, -3, -4, and -5) were isolated and characterized. Transcript levels of these genes did not decrease early after pollination (5-d-old fruits), but transcript content reduction of all of them, mainly of SlGA2ox2, was found later (from 10 d after anthesis). We conclude that pollination mediates fruit set by activating GA biosynthesis mainly through up-regulation of GA20ox. Finally, the phylogenetic reconstruction of the GA2ox family clearly showed the existence of three gene subfamilies, and the phylogenetic position of SlGA2ox1, -2, -3, -4, and -5 was established.
Fruit set has been defined as the changeover from the static condition of the flower ovary to the rapidly growing condition of the young fruit following ovary fertilization. In the case of tomato (Solanum lycopersicum), one of the most studied fleshy fruits, fruit growth takes place after fruit set in two consecutive phases: an active division, lasting about 7 to 10 DPA, and a cell expansion phase (Gillaspy et al., 1993). During the growth process the ovary wall develops into a pericarp composed of exocarp, mesocarp, and endocarp, while the placental parenchyma, supported by the columella, grows by division and expansion, enclosing the developing seeds and filling the locular cavities with a jelly-like homogenous tissue (locular tissue; Ho and Hewitt, 1986; Gillaspy et al., 1993).
GAs constitute a group of plant hormones that control developmental processes such as germination, shoot elongation, tuber formation, flowering, and fruit set, and growth in diverse species (Hedden and Kamiya, 1997; Olszewski et al., 2002). The metabolism of GA has been deeply investigated and is quite well understood (Sponsel and Hedden, 2004). In summary, ent-kaurene, synthesized from geranylgeranyl diphosphate by the action of two cyclases, is metabolized by the action of P450-dependent monoxygenases to GA12 and/or GA53, which in turn are metabolized by GA 20-oxidases and GA 3-oxidases, acting consecutively, to active GAs through two parallel pathways: the non-13-hydroxylation (leading to GA4) and the early 13-hydroxylation one (leading to GA1 and GA3 in some cases; Supplemental Fig. S1). Active GAs and their precursors can be irreversibly inactivated by GA 2-oxidases introducing a hydroxyl at the 2β position (Sponsel and Hedden, 2004). The existence of genes encoding GA deactivating enzymes catalyzing 16α,17-epoxidation in rice (Oryza sativa; Zhu et al., 2006) and formation of GA methyl esters in Arabidopsis (Arabidopsis thaliana; Varbanova et al., 2007) has been reported, although the importance of these reactions for GA homeostasis in other species is unknown. Most of the genes encoding all those enzymes have been cloned in many plant species (Hedden and Kamiya, 1997; Hedden and Phillips, 2000; Sponsel and Hedden, 2004) and their expression is regulated by endogenous and environmental factors (Yamaguchi and Kamiya, 2000; García-Martínez and Gil, 2002). GA 20-oxidases, GA 3-oxidases, and GA 2-oxidases are 2-oxoglutarate-dependent dioxygenases that have been found to be encoded by small gene families (e.g. in the case of Arabidopsis 5 GA20ox, 4 GA3ox, and 7 GA2ox), whose expression is temporarily and developmentally regulated (Hedden and Phillips, 2000). The GA2ox family is particularly complex since it is composed of two classes differing in their substrate specificity, C19-GAs and C20-GAs, respectively (Schomburg et al., 2003). In addition, some GA2ox enzymes using C19-GAs as substrates have multicatalytic activity, converting the GAs successively to 2β-hydroxylated metabolites and to GA catabolites (Supplemental Fig. S1; Thomas et al., 1999; Ubeda-Tomás et al., 2006).
Analysis of GAs has shown that seeded fruits of tomato contain mainly GAs from the early 13-hydroxylation biosynthetic pathway (Bohner et al., 1988; Fos et al., 2000) and that pollination induces an increase of GA content in the ovary (Mapelli et al., 1978; Koshioka et al., 1994), suggesting that these hormones are involved in fruit set and growth of tomato. This hypothesis is supported by results of GA application experiments to unpollinated ovaries (Sjut and Bangerth, 1982, 1983; Alabadí and Carbonell, 1998; Fos et al., 2000, 2001) and of inhibitors of GA biosynthesis to pollinated ovaries (Fos et al., 2000, 2001). There is, however, no demonstration on the nature of the active GA, nor on the possible changes in GA metabolism affected by pollination in relation to fruit set and early fruit growth in tomato.
The tomato ‘Micro-Tom’ (Scott and Harbaugh, 1989) has been proposed as a convenient model system to carry out research on the hormonal regulation of berry fruit development due to its small size, rapid growth, and easy transformation (Meissner et al., 1997; Eyal and Levy, 2002; Dan et al., 2006). The phenotype of this cultivar is the result of mutations in the genes Dwarf (encoding 6-deoxocatasterone dehydrogenase, of the brassinosteroid biosynthesis pathway), Self-Pruning (which controls the determinate/indeterminate phenotype), and Internode length reduction (probably similar to Miniature still uncharacterized; Martí et al., 2006). The dwarf phenotype of ‘Micro-Tom’ is not the result of GA deficiency (Martí et al., 2006). It has been found that pollinated ovaries of ‘Micro-Tom’ develop into normal fruits and that unpollinated ovaries respond to GA3 and auxin (but not to brassinosteroid) application (Serrani et al., 2007), showing that ‘Micro-Tom’ constitutes a good experimental system to investigate the role of hormones in fruit development.
In this work, using the tomato ‘Micro-Tom’, we have shown by application of different GAs and inhibitors of GA biosynthesis that tomato fruit set after pollination depends on GAs, and that GA1 is the active form to induce fruit development. Pollination increased the expression of genes encoding GA20ox, but not of those encoding GA3ox, supporting the hypothesis that GA 20-oxidase activity is limiting in unpollinated ovaries. Five members of the SlGA2ox family have also been isolated to investigate the effect of pollination on expression of genes of GA catabolism. No decrease in transcript levels was found for any of these genes early after pollination (at day 5 after anthesis), indicating that fruit set may not be induced by regulation of GA inactivation. Phylogenetic analysis of genes encoding GA2ox indicates the existence of three subfamilies denoted I, II, and III, the new five SlGA2ox being clustered within groups I and II, constituted by enzymes using C19-GAs as substrates.
RESULTS
Effect of Inhibitors of GA Biosynthesis on Growth of Pollinated Fruits
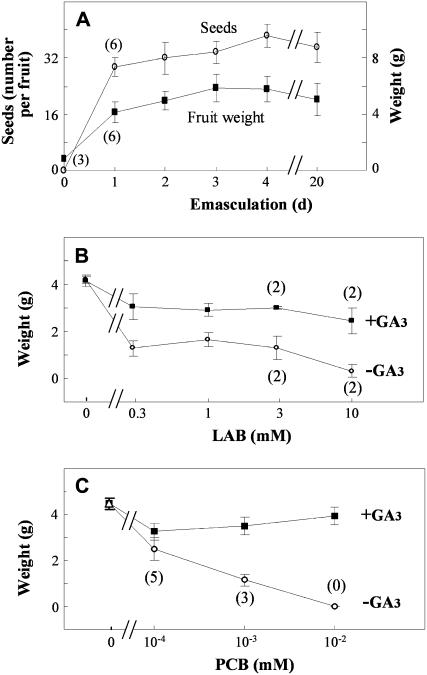
To investigate whether the development of pollinated fruits depends on GAs, two different kinds of inhibitors of GA biosynthesis were used: LAB 198999, an acylcyclohexanedione derivative which inhibits 2-oxoglutarate-dependent dioxygenases (Santes and García-Martínez, 1995), was applied to pollinated ovaries, and paclobutrazol, an inhibitor of P450-dependent monooxygenases (Hedden and Graebe, 1985), to the roots in the nutrient solution. In the case of LAB 198999, direct application to the ovaries was carried out 2 d after pollination, after removing stamen and petals, to facilitate absorption. This inhibitor was applied at that time because earlier application might prevent pollen germination or fertilization. It was shown previously that removal of those organs 2 d after pollination did not reduce the number of seeds per fruit nor the final fruit weight (Fig. 1A). Paclobutrazol was applied to the roots because direct treatment of pollinated ovaries the day equivalent to anthesis or later was not efficient. Paclobutrazol application was started when flowers on which the effect of the inhibitor was going to be determined were about 7 d before anthesis (estimated by flower bud size) to ascertain that it was transported in time to the pollinated ovary.
Figure 1.
Fruit set and growth inhibition of pollinated ovaries with inhibitors of GA biosynthesis and its reversal by GA3 application. A, Effect of time of emasculation and removal of petals, anthers, and style on number of seeds and fruit growth of pollinated ovaries (at day 0). B, Effect of different doses of LAB 198999. C, Effect of different doses of paclobutrazol (PCB). Pollination was carried out at day 0. LAB 198999 was applied directly to the ovary in 10 μL solution, 2 d after anthesis, after emasculation and petal removal. Paclobutrazol was applied to the roots in the nutrient solution, every 2 d, from 7 d before anthesis to 15 d after anthesis. GA3 (2,000 ng) was applied to the ovary in 10 μL solution at anthesis. Fruits were collected 20 d after treatment. Values are data from eight fruits ±se. One-hundred percent of fruits developed in all treatments, except those marked with figures in parentheses (number of fruits developed over eight treated).
LAB 198999 application (0.3–10 mm) reduced the weight of the fruit, an effect which was reversed by exogenous GA3. At the highest doses of inhibitor (3 and 10 mm) fruit set was also reduced, but could not be recovered by GA3 (Fig. 1B), probably due to nonspecific toxic effect of the inhibitor (necrotic spots appeared on the surface of the ovary) at those doses. In the case of paclobutrazol application, both fruit set and final fruit size decreased proportionally to the dose of inhibitor and at 10−2 m fruit set was 0% (Fig. 1C). This inhibition was fully reverted with GA3 application (Fig. 1C). Vegetative growth of plants treated with LAB 198999 was not affected (due probably to direct ovary application) and in the case of paclobutrazol the apical shoot length was only slightly reduced (due probably to application after flowering time, when most vegetative growth had already occurred). Interestingly, both kinds of inhibitors did not prevent the development of seeds in developed fruits (data not presented).
Effect of Inhibitors of GA Biosynthesis on GA Content of Pollinated Fruits
To assess the effect of modification of endogenous GA content in relation to early fruit development, GAs from the early 13-hydroxylation pathway were quantified in 10-d-old pollinated ovaries control or treated with 1 mm LAB 198999 (dose of inhibitor at which the effects are fully reverted by applied GA3; Fig. 1B). At that time, the weight of LAB 198999 treated ovaries was about half of control (Table I). This weight reduction was associated with significantly lower concentration (about half) of GA1 (the active GA), of its metabolite GA8 (about one tenth), and of GA29 (a metabolite of GA20, more than half; Table I). In contrast, LAB 198999 produced accumulation of all precursors of GA1 (GA53, GA44, GA19, and GA20; Table I). These results strongly support that fruit development in tomato depends on GAs, and specifically on GA1.
Table I.
Effect of LAB 198999 on weight and endogenous GA content (ng g fresh weight−1) of pollinated fruits
Fruits were collected 10 d after pollination (8 and a half days after 1 mm LAB 198999 application). Fruit weight data are means of 26 (−LAB) and 31 (+LAB) fruits, and GA data from three biological replicates (aliquots of about 5 g each) ±se.
| Weight | GA1 | GA8 | GA19 | GA20 | GA29 | GA44 | GA53 | |
|---|---|---|---|---|---|---|---|---|
| g fruit−1 | ||||||||
| −LAB | 1.04 ± 0.06 | 2.7 ± 0.8 | 31.4 ± 0.3 | 8.7 ± 0.4 | 23.5 ± 0.6 | 18.5 ± 2.6 | 2.7 ± 0.1 | <0.1 |
| +LAB | 0.47 ± 0.04 | 1.2 ± 0.0 | 3.3 ± 0.9 | 30.5 ± 0.9 | 50.5 ± 6.1 | 7.0 ± 1.2 | 3.4 ± 0.2 | 3.1 ± 1.6 |
Response of Unpollinated Ovaries to Application of Different Kinds of GAs
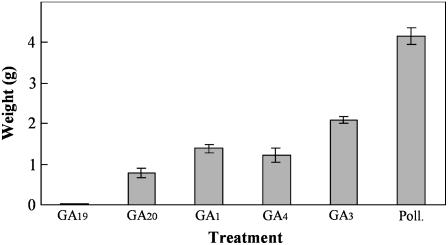
Diverse GAs from the early 13-hydroxylation pathway (GA1, GA3, GA19, and GA20) and GA4 (from the non-13-hydroxylation pathway) were tested for their activity to induce fruit set and growth of unpollinated ovaries. As in many other systems, GA3 was the most active followed by GA1 and GA4 (equally active), and GA20. Interestingly, GA19 (the immediate metabolic precursor of GA20) was completely inactive (Fig. 2). These results suggested that GA 20-oxidase activity is limiting in unpollinated ovaries.
Figure 2.
Response of unpollinated tomato ovaries to GA1, GA3, GA4, GA19, and GA20 (2,000 ng per ovary) application. Fruits were collected 20 d after treatment and values are means of eight fruits ±se. Values of pollinated ovaries are also included as control. Poll., Pollinated.
Effect of Pollination on Transcript Levels of Genes Encoding Enzymes of GA Biosynthesis
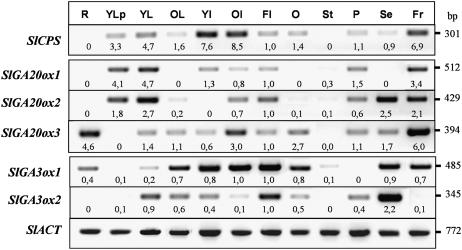
To test the last hypothesis we compared in unpollinated and pollinated ovaries transcript levels of SlCPS, SlGA20ox1, -2, and -3 and SlGA3ox1 and -2, genes previously cloned by Rebers et al. (1999) that encode three kinds of GA biosynthesis enzymes. The expression of those genes in diverse tomato organs is given in Figure 3. All the genes were expressed in aerial vegetative (leaves and internodes) and reproductive (flowers and their diverse parts) tissues. In roots we could only detect transcripts of SlGA20ox3 and SlGA3ox1. Transcripts of SlCPS, GA20ox3, and SlGA3ox1 and -2 were detected in ovaries of flowers at anthesis, and transcripts of all the analyzed genes, except of SlGA3ox2 also in pollinated 20-d-old fruits.
Figure 3.
Distribution of transcript levels of SlCPS, SlGA20ox1, -2, and -3, and SlGA3ox1 and -2 in different organs of tomato. Semiquantitative transcript analysis was carried out by RT-PCR, as described in “Materials and Methods,” using total RNA from roots (R), young leaves before flowering (YLp), young and old leaves from plants at flowering (YL, OL), young and old internodes (YI, OI), flowers (Fl), ovary at anthesis (O), stamens (St), sepals (Se), petals (Pe), and 20-d-old fruit (Fr). For each gene, figures below the blots mean normalized values of gene expression versus that of Actin (used as an internal control; flower expression set at 1.0). Data come from a representative experiment out of two biological replicates with similar results.
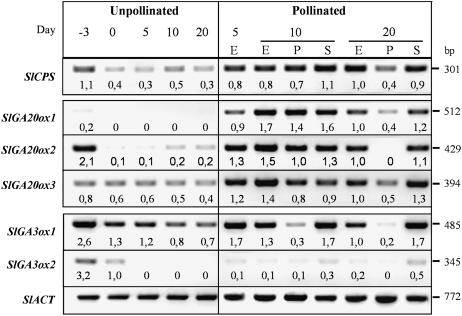
Expression of SlCPS was detected in unpollinated ovaries before anthesis (day 3) but decreased later on (from day 0 to 20 DPA; Fig. 4). In contrast, in entire (E) pollinated ovaries SlCPS transcript levels did not decrease and remained similar or higher than unpollinated ovaries before anthesis. Transcripts were present both in pericarp and developing seeds, more in the latter than in the former (Fig. 4).
Figure 4.
Effect of pollination on transcript levels of SlCPS, SlGA20ox1, -2, and -3, and SlGA3ox1 and -2 genes. Semiquantitative transcript analysis was carried out by RT-PCR, as described in “Materials and Methods,” using total RNA from unpollinated (days 0, 5, 10, and 20) and pollinated (day 5, 10, and 20) ovaries. E, Entire ovary; P, pericarp; S, seeds. For each gene, figures below the blots mean normalized values of gene expression versus that of Actin (used as an internal control; expression of entire 20-d-old pollinated fruits set at 1.0 for all the genes but for SlGA3ox2, where expression of day 0 unpollinated ovaries was used as reference). Data come from a representative experiment out of two biological replicates with similar results.
Almost undetectable expression of SlGA20ox1 was found in unpollinated ovaries (between −3 and 20 DPA). In the case of SlGA20ox2, high expression was detected before anthesis (day 3), but dropped to undetected or very low in unpollinated ovaries between day 0 and day 20 (Fig. 4). Interestingly, transcript levels of both SlGA20ox1 and -2 were very high in entire pollinated ovaries (5–20 DPA; at least 10-fold those of unpollinated ovaries). Transcript content could also be analyzed separately in pericarp and seeds of 10- and 20-d-old fruits. Transcripts were equally distributed in the pericarp and seeds at day 10, but were much more concentrated in seeds at day 20 (Fig. 4). SlGA20ox3 transcripts could be clearly detected and their levels did not vary in unpollinated ovaries (from day 3 to day 20). Interestingly, they increased also (about twice) in pollinated ovaries, particularly in developing seeds at day 20 (Fig. 4).
SlGA3ox1 transcript content was high in unpollinated ovaries before anthesis (day 3) and decreased from anthesis until day 20. Similar levels were found in unpollinated and pollinated ovaries until day 20 (Fig. 4). At day 10 and day 20 transcripts were concentrated in developing seeds (Fig. 4). In contrast, transcripts of SlGA3ox2, detected in ovaries before anthesis, were at very low levels or not detected in unpollinated ovaries after anthesis. In day 10 and day 20 pollinated ovaries SlGA3ox2 transcripts were barely detected and present mainly in the seeds (Fig. 4).
Cloning and Characterization of Genes Encoding Enzymes of GA Inactivation in Tomato
At the time of starting this work no GA 2-oxidase had been cloned in tomato, to our knowledge. Therefore, to know whether pollination increased active GA content by also altering GA inactivation, we isolated genes encoding GA2ox. Using reverse transcription (RT)-PCR and degenerated primers, followed by 5′ and 3′ RACE only one full-length cDNA clone could be isolated (SlGA2ox1, EF441351; see “Materials and Methods”). This cDNA was 1,281 bp long (including 88 and 143 bp in the 5′and 3′ untranslated regions, respectively) and encoded a protein of 349 amino acids.
Using BLAST search of EST databases we identified 18 sequences with high similarity to SlGA2ox1 and GA2ox from other species, which corresponded apparently to four additional different incomplete genes (gene 2, AW930043, BI935635, AW222239, BE434782, BE433301, BE435345; gene 3, AW030357, AI777086, BI921857, AW031637; gene 4, BI208568, AW931003, AW030225; and gene 5, AI899222, AI487548, AI488712, AW650238, AW650160). Full-length clones of these genes were isolated by 5′ and 3′ RACE, amplified, and named accordingly SlGA2ox2 (EF441352; 322 amino acids long), SlGA2ox3 (EF441353; 344 amino acids long), SlGA2ox4 (EF441354; 341 amino acids long), and SlGA2ox5 (EF441355; 346 amino acids long). Recently, the sequence of a clone similar to our SlGA2ox2 (EF017805) was also submitted to GeneBank.
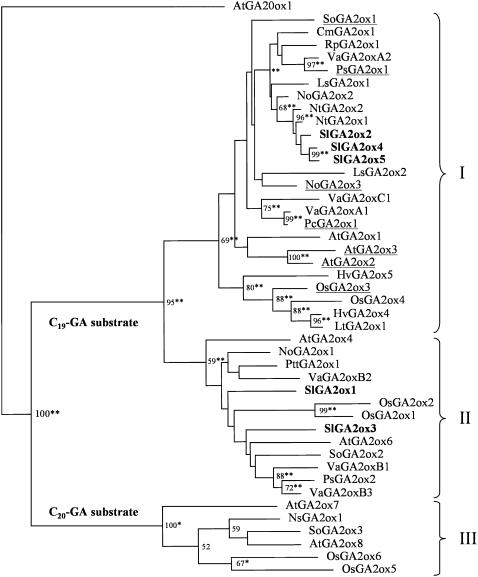
A phylogenetic analysis was carried out with the sequences of all published GA dioxygenase genes from tomato and those of the Arabidopsis genome, including the five putative GA2ox genes isolated in this work, previously published sequences of tomato GA20ox (three genes) and GA3ox (two genes), plus all sequences encoding GA dioxygenases (five GA20ox, four GA3ox, and seven GA2ox) in Arabidopis. Four groups corresponding to GA20ox, GA3ox, GA2ox using C19-GAs as substrate, and GA2ox using C20-GAs as substrate were found. The five SlGA2ox genes from tomato clustered with the group of GA2ox of Arabidopsis using C19-GAs as substrate, suggesting that all of them encode this kind of enzymes (Supplemental Fig. S2).
After subcloning the five SlGA2ox genes in the expression vector pET45b, the activity of the expressed proteins was analyzed using [14C]GA1, [14C]GA4, [14C]GA9, [14C]GA12, [14C]GA20, and [14C]GA53 as substrates. Separation of radioactive metabolites by HPLC showed that extracts from SlGA2ox1 metabolized [14C]GA1 and [14C]GA4 to compounds with the same retention times as [14C]GA8 and [14C]GA34, respectively; those from SlGA2ox3 metabolized [14C]GA1, [14C]GA4, and [14C]GA9 to compounds with the same retention times as [14C]GA8, [14C]GA34, and [14C]GA51, respectively, and those from SlGA2ox4 metabolized [14C]GA1, [14C]GA4, [14C]GA9, and [14C]GA20 to compounds with the same retention time as [14C]GA8, [14C]GA34, [14C]GA51, and [14C]GA29 (Supplemental Fig. S3). Activity of SlGA2ox5 extracts was very poor and only small peaks corresponding to putative [14C]GA34 and [14C]GA51 were found using [14C]GA4 and [14C]GA9 substrates, respectively (Supplemental Fig. S3). [14C]GA12 and [14C]GA53 were not metabolized in any case (Supplemental Fig. S3), confirming that SlGA2ox1, -3, -4, and -5 encoded C19 GA 2-oxidases. Expressed extracts from SlGA2ox2 did not metabolize any of the six labeled GAs used as substrates (data not presented), suggesting that the corresponding protein was probably inactive.
Phylogenetic Analysis of GA 2-Oxidases
To better locate the new SlGA2ox genes within the large GA2ox family, a phylogenetic analysis was performed with all of the full-length GA2ox genes found in the databases, using the outgroup sequence AtGA20ox1 to position the root of the tree. The analysis showed the existence of three large subfamilies of GA2ox (Fig. 5): groups I and II correspond to GA2ox using C19-GAs as substrate (the occurrence of these two groups was pointed out earlier by Elliott et al., 2001), and group III corresponds to GA2ox using C20-GAs as substrate. According to this phylogenetic tree, OsGA2ox5 and -6, and NsGA2ox1, for which catalytic properties have not been reported yet, would use C20-GAs as substrates. These subfamilies are similar to those described by Lee and Zeevaart (2005) in a previous analysis carried out with a selected number of sequences (20 versus 44 in this work). The topology of the root tree indicates that groups I and II are more closely related to each other than to group III. In other words, these data suggest that group III diverged from all other GA 2-dioxygenase genes before the split between groups I and II. Both monocot and dicot genes are present in each of the three groups, indicating that the gene duplication events that gave rise to these three subfamilies occurred before the split between monocots and dicots.
Figure 5.
Maximum-likelihood phylogenetic tree based on comparison of GA2ox protein sequences from different species. The tree was rooted using AtGA20ox1 as outgroup and branch lengths are proportional to the estimated sequence divergence. Bootstrap values above 50% are shown, whereas asterisks indicate statistical significance according to the weighted least-squares likelihood ration test (**, P < 0.01; *, P < 0.05). The three GA2ox subfamilies I, II, and II are indicated, and genes that have been shown to codify for multicatalytic enzymes are underlined. The five genes characterized in this study are shown in bold type. Accession numbers corresponding to the sequences in the tree are the following: AtGA20ox1, X83379; AtGA2ox1, AJ132435; AtGA2ox2, AJ132436 (Thomas et al., 1999); AtGA2ox3, AJ322437 (Thomas et al., 1999); AtGA2ox4, NM103695; AtGA2ox6, NM100121; AtGA2ox7, AC079284; AtGA2ox8, AL021960; CmGA2ox1, AJ315663; HvGA2ox4, AY551432; HvGA2ox5, AY551433; Ls2ox1, AB031206; Ls2ox2, AB031207; LtGA2ox1, DQ324114; NoGA2ox1, AY594291; NoGA2ox2, AY594292; NoGA2ox3, AY588978 (Ubeda-Tomás et al., 2006); NsGA2ox1, Ay242858; NtGA2ox1, AB125232; NtGA2ox2, AB125233; OsGA2ox1, AB059416; OsGA2ox2, AB092484; OsGA2ox3, AB092485 (Sakai et al., 2003); OsGA2ox4, AC132485; OsGA2ox5, BAC10398; OsGA2ox6, AL662958; PcGA2ox1, AJ132438 (Thomas et al., 1999); PsGA2ox1, AF056935 (Martin et al., 1999); PsGA2ox2, AF100954; PttGA2ox1, AY392094; RpGA2ox1, DQ641499; SlGA2ox1, EF441351; SlGA2ox2, EF441352; SlGA2ox3, EF441353; SlGA2ox4, EF441354; SlGA2ox5, EF441355; SoGA2ox1, AF506281 (Lee and Zeevaart, 2002); SoGA2ox2, AF506282; SoGA2ox3, AY935713; VaGA2oxA1, AB181372; VaGA2oxA2, AB181373; VaGA2oxB1, AB181374; VaGA2oxB2, AB181375; VaGA2oxB3, AB181376; VaGA2oxC1, AB181377.
Interestingly, the seven GA2ox reported in the literature as having multicatalytic activity were located in group I (underlined in Fig. 5; see also appropriate references in Fig. 5 legend). Certainly, not all GA2ox present in this group have been shown to be multicatalytic. Absence of annotation of this biochemical property in enzymes of group I may be due to: (1) the catalytic properties have not been investigated in these enzymes; (2) catabolite formation may have not been detected since it depends strongly on enzyme concentration and is adversely affected by dilution (Martin et al., 1999).
Amino acid sequence comparison of all GA2ox enzymes used to construct the phylogenetic tree of Figure 5 is given in Supplemental Figure S4. Interestingly, groups I and II differ in at least two specific amino acids at conserved regions that might be related to their possible different catalytic properties. For instance, within the sequence (N/T/S)GDXG(W/R/E/D/H)X(L/V/I)E(Y/H)(L/I)L (located between positions 90 and 100 of AtGA2ox1) the W present in all the sequences of group I (except in SlGA2ox2 that has an R) is substituted by a D/E in all the sequences of group II (except in VaGA2oxB3 that has an H). Also, within the sequence (Y/F)XX(F/L)(T/K)(W/R)X(E/D/Q)(Y/F)K (located between positions 294 and 303 of AtGA2ox1), the E present in all the sequences of group I (at position 296 of AtGA2ox1) is substituted by diverse nonacidic amino acids in all the enzymes of group II. According to these predictions (see Fig. 5; Supplemental Fig. S4) of the five genes isolated in this work, SlGA2ox1 and -3 would be monocatalytic (confirmed in this work) and SlGA2ox2, -4, and -5 would be multicatalytic (a prediction that we were unable to confirm; possible reasons for the absence of this kind of activity are given in the “Discussion”).
Effect of Pollination of Transcript Levels of Genes Encoding Enzymes of GA Inactivation in Tomato
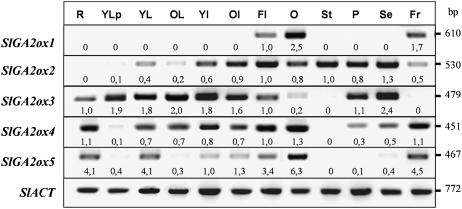
Distribution of SlGA2ox1 to -5 transcripts in diverse tomato organs is presented in Figure 6. SlGA2ox1 was expressed only in ovaries at anthesis and developing pollinated fruits. The other four genes were expressed to different extents in leaves (young and old), internodes (young and adult), and flowers at anthesis. In the roots we could only detect transcripts of SlGA2ox3, -4, and -5. In flowers at anthesis, SlGA2ox2 transcripts were present in all the organs (ovary, stamens, petals, and sepals), SlGA2ox3 mainly in petals and sepals, SlGA2ox4 in ovary, petals, and sepals, and those of SlGA2ox5 only in ovaries. Developing 20-d-old fruits contained transcripts of all GA2ox genes, except SlGA2ox3.
Figure 6.
Distribution of transcript levels of SlGA2ox1, -2, -3, -4, and -5 in different organs of tomato. Semiquantitative transcript analysis was carried out by RT-PCR, as described in “Materials and Methods,” using total RNA from roots (R), young leaves before flowering (YLp), young and old leaves from flowering plants (YL, OL), young and old internodes (YI, OI), flowers (Fl), ovary at anthesis (O), stamens (St), sepals (Se), petals (Pe), and 20-d-old fruit (Fr). For each gene, figures below the blots mean normalized values of gene expression versus that of Actin (used as an internal control; flower expression set at 1.0 for all the genes, except for SlGA2ox5, where expression of YI was used as reference). Data come from a representative experiment out of two biological replicates with similar results.
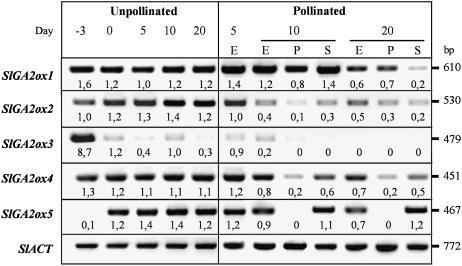
The effect of pollination on expression of SlGA2ox1 to -5 is shown in Figure 7. In unpollinated ovaries transcripts of all genes were present before or at the time of anthesis (day 3 and day 0). In unpollinated ovaries expression of all SlGA2ox remained high later on, except for SlGA2ox3 whose transcripts were at very low levels or undetected between day 0 and day 20 (in agreement with results presented in Fig. 6). In 5-d-old pollinated ovaries (a time at which fruit set and some growth had occurred already) transcript levels of the five SlGA2ox genes were similar to those of unpollinated ovaries. In contrast, in 10- and 20-d- old pollinated ovaries transcript levels of all SlGA2ox were lower than in unpollinated ovaries, particularly in the case of SlGA2ox2 and -3 (in the latter case transcripts were barely detected). An exception was SlGA2ox1 at day 10 where transcript levels were not reduced. Pericarp and seeds could be separated in 10- and 20-d-old fruits and therefore GA2ox transcript content were also analyzed in both organs at those times. SlGA2ox1 was always highly expressed in the pericarp and in seeds at day 10. In contrast, SlGA2ox4 and -5 were expressed mostly in the developing seeds and therefore they may not contribute to GA homeostasis in the pericarp.
Figure 7.
Effect of pollination on transcript levels of SlGA2ox1, -2, -3, -4, and -5 genes. Semiquantitative transcript analysis was carried out by RT-PCR, as described in “Materials and Methods,” using total RNA from unpollinated (days 0, 5, 10, and 20) and pollinated (days 5, 10, and 20) ovaries. E, Entire ovary; P, pericarp; S, seeds. For each gene, figures below the blots mean normalized values of gene expression versus that of Actin (used as an internal control; expression of unpollinated day 5 and 10 ovaries set at 1.0 for SlGA2ox1, -3, and -4, of pollinated day 5 ovaries for SlGA2ox2, and seeds from day 10 pollinated ovaries for SlGA2ox5). Data come from a representative experiment out of two biological replicates with similar results.
DISCUSSION
Fruit set and fruit growth of pollinated ‘Micro-Tom’ ovaries was reduced significantly, on a dose-effect response, by application of paclobutrazol, an inhibitor of GA biosynthesis that inhibits P450-dependent dioxygenases. The effect of paclobutrazol was fully counteracted by applied GA3 (Fig. 1C). LAB 198999, another inhibitor of GA biosynthesis that inhibits 2-oxoglutarate-dependent dioxygenases, also reduced fruit set and fruit growth, but the former effect could not be reverted by GA application (Fig. 1B), probably due to nonspecific toxic effect. These results support the hypothesis that tomato fruit development depends on GAs, as suggested previously (Fos et al., 2000, 2001).
The reduction of fruit growth (about 50%) by LAB 198999 was associated with a reduction of GA1 content to about 50% whereas GA8 content was reduced to 10% (Table I). At the same time, in LAB 198999 treated fruits there was accumulation of GA53, GA44, GA19, and GA20 (Table I). Since the early 13-hydroxylation is the main GA metabolic pathway in tomato (Bohner et al., 1988; Koshioka et al., 1994; Fos et al., 2000, 2001) this means: (1) that GA1 is the main active GA in tomato fruit development; (2) that the precursors of GA1 are not active per se but only after conversion to this active hormone. GA1 has been shown to be the active GA in shoot growth of many species such as pea (Pisum sativum; Ingram et al., 1984), lettuce (Lactuca sativa; Waycott et al., 1991), rice (Fujioka et al., 1988), spinach (Spinacia oleracea; Zeevaart et al., 1993), and Salix (Olsen et al., 1995). In contrast, GA4 is the main active hormone in others species like cucumber (Cucumis sativus; Nakayama et al., 1991) and Arabidopsis (Cowling et al., 1998). Application of GA4 is certainly capable of inducing tomato fruit development also (Fig. 2), but this hormone may have a minor physiological role because the non-13-hydroxylation pathway seems to be minor in this species. GA20 and GA1 were almost equally active to induce parthenocarpic fruit growth in tomato, while GA19 was completely inactive (Fig. 2). This suggests that unpollinated ovaries are capable of metabolizing GA20 but not GA19 to GA1 and, therefore, that the activity of GA 20-oxidase (that metabolizes GA19 to GA20) but not that of GA 3-oxidase (that metabolizes GA20 to GA1) is limiting in unpollinated tomato ovaries. Interestingly, in pat-2, a facultative parthenocarpic mutant of tomato, parthenocarpy is associated with a dramatic increase of GA20 and more GA1 and GA8 contents (Fos et al., 2000), due probably to enhanced activity of GA 20-oxidase.
The above-mentioned hypothesis was supported by results of comparing the effect of pollination on transcript levels of diverse SlGA20ox and SlGA3ox genes of tomato previously isolated by Rebers et al. (1999). SlGA3ox2 transcripts were almost undetected in unpollinated and pollinated ovaries, whereas SlGA3ox1 transcripts were present in unpollinated ovaries at day 0 and remained essentially constant in both unpollinated and pollinated ovaries at least until day 20 (Fig. 4). This supports the idea that GA 3-oxidase activity (encoded from SlGA3ox1) is present in ovaries before pollination and that pollination does not alter that activity. In contrast, SlGA20ox1 and -2 transcripts were at very low levels or undetected at day 0 and in 5- to 20-d-old unpollinated ovaries, but at high levels in 5- to 20-d-old pollinated ovaries. Transcript levels of SlGA20ox3, which were present in unpollinated ovaries, also increased upon pollination (Fig. 4). This suggests that GA 20-oxidase activity increases upon pollination, as indicated by previous GA application experiments (Fig. 2). However, we cannot decide, based on our data, whether the three SlGA20ox are or not equally important for fruit development regulation because transcripts of all of them were similarly distributed in the pericarp and seeds, at least until day 10 (Fig. 4). In any case, our results do not support a role for GA 3-oxidase activity for fruit development and are in contrast with the suggestions of Bohner et al. (1988) and Koshioka et al. (1994) based on endogenous GA content analyses that 3β-hydroxylation of GA20 is a rate-limiting step in GA1 biosynthesis after pollination in tomato.
Since transcript levels of SlCPS were higher in pollinated than in unpollinated ovaries, activity of earlier biosynthetic enzymes (e.g. copalyldiphosphate synthase [CPS]) might also contribute to the increase of GA content after pollination. CPS (formerly ent-kaurene synthetase A) activity is certainly present in extracts of tomato fruits (Bensen and Zeevaart, 1990). Arabidopsis CPS transcripts occur in actively growing tissues, particularly in developing flowers and seeds (Silverstone et al., 1997), and expression of PsCPS (locus LS) seems to play an important role on the regulation of GA biosynthesis in relation to seed development in pea (Ait-Ali et al., 1997). In contrast, overexpression of AtCPS in Arabidopsis, although increasing ent-kaurene production did not result in increase of active GAs (Fleet et al., 2003). Rebers et al. (1999) found that the expression of all the GA biosynthetic genes analyzed in this work (SlCPS, SlGA20ox, and SlGA3ox) change during flower bud development in tomato, with different patterns of mRNA accumulation, indicating a complex regulatory mechanism for controlling GA biosynthesis during flower development. However, no comparison of transcript levels in unpollinated and pollinated tomato ovaries was carried out. GA metabolism during fruit set and growth has also been investigated in pea. In this case, the increase of GA content upon pollination (Rodrigo et al., 1997) is also associated with an increase of PsGA20ox1 expression (Van Huizen et al., 1997). But in contrast to tomato, the presence of seeds seems also to up-regulate the expression of a GA3ox (PsGA3ox1; Ozga et al., 2003).
GA levels are a result of GA biosynthesis and inactivation (Hedden and Phillips, 2000). Therefore, modification of active GA levels may be due to simultaneous transcription alteration of genes encoding GA biosynthesis (e.g. GA20ox and/or GA3ox) and GA inactivating enzymes (GA2ox, GA epoxidases, and GA methyltransferases [GAMTs]). For instance, GA1 content decrease in the shoot during deetiolation in pea is due to down-regulation of PsGA3ox1, which controls the conversion of GA20 to GA1, and by up-regulation of PsGA2ox2, encoding a GA2ox that converts GA1 to inactive GA8 (Symons and Reid, 2003). Developing siliques of null mutants of GAMT1 and GAMT2 have higher GA1 and GA4 contents and their seeds are more resistant to ancymidol, suggesting that they also contain more active GAs (Varbanova et al., 2007). Since GA2ox are generally considered the main GA inactivating enzymes, to know whether the increase of GA1 upon pollination in tomato ovary is not only due to enhanced GA biosynthesis (through increase of GA20ox transcript levels, and may be SlCPS, as shown before), but also to reduction of catabolic activity, five cDNA clones encoding putative GA 2-oxidases from tomato (SlGA2ox1 to -5) were isolated. SlGA2ox1, -3, and -4 and -5 to a lesser extent were shown to encode active C19 GA2ox using different kinds of GAs as substrates (Supplemental Fig. S3). Expressed SlGA2ox2 extracts did not show activity with any of the six GAs used as substrate, suggesting that the corresponding protein was inactive in spite of carrying the purported amino acids binding Fe2+ and 2-oxoglutarae, and essentially all the amino acids conserved in GA2ox (Supplemental Fig. S4). A reason for SlGA2ox2 inactivity might be the presence of a mutation leading to the change of W (conserved in all GA2ox from group I) by an R at position 92 (Supplemental Fig. S4). This observation points out the possible importance this W residue has for GA2ox activity. Additionally, SlGA2ox2, -4, and -5 have a D at a site (position 44 of SlGA2ox2) where most GA2ox have a conserved G (Supplemental Fig. S4), which might also affect their activity.
Transcripts of the five SlGA2ox genes were detected in different tissues (Fig. 6), suggesting that their expression is developmentally regulated. All of them were expressed in unpollinated ovaries before and/or at the time of anthesis and also up to day 20 in unpollinated ovaries, at more or less extent. However, no decrease of expression was observed in any of the SlGA2ox genes in pollinated ovaries 5 d after anthesis, a time at which fruit set has already been established, as shown by the observation that a significant growth had occurred. This means that the effect of pollination on early fruit development may not be mediated by an effect on GA inactivation through GA2ox. However, we cannot discard a possible effect of GA2ox on later growth of tomato fruit (because transcripts of all SlGA2ox genes were lower in pollinated than in pollinated ovaries at day 10 and/or day 20), nor a possible role of other GA catabolic enzymes (e.g. GA epoxidases and GAMT) in GA homeostasis during fruit set and growth.
The phylogenetic analysis of GA2ox, using all the sequences available in data bank and AtGA20ox1 as outgroup (Fig. 5), indicates that a first split occurred between enzymes using C20-GAs as substrate (group III) and those using C19-GAs, and that divergence between groups I and II occurred more recently. The five SlGA2ox genes isolated in this work were distributed between groups I and II, and therefore, according to this prediction, should differ in their catalytic properties. While SlGA2ox1 and -3 presented monocatalytic activity, as expected, no multicatalytic activity could be demonstrated for SlGA2ox2, -4, and -5 (expressed SlGA2ox2 was completely inactive). Therefore, our results do not support the proposed hypothesis. However, since the three translated sequences of SlGA2ox2, -4, and -5 present changes in specific conserved amino acids that might affect activity, and it has been reported that detection of GA catabolites may be difficult and dependent on enzyme concentration (Martin et al., 1999), it may not be possible to completely discard that hypothesis before carrying out more biochemical work to substantiate it. Monocot and dicot genes are both present in each of the three groups, indicating that gene subfamilies I, II, and III were originated from gene duplications early in evolution. Finally, additional gene duplications occurred within each of the groups I and II as indicated by the presence of several duplicates of Arabidopsis and other species in those groups, whereas no further duplication seem to have occurred within the more ancestral group III (Fig. 5). Altogether, the data support the general hypothesis that acquisition of evolutionarily novel functions among GA dioxygenases is associated with gene duplication events, as previously shown for other gene families (Sanjuan and Marin, 2001).
The results of experiments of GA and inhibitors of GA biosynthesis application presented here, as well as of GA quantification analysis support the hypothesis that fruit set and early growth in tomato depend on GAs and that GA1 is the active hormone involved in these processes. Pollination increases the content of GAs in the ovary by increasing GA biosynthesis (through up-regulating GA20ox and SlCPS, but not GA3ox expression), not by reducing GA catabolic inactivation through GA2ox.
MATERIALS AND METHODS
Plant Material and Growth Conditions
Plants of tomato (Solanum lycopersicum) ‘Micro-Tom’ (seeds obtained originally from Dr. A. Levy) were used in the experiments. Plants (one per pot) were grown in 1 L pots with a mixture of peat:vermiculite (1:1), cultured in a greenhouse under 24°C (day)/20°C (night) conditions, and irrigated daily with Hoagland solution. Natural light was supplemented with Osram lamps (Powerstar HQI-BT, 400W) to get a 16 h light photoperiod.
Only one flower per truss, and the first two trusses were left per plant to prevent interaction between fruits at the same truss (Serrani et al., 2007).
Plant Hormone Applications
Application of GAs (GA1, GA4, GA19, and GA20, obtained from Prof. L. Mander, Australian National University, Canberra, Australia) and GA3 (Duchefa) was carried out to unpollinated ovaries in 10 μL of 5% ethanol, 0.1% Tween 80 solution. Flower emasculation was carried out 2 d before anthesis to prevent self-pollination. LAB 198999 (3,5-dioxo-4-butyryl-cyclohexane carboxylic acid ethyl ester; BASF) was applied in 10 μL of 5% ethanol, 0.1% Tween solution to pollinated ovaries, at different times after pollination, after removal of petals and stamens. Equal volumes of solvent solution was applied to control ovaries. Paclobutrazol (Duchefa) was applied to the roots in the nutrient solution.
Quantification of GAs
GAs were quantified following the protocol described in Fos et al. (2000). In summary, aliquots (about 3–5 g fresh weight) of frozen material were extracted with 80% methanol and, after removing the organic phase, the water fraction was partitioned against ethyl acetate and purified by QAE-Sephadex chromatography and C18 cartridges. The GAs where then separated by reverse-phase HPLC chromatography (4-μm C18 column, 15 cm long, 3.9 mm i.d.; NovaPak, Millipore) and appropriate fractions grouped for gas chromatography-single ion monitoring analysis after methylation and trimethylsililation. [17,17-2H]GA1, [17,17-2H]GA8, [17,17-2H]GA19, [17,17-2H]GA20, [17,17-2H]GA29, [17,17-2H]GA44, and [17,17-2H]GA53 (purchased from Prof. L. Mander) were added to the extracts as internal standards for quantification and [3H]GA20 and [3H]GA9 to monitor the separation of GAs after HPLC using a 10% to 100% methanol gradient. Quantification was carried out by gas chromatography-single ion monitoring using a gas chromatograph (model 5890, Hewlett-Packard) coupled to a mass-selective detector (model 5971A, Hewlett-Packard). The concentrations of GAs in the extracts were determined using the calibration curves methodology.
Isolation of cDNA Clones of GA2ox from Tomato
Total RNA was isolated from 20-d-old pollinated fruits using a phenol-chloroform method (Bartels and Thompson, 1983). Clones of SlGA2ox were isolated by RT-PCR using degenerated oligonucleotides. Two micrograms of total RNA were reverse transcribed with a first-strand cDNA synthesis kit (Amersham Biosciences) in 33 μL total volume reaction. PCR was performed taking 1 μL aliquot of cDNA solution in a 50 μL total volume reaction containing 0.2 mm of each dNTP, 2 mm MgCl2, 1× reaction buffer, 1 unit of NETZYME DNA Polymerase (Fermentas Gmbh), and 1 μm of degenerated primers A [5′-(GA)TXGGXTT(CT)GGXGA(AG)(CA)(CA)(AT)-3′] and B [5′-X(GC)CX(GC)(AC)(AG)AA(AG)TAXATCAT-3′]. Thermocycling conditions for amplification consisted of initial denaturation at 94°C for 2 min, followed by 40 cycles of 94°C/30 s, 45°C/60 s, and 72°C/60 s, and finally 10 min extension at 72°C. The products of an amplified band of about 250 bp, separated on 1% agarose gel electrophoresis, were purified (CONCERT Rapid Gel Extraction system, GIBCO-BRL), cloned in the pGEM-T Easy Vector (Promega), and sequenced. Six of these clones (out of 11 sequenced) were identical and homologous to GA2ox previously cloned from diverse species. Sequences of the 5′ and 3′ regions were obtained by RACE (RACE cDNA amplification kit, CLONTECH) using appropriate primers (Supplemental Table S1) and the following conditions for amplification: 95°C/5 min followed by five cycles of 94°C/30 s and 72°C/2.5 min, five cycles of 94°C/30 s and 70°C/2.5 min, and 30 cycles of 94°C/30 s and 68°C/2.5 min, and finally 10 min extension at 72°C. A full-length cDNA clone, named SlGA2ox1, was obtained by RT-PCR using appropriate primers (Supplemental Table S1) and the following thermocycling conditions: 94°C/2 min, followed by 40 cycles of 94°C/1 min, 57°C/2 min, and 72°C/3 min, and 10 min extension at 72°C, cloned in the pGEM-T Easy Vector and sequenced.
Additional GA2ox clones of tomato were identified by searching for tomato sequences homologous to GA2ox from diverse species (including Arabidopsis [Arabidopsis thaliana] and SlGA2ox1, previously cloned) in GenBank EST databases. Four groups coming from 18 ESTs corresponding to genes different to SlGA2ox1 were identified. Using this sequence information 5′ and 3′ regions were obtained by RACE, when necessary, as described before. Full-length cDNA clones (named SlGA2ox2, SlGA2ox3, SlGA2ox4, and SlGA2ox5) were amplified by RT-PCR using RNA from pollinated fruits (SlGA2ox2, -4, and -5) and mature leaves (SlGA2ox4), the primers given in Supplemental Table S1, and the thermocycling conditions described previously for SlGA2ox1 (but using as annealing temperatures of 50°C for SlGA2ox3 and -4, and 54°C for SlGA2ox2 and -5). Amplified products were cloned in pGEM-T Easy Vector and sequenced.
Heterologous Expression of cDNA Clones and Determination of Enzyme Activities
Coding cDNA sequences of SlGA2ox1, -2, -3, -4, and -5 were amplified by PCR, cloned using a Zero Blunt TOPO Cloning kit (Invitrogen), and inserted as a translational fusion into the pET45b prokariote expression vector (Novagen) using BamHI-HindIII (SlGA2ox1, -3, and -4) and NotI-XhoI (SlGA2ox2 and -5) sites. Recombinant clones were sequenced and expressed in BL21 (pLysS) D3 Escherichia coli cells (Novagen) following the manufacturer's instructions. Activity of expressed proteins from at least two sequenced PCR independent clones of each gene was determined enzymatically using appropriate cofactors [17-14C]GA1, [17-14C]GA4, [17-14C]GA9, [17-14C]GA12, [17-14C]GA20, and [17-14C]GA53 (333 Bq, 100–150 pmol; purchased from Dr. L. Mander, Australian National University, Canberra) as substrates, and 93 μL aliquots of cell lysates in a total 100 μL reaction volume as described elsewhere (García-Martínez et al., 1997). Metabolic products were separated by HPLC, detected using an on-line radioactive monitor (Radioflow Detector LB 508, Berthold Technologies), and identified by their retention times compared to pure GAs.
Semiquantitative RT-PCR
Total RNA was isolated from different tomato organs: roots, young and old leaves, young and old internodes, flowers, and separated flower organs at anthesis. Unpollinated and pollinated ovaries at 0, 5, 10, and 20 DPA were also collected and pericarp and seeds of 10- and 20-d-old pollinated ovaries separated for RNA extraction. RNA was treated with DNAse, according to manufacturer's protocol using an RNAeasy Plant mini kit (Quiagen). Then, 2 μg of total RNA were reverse transcribed with a first-strand cDNA synthesis kit (Amersham Biosciences) in 33 μL total volume reaction. PCRs were performed taking 1 μL aliquots of cDNA solution in a 50 μL total volume reaction containing 0.2 mm of each dNTP, 2 mm MgCl2, 1× reaction buffer, 1 unit of NETZYME DNA Polymerase (Fermentas Gmbh), and 1 μm of the appropriate pair of primers (Supplemental Table S2). PCR conditions for amplification of SlCPS, SlGA20ox1, -2, and -3 and SlGA3ox1 and -2 consisted of initial denaturation at 94°C for 2 min, followed by 32 cycles of 94°C/30 s, 57°C/60 s, and 72°C/60 s, and finally 10 min extension at 72°C. For amplification of SlGA2ox1, -2, -3, -4, and -5, 31 cycles were used with annealing temperatures of 60°C (SlGA2ox1) or 62°C (SlGA2ox2, -3, -4, and -5), and for SlCPS 33 cycles and 61°C. In the case of Actin annealing temperature of 60°C and 24 cycles were used. In all cases, the number of cycles was chosen to give amplified products within the linear synthesis reaction. Fifteen microliter aliquots of PCR products were separated on 1% agarose gel electrophoresis. The spots were stained with ethidium bromide, visualized under UV using a GeneGenius Bio Imaging system (Syngene), captured with the GeneSnap program (Syngene), and quantified with the GeneTools software (Syngene). Expression was normalized using Actin as internal control, by comparing expression ratios to that of the specific tissues indicated in the figure legends (set to 1.0).
The analyses were carried out in duplicate using biologically independent material with similar results. Only data from one representative replicate are given under “Results.”
Phylogenetic Analyses
Nucleotide sequences were translated into protein sequences using GeneDoc software (available at http://www.psc.edu/biomed/genedoc) and aligned with MUSCLE algorithm (freely available at http://www.drive5.com/muscle) using default parameters. Sequences were highly divergent, which led us to pursue the phylogenetic reconstruction using amino acid rather than nucleotide sequences. The best model of protein evolution was selected based on the Akaike criterion with the ProtTest on-line server (http://darwin.uvigo.es/software/prottest_server.html). The Jones-Taylor-Thornton evolutionary model (Jones et al., 1992) with evolution rates varying according to a Gamma distribution plus a class of invariant sites was judged optima in both phylogenetic analyses. In the 44 GA2ox dataset, the inferred parameters were α = 1.20 for the shape of the Gamma and P = 0.04 for the fraction of invariant sites, whereas for the 26-sequence dataset containing only tomato and Arabidopsis GA2ox, GA3ox, and GA20ox genes, the estimated values were α = 1.36 and P = 0.01. A maximum-likelihood tree was obtained with the proml implementation of the PHYLIP package version 3.66 (freely available at http://evolution.genetics.washington.edu/phylip.html) using the Hidden Markov model method of inferring different rates of evolution at different amino acid positions (Felsenstein and Churchill, 1996), with six discrete classes for the rates and prior probabilities chosen according to the above estimated parameters. To identify ancestral and derivate clusters in the 44 GA2ox dataset analysis, the outgroup AtGA20ox1 was used to root the tree, whereas in the other analysis, the tree was left unrooted.
To assess the statistical significance of each internal branch, 1,000 bootstrap pseudoreplicates of the protein alignments were generated using the seqboot implementation of the PHYLIP package version 3.66. The maximum-likelihood procedure was repeated for 100 of the pseudoreplicates (doing more pseudoreplicates would be computationally too intensive) and a consensus tree was obtained using the consense implementation of the same package, setting all parameters at their default values. The branch lengths of the tree were then estimated using the same maximum-likelihood method. A node is judged statistically significant if it is supported by a high bootstrap proportion, though the appropriate threshold value depends on many factors (Hillis and Bull, 1993). To have an additional criterion for clade selection, we performed a weighted least-squares likelihood ratio test (Sanjuan and Wrobel, 2005) on each node using the WeightLESS implementation (freely available at http://www.iopan.gda.pl/∼wrobel). To do that, we used the 1,000 pseudoreplicates to estimate the involved parameters, the distance matrix derived from the above Jones-Taylor-Thornton plus Gamma plus invariant class evolutionary model, and the above consensus tree.
Sequence data from this article can be found in the GenBank/EMBL data libraries under accession numbers EF441351 to EF441355.
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure S1. Scheme of GA metabolic pathways.
Supplemental Figure S2. Maximum-likelihood phylogenetic tree based on comparison of GA20ox, GA3ox, and GA2ox protein sequences from Arabidopsis and tomato.
Supplemental Figure S3. HPLC radioactivity traces of products of [14C]GA12, [14C]GA9, [14C]GA4, [14C]GA53, [14C]GA20, and [14C]GA1, incubated with heterologous expression products of SlGA2ox1, -3, -4, and -5 after 2 h incubation at 30°C.
Supplemental Figure S4. Alignment of amino acid sequences corresponding to GA 2-oxidases from groups I, II, and III used to construct the phylogenetic tree of Figure 5.
Supplemental Table S1. Primer sequences used to amplify full-length cDNA clones of SlGA2ox1, -2, -3, -4, and -5.
Supplemental Table S2. Primer sequences used for semiquantitative RT-PCR analysis of diverse GA metabolism genes of tomato.
Supplementary Material
Acknowledgments
We thank Dr. A. Levy for providing the tomato ‘Micro-Tom’ seeds, Dr. W. Rademacher for the gift of LAB 198999, Dr. H. Kawaide for providing SlCPS, SlGA20ox, and SlGA3ox cDNA clones, Dr. I. López-Díaz for help with EST searching, and Mrs. T. Sabater for help with GA analysis.
This work was supported by grants from the Ministerio de Ciencia y Tecnologia of Spain (grant nos. BIO2003–00151 and BIO2006–13437).
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantphysiol.org) is: José Luis García-Martínez (jlgarcim@ibmcp.upv.es).
The online version of this article contains Web-only data.
References
- Ait-Ali T, Swain SM, Reid JB, Sun TP, Kamiya Y (1997) The LS locus of pea encodes the gibberellin biosynthesis enzyme ent-kaurene synthase A. Plant J 11 443–454 [DOI] [PubMed] [Google Scholar]
- Alabadí D, Carbonell J (1998) Expression of ornithine decarboxylase is transiently increased by pollination, 2,4-dichlorophenoxyacetic acid, and gibberellic acid in tomato ovaries. Plant Physiol 118 323–328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartels D, Thompson RD (1983) The characterization of cDNA clones coding for wheat storage proteins. Nucleic Acids Res 11 2961–2977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bensen RJ, Zeevaart JAD (1990) Comparison of ent-kaurene synthetase A and B activities in cell-free extracts from young tomato fruits of wild-type and gib-1, gib-2, and gib-3 tomato plants. J Plant Growth Regul 9 237–242 [Google Scholar]
- Bohner J, Hedden P, Bora-Haber E, Bangerth F (1988) Identification and quantitation of gibberellins in frutis of Lycopersicon esculentum, and their relationship to fruit size in L. esculentum and L. pimpinellifolium. Physiol Plant 73 348–353 [Google Scholar]
- Cowling RJ, Kamiya Y, Seto H, Harberd NP (1998) Gibberellin dose-response regulation of GA4 gene transcript levels in Arabidopsis. Plant Physiol 117 1195–1203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dan Y, Yan H, Munyikwa T, Dong J, Zhang Y, Armstrong CL (2006) MicroTom, a high-throughput model transformation system for functional genomics. Plant Cell Rep 25 432–441 [DOI] [PubMed] [Google Scholar]
- Elliott RC, Ross JJ, Smith JJ, Lester DR, Reid JB (2001) Feed-forward regulation of gibberellin deactivation in pea. J Plant Growth Regul 20 87–94 [Google Scholar]
- Eyal E, Levy AA (2002) Tomato mutants as tools for functional genomics. Curr Opin Plant Biol 5 112–117 [DOI] [PubMed] [Google Scholar]
- Felsenstein J, Churchill GA (1996) A hidden Markov model approach to variation among sites in rate of evolution. Mol Biol Evol 13 93–104 [DOI] [PubMed] [Google Scholar]
- Fleet CM, Yamaguchi S, Hanada A, Kawaide H, David CJ, Kamiya Y, Sun TP (2003) Overexpression of AtCPS and AtKS in Arabidopsis confers increased ent-kaurene production but no increase in bioactive gibberellins. Plant Physiol 132 830–839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fos M, Nuez F, García-Martínez JL (2000) The gene pat-2, which induces natural parthenocarpy, alters the gibberellin content in unpollinated tomato ovaries. Plant Physiol 122 471–479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fos M, Proaño K, Nuez F, García-Martínez JL (2001) Role of gibberellins in parthenocarpic fruit development induced by the genetic system pat-3/pat-4 in tomato. Physiol Plant 111 545–550 [DOI] [PubMed] [Google Scholar]
- Fujioka S, Yamane H, Spray CR, Gaskin P, MacMillan J, Phinney BO, Takahashi N (1988) Qualitative and quantitative analyses of gibberellins in vegetative shoots on normal, dwarf-1, dwarf-2, dwarf-3, and dwarf-5 seedlings of Zea mays L. Plant Physiol 88 1367–1372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- García-Martínez JL, Gil J (2002) Light regulation of gibberellin biosynthesis and mode of action. J Plant Growth Regul 20 354–368 [DOI] [PubMed] [Google Scholar]
- García-Martínez JL, López-Díaz I, Sánchez-Beltrán MJ, Phillips AL, Ward DA, Gaskin P, Hedden P (1997) Isolation and transcript analysis of gibberellin 20-oxidase genes in pea and bean in relation to fruit development. Plant Mol Biol 33 1073–1084 [DOI] [PubMed] [Google Scholar]
- Gillaspy G, Ben-David H, Gruissem W (1993) Fruits: a developmental perspective. Plant Cell 5 1439–1451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedden P, Graebe JE (1985) Inhibition of gibberellin biosynthesis by paclobutrazol in cell-free homogenates of Cucurbita maxima endosperm an Malus pumila embryos. J Plant Growth Regul 4 111–122 [Google Scholar]
- Hedden P, Kamiya Y (1997) Gibberellin biosynthesis: enzymes, genes and their regulation. Annu Rev Plant Physiol Plant Mol Biol 48 431–460 [DOI] [PubMed] [Google Scholar]
- Hedden P, Phillips A (2000) Gibberellin metabolism: new insights revealed by the genes. Trends Pharmacol Sci 5 523–530 [DOI] [PubMed] [Google Scholar]
- Hillis DM, Bull JJ (1993) An empirical test of bootstrapping as a method for assessing confidence in phylogenetic analysis. Syst Biol 42 182–192 [Google Scholar]
- Ho L, Hewitt J (1986) Fruit development. In JG Atherton, J Rudish, eds, The Tomato Crop. Chapman and Hall, New York, pp 201–239
- Ingram TJ, Reid JB, Murfet IC, Gaskin P, Willis CL, MacMillan J (1984) Internode length in Pisum: the Le gene controls the 3β-hydroxylation of gibberellin A20 to gibberellin A1. Planta 160 455–463 [DOI] [PubMed] [Google Scholar]
- Jones DT, Taylor WR, Thornton JM (1992) The rapid generation of mutation data matrices from protein sequences. Comput Appl Biosci 8 275–282 [DOI] [PubMed] [Google Scholar]
- Koshioka M, Nishijima T, Yamazaki H, Liu Y, Nonaka M, Mander LN (1994) Analysis of gibberellins in growing fruits of Lycopersicon esculentum after pollination or treatment with 4-chlorophenoxyacetic acid. J Hortic Sci 69 171–179 [Google Scholar]
- Lee DJ, Zeevaart JAD (2002) Differential regulation of RNA levels of gibberellin dioxygenases by photoperiod in spinach. Plant Physiol 130 2085–2094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee DJ, Zeevaart JAD (2005) Molecular cloning of GA-Oxidase3 from spinach and its ectopic expression in Nicotiana sylvestris. Plant Physiol 138 243–254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mapelli SC, Frova C, Torti G, Soressi G (1978) Relationship between set, development and activities of growth regulators in tomato fruits. Plant Cell Physiol 19 1281–1288 [Google Scholar]
- Martí E, Gisbert C, Bishop GJ, Dixon MS, García-Martínez JL (2006) Genetic and physiological characterization of tomato cv. Micro-Tom. J Exp Bot 57 2037–2047 [DOI] [PubMed] [Google Scholar]
- Martin DN, Proebsting WM, Hedden P (1999) The SLENDER gene of pea encodes a gibberellin 2-oxidase. Plant Physiol 121 775–781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meissner R, Jacobson Y, Melamed S, Levyatuv S, Shalev G, Ashri A, Elkind Y, Levy AA (1997) A new model system for tomato genetics. Plant J 12 1465–1472 [Google Scholar]
- Nakayama M, Yamane H, Murofushi N, Takahashi N, Mander L, Seto H (1991) Gibberellin biosynthetic pathway and the physiologically active gibberellin in the shoot of Cucumis sativus L. J Plant Growth Regul 10 115–119 [Google Scholar]
- Olsen JE, Junttila O, Moritz T (1995) A localised decrease of GA1 in shoot tips of Salix pentandra seedlings precedes cessation of shoot elongation under short photoperiod. Physiol Plant 95 627–632 [Google Scholar]
- Olszewski N, Sun TP, Gubler F (2002) Gibberellin signalling, biosynthesis, catabolism, and response pathways. Plant Cell (Suppl) 14 S61–S80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozga JA, Yu J, Reinecke DM (2003) Pollination-, development-, and auxin-specific regulation of gibberellin 3β-hydroxylase gene expression in pea fruit and seeds. Plant Physiol 131 1137–1146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rebers M, Kaneta T, Kawaide H, Yamaguchi S, Yang Y-Y, Imai R, Sekimoto H, Kamiya Y (1999) Regulation of gibberellin biosynthesis genes during flower and early fruit development of tomato. Plant J 17 241–250 [DOI] [PubMed] [Google Scholar]
- Rodrigo MJ, Garcia-Martínez JL, Santes CM, Gaskin P, Hedden P (1997) The role of gibberellins A1 and A3 in fruit growth of Pisum sativum L. and the identification of gibberellins A4 and A7 in young seeds. Planta 201 446–455 [Google Scholar]
- Sakai M, Sakamoto T, Saito T, Matsuoka M, Tanaka H, Kobayashi M (2003) Expression of novel rice gibberellin 2-oxidase gene is under homeostatic regulation by biologically active gibberellins. J Plant Res 116 161–164 [DOI] [PubMed] [Google Scholar]
- Sanjuan R, Marin I (2001) Tracing the origin of the compensasome: evolutionary history of DEAH helicase and MYST acetyltransferase gene families. Mol Biol Evol 18 330–343 [DOI] [PubMed] [Google Scholar]
- Sanjuan R, Wrobel B (2005) Weighted least-squares likelihood ratio test for branch testing in phylogenies reconstructed from distance methods. Syst Biol 54 218–229 [DOI] [PubMed] [Google Scholar]
- Santes CM, García-Martínez JL (1995) Effect of the growth retardant 3,5-dioxo-4-butyryl-cyclohexane carboxylic acid ethyl ester, an acylcyclohexanedione compound, on fruit growth and gibberellin content of pollinated and unpollinated ovaries in pea. Plant Physiol 108 517–523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schomburg FM, Bizzell CM, Lee DJ, Zeevaart JAD, Amasino RM (2003) Overexpression of a novel class of gibberellin 2-oxidases decreases gibberellin levels and creates dwarf plants. Plant Cell 15 151–163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott JW, Harbaugh BK (1989) Micro-Tom—a miniature dwarf tomato. Florida Agr Expt Sta Cir 370 1–6 [Google Scholar]
- Serrani JC, Fos M, Atarés A, García-Martínez JL (2007) Effect of gibberellin and auxin on parthenocarpic fruit growth induction in the cv Micro-Tom of tomato. J Plant Growth Regul (in press)
- Silverstone AL, Chang CW, Krol E, Sun TP (1997) Developmental regulation of the gibberellin biosynthetic gene GA1 in Arabidopsis thaliana. Plant J 12 9–19 [DOI] [PubMed] [Google Scholar]
- Sjut V, Bangerth F (1982) Induced parthenocarpy—a way of changing the levels of endogenous hormones in tomato fruits (Lycopersicon esculentum Mill.). 1. Extractable hormones. Plant Growth Regul 1 243–251 [Google Scholar]
- Sponsel V, Hedden V (2004) Gibberellin biosynthesis and inactivation. In P Davies, ed, Plant Hormones: Biosynthesis, Signal Transduction, Action! Kluwer Academic Publishers, Dordrecht, The Netherlands, pp 63–94
- Symons GM, Reid JB (2003) Interactions between light and plant hormones during de-etiolation. J Plant Growth Regul 22 3–14 [Google Scholar]
- Thomas SG, Phillips AL, Hedden P (1999) Molecular cloning and functional expression of gibberellin 2-oxidases, multifunctional enzymes involved in gibberellin deactivation. Proc Natl Acad Sci USA 96 4698–4703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ubeda-Tomás S, García-Martínez JL, López-Díaz I (2006) Molecular, biochemical and physiological characterization of gibberellin biosynthesis and catabolism genes from Nerium oleander. J Plant Growth Regul 25 52–68 [Google Scholar]
- Van Huizen R, Ozga JA, Reinecke DM (1997) Seed and hormonal regulation of gibberellin 20-oxidase expression in pea pericarp. Plant Physiol 115 123–128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varbanova M, Yamaguchi S, Yang Y, McKelvey K, Hanada A, Borochov R, Yu F, Jikumaru Y, Ross Y, Cortes D, et al (2007) Methylation of gibberellins by Arabidopsis GAMT1 and GAMT2. Plant Cell 19 32–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waycott W, Smith VA, Gaskin P, MacMillan J, Taiz L (1991) The endogenous gibberellins of dwarf mutants of lettuce. Plant Physiol 95 1169–1173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi S, Kamiya Y (2000) Gibberellin biosynthesis: its regulation by endogenous and environmental signals. Plant Cell Physiol 41 251–257 [DOI] [PubMed] [Google Scholar]
- Zeevaart JAD, Gage DA, Talon M (1993) Gibberellin A1 is required for stem elongation in spinach. Proc Natl Acad Sci USA 90 7401–7405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y, Nomura T, Xu Y, Zhang Y, Peng Y, Mao B, Hanada A, Zhou H, Wang R, Li P, et al (2006) ELONGATED UPPERMOST INTERNODE encodes a cytochrome P450 monooxygenase that epoxidizes gibberellins in a novel deactivation reaction in rice. Plant Cell 18 442–456 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.