Abstract
Studies on long-term effects of plants grown at elevated CO2 are scarce and mechanisms of such responses are largely unknown. To gain mechanistic understanding on respiratory acclimation to elevated CO2, the Crassulacean acid metabolism Mediterranean invasive Opuntia ficus-indica Miller was grown at various CO2 concentrations. Respiration rates, maximum activity of cytochrome c oxidase, and active mitochondrial number consistently decreased in plants grown at elevated CO2 during the 9 months of the study when compared to ambient plants. Plant growth at elevated CO2 also reduced cytochrome pathway activity, but increased the activity of the alternative pathway. Despite all these effects seen in plants grown at high CO2, the specific oxygen uptake rate per unit of active mitochondria was the same for plants grown at ambient and elevated CO2. Although decreases in photorespiration activity have been pointed out as a factor contributing to the long-term acclimation of plant respiration to growth at elevated CO2, the homeostatic maintenance of specific respiratory rate per unit of mitochondria in response to high CO2 suggests that photorespiratory activity may play a small role on the long-term acclimation of respiration to elevated CO2. However, despite growth enhancement and as a result of the inhibition in cytochrome pathway activity by elevated CO2, total mitochondrial ATP production was decreased by plant growth at elevated CO2 when compared to ambient-grown plants. Because plant growth at elevated CO2 increased biomass but reduced respiratory machinery, activity, and ATP yields while maintaining O2 consumption rates per unit of mitochondria, we suggest that acclimation to elevated CO2 results from physiological adjustment of respiration to tissue ATP demand, which may not be entirely driven by nitrogen metabolism as previously suggested.
Rising atmospheric CO2 can have important effects on plant physiological processes, such as photosynthesis and respiration (Drake et al., 1997). At the global scale, these two processes are essential components of the carbon cycle, because 30% to 70% of the CO2 fixed by photosynthesis is released back to the atmosphere each year by plant respiration (Gonzalez-Meler et al., 2004; DeLucia et al., 2007). Thus, there is a need for better understanding on how tissue and whole-plant respiration rates are affected by elevated CO2 (i.e. from current ambient to doubling ambient levels), as small changes in terrestrial plant respiration could have a significant impact on the annual increment in atmospheric CO2 concentration (Valentini et al., 2000; Gonzalez-Meler et al., 2004; Schulze, 2006).
There is an apparent lack of consistency on the described effects of plant growth at elevated CO2 on leaf respiration rates as several studies have shown that these specific rates (area or mass basis) are either diminished, enhanced, or unaffected in plants grown at elevated CO2 (Amthor, 1991; Azcon-Bieto et al., 1994; Amthor, 1997; Drake et al., 1997; Poorter et al., 1997; Griffin et al., 2001; Davey et al., 2004; Gonzalez-Meler et al., 2004; Gonzalez-Meler and Taneva, 2005). In view of these results, it is unclear if the variety of long-term responses of respiration rates to plant growth at elevated CO2 represents a common underlying set of mechanisms or is based on species-specific responses. The described direct effects of CO2 on mitochondrial respiration and mitochondrial enzymes (Gonzalez-Meler et al., 1996; Bruhn et al., 2007) are not always translated to the tissue level (Gonzalez-Meler and Siedow, 1999; Davey et al., 2004; Gonzalez-Meler and Taneva, 2005). Therefore, respiratory responses to long-term growth at high CO2 may be more influenced by the integrated responses of carbon metabolism to elevated CO2, including processes such as photosynthesis, photorespiration, or energy demand from maintenance, growth, or phloem loading (Amthor, 1995; Gonzalez-Meler et al., 2004). Depending on the balance of these and other processes, acclimation of respiration (i.e. changes in respiratory machinery) and adjustment of respiratory rates in plants grown at elevated CO2 may occur independently (Gonzalez-Meler et al., 2004; Gonzalez-Meler and Taneva, 2005; Plaxton and Podestá, 2006).
Mitochondria are the cellular organelles ultimately responsible for oxidative phosphorylation but mitochondria are also involved in other metabolic processes (Krömer, 1995; Plaxton and Podestá, 2006). Investigations that include alterations of respiratory machinery, such as mitochondrial counts and mitochondrial enzymes, in addition to respiratory rates as influenced by growing conditions are lacking, specifically in the context of plant growth at elevated CO2 (e.g. Azcon-Bieto et al., 1994; Tissue et al., 2002). A few studies in leaves of C3 plants have shown that elevated CO2 can affect respiratory gene expression, enzyme content, and mitochondrial number when compared to ambient CO2 (e.g. Azcon-Bieto et al., 1994; Griffin et al., 2001; Ainsworth et al., 2006). In fact, total mitochondrial number can dramatically increase in plants exposed to elevated CO2, irrespective of the responses of respiration rates to high CO2 (Griffin et al., 2001, 2004; Tissue et al., 2002; Wang et al., 2004). If there is no direct relationship between total mitochondrial counts and tissue respiration rate, mitochondrial counts of C3 plants exposed to elevated CO2 may respond to factors other than production of ATP, such as generation of building blocks for growth and maintenance processes, changes in photorespiration, or alternative pathway rates or shifts in other anapleurotic functions, as these processes also require the mitochondrial compartment (Tjoelker et al., 1999; Bloom et al., 2002; Gonzalez-Meler et al., 2004; Plaxton and Podestá, 2006).
Decreases in photorespiratory activity of plants grown at high CO2 have been claimed as a significant factor contributing to the long-term acclimation of plant respiration to changes in atmospheric CO2, as photorespiration represents a major flow of mitochondrial metabolites at current CO2 levels (Amthor, 1997; Drake et al., 1999; Gonzalez-Meler et al., 2004). The fact that no clear acclimation of respiration rates and enzyme capacity has been reported for C4 plants grown at high CO2 (Azcon-Bieto et al., 1994; Drake et al., 1999; Davey et al., 2004) has strengthened the idea on the influence of photorespiration on mitochondrial features in C3 plants (Gonzalez-Meler et al., 2004). In contrast, obligate Crassulacean acid metabolism (CAM) plants grown at elevated levels of atmospheric CO2 could reduce photorespiratory activity during phase IV (last phase of the light period characterized by the fixation of exogenous CO2 via C3 photosynthesis), while having no effect during the decarboxylation metabolic phases when stomates are closed (Osmond, 1978; Lüttge, 2002). Unfortunately, information about the possible effects of growth at elevated CO2 on CAM respiratory metabolism is scarce (Drennan and Nobel, 2000).
In addition to tissue respiration rates and mitochondrial enzymes and counts, elevated CO2 can also affect the electron partitioning between the cytochrome and alternative pathways. Respiration through the alternative pathway bypasses at least two of the three sites of proton translocation, so respiration through this pathway does not support maintenance and growth processes as effectively as respiration through the cytochrome pathway (Vanlerberghe and McIntosh, 1997; Plaxton and Podestá, 2006). Elevated CO2 could differentially affect the activities of the alternative and cytochrome pathways without altering specific respiration rate, thereby affecting the efficiency at which substrate is converted into ATP (Gonzalez-Meler and Siedow, 1999; Gonzalez-Meler and Taneva, 2005). Changes in tissue composition, rates of respiration, or the relative contribution of alternative pathway to respiration can also establish the maintenance of respiratory energy demand as seen in plants grown at different temperature or various phosphorus supply (Gonzalez-Meler et al., 2001; Atkin and Tjoelker, 2003; Hartley et al., 2006). Respiratory acclimation to elevated CO2 may involve these different strategies in response to energy demand (ATP) by coupling (e.g. less alternative pathway) or uncoupling (e.g. more alternative pathway) respiration of photosynthate with rates of ATP formation.
In this study, we have investigated the long-term and acclimation responses of respiration rates to elevated CO2 in first-daughter cladodes of Opuntia ficus-indica, an invasive obligate CAM species in Mediterranean climate regions. It has been shown that this species enhances growth when exposed to elevated levels of atmospheric CO2 (Cui et al., 1993; Drennan and Nobel, 2000). Here, respiration rates and growth response observations have been combined with measurements of organelle counts, respiratory enzyme activities, and activities of the cytochrome and alternative pathways. For organelle measurements we used confocal microscopy to provide more replicated and accurate three-dimensional measurements of organelle density in whole cells of plants grown at either ambient or elevated CO2.
RESULTS
O. ficus-indica plant growth at elevated CO2 during 4 months increased second- and first-daughter cladode biomass production by 37% and 19%, respectively, when compared to control ones (Table I). Although 4- and 9-month plant growth at elevated CO2 did not result in a significantly higher total shoot biomass (i.e. basal cladode plus first-daughter cladode and second-daughter cladode biomass; 13%, P = 0.15), the biomass of new tissues produced at the elevated CO2 conditions significantly increased. The biomass of O. ficus-indica first-daughter cladodes increased by 20% when plants were exposed to elevated CO2 for 9 months when compared to the ambient counterparts (45.88 ± 2.60 g dry weight for ambient CO2 and 55.01 ± 2.12 g dry weight for elevated CO2, P = 0.04). Root biomass increased also by 20% in plants grown at elevated CO2 (P = 0.03; Table I). Plant growth at elevated CO2 had no effect on the leaf mass per unit area (LMA) of whole cladodes or of their photosynthetic tissue parts at all measured ages (Table I), despite the fact that LMA increased over time from 525 ± 13 for ambient and 545 ± 9 for elevated CO2 at 4 months of growth to 657 ± 15 g m−2 for ambient and 650 ± 19 g m−2 for elevated CO2 grown at 9 months of CO2 treatment. Our growth results are consistent with those obtained in other studies (Drennan and Nobel, 2000).
Table I.
Biomass and physiological parameters of O. ficus-indica plants grown at current ambient (A, 380 μg mL−1) or elevated (E, 780 μg mL−1) CO2 concentrations for 4 months in growth chambers
Total dry weight was obtained from second-, first-daughter, and basal cladodes; the rest of measurements are based on first-daughter cladodes developed during the CO2 treatments. The basal cladode mass was within 5% among all replicates after 4 months exposure to CO2 treatments. First-daughter cladode biomass in greenhouse-grown plants was also enhanced by 23% (P = 0.02) when plants were exposed to elevated CO2 (23.4 ± 1.8 g) when compared to the ambient ones (28.7 ± 1.0 g). Values shown are means ± se of at least five to nine replicates. Asterisk (*) denotes statistically significant differences between the two CO2 treatments (P < 0.05).
| Biomass and Physiological Parameters | CO2 during Growth
|
E/A | |
|---|---|---|---|
| A | E | ||
| Total dry weight (g plant−1) | |||
| Second-daughter cladode | 4.43 ± 0.16 | 6.54 ± 0.22* | 1.37* |
| First-daughter cladode | 19.2 ± 0.8 | 22.8 ± 0.8* | 1.19* |
| Total shoot | 68.1 ± 3.3 | 76.8 ± 3.4 | 1.13 |
| Root | 8.9 ± 0.8 | 10.7 ± 0.3* | 1.20* |
| Leaf mass area (g m−2) | |||
| Cladode segment | 525 ± 13 | 545 ± 9 | 1.04 |
| Photosynthetic tissue | 388 ± 5 | 377 ± 5 | 0.97 |
| Carbon (% dry weight) | |||
| Cladode segment | 36.2 ± 0.3 | 36.2 ± 0.4 | 1.00 |
| Photosynthetic tissue | 35.7 ± 0.5 | 35.5 ± 0.2 | 0.99 |
| Nitrogen (% dry weight) | |||
| Cladode segment | 1.61 ± 0.12 | 1.50 ± 0.09 | 0.93 |
| Photosynthetic tissue | 1.44 ± 0.07 | 1.31 ± 0.06 | 0.91 |
| Stomatal density (stomata mm−2) | 23.3 ± 0.4 | 19.1 ± 0.60* | 0.82* |
| Stomatal cell index (stomata photosynthetic cell−1) | 0.38 ± 0.01 | 0.32 ± 0.01* | 0.86* |
Tissue nitrogen concentration was analyzed on O. ficus-indica first-daughter cladodes grown at either ambient or elevated CO2 (Table I) as it is often seen to decrease when plants are exposed to elevated CO2 (Drake et al., 1997). However, total nitrogen concentration in tissues of first-daughter cladodes of O. ficus-indica exposed to elevated CO2 did not change significantly (7%–9%) both in cladode and in photosynthetic tissue segments, when compared to ambient-grown plants (P = 0.054 and 0.16, respectively; Table I). Long-term exposure of plants to elevated CO2 reduced stomatal density by 18% and stomatal index by 14% with respect to ambient plants (Table I), a common feature of plant growth at elevated CO2 (Drake et al., 1997; Drennan and Nobel, 2000).
Malate content on photosynthetic and nonphotosynthetic tissue segments of O. ficus-indica first-daughter cladodes was analyzed as a reference of CAM activity in response to the CO2 treatment. Our results showed that malate content increased for phase II, III, and IV in nonphotosynthetic tissues of plants grown at elevated CO2 (Table II). However, CO2 growth conditions did not alter malate levels (Table II) in photosynthetic tissues as previously described (Wang and Nobel, 1996). In both CO2 treatments, malate content was higher in nonphotosynthetic tissue (or water storage tissue) than in photosynthetic tissue, perhaps reflecting turgor maintenance in the water storage cells by osmotic adjustment (Smith and Lüttge, 1985; Smith et al., 1987; Goldstein et al., 1991).
Table II.
Malate content in whole, photosynthetic, and nonphotosynthetic tissue segments of first-daughter cladodes of O. ficus-indica plants grown at either ambient (A, 380 μg mL−1) or elevated (E, 780 μg mL−1) atmospheric CO2 concentration
Samples were collected after 4 months of CO2 treatment. Values are means ± se of three to seven replicates. Asterisk (*) denotes statistically significant differences between the two CO2 treatments (P < 0.05).
| Phase | CO2 during Growth | Malate Content
|
|
|---|---|---|---|
| Photosynthetic Tissue | Nonphotosynthetic Tissue | ||
| mmol malate g−1 dry weight | |||
| II | A | 1.69 ± 0.13 | 3.06 ± 0.12 |
| E | 1.71 ± 0.02 | 3.59 ± 0.06* | |
| III | A | 1.55 ± 0.07 | 3.01 ± 0.1 |
| E | 1.54 ± 0.80 | 3.61 ± 0.18* | |
| IV | A | 1.15 ± 0.06 | 2.56 ± 0.10 |
| E | 1.21 ± 0.04 | 3.12 ± 0.23* | |
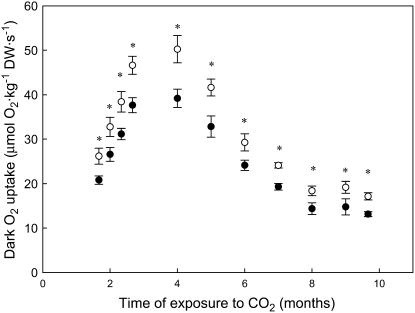
Dark respiration (oxygen uptake) rates expressed on a dry mass basis and measured at different developmental stages were reduced by 20% in cladode segments of first-daughter cladodes of O. ficus-indica plants grown at elevated CO2 when compared to ambient CO2-grown plants (Fig. 1; Table III). This reduction in respiration rates was also observed when rates were measured at two different measurement temperatures (20°C and 30°C) in O. ficus-indica plants grown at elevated CO2 for 4 months when compared to those exposed to ambient CO2 (Table III). In photosynthetic tissues of the first-daughter cladodes, elevated CO2 during growth also reduced dark oxygen uptake rates by 29%, compared to the 15% inhibition in the nonphotosynthetic tissue of the cladodes (Table III). Respiration rates observed in first-daughter cladodes were similar to those of aerial tissues of other CAM species (Adams et al., 1986a, 1986b).
Figure 1.
Dark oxygen uptake rates of cladode segments tissues of O. ficus-indica plants grown at either current ambient (○, 380 μg mL−1) or elevated (•, 780 μg mL−1) CO2 concentrations during ontogeny. Measurements were done at a common CO2 concentration. Values shown are means ± se of 10 to 15 replicates. Asterisk (*) denotes statistically significant differences between the two CO2 treatments (P < 0.05).
Table III.
Dark respiration rates of whole-cladode, photosynthetic, and nonphotosynthetic tissue segments of first-daughter cladodes of O. ficus-indica plants grown at either current ambient (A, 380 μg mL−1) or elevated (E, 780 μg mL−1) CO2 concentration
Cladode segments included the photosynthetic and nonphotosynthetic tissue. Measurements were made at 20°C and/or 30°C (T) at a common CO2 concentration. Values are means ± se of 10 to 13 replicates. Asterisk (*) denotes statistically significant differences between the two CO2 treatments (P < 0.05).
| Tissue Segments | T | Time in CO2 | Dark O2 Uptake
|
E/A | |
|---|---|---|---|---|---|
| A | E | ||||
| °C | months | μmol O2 kg−1 dry weight s−1 | |||
| Whole-cladode segment | 20 | 4 | 34.5 ± 2.06 | 27.5 ± 1.50* | 0.80* |
| 30 | 4 | 50.2 ± 3.07 | 39.2 ± 2.04* | 0.78* | |
| Photosynthetic tissue | 30 | 4 | 44.5 ± 1.57 | 31.7 ± 2.22* | 0.71* |
| 30 | 9 | 13.8 ± 0.80 | 10.1 ± 0.56* | 0.73* | |
| Nonphotosynthetic tissue | 30 | 4 | 52.2 ± 4.60 | 44.4 ± 2.05* | 0.85* |
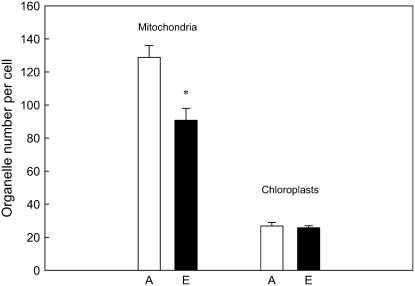
Plant growth at elevated CO2 resulted in a marked reduction (30%) in the number of functional mitochondria per cell in photosynthetic tissue when compared to the ambient CO2-grown plants (Figs. 2 and 3). In contrast, the number of functional chloroplast per cell was not changed by the CO2 treatment (Figs. 2 and 3). As a result, the mitochondrion-to-chloroplast ratio was reduced from 5 at ambient to 3.5 at elevated CO2 conditions (Fig. 2). The elevated CO2-induced relative reduction in active mitochondrial counts per unit of cell was independent of cladode age or length of exposure to elevated CO2 (Table IV). The observed organelle changes were not caused by other cell effects, as cell area was unaffected by the CO2 treatment (Table V). Finally, elevated CO2 during growth did not affect circularity index (i.e. mitochondrial axial dimensions; Table V).
Figure 2.
Number of mitochondria and chloroplasts per unit of cell in photosynthetic tissue segments of cladodes grown at either current ambient (A, 380 μg mL−1) or elevated (E, 780 μg mL−1) CO2 concentrations for 4 months. Values shown are means ± se of 11 to 13 plants for mitochondria and 9 to 12 plants for chloroplast counts. Asterisk (*) denotes statistically significant differences between the two CO2 treatments (P < 0.05).
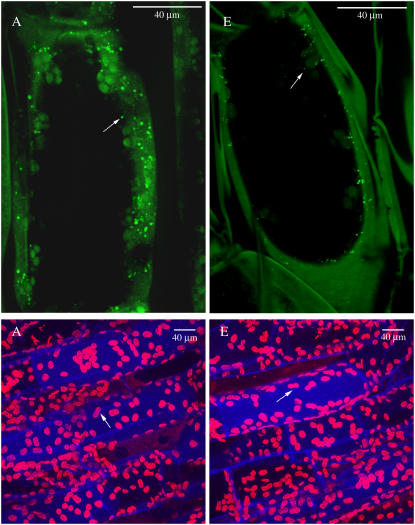
Figure 3.
Selected representative confocal images of photosynthetic cells showing mitochondria and chloroplasts of photosynthetic tissue cells of first-daughter cladodes grown at either current ambient (380 μg mL−1, A) or elevated (780 μg mL−1, E) CO2 concentrations. Shown cells are the result of the z axis projection of image slices scanned taken in intervals of 1 μm. Mitochondria and chloroplasts are indicated by arrows.
Table IV.
Number of mitochondria per cell of photosynthetic tissue of O. ficus-indica plants grown at current ambient (A, 380 μg mL−1) or elevated (E, 780 μg mL−1) CO2 concentrations during ontogeny
Values shown are means ± se (n = 7–13). Mean values for 4 months are presented in Figure 2. Asterisk (*) denotes statistically significant differences between the two CO2 treatments (P < 0.05).
| CO2 during Growth | Time of Exposure to CO2 (months)
|
|||
|---|---|---|---|---|
| 4 | 5 | 7 | 9 | |
| A | 129 ± 8 | 105 ± 3 | 83 ± 3 | 43 ± 2 |
| E | 91 ± 7* | 75 ± 4* | 55 ± 3* | 28 ± 2* |
Table V.
Photosynthetic cell area and mitochondrial size of first-daughter cladodes of O. ficus-indica plants grown at either current ambient (A, 380 μg mL−1) or elevated (E, 780 μg mL−1) CO2 concentrations for 4 months
Values shown are means ± se of 53 to 73 measurements of five plants. Values were not significantly different between the two CO2 treatments (P < 0.05).
| Organelle Parameters | CO2 during Growth
|
|
|---|---|---|
| A | E | |
| Photosynthetic cell area (μm2) | 16,202 ± 527 | 16,956 ± 576 |
| Mitochondrial size (μm2) | 0.23 ± 0.01 | 0.24 ± 0.01 |
Cytochrome c oxidase maximum activity reflects changes in the respiratory machinery related to oxidative phosphorylation. Cladode extracts of plants grown at elevated CO2 had a 30% reduction in the maximum activity of cytochrome c oxidase when compared to the ambient ones (Table VI). In both CO2 treatments, the maximum activity of cytochrome c oxidase was 2 times higher than the specific dark respiration rates from the same tissues (Table III and VI), denoting enough enzyme to support observed oxygen uptake rates (Table III).
Table VI.
Cytochrome c oxidase maximum activity of first-daughter cladodes of O. ficus-indica plants grown at either current ambient (A, 380 μg mL−1) or elevated (E, 780 μg mL−1) CO2 concentrations for 4 months
Measurements were made at 30°C and whole-cladode segments included the photosynthetic and nonphotosynthetic tissue. Values are means ± SEM of five to eight replicates. Asterisk (*) denotes statistically significant differences between the two CO2 treatments (P < 0.05).
| CO2 during Growth | Cytochrome c Oxidase Activity |
|---|---|
| μmol O2 kg−1 dry weight s−1 | |
| A | 107.2 ± 6.5 |
| E | 71.9 ± 3.7* |
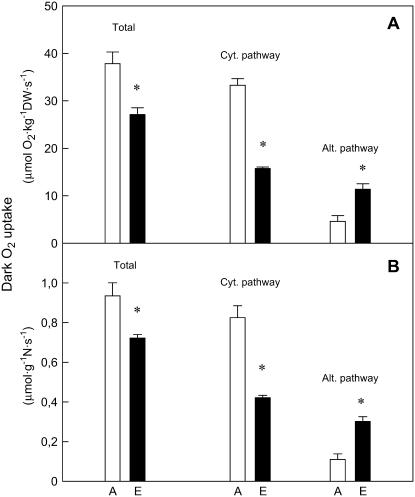
The in vivo activities of the cytochrome and alternative pathways of respiration are shown in Figure 4. Tissue segment sampling for oxygen fractionation during respiration experiments was during deacidification phase, when alternative pathway activity was shown to be more stable (Robinson et al., 1992). Oxygen isotope discrimination during respiration significantly increased in photosynthetic tissues of plants grown under elevated CO2 when compared to the ambient CO2 ones (19.9% ± 0.1% for ambient CO2 and 21.6% ± 0.1% for elevated CO2), indicating that the proportion of respiration through the nonphosphorylating alternative pathway increased as a consequence of plant growth at elevated CO2. As a result, the cytochrome pathway activity of photosynthetic tissues of plants grown at high CO2 was reduced by about 50% when compared to the control plants (Fig. 4A). In contrast, elevated CO2 significantly increased the activity of the alternative pathway in photosynthetic tissue by 2.5-fold when compared to ambient CO2-grown plants (Fig. 4A). The reduction in total respiratory rates and in the activity of the cytochrome pathway were also seen in plants grown at high CO2 when rates were expressed on tissue nitrogen basis (23% and 49%, respectively; Fig. 4B).
Figure 4.
Total, cytochrome (cyt.pathway) and alternative pathway (alt.pathway) respiration rates on a dry mass (A) and nitrogen (B) basis in photosynthetic tissue of first-daughter cladodes of O. ficus-indica plants grown at either ambient (A, 380 μg mL−1) or elevated CO2 (E, 600 μg mL−1) for 4 months. The discrimination value during respiration was 19.9% ± 0.1% for ambient CO2 and 21.6% ± 0.1% for elevated CO2. Oxygen isotope fractionation by SHAM-resistant respiration (i.e. cytochrome pathway discrimination) for both ambient and elevated CO2 was 19.2% ± 0.1%; oxygen isotope fractionation by cyanide-resistant respiration (i.e. alternative pathway discrimination) was 25% ± 0.1% for both CO2 treatments. KCN-resistant rates were 27.8 ± 3.1 and 33.4 ± 2.1 μmol O2 kg−1 dry weight s−1 for ambient and elevated CO2-grown plants, respectively; SHAM-resistant rates were 33.5 ± 3.0 and 25.3 ± 1.8 μmol O2 kg−1 dry weight s−1, for ambient and elevated CO2-grown plants. Residual respiration rates (i.e. SHAM and KCN simultaneously applied) were less than 8% of the uninhibited rate in all cases. Measurements were made at 25°C. Values are means ± SEM of three to four replicates. Asterisk (*) denotes statistically significant differences between the two CO2 treatments (P < 0.05).
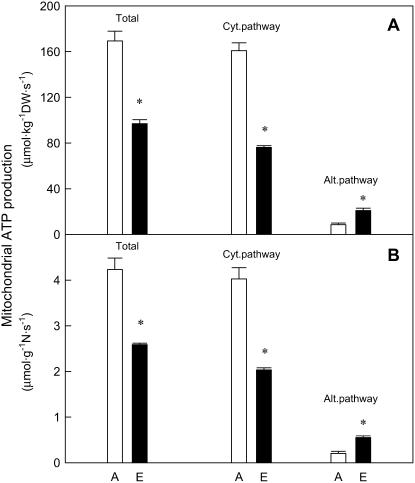
Growth at elevated CO2 reduced the calculated mitochondrial ATP production rates per unit of dry mass of photosynthetic tissue by 43% when compared to ambient CO2 counterparts (Fig. 5A). This was due to a 53% reduction in ATP synthesis via the cytochrome pathway that could not be compensated by a 2.5-fold increase in ATP yields via the alternative pathway in plants grown at elevated CO2 when compared with those grown at ambient CO2 (Fig. 5A). The contribution by the alternative pathway to total ATP yields increased from 5% at ambient to 21% at elevated CO2 conditions (Fig. 5A). Mitochondrial ATP production rates on a nitrogen concentration basis (measured in the same tissues as respiration and oxygen isotope discrimination) decreased by 38% in photosynthetic tissues of plants grown at elevated CO2 when compared to ambient CO2-grown plants (Fig. 5B). The increment of mitochondrial ATP production on tissue nitrogen basis via the alternative pathway could not compensate the decrease of mitochondrial ATP production via cytochrome pathway in response to elevated CO2 (Fig. 5B).
Figure 5.
Mitochondrial ATP production via total, cytochrome pathway, and alternative pathway in photosynthetic tissue of first-daughter cladodes of O. ficus-indica plants grown at either ambient (A, 380 μg mL−1) or elevated CO2 (E, 600 μg mL−1) for 4 months expressed either on a dry mass (A) and nitrogen (B) basis. Rates of ATP synthesis were obtained using the measured dry mass and nitrogen values for the same samples used for respiration and oxygen fractionation measurements. Measurements were made at 25°C. Values are means ± SEM of three to four replicates. Asterisk (*) denotes statistically significant differences between the two CO2 treatments (P < 0.05).
DISCUSSION
Long-Term Effects of Elevated CO2 on Respiration Rate
In this study, we analyzed long-term indirect and acclimation effects of respiration in an invasive CAM plant grown at elevated CO2 in relation to ontogeny and atmospheric CO2-induced alterations in growth, tissue nitrogen composition, and respiratory machinery. Although plant growth at elevated CO2 resulted in a consistent 20% to 30% reduction of respiration (Table III; Fig. 1), elevated CO2 increased first-, second-daughter cladode, and root biomass of O. ficus-indica (Table I) similar to biomass increases seen in other studies (Cui et al., 1993; Drennan and Nobel, 2000).
The inhibitory long-term effect of elevated CO2 on the rate of respiration was consistent throughout the 9 months of the study, despite the 3-fold variation in respiration rates seen with tissue age (Fig. 1). The magnitude in the reduction of dark respiration rates observed in O. ficus-indica first-daughter cladodes grown and developed in elevated CO2 (Table III; Fig. 1) was similar to those seen in C3 plants exposed to elevated CO2 (Amthor, 1997; Drake et al.,1997; Gonzalez-Meler et al., 2004). Ontogenic effects on respiration are large as energy demand for growth and maintenance processes vary at the tissue and whole-plant levels (Van der Werf et al., 1988; Amthor, 2000; Noguchi et al., 2001; Bouma, 2005). Specific costs for maintenance processes can also decline over time, often reflected by the decrease in nitrogen concentration with tissue age (Amthor, 1994; Carey et al., 1996). The reduction of respiration seen in plant tissues grown at elevated CO2 (Table III) may reflect a lower respiratory energy demand throughout the growing cycle of the cladodes, as it has been postulated for other species (Poorter et al., 1997; Amthor, 2000; Gonzalez-Meler et al., 2004). In some cases, reductions in respiration rate of plants grown at high CO2 are attributed to changes in LMA of tissues induced by accumulation of carbohydrates (Poorter et al., 1997; Curtis and Wang 1998), or to alterations in tissue chemistry, such as lower nitrogen concentration (Amthor, 1994; Wullschleger et al., 1995; Curtis and Wang, 1998; Drake et al., 1999; Gonzalez-Meler et al., 2004). However, neither LMA nor nitrogen concentration were significantly altered in tissues of first-daughter cladodes grown at high CO2 when compared to ambient plants (Table I), suggesting that the reduction seen in specific respiration rates of plants grown at high CO2 (Table III; Fig. 1) was due to other factors.
Long-Term Effects of Elevated CO2 on Respiratory Machinery
Changes in respiration rates in aging cladodes correlated well with concomitant changes in the number of metabolically active mitochondria per cell (Fig. 1; Table IV). Moreover, plant growth at elevated CO2 also resulted in a roughly 30% reduction in the active mitochondrial number per cell in photosynthestic tissues along the 9 months of the study (Table IV), closely matching the proportional reduction of respiration rates of the same tissues with age and growth CO2 (Table III). In contrast, chloroplast number per cell were unaltered regardless of the CO2 treatment (Fig. 2), suggesting that mitochondrial counts can change independently of chloroplasts, as it has been shown in C3 plants (Logan and Leaver, 2000).
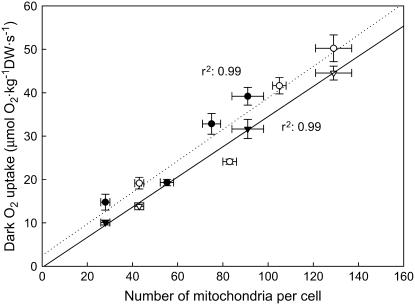
Other studies have observed that respiratory responses to elevated CO2 are not always coupled to changes in mitochondrial numbers or respiratory machinery (Gonzalez-Meler et al., 2004). For instance, Griffin et al. (2001, 2004), Tissue et al. (2002), and Wang et al. (2004) showed reductions in C3 leaf-specific respiration rates grown at elevated CO2 that were accompanied by increases in the number of total mitochondria per unit of cell area (analyzed using transmission electron microscopy [TEM]). This discrepancy between our results and those found in literature may indicate that respiration rates are more coupled to mitochondrial machinery in CAM plants than in C3 plants. Mitochondria of C3 plants are required to supply a large amount of reductant and carbon skeletons for the production of primary and secondary metabolites involved in photorespiration or other anaplerotic processes that may uncouple mitochondrial activity from the synthesis of ATP (Gonzalez-Meler et al., 2004; Plaxton and Podestá, 2006). However, the anaplerotic functions of mitochondria may be common in C3 and CAM plants, albeit different in magnitude, resulting in an unlikely explanation for the differential results between the C3 and CAM plants. The discrepancies in mitochondrial counts between C3 and CAM plants can also be explained by the different methodological approaches used in these studies. Here we counted only functionally active mitochondria that may be more coupled to respiration activity, as oppose to total number of mitochondria as explained above. In addition, the confocal microscope technique eliminates interferences between other organelles, such as chloroplasts, providing mitochondrial counts in a whole-cell view, a limitation of TEM approaches. This is especially important in view that elevated CO2 will likely alter mitochondrial association with organelles in C3 plants in response to changes in photorespiratory rates (Logan and Leaver, 2000). The discrepancy between total versus active number of mitochondria in plants grown at elevated CO2 conditions when compared to ambient plants can be an indication that mitochondrial biogenesis and/or turnover may be afftected by plant exposure to elevated CO2. Interestingly, Tissue et al. (2002) found that a 73% increase in total mitochondrial counts in Liquidambar styraciflua grown at elevated CO2 did not change respiration rates nor the maximum activity of cytochrome c oxidase. Changes in cytochrome c oxidase and functional mitochondrial counts found in this study (Fig. 2; Table VI) suggest that cytochrome c oxidase is a better indicator of metabolically active mitochondria and respiration rates (Fig. 6) than the total number of mitochondria measured using TEM.
Figure 6.
Estimated relationship between dark respiration rates and mitochondrial number in photosynthetic tissue (triangles) and cladode segments (circles) of first-daughter cladodes of O. ficus-indica plants grown at either ambient (○, ▵, 380 μg mL−1) or elevated (•, ▴, 780 μg mL−1) CO2 concentrations. Data on respiration rates are presented in Table III (for photosynthetic tissue) and Figure 1 (for cladode segment); data on mitochondrial counts are presented in Figure 2 and Table IV. Regression line for photosynthetic tissue (solid line) and cladode segment (dotted line) are also represented. Regression equation was y = 0.35x + 0.28 and y = 0.36x + 2.50 for photosynthetic tissue and cladode segment, respectively.
Our results show that changes in functional mitochondrial number in plants grown at elevated CO2 were associated with concomitant changes in the maximum activity of cytochrome c oxidase (Tables IV and VI). In fact, variation in respiration rates during ontogeny (Fig. 1) in plants grown at either ambient or elevated CO2 were correlated with changes in mitochondrial number (Table IV) and maximum activity of cytochrome c oxidase (Table VI). Azcon-Bieto et al. (1994), Hrubeck et al. (1985), and Tissue et al. (2002) also reported a correlation between the maximum activity of cytochrome c oxidase activity and respiration rates in trees, crops, sedges, and C3 and C4 grasses. These results suggest that the capacity of respiration is tightly regulated in plants (as suggested elsewhere; Gonzalez-Meler and Siedow, 1999; Atkin and Tjoelker, 2003) to maintain certain homeostasis between the capacity of respiration (mitochondrial counts, cytochrome c oxidase activity) and tissue-specific respiration rates, at least with respect to changes in atmospheric CO2 concentration.
In our study, the proportion of reduction in cytochrome c oxidase or active mitochondrial number matched that of respiration rates between plants grown at high and ambient CO2 (Tables IV and VI; Fig. 6), suggesting that the specific respiratory activity per unit of mitochondria was not affected by the CO2 treatment. The respiratory activity per unit of mitochondria can be calculated by using the data in Tables I to V (LMA, respiration rates, functional mitochondrial counts, and photosynthetic cell area) for ambient and elevated CO2. The calculated specific respiratory activity per unit of mitochondria in photosynthetic tissues of 4-month-old O. ficus-indica cladodes was 2.2 nmol O2 mitochondria−1 s−1 in both ambient and elevated CO2 treatments. To our knowledge, our results provide the first evidence for a homeostatic maintenance of mitochondrial-specific respiratory activity in response to a treatment in intact plant tissues.
The maintenance of homeostatic-specific oxygen uptake activity of mitochondria in response to the CO2 treatment suggests that the expected CO2-induced reduction in photorespiratory activity may have little impact on the reduction of respiratory machinery in plants grown at elevated CO2. Photorespiration becomes significant in CAM plants during phase IV due to changes in CO2/O2 balance as a consequence of malate exhaustion and stomatal opening (Lüttge, 2002). Reduced photorespiration rates in plants exposed to elevated CO2 could then reduce the need for the mitochondrial compartment in CAM plants, contributing to the long-term acclimation of plant respiration to elevated CO2 (as suggested for C3 plants in Amthor, 1997; Drake et al., l999; Gonzalez-Meler et al., 2004). Because malate concentration did not increase during phase IV in photosynthetic tissues (Table II), phase IV photorespiratory activity may be proportionally affected by changes in atmospheric CO2 in CAM plants. However, our results also showed that the magnitude in the reduction of cytochrome c oxidase activity and in active mitochondrial number was similar (about 30%) in plants grown at elevated CO2 when compared to the ambient CO2 ones, and that these reductions correlated to decreases in respiration rates (Tables III and VI; Fig. 2). Therefore, the active mitochondrial compartment seem to provide sufficient capacity to carry out the photorespiratory cycle during phase IV, maybe by increasing the concentration of photorespiratory enzymes in mitochondria as suggested in Armstrong et al. (2006). Our data also suggests that changes in respiratory active mitochondria in response to CO2 in O. ficus-indica are perhaps dominated by growth and maintenance processes.
Long-Term Effect of Elevated CO2 on Respiratory Pathways and ATP Yields
In contrast to the overall down-regulation of respiratory rates and machinery in plants grown at elevated CO2, the capacity of the alternative pathway (measured as KCN-resistant respiration, see Lennon et al., 1997; Ribas-Carbo et al., 2000; Gonzalez-Meler et al., 2001) increased in plants grown at high CO2 compared to control ones (Fig. 4 legend). The activity of the alternative pathway (measured with oxygen isotopes) also increased in plants grown at high CO2, although such increase in activity could not compensate the overall inhibition of the cytochrome pathway seen in plants grown at high CO2 when compared to the ambient ones (Fig. 4). It has been postulated that when the cytochrome pathway activity is restricted or saturated the alternative pathway will become more active (Vanlerberghe and McIntosh, 1997; Millenaar et al., 1998; Lambers et al., 2005). An enhanced activity of the alternative pathway under these conditions could reduce the endogenous generation of oxygen radical species from an overreduced ubiquinone pool (Maxwell et al., 1999; Lambers et al., 2005). However, some studies have shown that restricted activity of the cytochrome pathway in response to stress has not been always accompanied by increases in alternative pathway activity (Lennon et al., 1997; Gonzalez-Meler et al., 1999; Gonzalez-Meler et al., 2001). Because plant growth was enhanced at high CO2 in O. ficus-indica treatment and there was no observational evidence of a CO2 treatment-induced stress, our results indicate that an increased basal activity of the alternative pathway cannot always be related to a stress response.
Higher new biomass production in plants exposed to elevated CO2 was sustained with lower ATP yields when compared to ambient-grown plants (Table I; Fig. 5). The reduction in ATP yields was explained by the inhibition of the cytochrome pathway activity in elevated CO2 growing conditions, despite increases in ATP formation via the alternative pathway (Fig. 5A). Our results seem to support the idea that first-daughter cladodes of plants grown at elevated CO2 have reduced tissue maintenance and growth costs, thereby lowering the respiratory energy demand (Wullschleger et al., 1992; Wullschleger and Norby, 1992; Amthor, 1994; Carey et al., 1996; Griffin et al., 1996; Dvorak and Oplustilova, 1997; Will and Ceulemans, 1997; Wullschleger et al., 1997). It has been suggested that maintenance costs related to protein turnover, carbohydrate translocation, or ion gradient maintenance account for most of the respiratory energy demand of tissues (Bouma et al., 1994). Reductions in nitrogen concentration can also decrease energy demand for maintenance processes (Amthor, 1994; Carey et al., 1996). The marginal decrease seen in nitrogen concentration of cladodes grown at elevated CO2 compared to the ambient ones (Table I) could not account for all of the reduction seen in energy production as ATP yields per unit nitrogen mass were still lower in plants grown at elevated CO2 (Fig. 5B). ATP yields per unit nitrogen mass were held constant in nonstressed fully expanded leaves of Nicotiana tobaccum grown at different phosphorus supply (Gonzalez-Meler et al., 2001) or of Cornus florida grown at different atmospheric CO2 levels (Gonzalez-Meler and Taneva, 2005). These observations lead to the suggestion that acclimation responses of respiration to changes in environmental conditions in fully grown tissues are driven by changes in energy demand. When energy demand is not met by respiration, metabolic and growth stress can become apparent (Gonzalez-Meler et al., 2001; Gonzalez-Meler and Taneva, 2005). Following this rationale, and due to the fact that plant growth was enhanced in O. ficus-indica in response to high CO2 exposure, our results suggest that metabolic costs other than nitrogen-related processes (e.g. protein turnover, nitrogen compounds synthesis) were involved in reducing the energy demand of tissues of plants grown at elevated CO2 compared to the ambient ones.
In summary, respiration of the Mediterranean invasive O. ficus-indica was reduced in cladodes developed and grown at elevated CO2 conditions. The high CO2-induced reduction in the cladode and photosynthetic tissue respiration rates were sustained during tissue ontogeny. Contrary to some tree species (Griffin et al., 2001; Tissue et al., 2002), both the decrease in respiration rates during ontogeny and the CO2 reduction of respiration were positively correlated with the number of active mitochondria and the maximum activity of cytochrome c oxidase. Our results also showed that certain homeostasis is maintained between mitochondrial counts and respiration rates irrespectively of the CO2 treatment. This relationship was corroborated by the fact that the specific O2 uptake activity per unit of mitochondria was not affected by the CO2 treatment. However, the relative contribution of the mitochondrial respiratory pathways was altered by plant growth at elevated CO2. The activity and capacity of the alternative pathway were up-regulated in plants grown at high CO2 when compared to the ambient plants without evidence of physiological stress. The reduction of respiration in plants grown at elevated CO2 compared to ambient ones resulted from a lower energy demand from tissues not necessarily related to nitrogen costs or nitrogen metabolism.
MATERIALS AND METHODS
Plant Materials and Growth Conditions
Mature cladodes of Opuntia ficus-indica Miller were collected from the Marimurtra Botanical Garden, Blanes (Spain) and from the native region of Santa Margarita Ecological Reserve Flora, California. Basal cladodes were transplanted vertically in peat:vermiculite:perlite mixture (2:1:1) placed in 9 to 12 L plastic pots with one-third of their area below the soil surface and grown in environmental controlled growth chambers (2.32 m3 each one) or greenhouse rooms (approximately 183 m2 each one). The initial basal cladode biomass varied less than 3% among plants with the same mean and variance among treatments. Opuntia ficus-indica cladodes are modified stems with photosynthetic capacity and include two types of tissues: the photosynthetic tissue includes the cuticle, epidermis, hypodermis, and chlorenchyma cells; the internal nonphotosynthetic tissue is mainly a water reservoir.
Cladodes were grown at either current ambient (380 ± 30 μL L−1 in the growth chambers and the greenhouse room) or elevated CO2 (780 ± 20 μL L−1 in growth chambers or 600 ± 40 μL L−1 in the greenhouse room). Pure CO2 was supplied to the enriched chambers through flowmeters that were periodically adjusted to control the flow rate of CO2 (around 0.8 L min−1) and injected directly into the blower, ensuring thorough mixing with ambient air before entering the chamber. The injection and mixing systems were designed to minimize CO2 leakage into the ambient rooms. Air from the elevated rooms was sampled every 3 to 5 min for CO2 concentration and analyzed using either a VAISALA (Helsinki) or S151 infrared gas analyzer (IRGA, Qubit Systems). The CO2 concentration controlling systems were calibrated at least weekly.
Day/night temperatures were set at 25°C/20°C in chambers and 25°C/15°C for greenhouse rooms with a 12/12 h photoperiod and a photosynthetic photon flux density (measured on a horizontal surface at the top of the cladodes) of 450 to 500 μmol m−2 s−1 for growth chambers and 800 to 850 μmol m−2 s−1 for the greenhouse (supplemented by artificial lighting). Plants were watered at least twice a week with half-strength Hoagland solution. Plants were moved within rooms and among chambers weekly to minimize pseudoreplication issues.
Biomass, Carbon, and Nitrogen Analyses
First-daughter cladodes (developed and grown at the experimental conditions in greenhouse or growth chambers) were harvested for full biomass analyses at 4 and 9 months of growth at either ambient or elevated CO2. Collected plant material was separated between roots and shoots and dried in an air-forced oven at 70°C until no further weight change was measured. Dry samples were ground for total carbon and nitrogen concentration analysis using an elemental analyzer equipped with a zero-blank autosampler located at University of Illinois at Chicago (UIC; Costech Analytical).
Intermediate harvests were done to measure tissue parameters throughout the growth cycle at 1.5, 2, 3, 4, 7, and 9.5 months after treatment was implemented. For LMA measurements only the projected surface of the cladode was considered.
Stomatal Number
Optical microscopy on first-daughter cladodes grown in growth chambers was performed using a Olympus CHS according to Teare et al. (1971). Stomata was then observed at 40× and each final stomatal frequency was the mean of five randomly chosen microscopic fields for each replicate. Then using the two surfaces of the cladode, we calculated: (1) stomatal density according to Cui et al. (1993) and (2) stomatal index according to Peñuelas and Matamala (1990), but instead of expressing stomatal index by number of stomata/number of epidermical cells we made number of stomata/number of photosynthetic cells. This approach was taken because O. ficus-indica tissues are especially bulky with a very thick photosynthetic tissue that can represent about 60% of the whole-cladode segment weight.
Malate Content
Malate concentration in tissue segments of first-daughter cladodes collected from plants grown at either ambient or elevated CO2 in growth chambers were measured using a Waters 600 high performance liquid chromatograph (Waters Millipore) as described in Nogues et al. (2006). Plant samples were collected during the CAM's phase II (early morning phase characterized by CO2 fixation via PEP carboxylase and Rubisco), III (midday phase when CO2 proceeds from malate decarboxylation and fixation occurs via Rubisco), and IV and tissues sectioned using a thin blade. Samples were immediately frozen with liquid nitrogen and then stored at the freezer at −80°C until measurements were done. For malate extraction, plant samples were lyophilized and then ground to a fine powder (<10 μm). About 50 mg of the fine powder was suspended with 1 mL of distilled water in an Eppendorf tube (Eppendorf Scientific) mixed and centrifuged at 12,000g for 5 min at 5°C. After centrifugation, the supernatant was transferred to a new Eppendorf tube and then heated to 100°C for 3 min. Samples were placed on ice for 3 min and then centrifuged again at 12,000g for 5 min at 5°C. The supernatant was used for malate content analysis setting the HPLC UV detector at 214 nm. Samples were eluted through a column (Aminex HPX-87H, 300 mm × 7.8 mm) with 4 mm H2SO4 at a flow rate of 0.5 mL min−1 and 27 min retention time at 35°C. The recovery malate fraction after the extraction process was about 98%. To calculate malate content in tissues we also cochromatographed known concentrations of l-malic acid obtained commercially (Sigma-Aldrich).
Respiration Measurements
Dark O2 uptake rates of tissue segments of first-daughter cladodes collected from plants grown at either ambient or elevated CO2 in growth chambers were measured using a liquid-phase Clark-type oxygen electrode (Rank Brothers). Plant samples were collected during the light period and sectioned using a thin blade. Trial studies on dark showed that wound effects were negligible when sections were at least 2 mm thick and rates were measured after 10 to 20 min of sampling (data not shown). Tissue segments were first infiltrated in a Kitasato flask with 30 mm MES buffer + 0.2 mm CaCl2 (pH 6.2), put 45 to 50 min in the dark, and then washed three times before being introduced into the 6 mL Perspex cuvette with the same buffer (Gomez-Casanovas, 2006). To avoid oxygen-limiting conditions inside the cuvette, all measurements terminated before O2 reached about 50% to 60% of air saturation levels.
Analysis of Cell and Organelle Parameters
For mitochondrial counts, confocal microscopy was carried out on first-daughter cladodes grown in growth chambers using a Olympus IX70/Fluoview confocal laser-scanning microscope (Olympus) in fluorescent mode with a PlanApo 60×/1.4 oil immersion objective. Active mitochondria were stained by using Rhodamine 123 (Sigma-Aldrich), which selectively accumulates in mitochondria based on the membrane potential (Petit, 1992). Fresh tissue samples were incubated for 12 min in 250 mm Rhodamine 123 (Sigma Aldrich) at 37°C in the dark (previous trial studies indicated that less incubation time stained less brightly mitochondria and more incubation time quickly toxified tissues, and the staining rapidly disappeared). Excess dye was eliminated by washing the discs three times (2 min each) in 30 mm MES buffer + 0.2 mm CaCl2 at ambient temperature (Matzke and Matzke, 1986; Wu, 1987). Then tissue samples were mounted in distilled water on a glass slide and overlaid with a coverslip. The excitation wavelength for Rhodamine 123 and chlorophyll autofluorescence was set at 488 and 543 nm, respectively. A 505/25 nm band pass filter was used for the green channel with a 560 nm band pass filter for the red channel. Images were the result of the z axis projection of slices scanned taken in intervals of 1 μm. For chloroplast counts and cell area determination, confocal microscopy was performed using a Leica SPII confocal laser-scanning microscope (Leica Mycrosystems GmbH) in fluorescent mode, equipped with a PlanApO 40×/1.25 oil immersion objective. In this case, the excitation wavelength was set at 380 to 495 nm for chloroplast and at 647 to 747 nm for cell walls. Tissue samples for these analyses were collected during daytime, mounted in distilled water on a glass slide, and overlaid with a coverslip. Images obtained were examined with Image J 1.33u (Image J) and Photoshop 5.0 (Adobe Systems).
Confocal microscopy, as oppose to TEM, offers several advantages for mitochondrial and chloroplast counts. Plant mitochondrial heterogeneity depends on plants species, tissue, ontogeny, cellular type, and energetic state of mitochondria and they are often associated with other organelles and structures (Logan and Leaver, 2000). Contrary to TEM, confocal microscopy: (1) eliminates interferences from chloroplasts regardless of mitochondrial localization within the cytoplasm; (2) provides a global three-dimensional cell view so that mitochondrial morphology and mitochondrial-in-cell position is considered; and (3) Rhodamine 123 stains only metabolically functional mitochondria (Petit, 1992).
Mitochondrial size was analyzed using TEM, a technique best suited for ultrastructural features. Tissue samples were collected and immediately cut into 1 mm3 slices and fixed in 2% paraformaldehyde (v/w), 2.5% glutaraldehyde in 0.1 m cacodylate buffer (pH 7.4). After fixing, samples were washed in the same buffer four times (10 min each wash) at 4°C. The samples were then postfixed in 1% osmium tetraoxide and 0.8% K3Fe (CN)6 (in 0.1 m cacodylate buffer) for 12 h at 4°C, washed with milliQ water four times (10 min each) at 4°C, and dehydrated in acetone concentrated series (50%, 70%, 90%, 96%, and 100%). Samples were then embedded in Spurr resine at ambient temperature, polymerized at 60°C during 48 h, and finally cross sectioned with an ultracut-UCT ultramicrotome (Leica Mikrosysteme Gmbh). Images were obtained by using a JEOL JEM 1010.
Analysis of Cytochrome c Oxidase Activity
Tissue segments of first-daughter cladodes of plants grown in growth chambers were harvested at the beginning of the night period. Spines of the cladode segments were removed and tissue was sliced in 1 to 2 mm sections and immediately homogenized in a medium containing 25 mm HEPES buffer, 1 mm EDTA, 1% (w/v) polyvinylpyrrolidone, 0.2% (w/v) bovine serum albumin fraction V, and 15 mm Na ascorbate, using a Polytron (4–5 repetitions of 5 s each). The homogenate was then filtered and centrifuged at 3,000g to 4,000g for 5 min. For measurement, 1.8 mL of reaction medium was added to 0.2 mL of the supernatant into the O2 electrode cuvette (see above) as described in Azcon-Bieto et al. (1994).
Determination of Cytochrome and Alternative Pathways Using O2 Isotopes
Total, cytochrome, and alternative pathway activity was analyzed on first-daughter cladodes grown in greenhouse by the oxygen isotope fractionation method (as in Gonzalez-Meler et al., 2001) with modifications. For oxygen isotope measurements, tissue segments were collected during the light period (about 1.4–1.5 g fresh weight) when alternative pathway activity was stable (see Robinson et al., 1992) so comparison of measurements between CO2 treatments were more meaningful. Immediately after harvest, tissue segments (these segments just included photosynthetic tissue) were kept in the dark for 45 to 50 min in 10 mm TES buffer + 0.2 mm CaCl2 (pH 8.0) at 25°C before gas phase respiratory measurements were taken in a closed 4.96 mL stainless steel cuvette at 25°C. A CO2 absorber (ascarite II) was present during measurements to avoid inhibition of respiration as a consequence of the build up of CO2 in the closed cuvette during the course of the experiment (Gonzalez-Meler et al., 1996). Oxygen diffusion through bulky tissues can affect proper measurements of oxygen fractionation by mitochondrial respiration. To avoid diffusion limitation of oxygen inside the respiring bulky tissues, an artificial gas mixture containing 50% O2 in N2 was introduced into the gas cuvette at the beginning of the experiment and allowed to equilibrate with the tissue for 5 to 10 min before measurements began (M.A. Gonzalez-Meler, unpublished data). We performed trial studies on oxygen isotope fractionation and oxygen uptake to detect the effect of slicing the tissue, tissue thickness, and cuticle integrity. Tissue segments were at least 2 mm thick as thickness below 1.5 mm altered respiration and fractionation values. The effect of cuticule removal on respiration and oxygen fractionation values was negligible. The 18O/16O isotope ratios of the 100 μL gas sample was measured in continuous flow using a Finnegan Delta + XL isotope ratio mass spectometer. Calculations of oxygen isotope fractionation were made as described in Guy et al. (1989) with modifications (Gonzalez-Meler et al., 2001; Guy and Vanlerberghe, 2005).
The sampling system was checked regularly for leaks from external air by filling the cuvette with helium and sampling the cuvette for appearance of air over time. Leaks were always negligible. Over the course of the experiment, each sample consumed at least 30%, but no more than 50% of the initial oxygen. The r2 values of all unconstrained linear regressions between −lnf and ln (R/Ro; with at least five data points) were greater than the value 0.995 considered minimally acceptable (Ribas-Carbo et al., 2000). During inhibitor treatments, either 1 mm KCN (in 10 mm TES, pH 8.00) or 6 mm salicylhydroxamic acid (SHAM; in water from a 1 m stock in dymetilsulfoxide) were applied by sandwiching the leaf tissues between medical wipes soaked with the corresponding inhibitor and incubating in the dark for 45 to 50 min. The inhibitor concentrations were obtained from titrations carried out in a liquid phase oxygen electrode. All inhibitor and buffer stocks were freshly prepared before use. The CO2 absorber was removed in experiments requiring KCN to avoid recovery from inhibition. In addition, for KCN experiments, a piece of tissue wetted with KCN was present in the cuvette. Mitochondrial ATP production was calculated from the activities of the cytochrome and alternative pathways, assuming that electron flow through the alternative pathway promotes some synthesis of ATP via complex I and including some levels of proton leakage and considering 29/6 ATP formed for each O2 consumed through the cytochrome pathway and 11/6 ATP formed for each O2 consumed by the alternative oxidase (Amthor, 1994; Gonzalez-Meler et al., 2001).
Statistics
After checking for both homogeneity of variances and normal distribution of variables, significant differences between CO2 treatments were tested using Student's t test (P < 0.05), except for those experiments analyzing the effect of CO2 treatment during ontogeny when analysis of variance was performed using a Tukey's Honestly Significant Difference test (P < 0.05). In both cases, tests were implemented using Statgraphics Plus 5.0 software for Windows (Statistical Graphics Corporation).
Acknowledgments
We gratefully acknowledge Marimurtra Botanical Garden (Blanes, Spain), Santa Margarita Ecological Reserve Flora (California), and Dr. Erick De la Barrera (Universidad Nacional Autónoma de México) for providing O. ficus-indica plants. We also thank Dr. Sergey Oleynik (UIC) for the technical support with elemental and isotope analyses, Dr. Hormoz BassiriRad (UIC), and Dr. Simonneau and Mr. Josep Matas (Universitat de Barcelona [UB]) for their assistance in setting CO2 controls for the greenhouse and environmental controlled chambers experiments, respectively, and Dr. Carmen Lopez-Iglesias, Dr. Raquel Garcia Olivas, Dr. Maria Reixach, and Ms. Monica Roldan (UB) for their technical support.
This work was supported by the Spanish Government (grant no. BFI–2003–09680), Catalan Government (grant no. 2001–SGR–00094), University of Barcelona (grant no. ACES–UB–2005), and the National Science Foundation (grant no. IOB–0528069).
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantphysiol.org) is: Nuria Gomez-Casanovas (ngomezca8@bio.ub.edu and ngomezca@uic.edu).
Open Access articles can be viewed online without a subscription.
References
- Adams WW III, Nishida K, Osmond CB (1986. a) Quantum yields of CAM plants measured by photosynthetic O2 exchange. Plant Physiol 81 297–300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams WW III, Smith SD, Osmond CB (1986. b) Photoinhibition of the CAM succulent Opuntia basilaris growing in Death Valley: evidence from 77K fluorescence and quantum yield. Oecologia 71 221–228 [DOI] [PubMed] [Google Scholar]
- Ainsworth EA, Rogers A, Vodkin LO, Walter A, Schurr U (2006) The effects of elevated CO2 concentration on soybean gene expression: an analysis of growing and mature leaves. Plant Physiol 42 135–147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amthor JS (1991) Respiration in a future, higher-CO2 world. Plant Cell Environ 14 13–20 [Google Scholar]
- Amthor JS (1994) Plant respiration responses to the environment and their effect on the carbon balance. In RE Wilkinson, ed, Plant-Environment Interactions. Marcel Dekker, New York, pp 501–554
- Amthor JS (1995) Terrestrial higher-plant response to increasing atmospheric [CO2] in relation to the global carbon cycle. Glob Change Biol 1 243–274 [Google Scholar]
- Amthor JS (1997) Plant respiratory responses to elevated CO2 partial pressure. In LH Allen, MB Kirkham, DM Olszyk, CE Whitman, eds, Advances in Carbon Dioxide Effects Research. American Society of Agronomy Special Publication, Madison, Wisconsin, pp 35–77
- Amthor JS (2000) The McCree-de Wit-Penning de Vries-Thornley respiration paradigms: 30 years later. Ann Bot (Lond) 86 1–20 [Google Scholar]
- Armstrong AF, Logan DC, Tobin AK, O'Toole P, Atkin OK (2006) Heterogeneity of plant mitochondrial responses underpinning respiratory acclimation to the cold in Arabidopsis thaliana leaves. Plant Cell Environ 29 940–949 [DOI] [PubMed] [Google Scholar]
- Atkin OK, Tjoelker MG (2003) Thermal acclimation and the dynamic response of plant respiration to temperature. Trends Plant Sci 8 343–351 [DOI] [PubMed] [Google Scholar]
- Azcon-Bieto J, Gonzalez-Meler MA, Doherty W, Drake BG (1994) Acclimation of respiratory O2 uptake in green tissues of field grown native species after long-term exposure to elevated atmospheric CO2. Plant Physiol 106 1163–1168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloom AJ, Smart DR, Nguyen DT, Searles PS (2002) Nitrogen assimilation and growth of wheat under elevated carbon dioxide. Proc Natl Acad Sci USA 99 1730–1735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouma TJ (2005) Understanding plant respiration: separating respiratory components versus a process-based approach. In H Lambers, M Ribas-Carbo, eds, Plant Respiration: From Cell to Ecosystem, Series: Advances in Photosynthesis and Respiration. Springer-Verlag, Berlin, pp 177–194
- Bouma TJ, De Visser R, Janssen JHJA, De Kock PH, Van Leeuwen PH, Lambers H (1994) Respiratory energy requirements and rate of protein turnover in vivo determined by the use of an inhibitor of protein synthesis and a probe to asses its effect. Physiol Plant 92 585–594 [Google Scholar]
- Bruhn D, Wiskich JT, Atkin OK (2007) Contrasting responses by respiration to elevated CO2 in intact tissue and isolated mitochondria. Funct Plant Biol 34 112–117 [DOI] [PubMed] [Google Scholar]
- Carey EV, DeLucia EH, Ball T (1996) Stem maintenance and construction respiration in Pinus ponderosa grown in different concentrations of atmospheric CO2. Tree Physiol 16 125–130 [DOI] [PubMed] [Google Scholar]
- Cui M, Miller PM, Nobel PS (1993) CO2 exchange and growth of the Crassulacean Acid Metabolism plant Opuntia ficus-indica under elevated CO2 in Open Top Chambers. Plant Physiol 103 519–524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis PS, Wang Z (1998) A meta-analysis of elevated CO2 effects on woody plant mass, form and physiology. Oecologia 113 299–313 [DOI] [PubMed] [Google Scholar]
- Davey PA, Hunt S, Hymus GJ, DeLucia EH, Drake BG, Karnosky DF, Long SP (2004) Respiratory oxygen uptake is not decreased by an instantaneous elevation of CO2, but is increased with long-term growth in the field at elevated CO2. Plant Physiol 134 520–527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLucia EH, Drake JE, Thomas RB, Gonzalez-Meler M (2007) Forest carbon use efficiency: is respiration a constant fraction of gross primary production? Glob Change Biol 13 1157–1167 [Google Scholar]
- Drake BG, Azcon-Bieto J, Berry J, Bunce J, Dijkstra P, Farrar J, Gifford RM, Gonzalez-Meler MA, Koch G, Lambers H, et al (1999) Does elevated atmospheric CO2 concentration inhibit mitochondrial respiration in green plants? Plant Cell Environ 22 649–657 [Google Scholar]
- Drake BG, Gonzalez-Meler MA, Long SP (1997) More efficient plants: a consequence of rising atmospheric CO2? Annu Rev Plant Physiol Plant Mol Biol 48 609–639 [DOI] [PubMed] [Google Scholar]
- Drennan PM, Nobel PS (2000) Responses of CAM species to increasing atmospheric CO2. Plant Cell Environ 23 767–781 [Google Scholar]
- Dvorak V, Oplustilova M (1997) Respiration of woody tissues of Norway spruce in elevated CO2 concentrations. In GMJ Mohren, K Kramer, S Sabate, eds, Impacts of Global Change on Tree Physiology and Forests Ecosystems. Kluver Academic Publishers, Dordrecht, The Netherlands, pp 47–51
- Goldstein G, Andrade JL, Nobel PS (1991) Differences in water relations parameters for the chlorenchyma and the parenchyma of Opuntia ficus-indica under wet versus dry conditions. Funct Plant Biol 18 95–107 [Google Scholar]
- Gomez-Casanovas N (2006) Effects of long-term elevated CO2 on respiratory physiology in Opuntia ficus-indica plants. PhD thesis. University of Barcelona, Barcelona
- Gonzalez-Meler MA, Giles L, Thomas RB, Siedow JN (2001) Metabolic regulation of leaf respiration and alternative pathway activity in response to phosphate supply. Plant Cell Environ 24 205–215 [Google Scholar]
- Gonzalez-Meler MA, Ribas-Carbo M, Giles L, Siedow JN (1999) The effect of growth and measurement temperature on the activity of the alternative respiratory pathway. Plant Physiol 120 765–772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Meler MA, Ribas-Carbo M, Siedow JN, Drake BG (1996) Direct inhibition of plant mitochondrial respiration by elevated CO2. Plant Physiol 112 1349–1355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Meler MA, Siedow JN (1999) Inhibition of respiratory enzymes by elevated CO2: does it matter at the intact tissue and whole plant levels? Tree Physiol 19 253–259 [DOI] [PubMed] [Google Scholar]
- Gonzalez-Meler MA, Taneva L (2005) Integrated effects of atmospheric CO2 concentration on plant and ecosystem respiration. In H Lambers, M Ribas-Carbo, eds, Plant Respiration. Kluwer-Academic Publishers, Dordrecht, The Netherlands, pp 211–259
- Gonzalez-Meler MA, Taneva L, Trueman RJ (2004) Plant respiration and elevated atmospheric CO2 concentration: cellular responses and global significance. Ann Bot (Lond) 94 647–656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin KL, Anderson OR, Gastrich MD, Lewis JD, Lin G, Schusteri W, Seemann JR, Tissue DT, Turnbull MH, Whitehead D (2001) Plant growth in elevated CO2 alters mitochondrial number and chloroplast fine structure. Proc Natl Acad Sci USA 98 347–353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin KL, Anderson OR, Tissue DT, Turnbull MH, Whitehead D (2004) Variations in dark respiration and mitochondrial numbers within needles of Pinus radiata grown in ambient or elevated CO2 partial pressure. Tree Physiol 24 347–353 [DOI] [PubMed] [Google Scholar]
- Griffin KL, Winner WE, Strain BR (1996) Construction cost of loblolly and ponderosa pine leaves grown with varying carbon and nitrogen availability. Plant Cell Environ 19 729–738 [Google Scholar]
- Guy RD, Berry JA, Fogel ML, Hoering TC (1989) Differential fractionation of oxygen isotopes by cyanide-resistant and cyanide-sensitive respiration in plants. Planta 177 483–491 [DOI] [PubMed] [Google Scholar]
- Guy RD, Vanlerberghe GC (2005) Partitioning of respiratory electrons in the dark in leaves of transgenic tobacco with modified levels of alternative oxidase. Physiol Plant 125 171–180 [Google Scholar]
- Hartley IP, Armstrong AF, Murthy R, Barron-Gafford G, Ineson P, Atkin OK (2006) The dependence of respiration on photosynthetic substrate supply and temperature: integrating leaf, soil and ecosystem measurements. Glob Change Biol 12 1954–1968 [Google Scholar]
- Hrubeck TC, Robinson JM, Donaldson RP (1985) Effects of CO2 enrichment and carbohydrate content on the dark respiration of soybeans. Plant Physiol 79 684–689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krömer S (1995) Respiration during photosynthesis. Annu Rev Plant Physiol Plant Mol Biol 46 45–70 [Google Scholar]
- Lambers H, Robinson S, Ribas-Carbo M (2005) Regulation of respiration in vivo. In H Lambers, M Ribas-Carbo, eds, Plant Respiration: From Cell to Ecosystem, Series: Advaces in Photosynthesis and Respiration. Springer-Verlag, Berlin, pp 2–30
- Lennon AM, Neuenschwander UH, Ribas-Carbo M, Giles L, Ryals JA, Siedow JN (1997) The effects of salicylic acid and tobacco mosaic virus infection on the alternative oxidase of tobacco. Plant Physiol 115 783–791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logan DC, Leaver CJ (2000) Mitochondria-targeted GFP highlights the heterogeneity of mitochondrial shape, size and movement within living plant cells. J Exp Bot 51 865–871 [PubMed] [Google Scholar]
- Lüttge U (2002) CO2-concentrating: consequences in crassulacean acid metabolism. J Exp Bot 53 2131–2142 [DOI] [PubMed] [Google Scholar]
- Matzke MA, Matzke MJA (1986) Visualization of mitochondria and nuclei in living plant cells by the use of a potential-sensitive fluorescent dye. Plant Cell Environ 9 73–77 [Google Scholar]
- Maxwell DP, Wang Y, McIntosh L (1999) The alternative oxidase lowers mitochondrial reactive oxygen production in plant cells. Proc Natl Acad Sci USA 96 8271–8276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millenaar FF, Benschop JJ, Wagner AM, Lambers H (1998) The role of the alternative oxidase in stabilizing the in vivo reduction state of the ubiquinone pool and the activation state of the alternative oxidase. Plant Physiol 118 599–607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noguchi K, Go CS, Terashima I, Ueda S, Yoshinari T (2001) Activities of the cyanide-resistant respiratory pathway in leaves of sun and shade species. Aust J Plant Physiol 28 27–35 [Google Scholar]
- Nogues S, Tcherkez G, Streb P, Pardo A, Florence B, Bligny R, Ghashghaie J, Cornic G (2006) Respiratory carbon metabolism in the high mountain plant species Ranunculus glacialis. J Exp Bot 57 3837–3845 [DOI] [PubMed] [Google Scholar]
- Osmond CB (1978) Crassulacean Acid Metabolism: a curiosity in context. Annu Rev Plant Physiol 26 379–414 [Google Scholar]
- Peñuelas J, Matamala R (1990) Changes in N and S leaf content, stomatal density and specific leaf area of 14 plant species during the last three centuries of CO2 increase. J Exp Bot 41 1119–1124 [Google Scholar]
- Petit PX (1992) Flow cytometric analysis of Rhodamine 123 fluorescence during modulation of the membrane potential in plant mitochondria. Plant Physiol 98 279–286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plaxton W, Podestá F (2006) The functional organization and control of plant respiration. CRC Crit Rev Plant Sci 25 159–198 [Google Scholar]
- Poorter H, VanBerkel Y, Baxter R, DenHertog J, Dijkstra P, Gifford RM, Griffin KL, Roumet C, Roy J, Wong SC (1997) The effect of elevated CO2 on the chemical composition and construction costs of leaves of 27 C-3 species. Plant Cell Environ 20 472–482 [Google Scholar]
- Ribas-Carbo M, Lennon AM, Robinson SA, Giles L, Berry JA, Siedow JN (1997) The regulation of electron partioning between the cytochrome and alternative pathways in soybean cotyledon and root mitochondria. Plant Physiol 113 903–911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribas-Carbo M, Robinson S, Gonzalez-Meler MA, Lennon AM, Giles L, Siedow JN, Berry JA (2000) Effects of light on respiration and oxygen isotope fractionation in soybean cotyledons. Plant Cell Environ 23 983–990 [Google Scholar]
- Robinson SA, Yakir D, Ribas-Carbo M, Giles L, Osmond CB, Siedow JN, Berry JA (1992) Measurements of engagement of cyanide-resistant respiration in the Crassulacean Acid Metabolism plant Kalanchoë daigremontiana with the use of on-line oxygen isotope discrimination. Plant Physiol 100 1087–1091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulze ED (2006) Biological control of the terrestrial carbon sink. Biogeosciences. 3 147–166 [Google Scholar]
- Smith JAC, Lüttge U (1985) Day-night changes in leaf water relations associated with the rhythm of Crassulacean Acid Metabolism in Kalanchoe daigremontiana. Planta 163 272–282 [DOI] [PubMed] [Google Scholar]
- Smith JAC, Schulte PJ, Nobel PS (1987) Water flow and water storage in Agave deserti: osmotic implications of Crassulacean Acid Metabolism. Plant Cell Environ 10 639–648 [Google Scholar]
- Teare ID, Peterson CJ, Law AG (1971) Size and frequency of leaf stomata in cultivars of Triticum aestivum and other Triticum species. Crop Sci 11 496–498 [Google Scholar]
- Tissue DT, Lewis JD, Wullschleger SD, Amthor JS, Griffin KL, Anderson OR (2002) Leaf respiration at different canopy positions in sweetgum (Liquidambar styraciflua) grown in ambient and elevated concentrations of carbon dioxide in the field. Tree Physiol 22 1157–1166 [DOI] [PubMed] [Google Scholar]
- Tjoelker MG, Reich PB, Oleksyn J (1999) Changes in leaf nitrogen and carbohydrates underlie temperature and CO2 acclimation of dark respiration in five boreal species. Plant Cell Environ 22 767–778 [Google Scholar]
- Valentini R, Matteucci G, Dolman AJ, Schulze ED, Rebmann C, Moors EJ, Granier A, Gross P, Jensen NO, Pilegaard K, et al (2000) Respiration as the main determinant of carbon balance in European forests. Nature 404 861–865 [DOI] [PubMed] [Google Scholar]
- Van der Werf A, Kooijman A, Welschen R, Lambers H (1988) Respiration energy costs for the maintenance of biomass, for growth and for ion uptake in roots of Carex diandra and Carex acutiformis. Physiol Plant 72 483–491 [Google Scholar]
- Vanlerberghe GC, McIntosh L (1997) Alternative oxidase: from gene to function. Annu Rev Plant Physiol Plant Mol Biol 48 703–734 [DOI] [PubMed] [Google Scholar]
- Wang N, Nobel PS (1996) Doubling the CO2 concentration enhanced the activity of carbohydrate-metabolism enzymes, source carbohydrate production, photoassimilate transport, and sink strength for Opuntia ficus-indica. Plant Physiol 110 893–902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Anderson OR, Griffin KL (2004) Chloroplast numbers, mitochondrion numbers and carbon assimilation physiology of Nicotiana sylvestris as affected by CO2 concentration. Environ Exp Bot 51 21–31 [Google Scholar]
- Will RE, Ceulemans R (1997) Effects of elevated CO2 concentrations on photosynthesis, respiration and carbohydrate status of coppice Populus hybrids. Physiol Plant 100 933–939 [Google Scholar]
- Wu FS (1987) Localization of mitochondria in plant cells by vital staining with Rhodamine 123. Planta 17 346–357 [DOI] [PubMed] [Google Scholar]
- Wullschleger SD, Norby RJ (1992) Respiratory cost of leaf growth and maintenance in white oak saplings exposed to atmospheric CO2 enrichment. Can J For Res 22 1717–1721 [Google Scholar]
- Wullschleger SD, Norby RJ, Gunderson CA (1992) Growth and maintenance respiration in leaves of Liriodendron tulipifera L. saplings exposed to long-term carbon dioxide enrichment in the field. New Phytol 121 515–523 [Google Scholar]
- Wullschleger SD, Norby RJ, Hanson PJ (1995) Growth and maintenance respiration in stems of Quercus alba after four years of CO2 enrichment. Physiol Plant 93 47–54 [Google Scholar]
- Wullschleger SD, Norby RJ, Love JC, Runck C (1997) Energetic costs of tissue construction in yellow-poplar and white oak trees exposed to long-term CO2 enrichment. Ann Bot (Lond) 80 289–297 [Google Scholar]