Abstract
Bacterial and viral upper respiratory infections (URI) produce highly variable clinical symptoms that cannot be used to identify the etiologic agent. Proper treatment, however, depends on correct identification of the pathogen involved as antibiotics provide little or no benefit with viral infections. Here we describe a rapid and sensitive genotyping assay and microarray for URI identification using standard amplification and hybridization techniques, with electrochemical detection (ECD) on a semiconductor-based oligonucleotide microarray. The assay was developed to detect four bacterial pathogens (Bordetella pertussis, Streptococcus pyogenes, Chlamydia pneumoniae and Mycoplasma pneumoniae) and 9 viral pathogens (adenovirus 4, coronavirus OC43, 229E and HK, influenza A and B, parainfluinza types 1, 2, and 3 and respiratory syncytial virus. This new platform forms the basis for a fully automated diagnostics system that is very flexible and can be customized to suit different or additional pathogens. Multiple probes on a flexible platform allow one to test probes empirically and then select highly reactive probes for further iterative evaluation. Because ECD uses an enzymatic reaction to create electrical signals that can be read directly from the array, there is no need for image analysis or for expensive and delicate optical scanning equipment. We show assay sensitivity and specificity that are excellent for a multiplexed format.
Introduction
Upper respiratory tract infections (URI) can be caused by a variety of viruses, including rhinovirus, coronavirus, influenza A and B, parainfluenza, respiratory syncytial virus, adenovirus, metapneumovirus, and enterovirus; and by bacteria, including Chlamydia pneumoniae, Mycoplasma pneumoniae, Streptococcus pyogenes, S. pneumoniae, Bordetella pertussis, and Haemophilus influenzae [1], [2], [3]. Clinical symptoms of URI are highly variable and cannot be used to identify the etiologic agent or agents. Although the majority of URI are caused by viruses (69%; [3]), antibiotics are widely prescribed to treat symptoms. Antibiotics, which are of little or no benefit for viral infections, can contribute to the emergence and spread of resistant bacteria and to higher costs for health care [4], [5], [6].
The rapid identification of certain viruses and bacteria is necessary for proper treatment of infection. Patients with a history of rheumatic fever (Streptococcus), patients with bacterial or viral infections for which specific therapies are available, and patients whose URI symptoms persist for over two weeks, can benefit from rapid detection. Rapid and accurate identification of URI agents at the point of care can reduce the inappropriate prescription of antibiotics and help identify problematic infections early.
Traditional methods for the identification of URI pathogens, including culture and serology, are effective but are labor intensive and may require highly trained personnel and days to weeks to complete. Many rapid serological tests are not sensitive or specific enough to detect all infections. Enzyme immunoassays (EIA) generally show sensitivities of 85% and direct immunofluorescence (DIF) assays show sensitivities of approximately 60 to 80% [7]. Although viral culture is generally accepted as the gold standard for diagnosis, PCR can be more sensitive [8]. In one study, rhinovirus culture detected infection in 40% of patients while rhinovirus PCR detected infection in 51.5% of patients [3]. Similarly, an influenza virus surveillance study showed that 18% of samples were positive by culture and 28% were positive by TaqMan-PCR [9].
Because of their speed, specificity and sensitivity, genomic assays are complementary to serological assays and especially useful for identifying an unknown specimen where antibodies are not specific enough to differentiate closely related groups [10], [11], [12], [13], [14], [15], [16], [17], [18], [19], [20], [21], [22]. Reverse transcription-polymerase chain reaction (RT-PCR) is widely used for identifying virus and bacteria; however, a positive amplification is often verified by subsequent procedures that provide sequence information [8], [23], [24]. By using multiple probes against a single pathogen coupled with the proper amplification scheme, microarrays can serve as valuable tools for viral discovery, detection, genotyping and sequencing [13], [14], [21], [25], [26], [27], [28], [29], [30], [31], [32], [33].
While traditional assays for pathogen detection and typing represent the gold standard, they alone, cannot meet the future needs for rapid, sensitive, specific and simple methods. The majority of these approaches do not provide sequence information, utilize expensive equipment that cannot be easily transported, require a high level of expertise to operate equipment, require extended periods of time, or are not easily modified. Here we describe a rapid and sensitive genotyping assay and array for upper respiratory pathogen identification using standard amplification and hybridization techniques and electrochemical detection (ECD) on a semiconductor-based oligonucleotide microarray. This platform was particularly suitable for developing this assay because it supports custom probe synthesis, which permits oligonucleotides of interest to be tested empirically. Multiple, highly reactive probes that are specific for a particular organism can then be put on the array for further iterative evaluation. Content of the final assay can also be customized to suit diagnostic needs in different geographic areas. Finally, the ability to use electrochemical detection with semiconductor microchips eliminates the need for expensive and delicate optical scanning equipment [34], [35], [36]. Scanning of microarrays is typically labor intensive and a significant source of system noise that complicates the use of microarrays significantly; and has hampered the transition of these technologies into high throughput clinical scenarios by adding undue instrumentation cost and complexity. The ECD method of deriving signal from a microarray is extremely simple and provides a clear path to making microarray processing truly hands off throughout the entire process.
Results
URI pathogen genotyping assay
The assay was designed to identify 10 upper respiratory pathogens, with subtype identification in some cases (coronavirus and parainfluenza virus). We utilized multiple probes for each pathogen (approximately 30 to 100) that were designed from conserved regions of the respective genomes. Approximately 850 unique URI probes were included on the array with 12 to 13 replicates for each probe. The assumption for this methodology is that enough unique probes will produce specific signal in the presence of the labeled PCR products to result in a positive call. Multiple probes allow for genetic variability, especially with viral genomes, and also provide sequence information. In principle it is straightforward to substitute other organisms in these assays by synthesizing arrays with alternate probe specificity and alternate primer sets.
Amplification and labeling
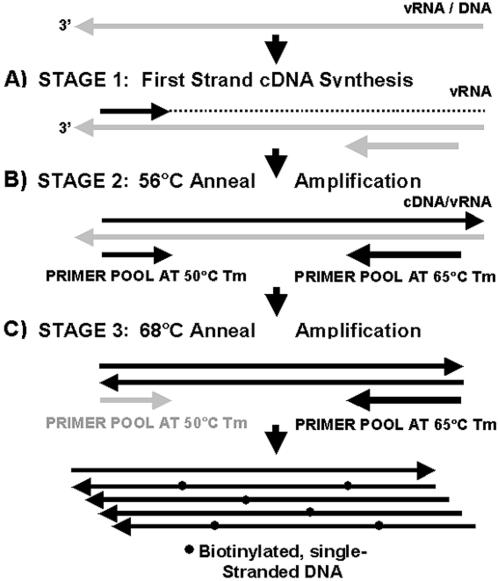
One-tube RT-PCR was used for target amplification and labeling because of its sensitivity and its application to both DNA and RNA targets (Fig. 1). Our PCR primers flanked conserved regions that had complementary sequences to the probes on the array. The combination of microarrays with RT-PCR allowed for the detection of specific signal from a complex reaction mix containing background NA. The PCR amplification/labeling reactions took approximately 3.5 hrs with a peltier thermal cycler; however the reaction time was reduced to less than two hours using a LightCycler (Roche). Single-strand target was produced by adjusting the melting temperature of the forward and reverse primer pools to approximately 50°C and 65°C respectively. This 15°C temperature differential between primer sets allowed for a 50°C reverse transcription step followed by exponential amplification at 56°C. Increasing the annealing temperature of the reaction to 68°C, with 30 additional rounds of amplification, resulted in a predominantly one-way reaction favoring reverse primer extension. Biotin labeling occurred during amplification by incorporation of biotinylated dCTP/dATP in the reaction mix. Biotinylated reverse primers were also employed as an alternate method for biotin labeling.
Figure 1. Strategy for amplification and labeling of pathogen-specific target.
The one-tube RT-PCR reaction includes three stages: A) Reverse transcription reaction to produce first strand cDNA for viral targets (vRNA); B) Low temperature (56°C), exponential amplification of target and C) High temperature (68°C), linear amplification to product single-strand, biotin-labeled target. Target is labeled by incorporation of biotin-14-dCTP during amplification or by the use of biotinylated reverse primers.
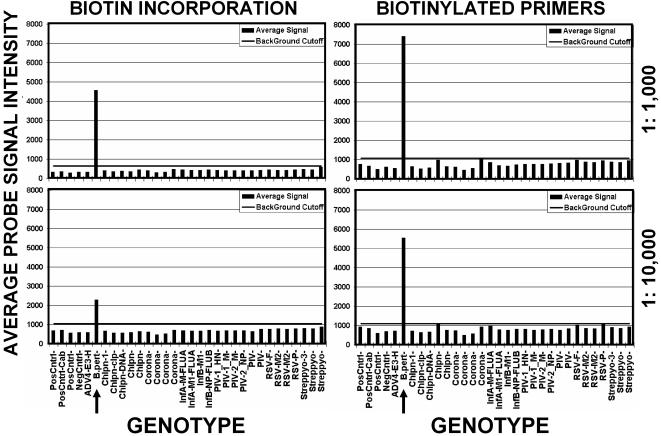
We compared the sensitivity and specificity of biotin labeling by incorporation during amplification versus biotinylated reverse primers (Fig. 2). Although using biotinylated reverse primers increased assay sensitivity, nonspecific signal increased due to binding of free biotinylated oligonucleotides to specific probes. This background could be eliminated during analysis by removing approximately 2% of the high and low signal for each genotype category prior to averaging.
Figure 2. Comparison of assay sensitivity with target labeled by biotin-incorporation and biotinylated primers.
B. pertussis (B.pert, arrow) genomic DNA at 15 ng/ul was diluted to 1∶1,000 (top panels) and 1∶10,000 (bottom panels) and labeled either by biotin-incorporation (left panels) or with biotinylated reverse primers (right panels). The average of type-specific probe group signals in picoamps is shown at the left and identity of pathogen type-specific probe groups is shown below panels. The horizontal bar indicates the assay cutoff, which was determined by the mean value of negative control probes plus 3 standard deviations of the mean.
Hybridization and electrochemical detection
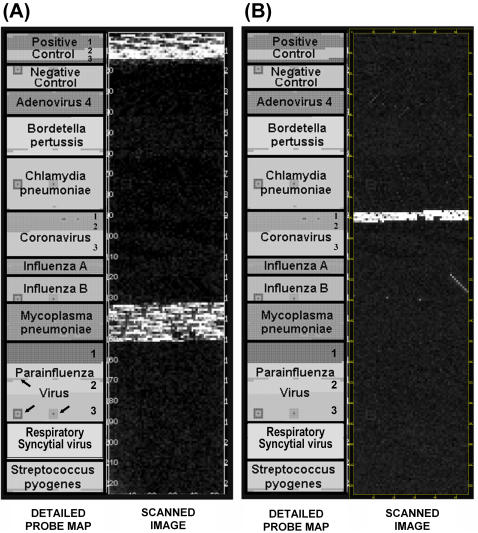
While the microarray can be used with fluorescence detection, we used electrochemical detection (ECD), which relies on a redox reaction to generate electrical current (pA) on the array for measurement. Pseudo-images of two hybridized targets are shown in Figure 3A (M. pneumoniae) and B (coronavirus 229E). These images are a representation of hybridization signals (i.e. current) derived from the raw data. This is in contrast to conventional microarray technology where the data are derived from the image and subsequently analyzed. Signal is predominately seen in the respective block of specific probes for each organism with scattered background signal. Variation of signal intensity within a pathogen-specific block of probes resulted from: 1) variable biotin labeling due to amplicon size and GC content; 2) variation in probe-target interaction due to genomic variability; and 3) predictable and un-predictable nucleotide base-pairing characteristics of probes. To overcome these issues, we determined a specific genotype based on the average signal intensity of all pathogen-specific probes, which includes 12 to 13 replicates of each probe. This was done rapidly using an Excel macro to generate bar graphs to identify the most likely candidate(s).
Figure 3. Pseudoimage of raw data from scanned chips illustrating Mycoplasma pneumoniae (A) and coronavirus 229E (B) detection.
The left panels (Probe Map) show the position of all pathogen-specific probes and include 3 sub-type probes for coronavirus and parainfluenza virus. Geometric shapes indicated by arrows are areas on the microarray dedicated to array quality control probes. Positive control target was included only with M. pneumoniae (A). Coronavirus probes are divided into three sections on the array: 1) Type 1, 2) Type 2, and 3) Type 3.
URI assay validation
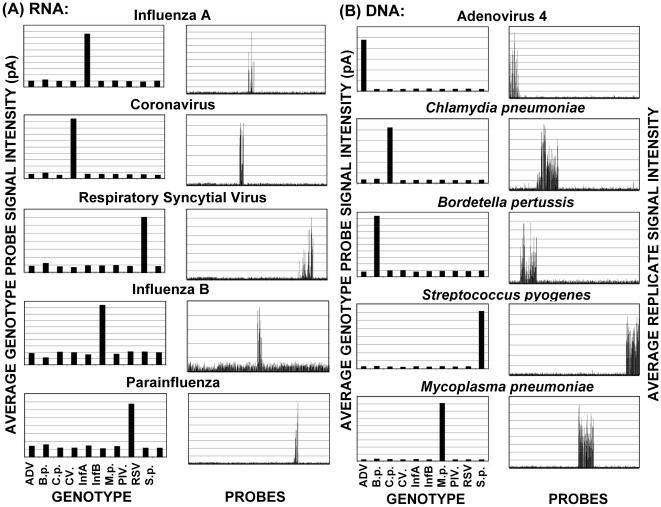
Results of array validation studies are shown in Figure 4. The prototype URI array contained probes representing two to six amplicons per organism (see Table 1) and two primer pools consisting of 18 primer pairs for RNA targets and 19 primer pairs for DNA targets (pools 1A and 2A respectively). Both pools contained two primer pairs for the Arabidopsis positive control (Cont.) spike-in nucleic acids for both DNA and RNA genome targets (spike-ins were predominately used for unknown samples and not for validation). Assays were designed to genotype each of the 10 URI pathogens with coronaviruses and parainfluenza viruses subdivided into three subtypes. Each of the 10 target genomes was successfully identified with little or no background cross-reactivity (Figs 4A and B).
Figure 4. URI chip validation studies showing average RNA genome (A) and DNA genome (B) target signal intensity for the 10 upper respiratory pathogens studied.
The left panels (GENOTYPE) show the average genotype-specific probe signal in picoamps that is used to determine a genome identity. The right panels (PROBES) illustrate the average probe signal intensities of 12 replicates for each probe and also illustrate signal specificity for both positive and negative probes. Pathogens are ADV (adenovirus 4), B.p. (Bordetella pertussis), C.p. (Chlamydia pneumoniae), CV (coronavirus), InfA (influenza A), InfB (influenza B), M.p. (Mycoplasma pneumoniae), PIV (parainfluenza virus), RSV (respiratory syncytial virus), and S.p. (Streptococcus pyogenes) at nucleic acid concentrations described in Table 2.
Table 1. Primer pools, amplicons and probes for the URI assay.
| Pool | Target | Forward | Reverse | Gene | Amplicon | A | B | C | Probes |
| 1 | B. pertussis | Bp_For_1 | Bp_Rev_1 | LysR TR | 427 | * | 8 | ||
| 1 | " | Bp_For_2 | Bp_Rev_2 | ABC T | 476 | * | * | * | 18 |
| 1 | " | Bp_For_3 | Bp_Rev_3 | ADH | 769 | * | 14 | ||
| 1 | " | Bp_For_4 | Bp_Rev_4 | LysR TR | 569 | * | 21 | ||
| 1 | " | Bp_For_5 | Bp_Rev_5 | tfdA | 552 | * | 10 | ||
| 1 | " | Bp_For_6 | Bp_Rev_6 | CAIB | 534 | * | * | 15 | |
| 1 | M. pneumoniae | Mp_For_1 | Mp_Rev_1 | dnaK | 564 | * | 29 | ||
| 1 | " | Mp_For_2 | Mp_Rev_2 | pdhA | 284 | * | * | * | 18 |
| 1 | " | Mp_For_3 | Mp_Rev_3 | tuf | 604 | * | * | 33 | |
| 1 | S. pyogenes | Sp_For_1 | Sp_Rev_1 | 3-oxoacyl | 510 | * | 19 | ||
| 1 | " | Sp_For_2 | Sp_Rev_2 | 23S rRNA | 463 | * | * | 26 | |
| 1 | " | Sp_For_3 | Sp_Rev_3 | ABC T | 421 | * | * | * | 24 |
| 1 | C. pneumoniae | Cp_For_1 | Cp_Rev_1 | clp endop | 491 | * | 23 | ||
| 1 | " | Cp_For_2 | Cp_Rev_2 | HB-2 | 528 | * | * | * | 25 |
| 1 | " | Cp_For_3 | Cp_Rev_3 | 29 kDa lip | 475 | * | 23 | ||
| 1 | " | Cp_For_4 | Cp_Rev_4 | RNA pol S | 526 | * | 22 | ||
| 1 | " | Cp_For_5 | Cp_Rev_5 | R-Isomer | 526 | * | * | 20 | |
| 1 | Adenovirus 4 | Adv_For_1 | Adv_Rev_1 | gp 12 | 531 | * | * | 55 | |
| 1 | " | Adv_For_2 | Adv_Rev_2 | gp 12 | 606 | * | * | * | ** |
| 2 | Coronavirus 229E | CV229E_F_1 | CV229E_R_1 | Matrix | 299 | * | * | 20 | |
| 2 | Coronavirus HK | CV HK_F_1 | CV HK_R_1 | HE | 767 | * | * | 22 | |
| 2 | Coronavirus OC43 | CVOC43_F_1 | CVOC43_R_1 | HE | 528 | * | 28 | ||
| 2 | " | CVOC43_F_2 | CVOC43_R_2 | N | 507 | * | * | 33 | |
| 2 | Influenza A | InfA_For_1 | InfA_Rev_1 | Matrix | 500 | * | * | 20 | |
| 2 | " | InfA_For_2 | InfA_Rev_2 | Matrix | 339 | * | 13 | ||
| 2 | Influenza B | InfB_For_1 | InfB_Rev_1 | NP | 516 | * | * | 29 | |
| 2 | " | InfB_For_2 | InfB_Rev_2 | Matrix | 506 | * | 28 | ||
| 2 | PIV 1 | PIV1_For_1 | PIV1_Rev_1 | Matrix | 488 | * | * | 28 | |
| 2 | " | PIV1_For_2 | PIV1_Rev_2 | HN | 532 | * | 23 | ||
| 2 | PIV 2 | PIV2_For_1 | PIV2_Rev_1 | Matrix | 576 | * | 28 | ||
| 2 | " | PIV2_For_2 | PIV2_Rev_2 | NP | 592 | * | * | 34 | |
| 2 | PIV 3 | PIV3_For_1 | PIV3_Rev_1 | Matrix | 840 | * | * | 35 | |
| 2 | " | PIV3_For_2 | PIV3_Rev_2 | HA | 517 | * | 24 | ||
| 2 | RSV | RSV_For_1 | RSV_Rev_1 | Matrix 2 | 440 | * | 18 | ||
| 2 | " | RSV_For_2 | RSV_Rev_2 | Matrix 2 | 496 | * | 22 | ||
| 2 | " | RSV_For_3 | RSV_Rev_3 | Phos | 412 | * | 23 | ||
| 2 | " | RSV_For_4 | RSV_Rev_4 | Fusion | 474 | * | * | 18 | |
| Cont. | Arabidopsis | RCA For | RCA Rev | RCA | 508 | * | * | * | 32 |
| Cont. | " | Cab For | Cab Rev | caB | 501 | * | * | * | 23 |
| Cont. | " | rbcL For | rbcL Rev | rcbL | 515 | * | 12 |
Primer pair included in the respective sub-pool.
Probes used for both amplicon 1 and 2.
Primer pool optimization
Initial studies were conducted with two primer pools consisting of 19 primer pairs for DNA targets and 18 primer pairs for RNA targets (Pools 1A and 2A; Table 1). Subsequent studies to determine primer interactions and optimal target signals on the array resulted in reducing primer pool sizes to increase assay sensitivity (Fig. 5). Primer pool 1B for DNA targets included 10 of the original primer pairs and pool 2B for RNA included 9 of the original primer pairs. A third primer pool for DNA targets, pool 1C, contained only 5 primer pairs (see Table 1). A comparison of DNA target primer pools A, B, and C, shown in Fig. 5, demonstrates the increased sensitivity of pools 1B and 1C over pool 1A in detecting increasing dilutions M. pneumoniae DNA. All pools produced sufficient PCR products for detection on arrays at a 1∶1,000 dilution of target DNA (1 pg). Pools B and C were effective on samples diluted 1∶10,000; but only pool C was effective at dilutions above 1∶10,000.
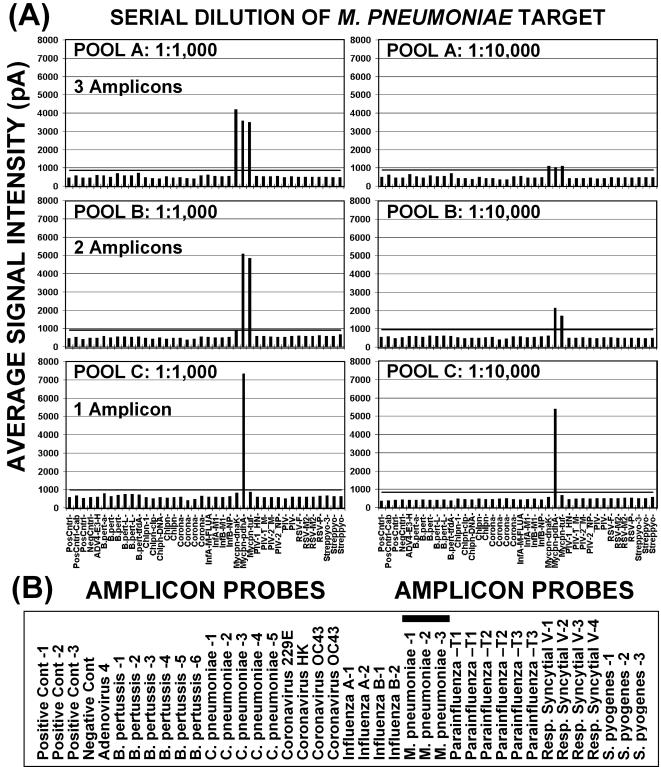
Figure 5. Comparison of assay sensitivity with primer pools 1A, 1B, and 1C.
Mycoplasma pneumoniae (M.p.) DNA, at 1 ng/ul, was serially diluted and amplified with primer pools 1A (3 M.p. amplicons), 1B (2 M.p. amplicons), and 1C (1 M.p. amplicon). Panels in (A) illustrate the sensitivity obtained with the 3 pools at M.p. DNA dilutions of 1∶1,000 (left) and 1∶10,000 (right). Average signal intensities in picoamps for each set of amplicon probes (vertical bars) are shown at the left. The horizontal bar within graphs indicates the assay cutoff (mean 3±SDM). The PCR amplicons analyzed for each pathogen are listed in panel (B) and the three M.p. amplicons are indicated by thick underline.
Primers for assay positive control A. thaliana RNA and DNA RT-PCR reaction spike-ins, originally consisted of three primer pairs: 1) A. thaliana RCA gene primers for DNA targets, 2) A. thaliana Cab gene primers for RNA targets, and 3) A.thaliana rcbL gene primers for both DNA and RNA targets. The primer set for the rcbL gene was subsequently removed from the final assay to improve performance.
Sensitivity and specificity studies
Using optimized primer pools (1C and 2B) and probe sequences, sensitivity studies were conducted on 10-fold serial dilutions of NA from each target organism. Starting NA concentrations were determined spectrophotometrically and from vendor specifications (Table 2). Exact concentrations of specific target organism NA was probably less because of host cell NA contamination and loss of material during NA isolation. In addition, when NA concentrations were based on PFU/CFU, the contribution of non-viable organism NA could not be determined. The URI genotyping assay could detect NA dilutions to approximately 1 to 10 genomes for 9 of 12 target categories (Table 2 and Figure 6). Less sensitivity was achieved for Bordetella pertussis and parainfluenza (PIV) types 1 and 3. The URI genotyping assay was also tested by including NA from the 5 organisms with DNA genomes and the 5 organisms with RNA genomes in separate PCR reactions, and then combining reactions for hybridization to arrays (Fig. 7). This did not result in the loss of a specific pathogen signal for the dilutions tested.
Table 2. Assay sensitivity tests for viral and bacterial URI pathogens.
| Sample | PFU-CFU/Reaction | Conc. ng/ul | Highest Dilution | Genomes Detected a | DNA-b RNA/Rx |
| Adenovirus 4 | 4,375a | 90 | 1∶10,000 | 1 | 10.0 pg |
| B. pertussis | 4,287,000 | 15 | 1∶10,000 | 429 | 3.0 pg |
| C. pneumoniae | 438a | 116 | 1∶100,000 | 1 | 2.3 pg |
| Coronavirus 229E | 4,375a | 128 | 1∶10,000,000 | 1 | 25.6 fg |
| Coronavirus HK | -c | - | - | - | - |
| Coronavirus OC43 | 43,750a | 760 | 1∶10,000 | 5 | 152 pg |
| Influenza A | ? | 4.7 | 1∶100,000 | (1) | 90 fg |
| Influenza B | 43,750a | 44.7 | 1∶1,000,000 | 1 | 89 fg |
| M. pneumoniae | 1,393,322 | 1 | 1∶1,000,000 | 1 | 2 fg |
| Parainfluenza Type 1 | 4,375,000a | 512 | 1∶100,000 | 44 | 10 pg |
| Parainfluenza Type 2 | - | - | - | - | - |
| Parainfluenza Type 3 | 43,750,000a | 40 | 1∶100,000 | 438 | 800 fg |
| Resp. Syncytial virus | 4,375a | 37 | 1∶100,000 | 1 | 740 fg |
| S. pyogenes | 9,775,345 | 16 | 1∶1,000,000 | 10 | 32 fg |
Based on culture titers provided by ATCC.
Based on nucleic acid concentrations.
Not tested (included for specificity).
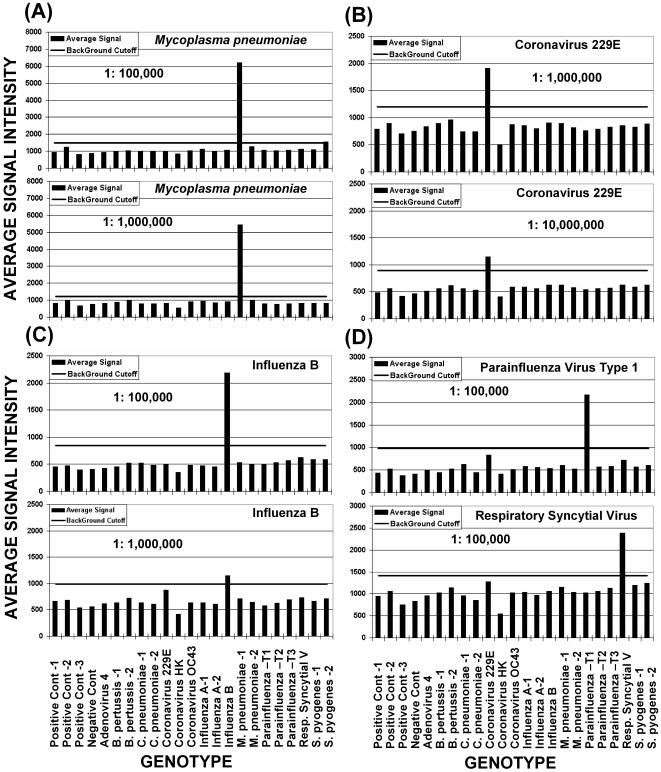
Figure 6. Results of sensitivity studies for Mycoplasma pneumoniae (A); coronavirus 229E (B); influenza B (C); and PIV 1, and RSV (D).
Vertical bars indicate the mean signal intensities in picoamps for positive genotypes (above cutoff bar) and negative genotypes (below cutoff bar). Horizontal bars indicate the assay cutoff, which is based on the mean of the negative control probes, plus 3 standard deviations of the mean. Relative probe electrochemical signal intensities in picoamps are shown to the left and probe identities are shown below. The identity of the pathogen tested and the dilution factor are shown on each graph. Beginning target nucleic acid concentrations are shown in Table 2.
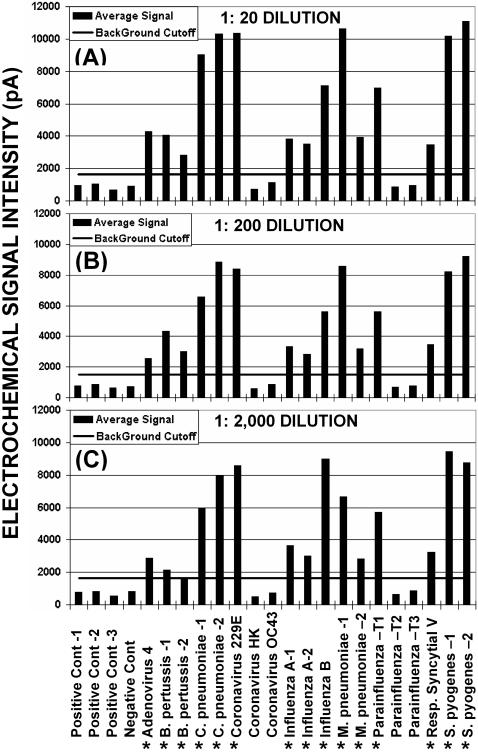
Figure 7. Example of sensitivity and specificity tests for multiple-organism assays.
Ten genotypes of interest (adenovirus 4, B. pertussis, C. pneumoniae, coronavirus 229E, influenza A, influenza B, M. pneumoniae, parainfluenza Type 1, respiratory syncytial virus, and S. pyogenes) were simultaneous amplified in two PCR reactions (5 DNA and 5 RNA targets) that were combined and hybridized on the same chip. Assays were run with pathogen nucleic acid dilutions of: (A) 1 to 20 (1∶20), (B) 1 to 200 (1∶200), and (C) 1 to 2,000 (1∶2000) that were based on the sample concentrations listed in Table 2. Vertical bars indicate average genotype probe signals in picoamps and horizontal bars indicate the assay cutoff (mean of negative control probes plus three standard deviations of the mean). Asterisks indicate the 10 target genomes that were amplified.
Signal specificity was determined by comparison of specific target signal to negative control probe signal and to signal from non-target probes. As shown in Figure 4A and B, very low signal (background) was seen on probes that were not specifically targeted. Probes for coronavirus and PIV represented three subtypes of each virus: coronavirus 229E, HK, and OC43; and PIV types 1, 2, and 3. When one subgroup was targeted, little signal was seen on probes for the other two subgroups as shown in Figures 6B, 6D and 7.
Discussion
A variety of multiplexed assays for the identification of mixed targets have been reported that use electrophoresis, molecular beacons, or enzymatic assays for detection [8], [37], [38]. Solid-phase PCR on microarrays has also been used for genotyping assays [39], [40]. This approach allows for either specific array probe extension and labeling for genome identification or on-chip isothermal, strand-displacement amplification. An assay combining a multiplexed RT-PCR platform with a library of photocleavable Masscode tags that are identified by mass spectrometry has been used to identify 22 respiratory pathogens [41]. Lin and colleagues [42] have used multiplexed RT-PCR combined with resequencing microarrays to identify multiple respiratory tract pathogens in a 12-hour assay. The majority of these approaches either do not provide sequence information, utilize expensive equipment that cannot be easily transported, require a high level of expertise to operate equipment or are not easily modified for additional pathogens.
In this study, we describe a rapid and sensitive genotyping assay for URI. This genotyping assay uses the same protocol to detect both bacteria and viruses that produce upper respiratory clinical symptoms; and it relies on the simultaneous examination of numerous loci to differentiate one organism from another. Microarray technologies can provide a significant amount of information at disparate loci in a highly redundant fashion that is helpful when discriminating between similar or different organisms or compensate for cases where genomic variation can result in false negative signals. In this array design, probes were developed to be species and sub-species-specific and then crosschecked against databases to minimize the possibility of cross-hybridization of target with other probes or with contaminating host NA. Multiple probes per pathogen (approximately 30 to 100) were used to overcome potential variability in pathogen genomes and also to provide sequence information at disparate loci. PCR primers were designed to flank the conserved regions that contained multiple complementary probe sequences. They also were designed such that the one-tube RT-PCR reaction produced biotin-labeled, single-strand target for hybridization to arrays. Decreasing the number of primer pairs per multiplex RT-PCR also increased assay sensitivity. Greatest sensitivity was achieved with one primer pair per genome of interest.
Equal labeling for GC-rich and AT-rich genomes was promoted by including both biotin-dCTP and biotin-dATP in PCR reactions. This is especially relevant for B. pertussis with a genome GC content of 67% and RSV with a GC content of 33%. Greater assay sensitivity was obtained by using 5′ biotinylated reverse primers in place of biotin-dCTP incorporation during amplification, as biotin-dCTP inhibits the PCR reactions (data not shown). However, with this increase in sensitivity, we also observed a slightly higher background signal and also a specific pattern of binding of free biotinylated primers to some probes. Removing microarray probes that bind to the free biotinylated primers can reduce this background artifact.
The arrays were validated by hybridizing labeled target that was generated from RNA or DNA from all viruses and bacteria represented on the upper respiratory chip, which includes influenza A and B; parainfluenza types 1, 2, and 3; adenovirus 4; respiratory syncytial virus; coronavirus 229E, HK, and OC43; Streptococcus pyogenes; Bordetella pertussis; Chlamydia pneumoniae; and Mycoplasma pneumonia. The arrays contain multiple probes per amplified region and multiple regions per organism that correspond to key distinguishing elements of each organism or subtype. However, to increase assay sensitivity, the final primer sets contained only one amplicon per organism. All virus and bacteria samples could be identified individually or in mixtures of 10 organisms within 5 hrs. Under optimal conditions, the assay was also sensitive, detecting 1 to 10 genomes, in most cases; and specific, detecting signal predominately on the pathogen probes of interest and not on probes for closely related species or serotypes as shown in Figures 4 through 7.
Studies to determine assay sensitivity were, in many cases, difficult to analyze because of complexities in quantifying target nucleic acid in a mixture with host nucleic acid. Most of the stock target bacterial DNA and viral RNA concentrations used in this study were based on PFU/CFU estimates provided by ATCC. Virus PFU were determined from cell culture lysates that would contain host cell nucleic acid. Given that one human cell contains approximately 7 pg of DNA, host cell nucleic acid contamination of samples could be very high. Thus, final results of assay sensitivity, based on PFU, could be underestimated in some cases.
In this paper, we demonstrate the usefulness of ECD and microchips as a diagnostic platform for genotyping URI. In addition, this type of microarray can easily be subdivided into multiple sub-arrays; e.g., 4 sectors of 2240 electrode features per array, which allows multiple assays on one array and provides significant cost savings. Also, because of the platform's flexibility, the array content and primer pools can easily be changed to reflect the needs of clinicians in different localities and different seasons. The high manufacturability of semiconductors and their adaptability to inexpensive electrochemical microarray readers, which are small and field deployable, make these assays much more powerful than simple Real Time-PCR systems. In essence, the array provides a much higher level of confidence in a result akin to DNA sequencing; and, in addition, upfront bioinformatics and judicious choice of probes and loci allow a greatly simplified and speedier analysis of complex organisms or mixtures thereof. Ghindilis et al. [35] have described electrochemical detection and the application of this technology on the CombiMatrix array platform. This platform is able to provide very similar sensitivity and reproducibility to state of the art fluorescent detection. Their analysis of data correlation between the electrochemical detection and standard fluorescent detection techniques demonstrated that the array-to-array correlation for both detection techniques is very high with an average correlation coefficient of 0.94 [35].
The use of multiplexed RT-PCR combined with electrochemical detection on micro-arrays, as described here, has several advantages over traditional assays, including RT-PCR alone: 1) The ElectraSense® ECD scanner is small, portable, and rugged and can easily be automated or incorporated as a “sub-system” into other genomic analysis systems; 2) The ECD scanner is faster, easier to use, and less expensive than fluorescence scanners; 3) Multiplexing is simpler because the array sorts out multiple signals that can arise from miss-priming in complex samples; 4) There is no need to sequence PCR products because the binding of multiple probes to specific targets provides sequence-like information; 5) This format is flexible, allowing for multiple probes, selection of the most reactive probes through iterative tests, and thus increased probability of making correct calls at low target concentrations and with rare isolates/subtypes. This platform also allows one to easily include the detection of single nucleotide polymorphisms and short stretches of sequence within an assay; and 6) A low level of technical expertise is needed to run assays and analyze data.
Disadvantages to using PCR for amplification are the possibility of contaminating reactions with amplicons from previous assays and amplifying genetic material from potential pathogens that are part of the normal flora. We are addressing amplicon contamination by developing a cartridge format for amplification, labeling, and hybridization of target. This cartridge contains the amplicons and is disposed of after analysis [36]. With respect to amplifying genetic material from pathogens that are part of the normal flora, PCR amplification is highly sensitive and is only qualitative at best. Therefore, the assay may (as with any standard bacteriological test) report the presence of pathogens in the absence of establishing a direct correlation with disease. As reported by Konno et al. [42], the flora of a healthy individual can contain pathogenic species (e.g., S. pyogenes, S. pneumoniae, H. influenzae and Moraxella catarrhalis). They detected these organisms in a group of healthy 0 to 6 year olds at approximately the same rate as in patients with acute upper respiratory infections. These organisms were also found in older healthy subjects (7 to 74 year olds) but at lower levels [43]; and they were also recovered at a higher rate in smokers than nonsmokers [44]. Clinicians must therefore verify the results of highly sensitive assays with patient symptoms that would suggest a bacterial infection. The inclusion of bacterial genetic markers for antibiotic resistance or virulence in microarray assays would also help guide the clinician in establishing an accurate diagnosis and appropriate treatment regimen.
Rapid identification of upper respiratory pathogens will significantly decrease the time and cost for the identification of potential lethal virus and bacterial strains and lead to better treatment and management of infections. Microarray and biosensor technologies show great promise for virus and bacteria detection and genotyping and are needed for rapid effective treatment, environmental monitoring and the detection of bioterrorism agents [22], [29]. Advances in microarray processing and simpler detection described here provide a path to migration of microarray technologies to clinical applications.
Materials and Methods
Viral and bacterial samples
Viral and bacterial samples were obtained from ATCC either as cultures (Influenza B: VR-823, PIV-1: VR-94, PIV-3: VR-93, Coronavirus OC43: VR-1558, Coronavirus 229E: VR-740, RSV-B: VR-1580, Adenovirus 4: VR-1572, and Chlamydia pneumoniae: VR-1355) or genomic DNA (Mycoplasma pneumoniae: 15531D). Dr. Wanda Lyon provided nucleic acid (NA) for additional samples, including Influenza A, Bordetella pertussis, and Streptococcus pyogenes. Viral RNA was extracted from clinical samples and archived cultures with an RNAeasy kit (Qiagen, Chatsworth, CA) or from active cultures (ATCC, Manassas, VA) with Trizol (Invitrogen, Carlsbad, CA) following the manufacturer's protocol and precipitated with ethanol and sodium acetate (Sigma, St. Louis, MO) in the presence of yeast tRNA (Invitrogen) and glycogen (Ambion, Foster City, CA). Genomic DNA from bacterial cultures was isolated with Qiagen DNeasy columns. Chlamydia pneumoniae DNA was isolated by resuspending the culture pellet in 475 µl Tris-EDTA, adding 25 µl 10% SDS (Sigma) and 2 µl or 20 mg/ml proteinase K (New England Biolabs, Beverly, MA) and incubating over night at 37°C. After heat inactivation at 70°C for 20 min, DNA was extracted once with phenyl/chloroform/isoamyl alcohol (25∶24∶1; Sigma), twice with chloroform (Sigma), and precipitated with 3M sodium acetate and ethanol. Concentrations of RNA and DNA were measured with a NanoDrop ND-1000 Spectrophotometer (NanoDrop Technologies, Wilmington, DE). Positive control Arabidopsis DNA (RCA at 1 ng/µl) and RNA (CAP at 1 ng/µl) for RT-PCR reaction verification was obtained from Invitrogen (SpotReport-3 Array Validation System). Positive control RNA and DNA were diluted (1∶20) in a solution of 20 µl yeast tRNA (1 mg/ml; Invitrogen) and 4.8 µl BSA (10 mg/ml; New England Biolabs) in 475.2 µl 10 mM Tris pH 7.4 (dilution buffer) (Sigma).
Microarray, probe and primer design
Microarrays used in this study were ElectraSense® 12K microarrays (CombiMatrix Corp. Mukilteo, WA) that contain 12,544 individually addressable electrodes linked by the semiconductor circuitry. Contact pads on the array allow for electrical connectivity with an external device to control custom synthesis of unique probes at each electrode, and electrochemical detection with the ElectraSense® microarray reader (CombiMatrix Corp). Each electrode has a distinct DNA probe above it, and each electrode can be read electronically or fluorescently to determine the level of hybridization for a specific DNA sequence.
Probe and primer design was achieved by aligning URI pathogen sequences obtained from GenBank and finding suitable regions for amplification and detection of each organism. A context database was then created for each organism to be typed. This was accomplished by collecting all available genomic sequences sharing the same genus or serotype as the sequence being investigated. Next, a structured BLAST database was created by incorporating genus and species information into the label of each context genomic sequence. For each organism to be typed, the gene sequences were extracted from the genomic record using the GenBank annotation. These gene sequences were then searched against the context database. All the matches between a gene of interest and a given context database genome were parsed to reflect each gene's conservation within that related genome. The specificity of each gene was reported in relation to the context database.
Genes were chosen for probe design based on their specificity within the organism's home clade. A combination of sub-type-specific, species-specific as well as clade-specific genes were chosen. The desired specificity for gene choice depended on what one wanted to discern within the topography of the organism's home-clade. In general, for each organism of interest, genes were chosen whose specificities included unique as well as conserved sequences. If discernment was to be made between two closely related organisms, more specific genes that would allow discrimination were chosen. Five to ten genes were initially selected for each organism of interest. For each gene chosen, isothermal Tm 60°C probes were designed in half-probe-length steps across each sequence. Only good-quality probes, as defined by convention, were accepted. For example, probes could not contain repeat region greater than 6 bp in length, significant secondary structure, or GC percentage less than 35% or greater than 65%.
After design, the probes were searched against the context database and their specificity was reported with respect to the context database. Regions within genomes that had the desired specificity were then selected. These regions were approximately 500 bp in length, and contained the probes that exhibited the desired specificity. The first and last probes of each region were designated as amplifying primers (the reverse primer was anti-sense), and the sequences in between were used as probes on the microarray. Where possible, PCR primers were chosen that were conserved within the clade. The forward primers were created with a Tm of approximately 50°C where as reverse primers were created with a Tm of approximately 65°C. This differential in temperature allowed for both amplification and single-strand target production. Bacterial and viral genes used for probe and primer selection are listed in Table 1.
Target amplification and labeling
Two methods were used for labeling NA during RT-PCR: biotin-14-dCTP incorporation (see Fig. 1) or biotinylated reverse primers (Integrated DNA Technologies, Coralville, IA). Twenty-five µl reaction mixes included 12.5 µl of reaction buffer (Invitrogen; SuperScript III One-Step RT-PCR kit with Platinum Taq), 2 µl of 5 mM MgSO4, 0.7 µl of 0.4 mM biotin-14-dCTP (Invitrogen) or biotin-11-dATP (PerkinElmer, Boston, MA), 2 µl primer pool (IDT, Coralville, IA), 1 µl Arabidopsis positive control mix, 0.5 µl enzyme mix, 2 µl RNA or DNA sample (diluted in positive control dilution mix (see above) and 4.3 µl dH2O. Thermal cycling parameters were: (50°C–30 min)×1 cycle; (94°C–3 min)×1 cycle; (94°C–30 sec, 56°C–45 sec, 72°C–45 sec)×40 cycles; (94°C–30 sec, 68°C–60 sec)×30 cycles and (72°C–5 min)×1 cycle. Amplification product was initially identified by loading 3.0 µl of each reaction product on 6% polyacrylamide gels (Invitrogen) and staining with SYBR Green I (Molecular Probes, Invitrogen). For ECD assay sensitivity, 10-fold serial dilutions of NA samples (10-1 to 10-8) were made in buffer containing 40.0 µg yeast tRNA (Invitrogen) and 9.6 µg BSA (NEB) per 1.0 ml of dH2O.
PCR optimization
Each PCR reaction component, including magnesium, biotin and primer pool concentration, was tested individually at various concentrations. Primer pairs were tested individually with their respective template, and each primer sequence was tested “in silico” (Clone Manager Suite, Version 7.1, Scientific and Educational Software Durham, NC) for interactions with multiplexed primers to eliminate cross reactivity. PCR temperatures and dwell times were adjusted for optimal sensitivity. The composition of primer pools was tested by varying the number of primer sets for each target organism (see Table 1).
Microarray hybridization and electrochemical detection
Initially, microarrays were incubated for 30 min at 45°C in 50 µl of a solution consisting of 5 ml of 2×hybridization solution (see below), 1 ml of 50×Denhardt's solution (Sigma) and 0.5 ml of 1% SDS (Sigma). For hybridization (see Fig. 8), PCR reactions from primer pools were combined and mixed 1∶1 with 2×hybridization buffer, which consisted of 6 ml of 20×SSPE (Ambion, Austin, TX), 0.1 ml of 10% Tween 20 (Sigma), 0.56 ml of 0.5 M EDTA (Ambion), 0.5 ml of 1% SDS (Sigma) and 3.84 ml of dH2O (Ambion). Microarray hybridization chambers were filled (50 µl volume) and sealed with tape. The arrays were incubated for 1 hr at 45°C with rotation in a hybridization oven (Fisher Scientific, Pittsburgh, PA) and washed for 5 min at 45°C with 3× SSPE with 0.05% Tween 20; twice with 2×PBS with 0.1% Tween 20 (PBST); and then blocked for 5 min with 5×PBS/Casein (BioFX Laboratories, Owing Mills, MD). For labeling, microarrays were incubated for 30 min with ExtrAvidin Peroxidase (Sigma) diluted 1∶1000 in BSA Peroxidase Stabilizer (BioFX). Arrays were washed twice with 2×PBST, and twice with pH4 Conductivity Buffer Substrate (BioFX). TMB Conductivity 1 Component HRP Microwell Substrate (BioFX) was added to the array, and it was scanned immediately with an ElectraSense® microarray reader (CombiMatrix Corp). This instrument measures μA at each of 12,544 electrodes on the array in 25 sec and outputs data in picoamps to a simple text file that can be used to create a pseudoimage or can be transferred to and graphed with an Excel macro.
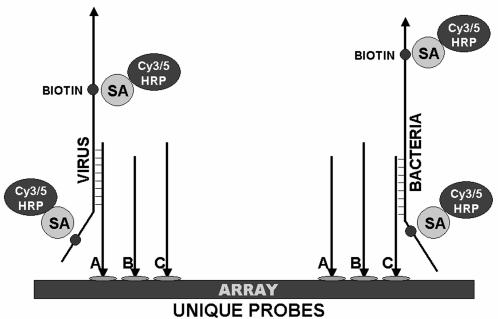
Figure 8. Hybridization and detection of viral and bacterial targets on microarrays.
Biotinylated, single-strand target is hybridized to complementary probes on the microarray (A, B and C) and labeled by the addition of streptavidin (SA)-horseradish peroxidase (HRP) (for electrochemical detection) or streptavidin-Cy3/Cy5 (for fluorescent detection).
Data analysis
For the analysis of raw intensity data from arrays, we developed a simple sorting and averaging algorithm in Excel. The organization of the data was keyed to the probe name. (e.g.: Case 1) PosCntrl-ATRCA|Probe-031; Case 2) PosCntrl-Cab|Probe-004; Case 3) Corona-229E|Probe-019; and Case 4) Corona-HK|Probe-001). Probe sequence information was organized and stored according to organism type and sub-type; with the sub-type section of the probe name occurring before the main delimiter, the bar (“|”), and the type section of the probe name appearing before the dash (“-“), when present. Thus the types for Cases 1 and 2 are “PosCntrl”, and the sub-type for Cases 3 and 4 are “Corona-229E” and “Corona-HK”, respectively. The data could be further divided into individual amplicon-specific probes. Once broken down into their groupings, the average signal for each type and sub-type was calculated, presented and graphed as a simple bar graph in picoamps. For an average signal to be considered significant, it had to be greater than three standard deviations above the mean signal of the negative controls. This calculation is termed the Assay Cut-off and is graphed as a horizontal line along the bar graph for simplicity of presentation and interpretation. Through this method of analysis, multiple positive results may be detected in a single assay.
Acknowledgments
We thank Mike Webster for help with microarray design and Luisa Dougan, Jonathan Adler, Dale Herauf, Michael Ottgen, and Jenna Gravley for synthesis and quality control of microarray chips. We also thank Brooke Anderson for help with figures.
Footnotes
Competing Interests: This is a research and development project funded by CombiMatrix Corporation (M.J.L., D.S., J.L.W., A.G.S., M.R., S.M., K.D., K.B., D.L.D., and A.M.). MQ and NI are employees of CHPPM-West at Fort Lewis, Washington and WJL is an employee of the US Air Force Research Laboratory, Wright-Patterson AFB, OH.
Funding: This is a research and development project funded by CombiMatrix Corporation (MJL, DS, JLW, AGS, MR, SM, KD, KB, DLD, and AM). MQ and NI are employees of CHPPM-West at Fort Lewis, Washington and WJL is an employee of the US Air Force Research Laboratory, Wright-Patterson AFB, OH.
References
- 1.Costa LF, Yokosawa J, Mantese OC, Oliveira TFM, Silveira HL, et al. Respiratory viruses in children younger than five years old with acute respiratory disease from 2001 to 2004 in Uberlandia, MG, Brazil. Mem Inst Oswaldo Cruz. 2006;101:301–306. doi: 10.1590/s0074-02762006000300014. [DOI] [PubMed] [Google Scholar]
- 2.Louie JK, Hacker JK, Gonzales R, Mark J, Maselli JH, et al. Characterization of viral agents causing acute respiratory infection in a San Francisco University medical center clinic during the influenza season. Clin Infect Dis. 2005;41:822–828. doi: 10.1086/432800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Makela MJ, Puhakka T, Ruuskanen O, Leinonen M, Saikku P, et al. Viruses and bacteria in the etiology of the common cold. J Clin Microbiol. 1998;36:539–542. doi: 10.1128/jcm.36.2.539-542.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dosh SA. Predictors of antibiotic prescribing for nonspecific upper respiratory infections, acute bronchitis, and acute sinusitis. J Fam Pract. 2000;49:407–414. [PubMed] [Google Scholar]
- 5.Liu HH. Overuse of antimicrobial therapy for upper respiratory infections and acute bronchitis: Who, why, and what can be done? Pharmacotherapy. 1999;19:371–373. doi: 10.1592/phco.19.6.371.31051. [DOI] [PubMed] [Google Scholar]
- 6.Mainous AG, III, MacFarlane LL, Connor MK, Green LA, Fowler K, et al. Survey of clinical pharmacists' knowledge of appropriateness of antimicrobial therapy for upper respiratory infections and acute bronchitis. Pharmacotherapy. 1999;19:388–392. doi: 10.1592/phco.19.6.388.31036. [DOI] [PubMed] [Google Scholar]
- 7.Cram P, Blitz SG, Monto A, Fendrick AM. Diagnostic testing for influenza: review of current status and implications of newer treatment options. Am J Man Care. 1999;5:1555–1561. [PubMed] [Google Scholar]
- 8.Templeton KE, Scheltinga SA, Beersma MFC, Kroes ACM, Claas ECJ. Rapid and sensitive method using multiplex real-time PCR for diagnosis of infections by influenza A and influenza B viruses, respiratory syncytial virus, and parainfluenza viruses 1, 2, 3, and 4. J Clin Microbiol. 2004;42:1564–1569. doi: 10.1128/JCM.42.4.1564-1569.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schwieger B, Zadow I, Heckler R, Timm H, Pauli G. Application of a fluoragenic PCR assay for typing and subtyping of influenza viruses in respiratory samples. J Clin Microbiol. 2000;38:1552–1558. doi: 10.1128/jcm.38.4.1552-1558.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Allwinn R, Preiser W, Rabenau H, Buxboum S, Sturmer M, et al. Laboratory diagnosis of influenza—virology or serology? Med Microbiol Immunol (Berl) 2002;191:157–160. doi: 10.1007/s00430-002-0137-y. [DOI] [PubMed] [Google Scholar]
- 11.Boivin G, Cote S, Dery P, Serres GD, Bergeron MG. Multiplex Real-time PCR assay for detection of influenza and human respiratory syncytial viruses. J Clin Microbiol. 2004;42:45–51. doi: 10.1128/JCM.42.1.45-51.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ecker DJ, Sampath R, Blyn LB, Eshoo MW, Ivy C, et al. Rapid identification and strain-typing of respiratory pathogens for epidemic surveillance. Proc Natl Acad Sci. 2005;102:8012–7. doi: 10.1073/pnas.0409920102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ellis JS, Zambon MC. Molecular diagnosis of influenza. Rev Med Virol. 2002;12:375–389. doi: 10.1002/rmv.370. [DOI] [PubMed] [Google Scholar]
- 14.Korimbocus J, Scaramozzino N, Lacroix B, Crance JM, Garin D, et al. DNA probe array for the simultaneous identification of herpesviruses, enteroviruses, and flaviviruses. J Clin Microbiol. 2005;43:3779–87. doi: 10.1128/JCM.43.8.3779-3787.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lin B, Vora GJ, Thach D, Waster E, Metzgar D, et al. Use of oligonucleotide microarrays for rapid detection and serotyping of acute respiratory disease-associated adenoviruses. J Clin Microbiol. 2004;42:3232–3239. doi: 10.1128/JCM.42.7.3232-3239.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lodes MJ, Suciu D, Elliott M, Stover AG, Ross M, et al. Use of semiconductor-based oligonucleotide microarrays for Influenza A virus subtype identification and sequencing. J Clin Microbiol. 2006;44:1209–1218. doi: 10.1128/JCM.44.4.1209-1218.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mentel R, Wegner U, Bruns R, Gurtler L. Real-time PCR to improve the diagnosis of respiratory syncytial virus infection. J Med Microbiol. 2003;52:893–896. doi: 10.1099/jmm.0.05290-0. [DOI] [PubMed] [Google Scholar]
- 18.Perkins SM, Webb DL, Torrance SA, El Saleeby C, Harrison LM, et al. Comparison of a real-time reverse transcriptase PCR assay and a culture technique for quantitative assessment of viral load in children naturally infected with respiratory syncytial virus. J Clin Microbiol. 2005;43:2356–62. doi: 10.1128/JCM.43.5.2356-2362.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Strålin K, Tornqvist E, Kaltoft MS, Olcen P, Holmberg H. Etiologic diagnosis of adult bacterial pneumonia by culture and PCR applied to respiratory tract samples. J Clin Microbiol. 2006;44:643–645. doi: 10.1128/JCM.44.2.643-645.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Taubenberger JK, Layne SP. Diagnosis of influenza virus: coming to grips with the molecular era. Mol Diagn. 2001;6:291–305. doi: 10.1054/modi.2001.28063. [DOI] [PubMed] [Google Scholar]
- 21.Wang D, Coscoy L, Zylberberg M, Avila PC, Boushey HA, et al. Microarray-based detection and genotyping of viral pathogens. PNAS. 2002;99:15687–15692. doi: 10.1073/pnas.242579699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zou S. A practical approach to genetic screening for influenza virus variants. J Clin Microbiol. 1997;35:2623–2627. doi: 10.1128/jcm.35.10.2623-2627.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McDonough EA, Barrozo CP, Russell KL, Metzgar D. A multiplex PCR for detection of Mycoplasma pneumoniae, Chlamydophila pneumoniae, Legionella pneumophila, and Bordetella pertussis in clinical specimens. Mol Cell Probes. 2005;19:314–322. doi: 10.1016/j.mcp.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 24.Metzgar D, Osuna M, Yingst S, Rakha M, Earhart K, et al. PCR analysis of Egyptian respiratory adenovirus isolates, including identification of species, serotypes, and coinfections. J Clin Microbiol. 2005;43:5743–5752. doi: 10.1128/JCM.43.11.5743-5752.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Amano Y, Cheng Q. Detection of influenza virus: traditional approaches and development of biosensors. Anal Bioanal Chem. 2005;381:156–184. doi: 10.1007/s00216-004-2927-0. [DOI] [PubMed] [Google Scholar]
- 26.Baxi MK, Baxi S, Clavijo A, Burton KM, Deregt D. Microarray-based detection and typing of foot-and-mouth disease virus. Vet J. 2006;172:473–81. doi: 10.1016/j.tvjl.2005.07.007. [DOI] [PubMed] [Google Scholar]
- 27.Bodrossy L, Sessitsch A. Oligonucleotide microarrays in microbial diagnosis. Curr Opin Microbiol. 2004;7:245–254. doi: 10.1016/j.mib.2004.04.005. [DOI] [PubMed] [Google Scholar]
- 28.Ivshina AV, Vodeiko GM, Kuznetsov VA, Volokhov D, Taffs R, et al. Mapping of genomic segments of influenza B virus strains by an oligonucleotide microarray method. J Clin Microbiol. 2004;42:5793–5801. doi: 10.1128/JCM.42.12.5793-5801.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kessler N, Ferraris O, Palmer K, Marsh W, Steel A. Use of the DNA flow-thru chip, a three-dimensional biochip, for typing and subtyping of influenza viruses. J Clin Microbiol. 2004;42:2173–2185. doi: 10.1128/JCM.42.5.2173-2185.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li J, Chen S, Evans DH. Typing and subtyping influenza virus using DNA microarrays and multiplex reverse transcriptase PCR. J Clin Microbiol. 2001;39:696–704. doi: 10.1128/JCM.39.2.696-704.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Roth SB, Jalava J, Ruuskanen O, Ruohola A, Nikkari S. Use of an oligonucleotide array for laboratory diagnosis of bacteria responsible for acute upper respiratory infections. J Clin Microbiol. 2004;42:4268–4274. doi: 10.1128/JCM.42.9.4268-4274.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sengupta S, Onodera K, Lai A, Melcher U. Molecular detection and identification of influenza viruses by oligonucleotide microarray hybridization. J Clin Microbiol. 2003;41:4542–4550. doi: 10.1128/JCM.41.10.4542-4550.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang D, Urisman A, Liu Y-T, Springer M, Ksiazek TG, et al. Viral discovery and sequence recovery using DNA microarrays. PLoS Biology. 2003;1:257–260. doi: 10.1371/journal.pbio.0000002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dill K, Montgomery DD, Ghindilis AL, Schwarzkopf KR, Ragsdale SR, et al. Immunoassays based on electrochemical detection using microelectrode arrays. Biosensors and Bioelectronics. 2004;20:736–742. doi: 10.1016/j.bios.2004.06.049. [DOI] [PubMed] [Google Scholar]
- 35.Ghindilis AL, Smith MW, Schwarzkopf KR, Roth K, Peyvan K, et al. CombiMatrix Oligonucleotide Arrays: Genotyping and Gene Expression Assays Employing Electrochemical Detection. Biosens Bioelectron. 2007;22:1853–60. doi: 10.1016/j.bios.2006.06.024. [DOI] [PubMed] [Google Scholar]
- 36.Liu RH, Lodes MJ, Nguyen T, Siuda T, Slota M, et al. Validation of a fully integrated microfluidic array device for influenza A subtype identification and sequencing. Anal Chem. 2006;78:4184–4193. doi: 10.1021/ac060450v. [DOI] [PubMed] [Google Scholar]
- 37.Grondahl B, Puppe W, Hoppe A, Kuhne I, Weigl JAI, et al. Rapid identification of nine microorganisms causing acute respiratory tract infections by single-tube multiplex reverse transcription-PCR; feasibility study. J Clin Microbiol. 1999;37:1–7. doi: 10.1128/jcm.37.1.1-7.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Syrmis MW, Whiley DM, Thomas M, Mackay IM, Williamson J, et al. A sensitive, specific, and cost-effective multiplex reverse transcriptase-PCR assay for the detection of seven common respiratory viruses in respiratory samples. J Molecular Diag. 2004;6:125–131. doi: 10.1016/S1525-1578(10)60500-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mitterer G, Huber M, Leidinger E, Kirisits C, Lubitz W, et al. Microarray-based identification of bacteria in clinical samples by solid-phase PCR amplification of 23S ribosomal CAN sequences. J Clin Microbiol. 2004;42:1048–1057. doi: 10.1128/JCM.42.3.1048-1057.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Westin L, Xu X, Miller C, Wang L, Edman CF, et al. Anchored multiplex amplification on a microelectronic chip array. Nature Biotech. 2000;18:199–204. doi: 10.1038/72658. [DOI] [PubMed] [Google Scholar]
- 41.Briese T, Palacios G, Kokoris M, Jabado O, Liu Z, et al. Diagnostic system for rapid and sensitive differential detection of pathogens. Emerg Infect Dis. 2005;11:310–313. doi: 10.3201/eid1102.040492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lin B, Wang Z, Vora GJ, Thornton JA, Schnur JM, et al. Broad-spectrum respiratory tract pathogen identification using resequencing DNA microarrays. Genome Res. 2006;16:527–535. doi: 10.1101/gr.4337206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Konno M, Baba S, Mikawa H, Hara K, Matsumoto F, et al. Study of upper respiratory tract bacterial flora: first report. Variations in upper respiratory tract bacterial flora in patients with acute upper respiratory tract infection and healthy subjects and variations by subject age. J Infect Chenother. 2006;12:83–96. doi: 10.1007/s10156-006-0433-3. [DOI] [PubMed] [Google Scholar]
- 44.Brook I, Gober AE. Recovery of potential pathogens and interfering bacteria in the nasopharynx of smokers and nonsmoters. Chest. 2005;127:2072–2075. doi: 10.1378/chest.127.6.2072. [DOI] [PubMed] [Google Scholar]