Abstract
A 1.8-kb cDNA clone was isolated from a Bothrops jararaca venom gland cDNA library that encodes a 256-aa precursor for bradykinin-potentiating peptides (angiotensin-converting enzyme inhibitors) and a C-type natriuretic peptide (CNP). The seven bradykinin-potentiating peptides are aligned tandemly after the hydrophobic signal peptide sequence, followed by a putative intervening sequence and a CNP at the C terminus. Northern blot analysis indicated the predominant expression of a 1.8-kb mRNA in the venom glands as well as in the spleen and the brain. Two lower intensity mRNA bands of 3.5 kb and 5.7 kb also hybridized to the cDNA clone. Radioimmunoassay for the CNP was performed using the antiserum against rat CNP. The presence of CNP immunoreactivity was detected in the low molecular weight fraction of the Bothrops jararaca venom.
The angiotensin-converting enzyme (ACE, EC 3.4.15.1) is the cytoplasmic membrane peptidase of endothelial cells responsible both for the conversion of angiotensin I into angiotensin II (1) and for bradykinin degradation (2, 3). This enzyme has been the critical metabolic target used by the pharmaceutical industry to generate antihypertensive drugs through the development of specific ACE inhibitors (ACEIs). Several ACEIs are currently used to treat human hypertension (4, 5). The anti-hypertensive effect of the ACEIs is not only explained by the preclusion of the hypertensive effect of angiotensin II but also by the potentiating hypotensive effect of the circulating bradykinin (3).
The bradykinin-potentiating oligopeptides (BPPs) present in B. jararaca (Bj) venom were the first naturally occurring ACEIs described (6, 7). The structure-activity studies of the BPPs and analogs were essential for the development of the nonpeptidic ACEIs (7). Thirteen Bj-BPPs, containing from 5 to 13 amino acid residues, have been purified and sequenced so far (8, 9). Except for BPP-Va, they all share characteristics, including (i) a pyroglutamyl residue at the N terminus, (ii) a high content of proline residues, and (iii) the tripeptide Ile-Pro-Pro at the C terminus. However, the gene encoding their precursor(s), as well as the processing enzyme(s), which give rise to mature BPPs, have not yet been identified. In this study we report the isolation of a B. jararaca clone from a venom gland cDNA library encoding seven BPPs, aligned in tandem. Surprisingly, this cDNA also encodes, at the C terminus, a polypeptide of 22 aa, which is homologous to the C-type natriuretic peptide (CNP) found in the brain and endothelial cells of mammals.
MATERIALS AND METHODS
Materials.
Restriction endonucleases and DNA-modifying enzymes were obtained from Takara Shuzo (Kyoto). Recombinant Pfu DNA polymerase was from Stratagene. Oligonucleotides were provided by Greiner (Tokyo). Digoxigenin-labeled dUTP, alkaline phosphatase-labeled anti-digoxigenin antibody, and blocking reagent were purchased from Boehringer Mannheim. Hybond-N nylon filters were from Amersham. BPP-Va was synthesized by solid-phase method by Luiz Juliano (Escola Paulista de Medicina, São Paulo, Brazil). Rat CNP-22 and the respective antiserum were from Peninsula Laboratories.
cDNA Library Construction and Screening.
Poly(A)+ RNA was prepared from the venom glands of a single B. jararaca using a Fast Track mRNA isolation kit (Invitrogen). cDNA was synthesized, cloned, and packed using the ZAP-cDNA synthesis kit and the ZAP-cDNA Gigapack II Gold Packaging Extract (Stratagene). To obtain a long, specific probe, an insert (coding region of a cDNA named NM29) was amplified by PCR using the sense (5′-ATGCCATGGTCCTCTCCCGCCT-3′) and antisense (5′-ATCAAGCTTCAGCAGCCCAGGCCG-3′) primers, the Pfu DNA polymerase, and digoxigenin-labeled dUTP. The locations of the primers are bp 173–190 and 928–946 for the sense and the antisense primers, respectively (see Fig. 1). The B. jararaca venom gland cDNA library was screened as follows: ≈104 recombinant phages were transferred to Hybond-N nylon filters and screened using the digoxigenin-labeled DNA probe. Prehybridization of the filters was performed for 1 h at 65°C in 500 mM phosphate buffer (pH 7.2), 7% SDS, and 1 mM EDTA, followed by hybridization for 16 h under the same conditions. The filters were washed three times in 40 mM phosphate buffer (pH 7.2), and 1% SDS at 65°C. The detection of positive plaques was performed by incubation with alkaline phosphatase-labeled anti-digoxigenin antibodies (1:10,000) in 100 mM Tris·HCl (pH 7.5), 150 mM NaCl, and 0.2% Tween 20, and visualized with a chemiluminescent substrate (CSPD, Tropix, Bedford, MA). The filters were exposed to x-ray film for 20 min at room temperature.
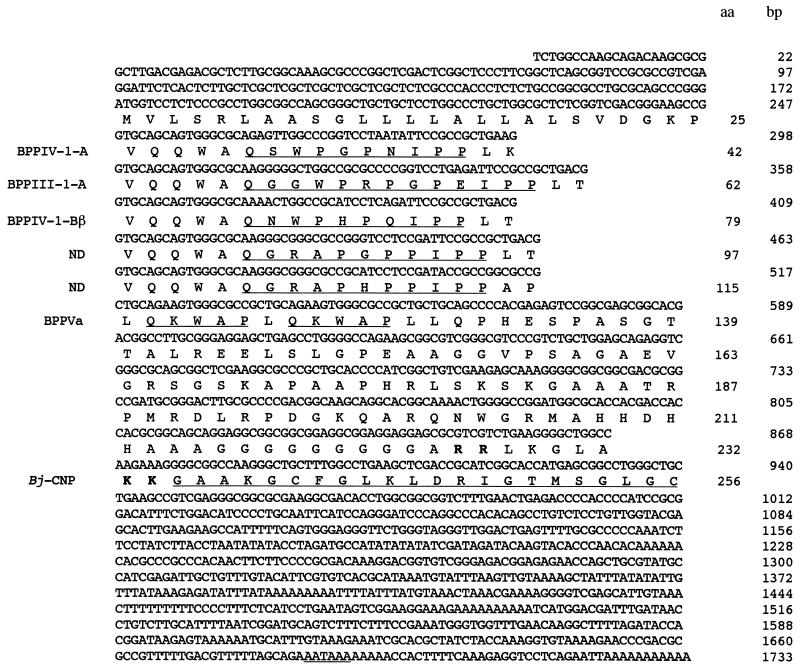
Figure 1.
Nucleotide and deduced amino acid sequences of a full-length cDNA clone (NM96) encoding BPPs and Bj-CNP. The 1733-nt sequence contains one major open reading frame encoding a 256-aa precursor of prepro-BPPs-Bj-CNP composed of 7 tandem stretches, each containing the sequence of a BPP isoform (underlined) and one sequence of the Bj-CNP. The BPP-encoding region is aligned in repetitive amino acid sequences corresponding to the first five BPPs. The left side of each repetitive sequence shows the five conserved amino acid residues of the intervening sequences. Numberings of base pairs (bp) of the cDNA clone and of the amino acids (aa) and in the preproprotein are indicated at right. The dibasic pairs of residues at positions amino acids 226–227 and amino acids 233–234 are in boldface type, and the poly(A) addition signal is underlined. The BPPs are identified by the numbers given in ref. 8.
DNA Sequencing.
A series of nested deletions of the clone were obtained using the kit of Takara Shuzo (Kyoto). The nucleotide sequence was determined by the dideoxy chain-termination method using the Texas Red-labeled primer and the Hitachi (Tokyo) SQ-5500 DNA sequencer with an automated electrophoresis detection system.
Sequence Alignment.
Homologies of DNA sequences and of amino acid sequences were analyzed using the dnasis program (Hitachi).
Northern Blot Analysis.
The tissues were obtained from one adult specimen B. jararaca sacrificed with ether. The brain, heart, lungs, liver, spleen, kidneys, and venom glands were dissected and rapidly immersed in liquid nitrogen until further processing. The tissues were homogenized and total RNA was isolated by a single-step method using guanidinium thiocyanate acid-phenol-chloroform extraction (10). Total RNA (10 μg) of B. jararaca whole tissues were submitted to electrophoresis in denaturing agarose gels (1.7% formaldehyde) and transferred to nylon membranes (11). The RNA was fixed on the membrane by UV crosslinking. Membranes were prehybridized overnight at 42°C in 50% formamide, 25 mM K2PO4 (pH 7.4), 5× SSC, 0.02% SDS, 5× Denhardt’s solution, 50 μg/ml herring sperm DNA, and 10% dextran sulfate (11). Hybridizations with the radiolabeled cDNAs were performed for 16 h at 42°C, adding the probe to the prehybridization solution (1.5 × 106 cpm/ml). The cDNA was radiolabeled with [α-32P]dATP using the random primer procedure (12). The blots were washed using high stringency conditions: four washes at 65°C with 2× SSC/0.1% SDS for 15 min, and three washes at 65°C with 0.1× SSC/0.1% SDS for 10 min. The blots were exposed to x-ray film for a suitable time. The intensities of the bands were measured using a densitometer.
Isolation of Low Molecular Weight Fraction of the B. jararaca Venom.
Crude venom (900 mg) was dissolved in 2.5 ml of 50 mM ammonium bicarbonate buffer (pH 8.0) and loaded onto a Sephadex G-25 column (115 × 1.2 cm, 130 ml). The material was eluted using the same buffer at a flow rate of 45 ml/h, and fractions of 2.0 ml were collected. The low Mr fraction (LMr) containing the bradykinin-potentiating activity was eluted between 76 and 112 ml. The other fractions were discarded.
Bradykinin Potentiation on Isolated Guinea Pig Ileum.
About 15 cm of the ileum distal of the guinea pigs of either sex (150–250 g body weight) were removed immediately after death and washed with Tyrode solution (137 mM NaCl/2.7 mM KCl/1.36 mM CaCl2/0.49 mM MgCl2/0.36 mM NaH2PO4/11.9 mM NaHCO3/5.04 mM d-glucose). Cut segments of 4.5 cm of the isolated ileum were mounted isotonically, under 1-g load, in 5 ml muscle bath containing Tyrode solution maintained at 37°C and bubbled with air. Recordings were made on a Gould 2600 polygraph (Gould, Cleveland). The bradykinin potentiation assays were performed according to Shimuta et al. (13).
Relaxing Activity on Isolated Rabbit Aortic Strips.
Thoracic aortas were isolated from 2-month-old female rabbits and cut into 5-mm strips. Arterial rings were mounted in an organ chamber and equilibrated at 37°C for 1 h in Krebs–Ringer solution (120 mM NaCl/4.8 mM KCl/1.2 mM CaCl2/1.3 mM MgSO4/25 mM NaHCO3/1.2 mM K2HPO4/5.8 mM d-glucose) gassed with 95% O2/5% CO2. Strips under 2 g of resting tension were conditioned by two contraction/relaxation cycles with 40 mM KCl, contracted by a third application of 40 mM KCl, and then relaxed with different combinations of rat synthetic CNP. Aortic strips were fixed to a UL5 microbalance and screwed to a UC2 isometric force transducer, which was connected to a model 50 amplifier (Gould). Changes in isometric tension were recorded on a Gould 2600 polygraph. The natriuretic peptide activity was assayed according to Schweitz et al. (14).
Radioimmunoassay.
A radioimmunoassay (15), using an antiserum developed against the rat CNP, was applied to the crude and to the partially purified B. jararaca venom.
RESULTS AND DISCUSSION
Sequence Analysis of the NM96 cDNA Clone.
During the analysis of a B. jararaca venom gland cDNA library, we serendipitously isolated a cDNA clone, NM29, which had 1743 bp in length and encoded a large plurifunctional prepropolypeptide containing several copies of BPPs and one copy of a CNP. We anticipated that the gene corresponding to this cDNA extended beyond the 5′ end of NM29. Thus, the coding region of this clone was used as a probe to isolate a complete cDNA clone from the same library. The screening, by hybridization, indicated that 16% of the clones were positive. Fourteen clones were analyzed and a single clone, NM96, was found to contain the longest sequence in its 5′ region, although its noncoding 3′ region was found to be shorter than that of NM29. The nucleotide sequence of NM96 showed that the insert is 1733 bp in length, with a predicted open reading frame of 768 nt encoding a precursor protein of 256 amino acid residues (Fig. 1). The 5′ and 3′ untranslated regions are 172 and 793 bp long, respectively. A single AATAAA polyadenylation signal was found 32 nt upstream of the poly(A)-tail. A search for nucleotide sequence homology revealed a segment of about 240 bp at the 5′ end (base pairs 22–261), which includes the sequence coding for the signal peptide, showing 62.1% homology (noncoding region) and 74.1% homology (coding region) to the corresponding regions of the cDNA encoding the sarafotoxins. The preprosarafotoxins, with 12 successive sequences of 6 different sarafotoxin isoforms were found in the venom of an Asiatic snake from the genus Atractapis. Sarafotoxins represent another class of vasoactive peptides homologous to endothelin (16).
Analysis of the Prepropolypeptide Encoded by cDNA NM96.
The preprobradykinin-potentiating polypeptide is composed of four distinct domains: a putative signal peptide of ≈22 aa located at the N terminus of the precursor, a BPP progenitor sequence of 101 aa (amino acids 26–127) containing seven BPPs, a linker sequence (amino acids 128–225) with unknown function, followed by a peptide of 31 amino acid residues, which is homologous to the CNP (17). The BPP domain is rich in tryptophan (9.2%), proline (26.2%), and glutamine (18.5%) compared with globular proteins, which have an abundance of 1.2%, 4.5%, and 3.9% of these amino acids, respectively (18). The BPP domain comprises five copies of BPPs of 10–13 amino acid residues, flanked by conserved sequences of 5 aa (VQQWA), and dual repeats of the trimmed BPP motif of 5 aa (Fig. 1). Each BPP possesses a glutamine residue at the N terminus and a proline residue at the C terminus. Four of the six types of BPPs (BPP IV-1-A, BPP III-1-A, BPP IV-1-Bβ, and BPP-Va) present in this precursor are identical to those previously reported (8). The other two putative BPPs have not yet been described, and their complete characterization as bradykinin-potentiating peptides and as ACE inhibitors should be confirmed. Differing from the deciphered sequences, the amino-terminal residues of these peptides are pyroglutamates. For the completion of the BPPs’ biosynthesis, the N-terminal glutamine residues might be circularized by the action of a pyroglutaminyl cyclase. Recently, we isolated a cDNA encoding a putative pyroglutaminyl cyclase from the cDNA library of the B. jararaca venom glands (unpublished results). The putative processing mechanism leading to the release of the mature BPPs does not seem to follow the well known bioactive peptide processing pathway, which relies on the action of Kex2-like serine proteases, acting toward dibasic amino acid residues (19). No typical prohormone processing signals flanking the BPP sequences were found. The presence of Lys-24, immediately preceding the proximal BPP (BPP IV-1-A), might serve as recognition for the signal peptidase. The 31 aa at the C-terminal coding region of the precursor (Bj-CNP31) is highly homologous to the human and porcine CNP53 (17). It contains two typical processing signals, the dibasic pairs Arg-226/Arg-227 and Lys-233/Lys-234, separated from another by five amino acid residues (Fig. 2). The similarities between the mammalian CNP53 and the Bj-CNP31 includes the presence of a dibasic pair (Arg-Lys for the human- and porcine-CNP53, and Arg-Arg for the Bj-CNP31), five residues before the second processing signal and the Lys-Lys, which precedes Bj-CNP22, H-CNP22, and P-CNP22 (Fig. 2). Thus, the mature Bj-CNP22 could be liberated either by the two-steps mechanism involving cleavage on the carboxyl side of the Lys-233 followed by the removal of Lys-234 by an aminopeptidase, or by a single event at the carboxyl side of Lys-234. Similar to the formation of peptide hormones and neuropeptides in mammalian neuroendocrine cells, the processing of the Bj-CNP could be performed by a Kex2-like serine protease (19), an enzyme not yet described in the B. jararaca venom gland. In mammals the precursor forms of CNP are highly conserved. Indeed, the amino acid homology between the rat and the pig precursors is 97% (23), and between human and pig prepro-CNP is 96% (24). However, the precursor of Bj-CNP of the venom gland has no homology to the mammalian counterparts, except for the C-terminal 22-aa region, which is remarkably homologous to the mammalian CNP22: 19 out of the 22 amino acid residues are shared (Fig. 2). Another snake venom peptide, Dendroaspis angusticeps A-type natriuretic peptide (DNP), which is homologous to natriuretic peptides, was detected by Schweitz et al. (14). They isolated and sequenced a peptide of 38 amino acid residues from the venom of Green Mamba (Dendroaspis angusticeps), which is homologous to the mammalian ANP. However, the Bj-CNP indicates a much higher homology to mammalian CNPs in contrast to DNP, which shared only 11 amino acids (Fig. 2).
Figure 2.
Compared amino acid sequence of the C-terminal region of the CNP precursors of several vertebrates. DNP, Dendroaspis ANP (14); BJ, Bothrops jararaca; H-CNP, human CNP (17); P-CNP, pig CNP (17); C-CNP, chicken CNP (20); B-CNP, bullfrog CNP (21); E-CNP, eel CNP (22).
Northern Blot Analysis of Tissue Total RNAs.
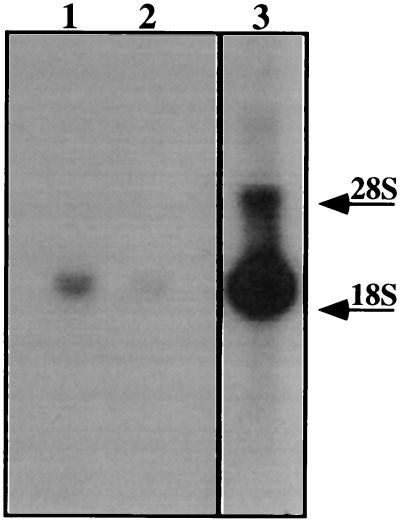
The Northern blot analysis, using the complete sequence of the BPPs-Bj-CNP precursor clone and performed under high stringency, indicated a very high expression of mRNAs of 1.8, 3.5, and 5.7 kb in total RNA from the B. jararaca venom glands (Fig. 3, lane 3). The 1.8-kb mRNA was clearly predominant and its expression was found to be about 6-fold and 90-fold higher than the 3.5-kb mRNA and the 5.7-kb mRNAs, respectively, as determined by densitometry. The presence of the 1.8-kb mRNA was also detected in 10 μg of total RNA from brain and spleen, but not in other tissues (heart, lung, liver, kidney, or skeletal muscles). The signals of the 1.8-kb mRNAs in spleen and brain were, respectively, 10-fold and 98-fold lower than in the venom gland. These results suggest the presence of the BPPs-Bj-CNP precursor encoding mRNA in the brain and the spleen of this animal. There have been no reports on the presence of mature BPPs in other tissues except in the B. jararaca venom gland. On the other hand, CNP has been identified in brain (25), and more recently, in endothelial cells (26) and macrophages (27). Whether or not the 1.8-kb mRNA is the mRNA encoding the precursor of the CNP of B. jararaca is a question that remains to be answered.
Figure 3.
Northern blot analysis of the total RNA isolated from the B. jararaca whole tissue. Total RNA (10 μg) of B. jararaca spleen (lane 1), brain (lane 2), and venom gland (lane 3) were hybridized to the radiolabeled cDNA of the BPP-Bj-CNP precursor. Exposure times were 2 h for the gland RNA and 5 h for the other tissues. The size standards on the right show the migration of rRNAs.
Biological Activities of the LMr Fraction of the B. jararaca Venom.
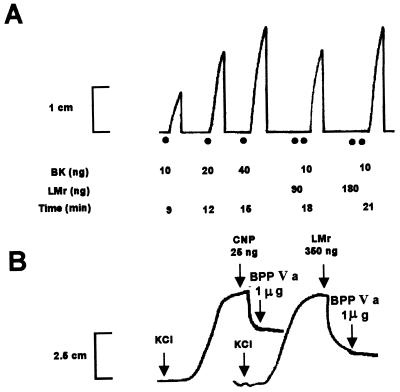
Direct evidence for the presence of the natriuretic peptide in the crude B. jararaca venom, both by the detection of its biological activity and by radioimmunoassay (RIA), was not possible due to the presence of phospholipases and proteases in the snake venom (28), which cause damage to the tissues used in the biological assays, or affect the immunoglobulins of the RIA. Thus, the bioassays and the RIA were performed in the LMr of the B. jararaca venom. The presence of mature Bj-CNP in the LMr fraction was detected by its relaxing activity on isolated rabbit aorta, in the same LMr fraction containing the bradykinin-potentiating activity of the snake venom (Fig. 4 A and B). The results presented in B show that the BPP-Va neither has intrinsic activity nor does it potentiate the vaso-relaxing activity of the rat CNP-22. The RIA for CNP confirmed this result, indicating the presence of 72 ± 15 ng of CNP-immunoreactive material per mg of protein.
Figure 4.
Biological activities of the LMr fraction of the B. jararaca venom. (A) The bradykinin potentiation on isolated guinea pig ileum indicates that 90 ng of the LMr fraction of the B. jararaca venom was necessary to double the effect of 10 ng of bradykinin. (B) The relaxing effects of either 25 ng of synthetic CNP or 350 ng of the LMr fraction of the B. jararaca venom on isolated rabbit aortic strips, previously contracted by KCl, were not affected by 1 μg of synthetic BPP-Va.
The results presented here indicate that at least two distinct classes of bioactive peptides, BPPs and Bj-CNP, displaying synergistic effects on blood pressure, most likely contribute to the cardiovascular effects caused by the B. jararaca envenoming. Thus, the hypotension provoked by the B. jararaca envenoming could be, in part, explained by the rapid diffusion of toxic substances due to the local vasodilation and increased capillary permeability (29). Once in the circulatory system, a B. jararaca kininogenase releases bradykinin from plasma kininogen (30) causing vasodilation, which is highly potentiated by the ACE inhibitory effect of the BPPs. In addition, the BPPs could increase the sensitivity of the bradykinin receptor of the smooth muscle (13, 31, 32) as well as activate the local release of bradykinin (33, 34), which aggravates the local and systemic vasodilation. Natriuretic peptides provide another means of achieving hypotension (35). The amount of the immunoreactive Bj-CNP that may be injected into the prey by a single snake bite (29) may contain 100–150 ng of Bj-CNP, which is more than 100-fold higher than the concentration of the ANP in the circulation (15).
Acknowledgments
We are grateful to Prof. J. Antunes Rodrigues (FMRP–University of São Paulo) for performing the RIA for the natriuretic peptide. We are also grateful to Patricia H. C. S. do Couto for technical assistance. This work was supported by Fundação de Amparo e Pesquisa do Estado de São Paulo, Conselho Nacional de Pesquisa, and by a grant-in-aid from the Shigaku-Shinko-Zaidan.
Footnotes
Abbreviations: BPPs, bradykinin-potentiating peptides; CNP, C-type natriuretic peptide; Bj-CNP, Bothrops jararaca CNP; DNP, Dendroaspis angusticeps A-type natriuretic peptide; ACE, angiotensin-converting enzyme; ACEI, ACE inhibitor; LMr, low Mr fraction.
Data deposition: The sequence reported in this paper has been deposited in the DDBJ, GenBank, and EMBL databases (accession no. D85843D85843).
References
- 1.Skews L T, Kahn J R, Shumway N P. J Exp Med. 1956;103:295–299. doi: 10.1084/jem.103.3.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yang H Y T, Erdos E G, Levin Y. Biochim Biophys Acta. 1970;214:374–376. doi: 10.1016/0005-2795(70)90017-6. [DOI] [PubMed] [Google Scholar]
- 3.Linz W, Wiemer G, Gohlke P, Unger T, Schølkens B A. Pharmacol Rev. 1995;47:25–49. [PubMed] [Google Scholar]
- 4.Unger T, Gohlke P. Cardiovasc Res. 1994;28:478–482. doi: 10.1093/cvr/28.2.146. [DOI] [PubMed] [Google Scholar]
- 5.Bönner G. In: Hypertension: Pathophysiology, Diagnosis and Management. Laragh J H, Baenner B N, editors. New York: Raven; 1995. pp. 2877–2893. [Google Scholar]
- 6.Ferreira S H. Br J Pharmacol. 1965;24:163–169. doi: 10.1111/j.1476-5381.1965.tb02091.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ondetti M A, Cushman D W. Annu Rev Biochem. 1983;51:283–308. doi: 10.1146/annurev.bi.51.070182.001435. [DOI] [PubMed] [Google Scholar]
- 8.Stewart J M. Handb Exp Pharmacol. 1979;25:227–265. [Google Scholar]
- 9.Cintra A C O, Vieira C A, Giglio J R. J Protein Chem. 1990;9:221–227. doi: 10.1007/BF01025312. [DOI] [PubMed] [Google Scholar]
- 10.Puissant C, Houdebine L. BioTechniques. 1990;8:148–149. [PubMed] [Google Scholar]
- 11.Ausubel F M, Brent R, Kingston R E, Moore D D, Seidman J G, Smith J A, Struhl K. Current Protocols in Molecular Biology. New York: Wiley Interscience; 1994. Suppl. 21, p. 4.9.7. [Google Scholar]
- 12.Harris D E, Warshaw D M, Periasamy M. Gene. 1992;112:265–266. doi: 10.1016/0378-1119(92)90388-6. [DOI] [PubMed] [Google Scholar]
- 13.Shimuta S I, Sabia E B, Paiva A C M, Paiva T B. Eur J Pharmacol. 1982;70:551–558. doi: 10.1016/0014-2999(81)90366-6. [DOI] [PubMed] [Google Scholar]
- 14.Schweitz H, Vigne P, Moinier D, Frelin C, Lazdunski M. J Biol Chem. 1992;267:13928–13932. [PubMed] [Google Scholar]
- 15.Gutkowska J, Horky K, Thibault G, Januszewicz P, Cantin M, Genest J. Biochem Biophys Res Commun. 1984;125:315–323. doi: 10.1016/s0006-291x(84)80370-8. [DOI] [PubMed] [Google Scholar]
- 16.Ducancel F, Matre V, Dupont C, Lajeunesse E, Vollberg Z, Bdolah A, Kochva E, Boulain J C, Menez A. J Biol Chem. 1993;268:3052–3055. [PubMed] [Google Scholar]
- 17.Tawaragi Y, Fuchimura K, Tanaka S, Minamino N, Kangawa K, Matsuo H. Biochem Biophys Res Commun. 1991;175:645–651. doi: 10.1016/0006-291x(91)91614-i. [DOI] [PubMed] [Google Scholar]
- 18.Poorman R A, Tommasselli A G, Heinrikson R L, Kézdi F J. J Biol Chem. 1991;266:14554–14561. [PubMed] [Google Scholar]
- 19.Lipkind G, Gong Q, Steiner D F. J Biol Chem. 1995;270:13277–13284. doi: 10.1074/jbc.270.22.13277. [DOI] [PubMed] [Google Scholar]
- 20.Arimura J J, Minamino N, Kangawa K, Matsuo H. Biochem Biophys Res Commun. 1991;174:142–148. doi: 10.1016/0006-291x(91)90497-u. [DOI] [PubMed] [Google Scholar]
- 21.Yoshihara A, Kozawa H, Minamino N, Kangawa K, Matsuo H. Biochem Biophys Res Commun. 1990;173:591–598. doi: 10.1016/s0006-291x(05)80076-2. [DOI] [PubMed] [Google Scholar]
- 22.Takei Y, Takahashi A, Watanabe T X, Nakajima K, Sakakibara S, Takao T, Shimonishi Y. Biochem Biophys Res Commun. 1990;170:883–891. doi: 10.1016/0006-291x(90)92174-x. [DOI] [PubMed] [Google Scholar]
- 23.Kojima M, Minamino N, Kangawa K, Matsuo H. FEBS Lett. 1990;276:209–213. doi: 10.1016/0014-5793(90)80544-s. [DOI] [PubMed] [Google Scholar]
- 24.Ogawa Y, Nakao K, Nakagawa O, Komatsu T, Hosoda K, Suga S, Arai H, Nagata K, Yoshida N, Imura H. Hypertension. 1992;19:809–813. doi: 10.1161/01.hyp.19.6.809. [DOI] [PubMed] [Google Scholar]
- 25.Minamino N, Kangawa K, Matsuo H. Biochem Biophys Res Commun. 1990;179:973–979. doi: 10.1016/0006-291x(90)92187-5. [DOI] [PubMed] [Google Scholar]
- 26.Suga S, Nakao K, Itoh H, Komatsu Y, Ogawa Y, Hama N, Imura H. J Clin Invest. 1992;90:1145–1149. doi: 10.1172/JCI115933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ishizuka Y, Kangawa K, Minamino N, Ishi K, Takano S, Eto T, Matsuo H. Biochem Biophys Res Commun. 1992;189:697–704. doi: 10.1016/0006-291x(92)92257-x. [DOI] [PubMed] [Google Scholar]
- 28.Stocker K F. In: Medical Use of Snake Venom Proteins. Stocker K F, editor. Boca Raton, FL: CRC; 1990. pp. 33–56. [Google Scholar]
- 29.Wen Fan H, Cardoso J L. In: Clinical Toxicology of Animal Venoms. Meier J, White J, editors. Boca Raton, FL: CRC; 1995. pp. 667–687. [Google Scholar]
- 30.Rocha e Silva M, Beraldo W T, Rosenfeld G. Am J Physiol. 1949;156:261–273. doi: 10.1152/ajplegacy.1949.156.2.261. [DOI] [PubMed] [Google Scholar]
- 31.Camargo A C M, Ferreira S H. Br J Pharmacol. 1971;42:305–307. doi: 10.1111/j.1476-5381.1971.tb07113.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Greene L J, Camargo A C M, Krieger E M, Stewart J M, Ferreira S H. Circ Res. 1972;30:62–71. [PubMed] [Google Scholar]
- 33.Wiemer G, Schølkens B A, Becker R H A, Busse R. Hypertension. 1991;18:558–563. doi: 10.1161/01.hyp.18.4.558. [DOI] [PubMed] [Google Scholar]
- 34.Hecker M, Porsti I, Bara A T, Busse R. Br J Pharmacol. 1994;111:238–244. doi: 10.1111/j.1476-5381.1994.tb14050.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Espiner E A. J Intern Med. 1994;235:527–541. doi: 10.1111/j.1365-2796.1994.tb01261.x. [DOI] [PubMed] [Google Scholar]