Abstract
Background
Static magnets are marketed with claims of effectiveness for reducing pain, although evidence of scientific principles or biological mechanisms to support such claims is limited. We performed a systematic review and meta-analysis to assess the clinical evidence from randomized trials of static magnets for treating pain.
Methods
Systematic literature searches were conducted from inception to March 2007 for the following data sources: MEDLINE, EMBASE, AMED (Allied and Complementary Medicine Database), CINAHL, Scopus, the Cochrane Library and the UK National Research Register. All randomized clinical trials of static magnets for treating pain from any cause were considered. Trials were included only if they involved a placebo control or a weak magnet as the control, with pain as an outcome measure. The mean change in pain, as measured on a 100-mm visual analogue scale, was defined as the primary outcome and was used to assess the difference between static magnets and placebo.
Results
Twenty-nine potentially relevant trials were identified. Nine randomized placebo-controlled trials assessing pain with a visual analogue scale were included in the main meta-analysis; analysis of these trials suggested no significant difference in pain reduction (weighted mean difference [on a 100-mm visual analogue scale] 2.1 mm, 95% confidence interval –1.8 to 5.9 mm, p = 0.29). This result was corroborated by sensitivity analyses excluding trials of acute effects and conditions other than musculoskeletal conditions. Analysis of trials that assessed pain with different scales suggested significant heterogeneity among the trials, which means that pooling these data is unreliable.
Interpretation
The evidence does not support the use of static magnets for pain relief, and therefore magnets cannot be recommended as an effective treatment. For osteoarthritis, the evidence is insufficient to exclude a clinically important benefit, which creates an opportunity for further investigation.
Magnets produce energy in the form of magnetic fields. Two main types of magnets exist: static or permanent magnets, in which the magnetic field is generated by the spin of electrons within the material itself, and electromagnets, in which a magnetic field is generated when an electric current is applied. Most magnets that are marketed to consumers for health purposes are static magnets of various strengths, typically between 30 and 500 mT. Magnets have been incorporated into arm and leg wraps, mattress pads, necklaces, shoe inserts and bracelets.1
Static magnets represent a multi-billion-dollar industry.2 They are marketed with claims of effectiveness for reducing pain of various origins. One survey suggested that about 28% of patients with rheumatoid arthritis, osteoarthritis or fibromyalgia use magnets or copper bracelets for pain relief.3 However, evidence for the scientific principles or biological mechanisms to support such claims is limited. According to one proposed mechanism, nociceptive C-fibres have a lower threshold potential, and magnetic fields selectively attenuate neuronal depolarization by shifting the membrane resting potential.4 Another theory suggests that magnetic fields promote an increase in blood flow through the skin and the subcutaneous and muscular tissues, which reduces the pain.5
In this systematic review and meta-analysis, we assessed the clinical evidence from randomized controlled trials of static magnets for treating pain.
Methods
Data search
The following databases were searched from inception to March 2007: MEDLINE, EMBASE, AMED (Allied and Complementary Medicine Database), CINAHL, Scopus, the Cochrane Library and the UK National Research Register. The search strategy was designed to retrieve all articles on the topic (using the terms “static,” “permanent,” “magnet” and “pain” and derivatives of these, according to the following strategy: “static” OR “permanent” AND “magnet” AND “pain”). In addition, we hand-searched conference proceedings (published in the journal FACT: Focus on Alternative and Complementary Therapies, 1996–2006), relevant medical journals (specifically, Phytomedicine, 1994–2006; Alternative and Complementary Therapies, 1995–2006; and Forschende Komplementärmedizin Klassische Naturheilkunde [Complementary medicine Research and Classical Naturopathy], 1994–2006) and our own collection of papers. We also searched the bibliographies of all retrieved articles by hand. There were no restrictions on the language of publication. For all relevant trials lacking data, we attempted to contact the corresponding author by email or regular mail for further information.
Data selection
For our analysis, we included only trials that were reported as randomized with a control consisting of nonmagnetic placebo or device with weak magnetic field strength and that had pain as an outcome measure. There were no restrictions on the condition causing the pain. The magnets had to be described as static or permanent, and only trials with human patients were considered. The titles and abstracts of the identified articles were independently assessed, and hard copies of all potentially relevant articles were obtained (by E.M.B. or E.E.or both) for further evaluation.
Validity assessment
Methodological quality was evaluated with the Jadad scoring system.6 The Jadad score was assessed independently by 2 of us (E.M.B and E.E.). Allocation concealment was assessed with use of the classification of the Cochrane Collaboration.7
Data extraction
Data were extracted systematically and independently (by E.M.B. and E.E.). We extracted data on study design, study quality, sample size, magnet strength, exposure, comparator and results. Any differences in extracted data, which were due mostly to reading errors, were resolved by discussion.
Quantitative data synthesis
The mean change in pain, as measured on a 100-mm visual analogue scale relative to baseline, was defined as the primary outcome and was used to assess the difference between static magnets and placebo. In the primary analysis, only randomized placebo-controlled trials were assessed on the basis of data from the end of the treatment period. Means and 95% confidence intervals (CIs) were calculated using standard meta-analysis software (RevMan 4.27, Update Software Ltd., Oxford, UK). Summary estimates of treatment effect were calculated using the more conservative approach of a random-effects model. Differences compared with placebo were considered relevant in the context of this study. The χ2 test and the Higgins I2 test were used to assess heterogeneity.7 We attempted to assess publication bias using a funnel plot, whereby effect estimates of the common outcome measure were plotted against sample size. Post hoc sensitivity analyses were performed to test the robustness of the overall effect.
Results
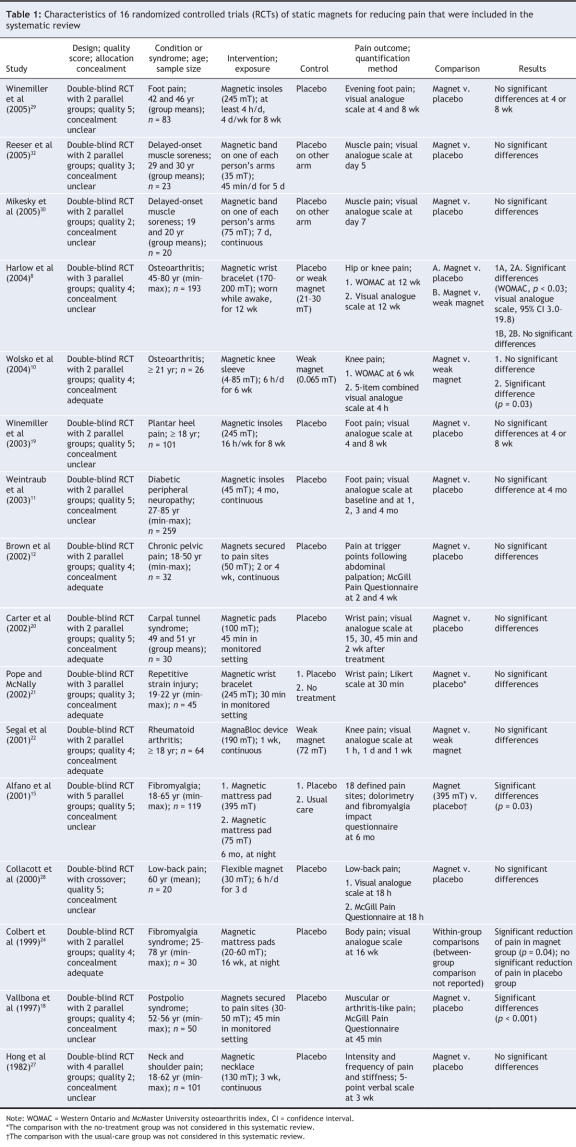
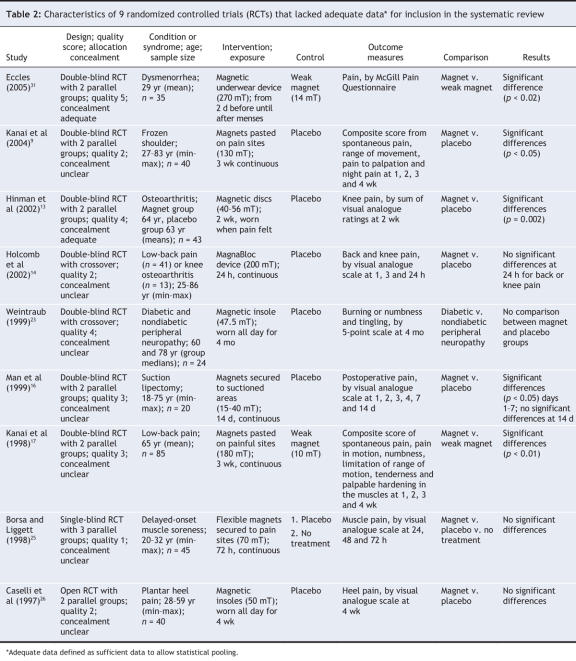
Twenty-nine potentially relevant trials8–36 were identified (Table 1 and Table 2). All trials were published in English, and all except 2 randomized controlled trials were double-blinded. Four studies33–36 were excluded, 3 because they were reported as abstracts only and could not be fully appraised and 1 because it compared 2 strong magnetic fields (Figure 1). In 2 other cases, additional information was requested but was not received.13,16 Four randomized controlled trials assessed patients with peripheral joint osteoarthritis, and 3 were available for each of low-back pain, delayed-onset muscle soreness and foot pain. There was no other condition for which more than 2 randomized trials were available (Table 1 and Table 2). Five trials8,10,17,31,22 used weak magnets, most of them below the assumed therapeutic strength (believed to be 30 mT),8 as the control.
Table 1
Table 2
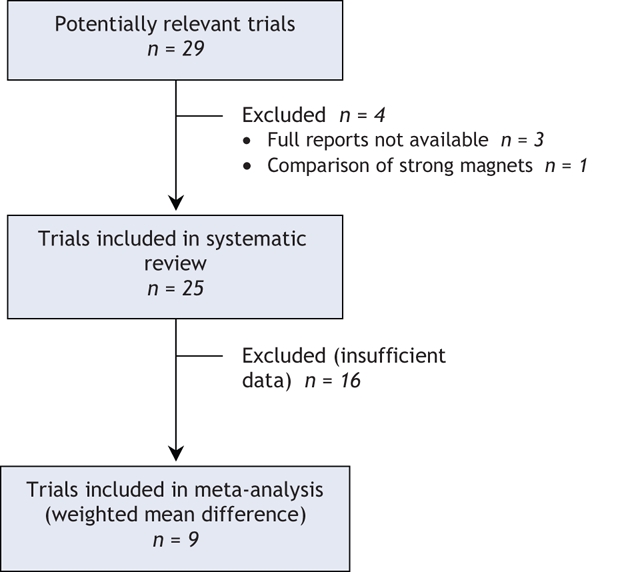
Figure 1: Selection of studies for meta-analysis. In addition to the 9 studies included in the meta-analysis of weighted mean difference, 16 studies were analyzed by standardized mean difference.
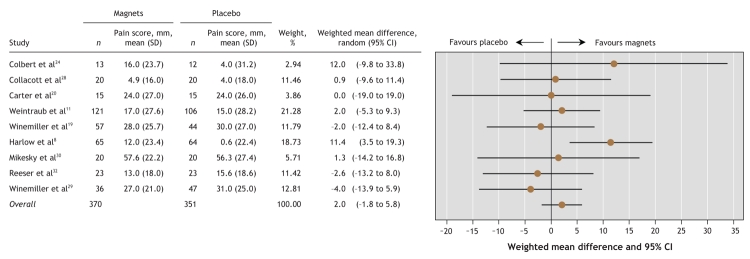
Meta-analysis of the 9 trials that assessed pain on a 100-mm visual analogue scale (Figure 2) indicated no significant difference in pain reduction between the magnet and placebo groups (weighted mean difference 2.1 mm, 95% CI –1.8 to 5.9 mm, p = 0.29). The χ2 test for heterogeneity indicated that the observed differences between trial results were unlikely to have been caused by chance (χ2 = 9.03, degrees of freedom [df] = 8, p = 0.34; I2 = 11.4%).
Figure 2: Effects of static magnets for treating pain. The outcome is the weighted mean difference in pain reduction (relative to baseline) on a 100-mm visual analogue scale, with 95% confidence intervals (CIs). The vertical line represents no difference between magnets and placebo. SD = standard deviation.
However, the issue of clinical heterogeneity remains. In particular, differences in the conditions causing the pain and differences in the duration of the intervention contributed to this clinical heterogeneity. A post hoc sensitivity analysis, excluding 3 short-term randomized trials20,28,32 with intervention periods between 45 minutes and 18 hours, suggested no significant difference between the magnet and placebo groups (weighted mean difference on a 100-mm visual analogue scale 2.9 mm, 95% CI –2.5 to 8.3 mm, p = 0.29; χ2 = 7.92, df = 5, p = 0.16; I2 = 36.8%). Another sensitivity analysis of randomized controlled trials assessing only musculoskeletal pain conditions with intervention periods between 2 and 4 months8,19,24,29 also suggested no significant difference (weighted mean difference 3.5 mm, 95% CI –5.5 to 12.4 mm, p = 0.45; χ2 = 7.67, df = 3, p = 0.05; I2 = 60.9%). Across all trials (Table 1 and Table 2), there was evidence of no effect for intervention periods between 30 minutes and 1 week. Assessment of publication bias using a funnel plot was attempted, but too few studies were available to allow any meaningful judgment.37 Analysis of the 16 trials that assessed pain using various scales (Table 1) suggested significant statistical heterogeneity among the trials, and pooling these data was therefore considered unreliable (standardized mean difference 0.23 mm, 95% CI 0.04 to 0.42 mm, p = 0.02; χ2 = 30.77, df = 15, p = 0.009; I2 = 51.2%).
Osteoarthritis was assessed in 4 double-blind randomized controlled trials8,10,13,14 (total sample size 275; Table 1 and Table 2). Two small trials (n = 26 and 43, respectively) reported some positive effects of static magnets relative to placebo and weak magnets.10,13 This finding was confirmed in a larger trial,8 which reported pain reductions (relative to placebo) on the Western Ontario and McMaster osteoarthritis index and a visual analogue scale. In these 3 trials,8,10,13 treatments lasting 2 to 12 weeks were associated with positive effects, whereas a small study of continuous 24-hour magnet treatment14 did not report such effects (Table 1 and Table 2). Low-back pain was assessed in 3 double-blind randomized controlled trials14,17,28 (total sample size 146). One of these trials (n = 85) suggested beneficial effects relative to a weak magnet,17 whereas the 2 smaller trials14,28 showed no significant differences relative to placebo. For each of delayed-onset muscle soreness and foot pain, 3 randomized controlled trials could be included (total sample sizes 88 and 224, respectively). All of these trials reported no significant differences on visual analogue scales for pain (relative to placebo) for magnet field strengths between 50 and 245 mT.
Interpretation
Overall, the meta-analysis suggested no significant effects of static magnets for pain relief relative to placebo. Peripheral joint osteoarthritis was the one condition for which the evidence appeared encouraging. For all other conditions, there was no convincing evidence to suggest that static magnets might be effective for pain relief. Given the possibility of small effects, if any, that cannot be excluded on the basis of the evidence, further study is warranted. However, whether additional time and funds should be devoted to this question is a matter of debate.
The strengths of our systematic review pertain to its rigour in terms of searching the literature, the inclusion and exclusion criteria, and the data assessment. Our analyses of data from randomized controlled trials have yielded a relatively robust indication of the effects of magnets on pain outcomes, although further trials are still required. We searched databases with a focus on the US and European literature, as well as specialist data sources, and included hand searches in relevant journals, with no restriction in terms of publication language. However, there remains a possibility that our search was incomplete.38–41
The limitations of our study pertain to the lack of rigour of the original studies, and (although the forest plot [Figure 2] indicates overlap of confidence intervals for all studies) to the heterogeneity of the trials. Clinical heterogeneity was evident in differences in the conditions causing pain and in the duration of the interventions. Two post hoc sensitivity analyses exploring these issues confirmed the results of the overall analysis. Another reason for clinical heterogeneity was the variation in magnet strength in the original studies, from 4 to 395 mT. Across all trials there was no convincing indication that high-strength magnets performed any better than low-strength magnets. Positive and negative studies were spread across magnet strengths, which suggests neither an optimal magnet strength nor a “window of time” when magnet therapy is effective for treating pain.
The success of blinding in magnet and placebo groups was not assessed in 18 of the randomized controlled trials.9,13–18,20–26,28,30–32 Nonspecific effects may have contributed to the observed effects and may even have been the main factor contributing to the findings in some trials. Six trials8,10,11,19,27,29 established that equal proportions of participants in the magnet and placebo groups believed they had been given magnetic devices; the 2 groups could thus be assumed to have similar expectations of pain relief. In 3 of 11 trials indicating a significant beneficial effect,8,10,11 blinding was demonstrated to have been adequate throughout the study. Among the trials of peripheral joint osteoarthritis, 2 trials8,10 reported adequate blinding. Also, the trials had mixed Jadad quality scores and largely suffered from a lack of adequate allocation concealment (Table 1 and Table 2). Most of the samples were small, with 17 of the randomized controlled trials assessing 50 or fewer patients (Table 1 and Table 2). Therefore, the possibility of a type 2 error cannot be excluded. However, across all trials with sample sizes above 100, there was no evidence of a convincing effect in favour of magnets. Future studies should be large enough to have an 80% chance of detecting possible effects, should include well-defined patient samples and should pay particular attention to the design of the placebo or sham magnet. From the existing evidence, the ideal magnet strength and treatment duration are unclear.
Static magnets are generally considered safe. Adverse effects are rare, but reddening of the skin on the area of application has been observed.1 Pacemakers, insulin pumps and other devices adversely affected by magnetic fields are considered contraindications for the use of static magnets.1
In conclusion, the evidence does not support the use of static magnets for pain relief, and such magnets therefore cannot be recommended as an effective treatment. For osteoarthritis, the evidence is insufficient to exclude a clinically important benefit, which creates an opportunity for further investigation.
Acknowledgments
We thank Hitoshi Yamashita PhD, Tsukuba College of Technology Clinic, Tsukuba, Japan, for the translation from Japanese to English.
Footnotes
Une version française de ce résumé est disponible à l'adresse www.cmaj.ca/cgi/content/full/177/7/736/DC1
This article has been peer reviewed.
Contributors: All of the authors made substantial contributions to the conception and design of the study, the acquisition, analysis and interpretation of data, and the drafting and revising of the article critically for important intellectual content. All gave final approval for the version to be published.
Competing interests: None declared.
Correspondence to: Dr. Max H. Pittler, Complementary Medicine, Peninsula Medical School, Universities of Exeter and Plymouth, 25 Victoria Park Rd., Exeter EX2 4NT, UK; fax +44 1392 427562; max.pittler@pms.ac.uk
REFERENCES
- 1.Ernst E, Pittler MH, Wider B, et al. Complementary and alternative therapies for pain management. London: Mosby/Elsevier; 2007.
- 2.Magnets and magnet materials. Norwalk (CT): BCC Research; 2006. Available for purchase through Market Research.com (www.marketresearch.com/product/display.asp?productid=1354460&xs=r).
- 3.Rao JK, Mihaliak K, Kroenke K, et al. Use of complementary therapies for arthritis among patients of rheumatologists. Ann Intern Med 1999;131:409-16. [DOI] [PubMed]
- 4.Lednev VV. Possible mechanism of weak magnetic fields on biological systems. Bioelectromagnetics 1991;12:71-5. [DOI] [PubMed]
- 5.Trock DH. Electromagnetic fields and magnets: investigational treatment for musculoskeletal disorders. Rheum Dis Clin North Am 2000;26:51-62. [DOI] [PubMed]
- 6.Jadad AR, Moore RA, Carroll D, et al. Assessing the quality of reports of randomized clinical trials: Is blinding necessary? Control Clin Trials 1996;17:1-12. [DOI] [PubMed]
- 7.Higgins JPT, Green S, editors. Cochrane handbook for systematic reviews of interventions 4.2.5 [Cochrane review]. In: The Cochrane Library; Issue 3, 2005. Oxford: Wiley Interscience.
- 8.Harlow T, Greaves C, White A, et al. Magnetic bracelets for relieving pain in lower-limb osteoarthritis: a randomised controlled trial. BMJ 2004;329:1450-4. [DOI] [PMC free article] [PubMed]
- 9.Kanai S, Taniguchi N, Kawamoto M, et al. Effect of static magnetic field on pain associated with frozen shoulder. Pain Clinic 2004;16:173-9.
- 10.Wolsko PM, Eisenberg DM, Simon LS, et al. Double-blind placebo-controlled trial of static magnets for the treatment of osteoarthritis of the knee: results of a pilot study. Altern Ther Health Med 2004;10:36-43. [PubMed]
- 11.Weintraub MI, Wolfe GI, Barohn RA, et al. Static magnetic field therapy for symptomatic diabetic neuropathy: a randomised, double-blind, placebo-controlled trial. Arch Phys Med Rehabil 2003;84:736-46. [DOI] [PubMed]
- 12.Brown CS, Ling FW, Wan JY, et al. Efficacy of static magnetic field therapy in chronic pelvic pain: a double-blind pilot study. Am J Obstet Gynecol 2002;187: 1581-7. [DOI] [PubMed]
- 13.Hinman MR, Ford J, Heyl H. Effects of static magnets on chronic knee pain and physical function: a double-blind study. Altern Ther Health Med 2002;8:50-5. [PubMed]
- 14.Holcomb RR, Parker RA, Harrison MS. Biomagnetics in the treatment of human pain-past, present, future. Available: www.holcombhealthcare.com/reports/pub-bio.html (accessed 2006 Aug 29).
- 15.Alfano AP, Taylor AG, Forresman PA, et al. Static magnetic fields for treatment of fibromyalgia: a randomized controlled trial. J Alternat Complement Med 2001;7:53-64. [DOI] [PubMed]
- 16.Man D, Man B, Plosker H. The influence of permanent magnetic field therapy on wound healing in suction lipectomy patients: a double-blind study. Plast Reconstr Surg 1999;104:2261-8 [DOI] [PubMed]
- 17.Kanai S, Okano H, Susuki R, et al. Therapeutic effectiveness of static magnetic fields for low back pain monitored with thermography and deep body thermometry. J Jpn Soc Pain Clin 1998;5:5-10.
- 18.Vallbona C, Hazlewood CF, Jurida G. Response of pain to static magnetic fields in postpolio patients: a double-blind pilot study. Arch Phys Med Rehabil 1997;78:1200-3. [DOI] [PubMed]
- 19.Winemiller MH, Billow RG, Laskowski ER, et al. Effect of magnetic vs sham-magnetic insoles on planter heel pain. JAMA 2003;290:1474-8. [DOI] [PubMed]
- 20.Carter R, Hall T, Asby CB, et al. The effectiveness of magnet therapy for treatment of wrist pain attributed to carpal tunnel syndrome. J Fam Pract 2002;51:38-40. [PubMed]
- 21.Pope KW, McNally RJ. nonspecific placebo effects explain the therapeutic benefit of magnets. Sci Rev Altern Med 2002;6:13-6.
- 22.Segal NA, Toda Y, Huston J, et al. Two configurations of static magnetic fields for treating rheumatoid arthritis of the knee: a double blind clinical trial. Arch Phys Med Rehabil 2001;82:1453-60. [DOI] [PubMed]
- 23.Weintraub MI. Magnetic bio-stimulation in painful diabetic peripheral neuropathy: a novel intervention — a randomized, double-placebo crossover study. Am J Pain Manage 1999;9:8-17.
- 24.Colbert AP, Banerji M, Pilla AA. Magnetic mattress pad use in patients with fibromyalgia: a randomised double-blind pilot study. J Back Musculoskel Rehab 1999;13:19-31.
- 25.Borsa PA, Liggett CL. Flexible magnets are not effective in decreasing pain perception and recovery time after muscle microinjury. J Athl Train 1998;33:150-5. [PMC free article] [PubMed]
- 26.Caselli MA, Clark N, Lazarus S, et al. Evaluation of magnetic foil and PPT insoles in the treatment of heel pain. J Am Podiatr Med Assoc 1997;87:11-6. [DOI] [PubMed]
- 27.Hong CZ, Lin JC, Bender LF, et al. Magnetic necklace: its therapeutic effectiveness on neck and shoulder pain. Arch Phys Med Rehabil 1982;63:462-6. [PubMed]
- 28.Collacott EA, Zimmerman JT, White DW, et al. Bipolar permanent magnets for the treatment of chronic low back pain. JAMA 2000;283:1322-5. [DOI] [PubMed]
- 29.Winemiller MH, Billow RG, Laskowski ER, et al. Effect of magnetic vs sham-magnetic insoles on nonspecific foot pain in the workplace: a randomized, double-blind, placebo-controlled trial. Mayo Clin Proc 2005;80:1138-45. [DOI] [PubMed]
- 30.Mikesky AE, Hayden MW. Effect of static magnetic therapy on recovery from delayed onset muscle soreness. Phys Ther Sport 2005;6:188-94.
- 31.Eccles NK. A randomized, double-blinded, placebo-controlled pilot study to investigate the effectiveness of a static magnet to relieve dysmenorrhea. J Alternat Complement Med 2005;11:681-7. [DOI] [PubMed]
- 32.Reeser JC, Smith DT, Fischer V, et al. Static magnetic fields neither prevent nor diminish symptoms and signs of delayed onset muscle soreness. Arch Phys Med Rehabil 2005;86:565-70. [DOI] [PubMed]
- 33.Coghill R, Hails S, Connors C. Effects of a static magnetic field device on menstrual pain: a double-blind, placebo-controlled clinical trial [abstract]. Available: www.natural-period-pain-relief.com/uk_research.htm (accessed 2006 Aug 29).
- 34.Harper DW, Wright EF. Magnets as analgesics. Lancet 1977;2:47. [DOI] [PubMed]
- 35.Langford J, McCarthy PW. Randomised controlled clinical trial of magnet use in chronic low back pain; a pilot study. Clin Chiropractic 2005;8:13-9.
- 36.MacKrodt K. A comparative study of static magnetic field (SMF) therapy against transcutaneous electrical nerve stimulation (TENS) therapy on mechanical back pain and neck pain. Project summary available through: www.nrr.nhs.uk/ViewDocument.asp?ID=N0355127318 (accessed 2007 Aug 21).
- 37.Ioannidis JP, Trikalinos TA. The appropriateness of asymmetry tests for publication bias in meta-analyses: a large survey. CMAJ 2007;176:1091-6. [DOI] [PMC free article] [PubMed]
- 38.Dickersin K. The existence of publication bias and risk factors for its occurrence. JAMA 1990;263:1385-9. [PubMed]
- 39.Egger M, Davey Smith G. Bias in location and selection of studies. BMJ 1998;316:61-6. [DOI] [PMC free article] [PubMed]
- 40.Ernst E, Pittler MH. Alternative therapy bias [letter]. Nature 1997;385:480. [DOI] [PubMed]
- 41.Pittler MH, Abbot NC, Harkness EF, et al. Location bias in controlled clinical trials of complementary/alternative therapies. J Clin Epidemiol 2000;53:485-9. [DOI] [PubMed]