Abstract
Background
Previous work examining ethanol’s autonomic effects has found contrasting patterns of age-related differences in ethanol-induced hypothermia between adolescent and adult rats. Most studies have found adolescents to be less sensitive than adults to this effect, although other work has indicated that adolescents may be more sensitive than adults under certain testing conditions. To test the hypothesis that adolescents show more ethanol hypothermia than adults when the amount of disruption induced by the test procedures is low, but less hypothermia when the experimental perturbation is greater, the present study examined the consequences of manipulating the amount of perturbation at the time of testing on ethanol-induced hypothermia in adolescent and adult rats.
Methods
The amount of test disruption was manipulated by administering ethanol through a chronically indwelling gastric cannula (low perturbation) versus via intragastric intubation (higher perturbation) in Experiment 1 or by either familiarizing animals to the handling and injection procedure for several days pretest or leaving them unmanipulated before testing in Experiment 2.
Results
The results showed that the handling manipulation, but not the use of gastric cannulae, altered the expression of ethanol-induced hypothermia differentially across age. When using a familiarization protocol sufficient to reduce the corticosterone response to the handling and injection procedure associated with testing, adolescents showed greater hypothermia than adults. In contrast, the opposite pattern of age differences in hypothermia was evident in animals that were not manipulated before the test day. Surprisingly, however, this difference across testing circumstances was driven by a marked reduction in hypothermia among adults who had been handled before testing, with handling having relatively little impact on ethanol hypothermia among adolescents.
Conclusions
Observed differences between adolescents and adults in the autonomic consequences of ethanol were dramatically influenced by whether animals were familiarized with the handling/injection process before testing. Under these circumstances, adolescents were less susceptible than adults to the impact of experimental perturbation on ethanol-induced hypothermia. These findings suggest that seemingly innocuous aspects of experimental design can influence conclusions reached on ontogenetic differences in sensitivity to ethanol, at least when indexed by ethanol-induced hypothermia.
Keywords: Adolescence, Autonomic Nervous System, Ethanol, Hypothermia, Handling
During adolescence, organisms undergo dramatic changes in behavior and brain structure that may increase their vulnerability to drug use and abuse (for a review, see Spear, 2000). According to the Monitoring the Future Study, binge ethanol use (the consumption of 5 or more drinks in a row) increases steadily during adolescence, with a similar trend evident in ethanol abuse (Johnston et al., 2003). Additional research from the U.S. Department of Health and Human Services has indicated that as many as 48.7% of Americans between the ages of 18 and 25 reported binge drinking in 2001 (U.S. Department of Health and Human Services, 2002). Such ethanol exposure during adolescence may be associated with damage in certain forebrain regions (Crews et al., 2000) and long-lasting effects that persist into adulthood, including cognitive deficits (Tapert and Schweinsburg, 2005). Precisely why adolescents are more likely than adults to experiment with and abuse ethanol is not fully understood. One potential contributing factor is that adolescents may be less sensitive to ethanol effects that normally serve as cues to limit intake, perhaps permitting increased consumption relative to more mature animals (see Doremus et al., 2003; Ristuccia and Spear, 2005 for a discussion). Given that this issue is very difficult to investigate ethically in human adolescents, various animal models have been used.
Ontogenetic increases in ethanol sensitivity through adolescence have been reported in laboratory animals using a variety of response measures, including ethanol-induced motor impairment (e.g., White et al., 2002a, 2002b), sedation (Silveri and Spear, 1998), dysphoria (Shram et al., 2005), anxiolysis (Varlinskaya and Spear, 2002), and analgesia (Hernandez and Spear, in preparation). In contrast to the developmental insensitivities seen with these ethanol effects, adolescents have been found to exhibit greater sensitivity to ethanol-induced impairments in memory function (Markwiesse et al., 1998; White et al., 2000) and brain plasticity (Swartzwelder et al., 1995), as well as to ethanol-induced social facilitation (Varlinskaya and Spear, 2002).
When examining the ontogeny of ethanol’s effects on the autonomic nervous system indexed through assessment of ethanol-induced hypothermia, however, the available data are more mixed. Some studies have found adolescent rats to exhibit less hypothermia than mature animals following injected or intubated ethanol (Ristuccia and Spear, 2004; Silveri and Spear, 2000). Brasser and Spear (2002) also found adolescents to show less ethanol-induced hypothermia than adult rats following intragastric (i.g.) administration of 2.0 g/kg of ethanol, although adolescents conversely exhibited a greater hypothermic response than adults at a high, sedating dose (4.0 g/kg). More marked ethanol-induced hypothermia also emerged in adolescent relative to adult rats in response to a 4-hour long ethanol vapor inhalation session (Ristuccia and Spear, 2005). That study also found adolescents to develop chronic tolerance to ethanol-induced hypothermia at a slower rate than adults, findings that contrast with those previously reported when ethanol was administered via chronic intubations (Swartzwelder et al., 1998).
One possible explanation for these contrasting ontogenetic findings across studies—and even within the same laboratory (e.g., Ristuccia and Spear, 2004 vs 2005)—may be the amount of perturbation associated with the experimental procedures. In Ristuccia and Spear (2005), the animals underwent minimal handling immediately before and during daily ethanol exposure, core body temperature was assessed using indwelling telemetry probes, and ethanol was administered via a minimally invasive route, vapor inhalation. However, in Silveri and Spear (2000), ethanol was injected intraperitoneally (i.p.) and temperatures were taken with a rectal probe, whereas Brasser and Spear (2002) and Ristuccia and Spear (2004) intubated the animals i.g. even though temperatures were recorded using telemetry probes. These procedures presumably were more invasive and hence more disruptive than when ethanol was administered via vapor inhalation. Although pharmacokinetic differences could also contribute to the differing findings obtained across routes of administration, the relative invasiveness of the test procedures seems a potentially likely contributor to the differences in the hypothermic response observed across studies.
Therefore, the experiments presented here examined whether manipulating the amount of perturbation at the time of test differentially influenced expression of ethanol-induced hypothermia in adolescent versus adult rats. Experimental perturbation was manipulated using indwelling gastric cannulae versus i.g. intubation (Experiment 1) and via either familiarizing animals with the handling and injection procedure before testing or not manipulating them between surgery and testing (Experiment 2). Based on the findings of previous studies, it was anticipated that greater disruption at the time of test would diminish ethanol sensitivity of adolescents but not adults, with the result that adolescents would show less hypothermia than adults after ethanol was administered when the amount of test disruption was high but greater sensitivity than adults when ethanol was administered under presumably less disrupting test circumstances. Results supported the adolescent/adult differences predicted, although surprisingly it was the adult animals rather than the adolescents whose hypothermic reaction to ethanol was differentially influenced by variations in test circumstances.
EXPERIMENT 1
This experiment was designed to vary the disruptive effect of i.g. exposure to ethanol or water to test the hypothesis that adolescent rats would show less ethanol-induced hypothermia than adult rats when the perturbation associated with the ethanol exposure protocol was high but more hypothermia than adults when the perturbation was relatively low. To accomplish this, half of the animals were challenged with ethanol using chronically indwelling gastric cannulae while the others were challenged via i.g. intubations. Administration through the cannula required minimal handling relative to the more extensive handling and restraint necessary for the insertion of the gavage needle into the animal’s oral cavity for i.g. intubation. In this experiment as well as in Experiment 2b, indwelling telemetry probes were used to assess body temperature so as to avoid the stress associated with measuring temperature rectally.
Methods
The design of this experiment was a 2 age (adolescent or adult)×2 route of administration (i.g. intubation or gastric cannula)×2 drug (ethanol or water) factorial. Eight male Sprague–Dawley rats were used in each experimental condition (N = 564), with no more than 1 animal per litter placed in each condition. All animals were surgically implanted with gastric cannulae, although at the time of testing only half of the animals received the challenge via the gastric cannula, whereas the remaining animals were exposed to ethanol or water via i.g. intubation. All procedures used in this experiment were conducted in accordance with protocols approved by the Binghamton University Institutional Animal Care and Use Committee.
Subjects
Animals were bred in a vivarium at Binghamton University. One day after birth, litters were culled to 10 pups (6 males and 4 females whenever possible) and then on postnatal day 21 (P21) were weaned and pair housed with a same-sex littermate. Throughout the experiment, all animals were housed in a colony room with an ambient temperature between 20 and 22 °C and, unless otherwise stated, were maintained on ad libitum food and water.
Surgery
Because housing condition did not significantly impact ethanol-induced hypothermia in Ristuccia and Spear (2004), animals of both ages were isolated in flat-bottom breeder tubs 1 day before surgery and remained in these housing conditions throughout the experiment. Surgeries were conducted on P30 for adolescents and P65–70 for adults. On the day before implantation, animals were isolate housed and deprived of food for 24 hours to deplete stomach contents and hence facilitate surgery. For implantation of gastric cannulae, animals were first anesthetized with isoflurane anesthesia and, when they were unresponsive, an incision (approximately 3 cm long) was made through the skin and the muscle of the abdominal wall. Once the stomach was exposed, a small incision was made and a flanged piece of polyethylene PE50 tubing was inserted into the stomach. The incision in the stomach was then closed around the tubing using a “purse-string” stitch. The tubing was tunneled subcutaneously and externalized through a small incision at the top of the head. Before closing the abdominal wall, all animals were also implanted with an indwelling telemetry probe to measure body temperature (model # TA10TA-F20; Data Sciences International, St. Paul, MN). The implant was inserted into the peritoneal cavity through the existing incision. The peritoneum was then sutured closed and the skin was closed with wound clips.
Procedure
Immediately after surgery, implanted animals were returned to ad libitum food and water. All animals were moved to the telemetry recording room and their cages placed on top of RLA1020 telemetry signal-receiving plates (Data Sciences International) and allowed to recover for 3 days before testing. During this time, gastric cannulae were flushed daily to ensure cannula patency. For flushing the cannula, each animal was removed from its cage and a 23-gauge needle inserted into the externalized end of the PE50 tubing before injecting approximately 1 ml of tap water through the tubing. Telemetry probes were magnetically activated 1 day before testing when the cannula was flushed for the last time. On test day, half of the animals were given 2.0 g/kg of 18.9% ethanol while the remaining animals received isovolumetric water, with half of the animals in each challenge condition receiving their solution through the gastric cannula while the other half were given i.g. intubations. All animals remained in their home cages during the experimental period.
Telemetry Data Collection and Analysis
Telemetry data were analyzed using the Data Quest A.R.T. 2.3 Analysis package, Microsoft Excel 2003, and Statsoft Statistica 6.1. Temperature and activity data were sampled for 10 seconds once every 10 minutes for 30 minutes prechallenge and for 5.5 hours thereafter. For analysis, telemetry data were collapsed into 30 minutes time bins, each created by taking the means of 3 consecutive samples and identified by the timing of the middle sample in each bin (i.e., the “40 minutes” time bin consisted of the mean of the samples collected 30, 40, and 50 minutes post injection). All samples were expressed as elapsed time from injection (time 0), with the first time bin (“−20”) encompassing the mean of the 3 samples recorded before injection. Temperature data were binned up to 320 minutes after injection, with the last sample labeled “310.” These data were analyzed by means of a time×age×route of administration×drug analysis of variance (ANOVA) with time treated as a repeated measure.
Results
Body Weight
The analysis of body weight on test day indicated no significant main effects or interactions of drug or route of administration, although of course adolescents weighed less (125.03 ± 2.24 g) than adults (347.63 ± 32.85 g) [age effect: F(1, 56) = 1,243.8, p<0.001].
Temperature
The ANOVA of the prechallenge temperature data (i.e., the time bin labeled “−20”) revealed no significant main effects or interactions of age, drug, or route of administration (p>0.05). Therefore, no baseline differences were detected among the animals in any of the conditions.
The analysis of the temperature data collected after ethanol intubation indicated main effects of time [F(10, 560) = 10.94, p<0.001] and drug [F(1, 56) = 47.07, p<0.001], tempered by a significant time×drug interaction [F(10, 560) = 22.34, p<0.001], with ethanol-treated animals having lower body temperatures than water-treated animals during the time bins from 10 to 220 minutes post administration (see Fig. 1). In this analysis, no significant main effects or interactions involving age or route of administration emerged. Thus, regardless of whether ethanol was administered via i.g. intubations or gastric cannulae, the 2.0 g/kg challenge dose of ethanol was found to induce marked hypothermia in both adolescents and adults.
Fig. 1.
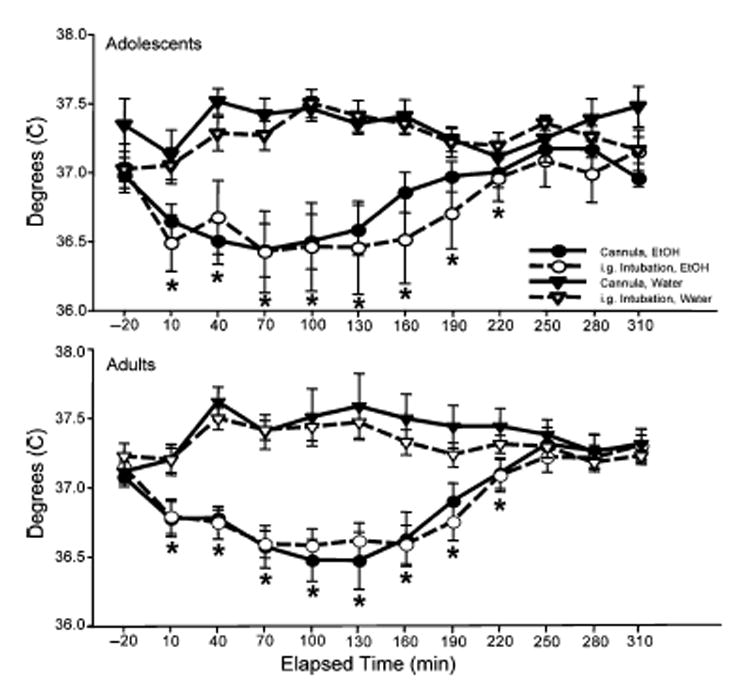
Adolescent (top) and adult (bottom) body temperature recorded by telemetry implants in Experiment 1 and expressed as °C. Data are shown in 30 min time bins with the point of ethanol administration labeled as “10.” *Significant difference (p<0.05) between animals of both ages that were administered ethanol and those that were administered tap water (based on Fischer’s post hoc analysis of the significant time×drug interaction).
EXPERIMENT 2
The results of Experiment 1 revealed similar hypothermic reactions to ethanol in both adolescent and adult rats not only when administered via chronically indwelling gastric cannulae but also when given via gavage. The latter result was surprising, given previous findings of age differences in hypothermic responses following i.g. ethanol administration (Brasser and Spear, 2002; Ristuccia and Spear, 2004). Given that all animals in Experiment 1 were implanted with gastric cannulae, it is possible that aspects of the cannula implantation surgery or the daily handling for cannula flushing may have induced sufficient commonalities across administration mode to obscure their differential impact. To better assess the influence of experimental perturbation on ethanol-induced hypothermia, a different strategy was used in Experiment 2, whereby some animals were pre-exposed to the handling/injection process to habituate them to this test procedure before test day, whereas other animals were unmanipulated before testing. Intraperitoneal injections were used instead of i.g. intubations in this experiment because of previous work suggesting that animals take notably longer to adapt behaviorally to the i.g. intubation process than to i.p. injections (unpublished pilot data). Experiment 2a was conducted to determine whether such pretest familiarization was indeed effective in attenuating responsiveness to handling/injection as indexed by a reduced corticosterone response to the injection process on test day. Based on the positive results from Experiments 2a, 2b then used such pretest familiarization to examine the hypothesis that differences observed between adolescents and adults in sensitivity to ethanol’s hypothermic effects are in part a function of the amount of perturbation associated with the injection process on test day. It was anticipated that, when experimental perturbation was minimized through pretest familiarization with the injection procedure, adolescents would show greater ethanol-induced hypothermia than adults, with the opposite pattern evident when animals were not familiarized before testing.
Methods (Experiment 2a)
The design of this study was a 2 age (adolescent or adult)×2 pretest handling (handled or nonhandled)×2 sacrifice condition (sacrificed directly from the home cage or 30 minutes following handling/injection) factorial, with 8 group-housed male Sprague–Dawley rats placed into each experimental condition as specified by this factorial design (N = 64), and no more than 1 animal per litter assigned to any experimental group.
Procedure
Beginning on P27 (adolescent) or P65–70 (adult) and continuing for 7 days, half of the animals were taken from their home cages and handled for approximately 5 to 10 minutes daily. This procedure included injecting the animal with saline (i.p.) and allowing the animal to familiarize itself with the guillotine that was eventually used to sacrifice the animals. The remaining animals were not manipulated until test day. No surgery was performed on the animals in these experiments.
On test day, 1 animal from each cage was removed and immediately sacrificed by decapitation (home cage control). Immediately thereafter, animals in the test condition group were handled and injected i.p. with saline and then returned to the home cage until sacrificed 30 minutes post injection. Immediately after killing, trunk blood samples were collected in heparinized capillary tubes, centrifuged for 20 minutes at approximately 800×g and frozen at −80 °C until the time of analysis of plasma corticosterone using a radioimmunoassay kit from ICN Biomedical (Orangeburg, NY). The resulting data were then analyzed with a 2-age×2 pretest handling×2 sacrifice condition ANOVA.
Results (Experiment 2a)
The analysis of plasma corticosterone concentrations indicated no significant main effects or interactions of age, but significant main effects of handling [F(1, 52) = 8.32, p<0.01] and sacrifice condition [F(1, 52) = 53.83, p<0.001], both tempered by a significant interaction of these variables [F(1, 52) = 6.60, p<0.05]. As shown in Fig. 2, animals had significantly higher plasma corticosterone concentrations 30 minutes after injection than when sacrificed directly from the home cage, although the increases seen in response to the injection protocol were significantly lower in animals that had received prior familiarization with the handling/injection procedure than in animals not previously manipulated. Thus, the pretest handling/injection procedure was found to be sufficient to attenuate the elevation in corticosterone levels following the injection protocol in animals at both ages. These data document the efficacy of this familiarization procedure for use in Experiment 2b to test the hypothesis that adolescents will show a greater hypothermic response to ethanol challenge than adults when perturbations associated with the injection/test process are low, but less hypothermia than adults when the relative disruption at the time of test is higher.
Fig. 2.
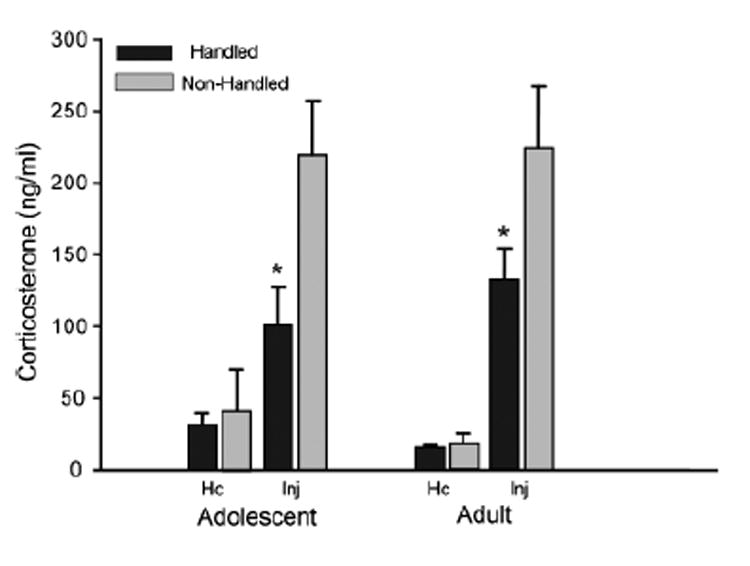
Plasma corticosterone concentrations (ng/mL) from adolescents (left) and adults (right), collected either immediately from animals taken directly out of the homecage (Hc) or 30 min after saline injection (Inj) in Experiment 2a. *Significantly lower (p<0.05) corticosterone level in handled than nonhandled animals at the same age (based on Fischer’s post hoc analysis of the significant handling×killing condition interaction).
Methods (Experiment 2b)
The design of this study was a 2 age (adolescent or adult)×2 pretest handling (handled or nonhandled)×2 drug (ethanol or saline) factorial, with 8 male Sprague–Dawley rats from 8 different litters placed into each experimental condition as specified by this factorial design (N = 64). All animals were reared identically to those in Experiment 1, including pair housing until the time of telemetry implantation.
Procedure
Adolescents were implanted with telemetry probes on P27 and adults on P65–70 using surgical procedures similar to Experiment 1, after which all animals were isolate housed for the remainder of the experiment. Beginning on the day after surgery, half of the animals at each age underwent 5 days of handling/injection. This procedure included removing the animal from its cage, placing it on the experimenter’s arm, and gently stroking it for approximately 5 minutes before weighing the animal and injecting it with saline i.p. at a volume equivalent to that of the ethanol and saline challenges to be given on test day. Nonhandled animals were not manipulated until test day. On the sixth day after surgery, both handled and nonhandled animals were injected i.p. with either 2.0 g/kg of 18.9% ethanol or isovolumetric saline. All solutions were warmed to approximately 38 °C before injection. The procedures used to collect and analyze telemetry data were identical to those used in Experiment 1.
Results (Experiment 2b)
Body Weight
Body weight data were analyzed by calculating overall weight gain throughout the experimental procedure (weight on test day—presurgical weight). The analysis of the body weight gain data indicated a main effect of handling [F(1, 55) = 8.41, p<0.01], with handled animals gaining slightly but significantly less weight than nonhandled animals (32.22 ± 3.52 vs 39.32 ± 2.89 g). There was also a main effect of age [F(1, 55) = 185.14, p<0.001], with adolescent rats, as expected, gaining significantly more weight than adults (50.91 ± 0.97 vs 20.03 ± 2.30 g) across the 9-day period.
Temperature
No significant baseline temperature differences were found between any of the experimental groups in the ANOVA of the temperature data collected immediately before challenge with ethanol or saline (p>0.05). When temperature data collected from 0 to 320 minutes post injection were analyzed, there was a significant main effect of drug [F(1, 55) = 21.01, p<0.01] as well as significant handling×drug [F(1, 55) = 8.39, p<0.001] and age×handling [F(1, 55) = 7.18, p<0.01] interactions, all tempered by a significant interaction of these 3 variables [F(1, 55) = 6.15, p<0.05]. Post hoc analysis of data collapsed across time to explore the locus of this interaction showed no difference between handled and nonhandled adolescents in either drug condition, although ethanol-treated adolescents had significantly lower body temperatures than those injected with saline (see Fig. 3). There was likewise no difference in body temperature among handled and nonhandled adults injected with saline. However, when injected with ethanol, only the nonhandled adults showed a hypothermic response. Nonhandled adults injected with ethanol displayed greater hypothermia than their adolescent counterparts and had lower temperatures than adults in all other treatment conditions. In contrast, handled adolescents displayed greater hypothermia than handled adults following ethanol exposure.
Fig. 3.
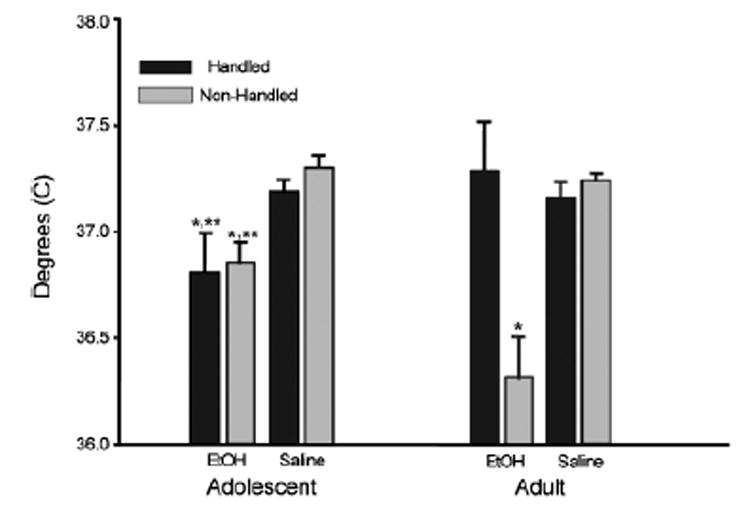
Adolescent (left) and adult (right) body temperature from Experiment 2b collapsed across the 10 to 320 min postinjection time bins and expressed as °C. **Significant differences (p<0.05) from same-aged saline-injected animals from the same handling condition and **significant differences in adult-handled animals injected with ethanol (based on Fischer’s post hoc analysis of the significant age×handling×drug interaction).
There was also a significant main effect of time [F(10, 550) = 4.28, p<0.001] that was tempered by separate interactions with age [F(10, 550) = 6.00, p<0.001] and drug [F(10, 550) = 8.99, p<0.001]. Although it is difficult to see these differences in Fig. 4, post hocs conducted on data collapsed across handling and drug condition to explore age×time effects revealed that adolescents had lower temperatures than adults during the 10 and 40 minutes postinjection time bins (e.g., 36.93 ± 0.13 vs 37.15 ± 0.12 °C at 40 minutes post injection) but higher temperatures in the 160, 220, 250, and 310 minutes postinjection time bins (e.g., 37.17 ± 0.07 vs 36.98 ± 0.12 °C at 220 minutes post injection). The observed increases in adolescent body temperatures relative to that of adults during the middle part of the light phase of the diurnal cycle are reminiscent of age differences in the circadian patterning of temperature observed previously (Ristuccia and Spear, 2004, 2005). Post hocs on data collapsed across handling and age to explore the time×drug interaction revealed significant ethanol-induced hypothermia beginning with the 10 minutes postinjection time bin and ending with the 250 minute bin.
Fig. 4.
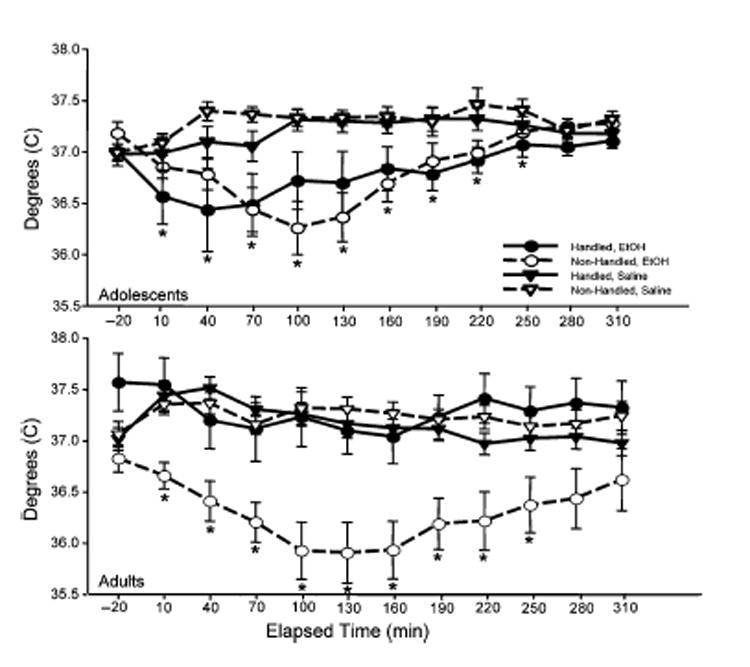
Adolescent (top) and adult (bottom) temperature data from Experiment 2b expressed as °C. Data are shown in 30 min time bins, with the time point of ethanol administration labeled as “10.”
GENERAL DISCUSSION
These experiments were designed to manipulate the amount of test-associated perturbation to test the hypothesis that adolescents show a greater hypothermic response to ethanol than adults when animals are minimally perturbed by the testing situation but an attenuated hypothermic response relative to adults when the test procedure involves greater disruption of the animals. Efforts to test this hypothesis using implanted gastric cannulae in Experiment 1 proved unsuccessful for several potential reasons. It is possible that the daily handling of all animals associated with the cannula flush procedure may have partially habituated the animals to handling by the experimenter, potentially reducing the relative amount of perturbation associated with both administration routes. Alternatively, the use of a gastric cannula to deliver ethanol may not have sufficiently reduced the amount of perturbation relative to i.g. intubations. It is also possible that the dual implants (gastric cannula and telemetry probe) to which all animals in Experiment 1 were exposed may have been disruptive enough to have masked age differences in the hypothermic response.
Experiment 2b used a different strategy to test this hypothesis using a pretest familiarization procedure that was shown in Experiment 2a to reduce the animals’ responses to the test day injection procedure (as indexed by a reduced corticosterone response). By varying whether animals were familiarized to the handling/injection procedure before testing, the results of Experiment 2b did reveal the expected pattern of age differences—i.e., in animals that underwent the pretest familiarization procedure, adolescents did in fact show a greater hypothermic response to ethanol than adults, whereas among nonfamiliarized animals, adolescents showed less ethanol-induced hypothermia than adults. Contrary to expectations, however, this relative age difference in the hypothermic response to ethanol was driven by adult animals, with pretest familiarization effectively eliminating the otherwise marked hypothermic response to ethanol injection seen in nonhandled adults. Adolescents, on the other hand, remained relatively unaffected by the pretest manipulation, exhibiting moderate levels of ethanol-induced hypothermia regardless of whether they had undergone pretest handling/injection.
The pattern of findings obtained in Experiment 2b was consistent with previous studies that examined ethanol-induced hypothermia in adolescent and adult rats. In experiments where the amount of experimental perturbation was relatively high, either because ethanol was administered through i.g. intubations (Brasser and Spear, 2002; Ristuccia and Spear, 2004; Swartzwelder et al., 1998) or via i.p. injections with no prior familiarization with the procedure (Silveri and Spear, 2000; nonhandled animals in Experiment 2b), adolescent rats showed significantly less ethanol-induced hypothermia than adults. Conversely, when the disruption to the animals at the time of testing was relatively low, either because ethanol was administered noninvasively through vapor inhalation (Ristuccia and Spear, 2005) or via prior pretest familiarization (animals in the handling group in Experiment 2b), adolescents showed a greater hypothermic response to ethanol than adults. Thus, previously reported differences in the relative sensitivity of adolescents versus adults to ethanol-induced hypothermia may have been driven at least in part by the amount of disruption associated with the test procedures and the resulting effect on adult hypothermia, although this does not rule out possible pharmacokinetic or dose-related age differences in ethanol effects as well.
The insensitivity of adolescents to the perturbation-related variations in ethanol responsivity that was evident in adults was unexpected, given reports that adolescent animals are sometimes more sensitive to stressors than adults (e.g., Stone and Quartermain, 1997). While it is possible that this adolescent insensitivity could reflect a relatively high basal stress level among adolescents that might mask consequences of minor perturbations such as those associated with the administration protocol, the present studies provide no evidence to support this suggestion, with no age differences in basal corticosterone concentrations seen in Experiment 2a among animals that were sacrificed directly from their home cages.
While the exact cause of the difference in the hypothermic response to alcohol between adults that were or were not familiarized to the handling/injection process before testing is not known, there are several possibilities. It is unlikely that these results are related to a familiarization-related alteration in stress-induced hyperthermia (e.g., Briese and Cabanac, 1991), given that the results obtained were in contrast to those that would be expected if pretest familiarization had reduced the hyperthermic reaction to the test procedure. That is, if stress-induced hyperthermia following injection had masked the ethanol-induced hypothermia, one would expect that it would be the nonhandled adults that would show the attenuated hypothermic response, rather than the adults that were handled.
An alternative possibility for the pronounced effect of handling on expression of ethanol-induced hypothermia among the adult animals may have been related to the emergence of acute, within-session tolerance to ethanol. In previous research examining responsiveness during an initial ethanol challenge, young animals through adolescence showed considerable within-session (acute) tolerance to ethanol, at least when indexed on ethanol-induced sedative (Silveri and Spear, 1998, 2004a, 2004b) and social suppressive (Varlinskaya and Spear, 2006) effects. No acute tolerance was evident in adults under those testing circumstances (Silveri and Spear, 1998, 2004a, 2004b; Varlinskaya and Spear, 2006), except when the testing for acute tolerance was conducted 1 day following handling and saline injection (Silveri and Spear, 2004a, 2004b). To the extent that the pretest handling procedure used in Experiment 2b may likewise have facilitated expression of acute tolerance in adults when indexed in terms of hypothermia, attenuation of the hypothermic response to ethanol would have been expected among the handled adults, as was observed.
A potential handling-induced induction of acute tolerance in adults could serve to attenuate some of the negative effects of ethanol. Prior work has indicated that the hypothermic effects of ethanol may be aversive, with animals showing the greatest hypothermic reactions to ethanol also showing greater ethanol-induced taste aversions (Cunningham et al., 1992). Indeed, findings that handling prevented the formation of ethanol-induced conditioned place aversions in mice while having no impact on conditioned place preference (Bechtholt et al., 2004) were reminiscent of data from Experiment 2b showing a handling-associated attenuation in ethanol’s hypothermic effects in adult rats. However, when mice from various inbred strains were examined, ethanol-induced hypothermia did not correlate with propensity to develop ethanol taste aversions as strongly as did withdrawal sensitivity or homecage ethanol preference, indicating that other factors may also play a role in ethanol hedonics (Broadbent et al., 2002).
Regardless of the mechanisms underlying these effects, the finding that pretest familiarization to the handling/injection procedure dramatically altered the observed expression of ethanol-induced hypothermia in adult rats suggests that relatively minor manipulations that seem innocuous to experimenters may sometimes have a profound effect on experimental outcome. Indeed, there have been other occasional reports that manipulations associated with drug administration may themselves alter behavior. For example, as mentioned above, the handling necessary for the intracerebroventricular administration of ethanol was found to disrupt the formation of ethanol-conditioned place aversions, but not conditioned place preferences (Bechtholt et al., 2004). That adolescents were relatively unaffected in the current experiment by a pretest manipulation effective in altering ethanol responsiveness of adults indicates that animals of different ages may be differentially sensitive to these effects. Taken together, these results support the value of the inclusion of minimally manipulated animals where possible to determine the effect of the experimental procedures per se on dependent measures of interest. Saline or vehicle-injected control animals may provide necessary but not sufficient control conditions in some cases.
Acknowledgments
The authors would like to thank Judith Sharp for her help in conducting the corticosterone assays.
This research was supported by NIAAA grants R37-AA12525 to LPS and F31 AA16048 to RCR.
References
- Bechtholt AJ, Gremel CM, Cunningham CL. Handling blocks expression of conditioned place aversion but not conditioned place preference produced by ethanol in mice. Pharmacol Biochem Behav. 2004;79:739–744. doi: 10.1016/j.pbb.2004.10.003. [DOI] [PubMed] [Google Scholar]
- Brasser SM, Spear NE. Physiological and behavioral effects of acute ethanol hangover in juvenile, adolescent, and adult rats. Behav Neurosci. 2002;116:305–320. doi: 10.1037//0735-7044.116.2.305. [DOI] [PubMed] [Google Scholar]
- Briese E, Cabanac M. Stress hyperthermia: physiological arguments that it is a fever. Physiol Behav. 1991;49:1153–1157. doi: 10.1016/0031-9384(91)90343-m. [DOI] [PubMed] [Google Scholar]
- Broadbent J, Muccino KJ, Cunningham CL. Ethanol-induced conditioned taste aversion in 15 inbred mouse strains. Behav Neurosci. 2002;116:138–148. [PubMed] [Google Scholar]
- Crews FT, Braun CJ, Hoplight B, Switzer RC, III, Knapp DJ. Binge ethanol consumption causes differential brain damage in young adolescent rats compared with adult rats. Alcohol Clin Exp Res. 2000;24:1712–1723. [PubMed] [Google Scholar]
- Cunningham CL, Niehus JS, Bachtold JF. Ambient temperature effects on taste aversion conditioned by ethanol: contribution of ethanol-induced hypothermia. Alcohol Clin Exp Res. 1992;16:1117–1124. doi: 10.1111/j.1530-0277.1992.tb00707.x. [DOI] [PubMed] [Google Scholar]
- Doremus TL, Brunell SC, Varlinskaya EI, Spear LP. Anxiogenic effects during withdrawal from acute ethanol in adolescent and adult rats. Pharmacol Biochem Behav. 2003;75:411–418. doi: 10.1016/s0091-3057(03)00134-5. [DOI] [PubMed] [Google Scholar]
- Hernandez M, Spear LP. Ethanol-induced analgesia in adolescent and adult rats (in preparation) [Google Scholar]
- Johnston LD, O’Malley PM, Bachman JG, Schulenberg JE. Monitoring the Future National Survey Results on Drug Use, 1975–2003: Volume I, Secondary School Students (NIH Publication No 04-5507) National Institute on Drug Abuse; Bethesda, MD: 2003. [Google Scholar]
- Markwiese BJ, Acheson SK, Levin ED, Wilson WA, Swartzwelder HS. Differential effects of ethanol on memory in adolescent and adult rats. Alcohol Clin Exp Res. 1998;22:416–421. [PubMed] [Google Scholar]
- Ristuccia RC, Spear LP. Adolescent ethanol sensitivity: hypothermia and acute tolerance. Ann NY Acad Sci. 2004;1021:445–447. doi: 10.1196/annals.1308.061. [DOI] [PubMed] [Google Scholar]
- Ristuccia RC, Spear LP. Sensitivity and tolerance to autonomic effects of ethanol in adolescent and adult rats during repeated vapor inhalation session. Alcohol Clin Exp Res. 2005;29:1809–1820. doi: 10.1097/01.alc.0000183010.72764.cd. [DOI] [PubMed] [Google Scholar]
- Shram MJ, Funk D, Chau V, Li Z, Lê AD. Age differences in the aversive properties of alcohol; Poster presented at the annual meeting of the Research Society on Alcoholism; Santa Barbara, CA. 2005. [Google Scholar]
- Silveri MM, Spear LP. Decreased sensitivity to the hypnotic effects of ethanol early in ontogeny. Alcohol Clin Exp Res. 1998;20:670–676. doi: 10.1111/j.1530-0277.1998.tb04310.x. [DOI] [PubMed] [Google Scholar]
- Silveri MM, Spear LP. Ontogeny of ethanol elimination and ethanol-induced hypothermia. Alcohol. 2000;20:45–53. doi: 10.1016/s0741-8329(99)00055-5. [DOI] [PubMed] [Google Scholar]
- Silveri MM, Spear LP. Characterizing the ontogeny of ethanol-associated increases in corticosterone. Alcohol. 2004a;32:145–155. doi: 10.1016/j.alcohol.2003.12.004. [DOI] [PubMed] [Google Scholar]
- Silveri MM, Spear LP. The effects of NMDA and GABAA pharmacological manipulations on acute and rapid tolerance to ethanol during ontogeny. Alcohol Clin Exp Res. 2004b;28:884–894. doi: 10.1097/01.alc.0000128221.68382.ba. [DOI] [PubMed] [Google Scholar]
- Spear LP. The adolescent brain and age-related behavioral manifestations. Neurosci Biobehav Rev. 2000;24:417–463. doi: 10.1016/s0149-7634(00)00014-2. [DOI] [PubMed] [Google Scholar]
- Stone EA, Quartermain D. Greater behavioral effects of stress in immature as compared to mature male mice. Physiol Behav. 1997;63:143–145. doi: 10.1016/s0031-9384(97)00366-1. [DOI] [PubMed] [Google Scholar]
- Swartzwelder HS, Richardson RC, Markwiese-Foerch B, Wilson WA, Little PJ. Developmental differences in the acquisition of tolerance to ethanol. Alcohol. 1998;15:311–314. doi: 10.1016/s0741-8329(97)00135-3. [DOI] [PubMed] [Google Scholar]
- Swartzwelder HS, Wilson WA, Tayyeb MI. Age-dependent inhibition of long-term potentiation by ethanol in immature versus mature hippocampus. Alcohol Clin Exp Res. 1995;19:1480–1485. doi: 10.1111/j.1530-0277.1995.tb01011.x. [DOI] [PubMed] [Google Scholar]
- Tapert SF, Schweinsburg AD. The human adolescent brain and alcohol use disorders. In: Galanter M, editor. Recent Developments in Alcoholism. Vol. 17. Kluwer Academic/Plennum; New York, NY: 2005. pp. 177–189. [DOI] [PubMed] [Google Scholar]
- U.S. Department of Health and Human Services. Substance Abuse and Mental Health Services Administration (SAHMSA Publication No 02-3758) U.S Department of Health and Human Services; Washington, DC: 2002. [Google Scholar]
- Varlinskaya EI, Spear LP. Acute effects of ethanol on social behavior of adolescent and adult rats: role of famillarity of the test situation. Alcohol Clin Exp Res. 2002;26:1502–1511. doi: 10.1097/01.ALC.0000034033.95701.E3. [DOI] [PubMed] [Google Scholar]
- Varlinskaya EI, Spear LP. Ontogeny of acute tolerance to ethanol-induced social inhibition in Sprague–Dawley rats. Alcohol Clin Exp Res. 2006;30:1833–1844. doi: 10.1111/j.1530-0277.2006.00220.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White AM, Bae JG, Truesday MC, Ahmad S, Wilson WA, Swartzwelder HS. Chronic-intermittent ethanol exposure during adolescence prevents normal developmental changes in sensitivity to ethanol-induced motor impairments. Alcohol Clin Exp Res. 2002a;26:960–968. doi: 10.1097/01.ALC.0000021334.47130.F9. [DOI] [PubMed] [Google Scholar]
- White AM, Ghia AJ, Levin ED, Swartzwelder HS. Binge pattern ethanol exposure in adolescent and adult rats: differential impact on subsequent responsiveness to ethanol. Alcohol Clin Exp Res. 2000;24:1251–1256. [PubMed] [Google Scholar]
- White AM, Truesdale MC, Bae JG, Ahmad S, Wilson WA, Best PJ, Swartzwelder HS. Differential effects of ethanol on motor coordination in adolescent and adult rats. Pharmacol Biochem Behav. 2002b;73:673–677. doi: 10.1016/s0091-3057(02)00860-2. [DOI] [PubMed] [Google Scholar]


