Abstract
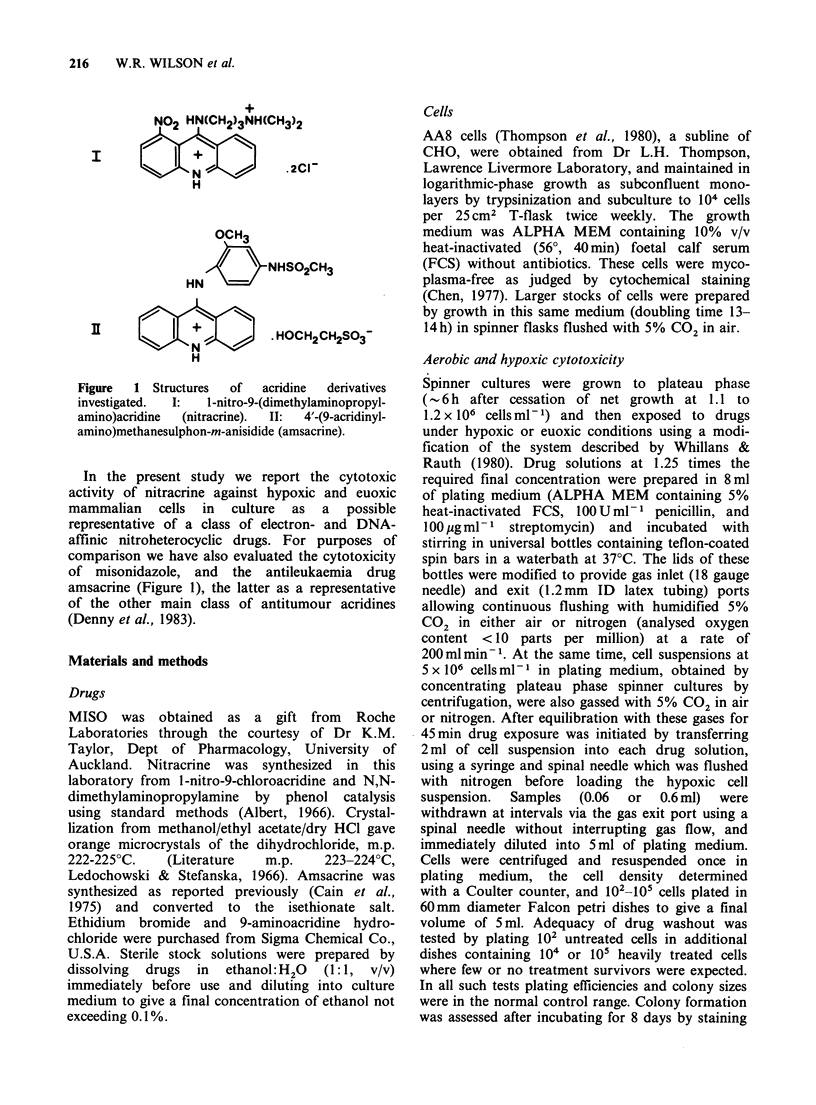
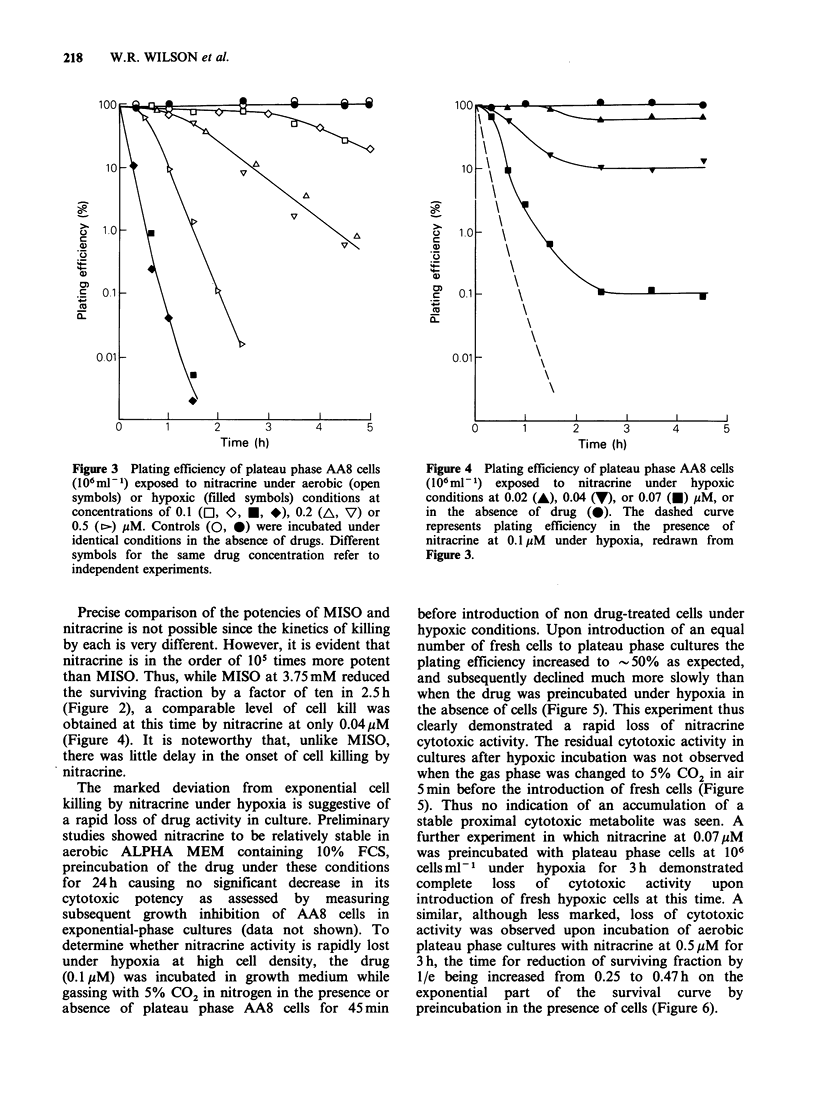
Hypoxic cells in solid tumours are resistant to ionizing radiation and may be refractory to treatment by many chemotherapeutic agents. For these reasons the identification of drugs with selective toxicity towards hypoxic cells is an important objective in cancer chemotherapy. Nitroimidazoles such as misonidazole demonstrate such hypoxia-selective toxicity but have very low dose potency. The 1-nitroacridine derivative 1-nitro-9-(dimethylaminopropylamino)acridine (nitracrine) binds reversibly to DNA but also forms covalent adducts with DNA in vivo. We have found nitracrine to be selectively toxic to the Chinese hamster ovary cell line AA8 under hypoxic conditions in culture, with a potency approximately 100,000 times higher than that of misonidazole. The effect of oxygen is not a simple dose-modifying one in this system, probably in part because of rapid metabolic inactivation of nitracrine under hypoxic conditions. Viscometric studies with the mini col E1 plasmid PML-21 confirmed that nitracrine binds to DNA by intercalation, and provided an unwinding angle of 16 degrees (relative to 26 degrees for ethidium). It is proposed that the cytotoxicity of nitracrine under hypoxia is due to reductive metabolism to form an alkylating species, but that intercalation of the chromophore may enhance reactivity towards DNA and hence contribute to the marked enhancement of potency with respect to simple nitroheteroaromatic drugs.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams G. E., Stratford I. J., Wallace R. G., Wardman P., Watts M. E. Toxicity of nitro compounds toward hypoxic mammalian cells in vitro: dependence on reduction potential. J Natl Cancer Inst. 1980 Mar;64(3):555–560. [PubMed] [Google Scholar]
- Baguley B. C., Denny W. A., Atwell G. J., Cain B. F. Potential antitumor agents. 34. Quantitative relationships between DNA binding and molecular structure for 9-anilinoacridines substituted in the anilino ring. J Med Chem. 1981 Feb;24(2):170–177. doi: 10.1021/jm00134a009. [DOI] [PubMed] [Google Scholar]
- Brown J. M. Evidence for acutely hypoxic cells in mouse tumours, and a possible mechanism of reoxygenation. Br J Radiol. 1979 Aug;52(620):650–656. doi: 10.1259/0007-1285-52-620-650. [DOI] [PubMed] [Google Scholar]
- Brown J. M. The mechanisms of cytotoxicity and chemosensitization by misonidazole and other nitroimidazoles. Int J Radiat Oncol Biol Phys. 1982 Mar-Apr;8(3-4):675–682. doi: 10.1016/0360-3016(82)90711-8. [DOI] [PubMed] [Google Scholar]
- Cain B. F., Atwell G. J., Denny W. A. Potential antitumor agents. 16.4'-(Acridin-9-ylamino)methanesulfonanilides. J Med Chem. 1975 Nov;18(11):1110–1117. doi: 10.1021/jm00245a013. [DOI] [PubMed] [Google Scholar]
- Cain B. F., Baguley B. C., Denny W. A. Potenial antitumor agents. 28. Deoxyribonucleic acid polyintercalating agents. J Med Chem. 1978 Jul;21(7):658–668. doi: 10.1021/jm00205a013. [DOI] [PubMed] [Google Scholar]
- Chen T. R. In situ detection of mycoplasma contamination in cell cultures by fluorescent Hoechst 33258 stain. Exp Cell Res. 1977 Feb;104(2):255–262. doi: 10.1016/0014-4827(77)90089-1. [DOI] [PubMed] [Google Scholar]
- Filipski J., Marczyński B., Chorazy M. Complexes of derivatives of 1-nitro-9-aminoacridine with DNA. Acta Biochim Pol. 1975;22(2):119–129. [PubMed] [Google Scholar]
- Filipski J., Marczyński B., Sadzińska L., Chalupka G., Chorazy M. Interactions of some nitro-derivatives of substituted 9-aminoacridine with DNA. Biochim Biophys Acta. 1977 Sep 6;478(1):33–43. doi: 10.1016/0005-2787(77)90241-6. [DOI] [PubMed] [Google Scholar]
- Gniazdowski M., Szmigiero L., Slaska K., Jaros-Kamińska B., Ciesielska E. Influence of thiols on inhibition of ribonucleic acid synthesis in vitro by a 1-nitro-9-aminoalkylacridine derivative, C-283. Mol Pharmacol. 1975 May;11(3):310–318. [PubMed] [Google Scholar]
- Hershfield V., Boyer H. W., Chow L., Helinski D. R. Characterization of a mini-ColC1 plasmid. J Bacteriol. 1976 Apr;126(1):447–453. doi: 10.1128/jb.126.1.447-453.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirst D. G., Denekamp J. Tumour cell proliferation in relation to the vasculature. Cell Tissue Kinet. 1979 Jan;12(1):31–42. doi: 10.1111/j.1365-2184.1979.tb00111.x. [DOI] [PubMed] [Google Scholar]
- Konopa J., Pawlak J. W., Pawlak K. The mode of action of cytotoxic and antitumor 1-nitroacridines. III. In vivo interstrand cross-linking of DNA of mammalian or bacterial cells by 1-nitroacridines. Chem Biol Interact. 1983 Feb;43(2):175–197. doi: 10.1016/0009-2797(83)90094-7. [DOI] [PubMed] [Google Scholar]
- Kwaśniewska-Rokicińska, Winkler A. The effect of 1-nitro-9-aminoacridine on Yoshida sarcoma sensitive and resistant to alkylating agents. Arch Immunol Ther Exp (Warsz) 1969;17(3):376–379. [PubMed] [Google Scholar]
- McGhee J. D., von Hippel P. H. Theoretical aspects of DNA-protein interactions: co-operative and non-co-operative binding of large ligands to a one-dimensional homogeneous lattice. J Mol Biol. 1974 Jun 25;86(2):469–489. doi: 10.1016/0022-2836(74)90031-x. [DOI] [PubMed] [Google Scholar]
- Moore B. A., Palcic B., Skarsgard L. D. Radiosensitizing and toxic effects on the 2-nitroimidazole Ro-07-0582 in hypoxic mammation cells. Radiat Res. 1976 Sep;67(3):459–473. [PubMed] [Google Scholar]
- Ozols R. F., Locker G. Y., Doroshow J. H., Grotzinger K. R., Myers C. E., Young R. C. Pharmacokinetics of adriamycin and tissue penetration in murine ovarian cancer. Cancer Res. 1979 Aug;39(8):3209–3214. [PubMed] [Google Scholar]
- Pawlak J. W., Konopa J. In vitro binding of metabolically activated [14C]-ledakrin, or 1-nitro-9-14C-(3'-dimethylamino-N-propylamino) acridine, a new antitumor and DNA cross-linking agent, to macromolecules of subcellular fractions isolated from rat liver and HeLa cells. Biochem Pharmacol. 1979 Dec 1;28(23):3391–3402. doi: 10.1016/0006-2952(79)90078-9. [DOI] [PubMed] [Google Scholar]
- Pawlak K., Matuszkiewicz A., Pawlak J. W., Konopa J. The mode of action of cytotoxic and antitumor 1-nitroacridines. I. The 1-nitroacridines do not exert their cytotoxic effects by physicochemical binding with DNA. Chem Biol Interact. 1983 Feb;43(2):131–149. doi: 10.1016/0009-2797(83)90092-3. [DOI] [PubMed] [Google Scholar]
- Radzikowski C., Ledóchowski Z., Ledóchowski A., Wrzolek S., Hrabowska M., Konopa J. The evaluation of antitumor properties of acridine derivatives on the basis of the results from some in vivo and in vitro tests. Arch Immunol Ther Exp (Warsz) 1967;15(1):126–128. [PubMed] [Google Scholar]
- THOMLINSON R. H., GRAY L. H. The histological structure of some human lung cancers and the possible implications for radiotherapy. Br J Cancer. 1955 Dec;9(4):539–549. doi: 10.1038/bjc.1955.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tannock I. F. The relation between cell proliferation and the vascular system in a transplanted mouse mammary tumour. Br J Cancer. 1968 Jun;22(2):258–273. doi: 10.1038/bjc.1968.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tannock I., Guttman P. Response of Chinese hamster ovary cells to anticancer drugs under aerobic and hypoxic conditions. Br J Cancer. 1981 Feb;43(2):245–248. doi: 10.1038/bjc.1981.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tannock I. Response of aerobic and hypoxic cells in a solid tumor to adriamycin and cyclophosphamide and interaction of the drugs with radiation. Cancer Res. 1982 Dec;42(12):4921–4926. [PubMed] [Google Scholar]
- Taylor Y. C., Bump E. A., Brown J. M. Studies on the mechanism of chemosensitization by misonidazole in vitro. Int J Radiat Oncol Biol Phys. 1982 Mar-Apr;8(3-4):705–708. doi: 10.1016/0360-3016(82)90717-9. [DOI] [PubMed] [Google Scholar]
- Taylor Y. C., Rauth A. M. Sulphydryls, ascorbate and oxygen as modifiers of the toxicity and metabolism of misonidazole in vitro. Br J Cancer. 1980 Jun;41(6):892–900. doi: 10.1038/bjc.1980.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teicher B. A., Lazo J. S., Sartorelli A. C. Classification of antineoplastic agents by their selective toxicities toward oxygenated and hypoxic tumor cells. Cancer Res. 1981 Jan;41(1):73–81. [PubMed] [Google Scholar]
- Thompson L. H., Fong S., Brookman K. Validation of conditions for efficient detection of HPRT and APRT mutations in suspension-cultured Chinese hamster ovary cells. Mutat Res. 1980 Feb;74(1):21–36. doi: 10.1016/0165-1161(80)90188-0. [DOI] [PubMed] [Google Scholar]
- Wang J. C. The degree of unwinding of the DNA helix by ethidium. I. Titration of twisted PM2 DNA molecules in alkaline cesium chloride density gradients. J Mol Biol. 1974 Nov 15;89(4):783–801. doi: 10.1016/0022-2836(74)90053-9. [DOI] [PubMed] [Google Scholar]
- Waring M. J. DNA-binding characteristics of acridinylmethanesulphonanilide drugs: comparison with antitumour properties. Eur J Cancer. 1976 Dec;12(12):995–1001. doi: 10.1016/0014-2964(76)90066-9. [DOI] [PubMed] [Google Scholar]
- Whillans D. W., Rauth A. M. An experimental and analytical study of oxygen depletion in stirred cell suspensions. Radiat Res. 1980 Oct;84(1):97–114. [PubMed] [Google Scholar]
- Wilson W. R., Baguley B. C., Wakelin L. P., Waring M. J. Interaction of the antitumor drug 4'-(9-acridinylamino)methanesulfon-m-anisidide and related acridines with nucleic acids. Mol Pharmacol. 1981 Sep;20(2):404–414. [PubMed] [Google Scholar]
- Wilson W. R., Giesbrecht J. L., Hill R. P., Whitmore G. F. Toxicity of 4'-(9-acridinylamino)methanesulfon-m-anisidide in exponential- and plateau-phase Chinese hamster cell cultures. Cancer Res. 1981 Jul;41(7):2809–2816. [PubMed] [Google Scholar]


