Abstract
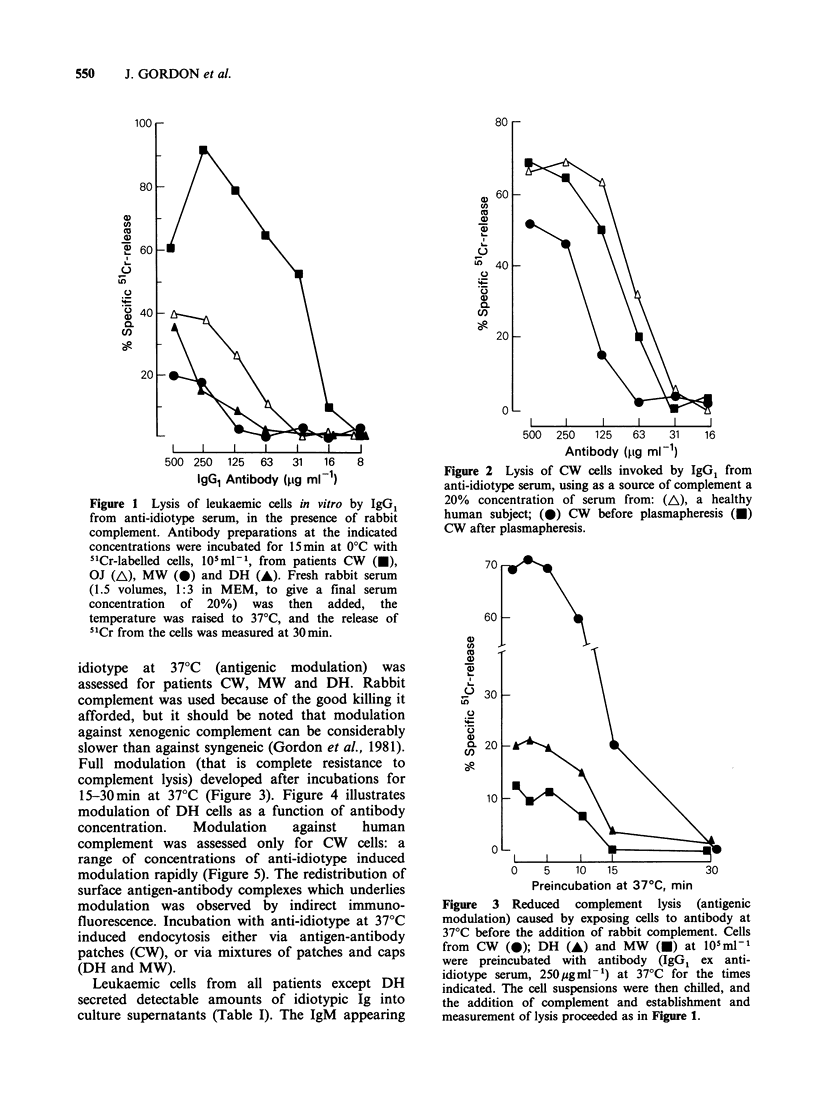
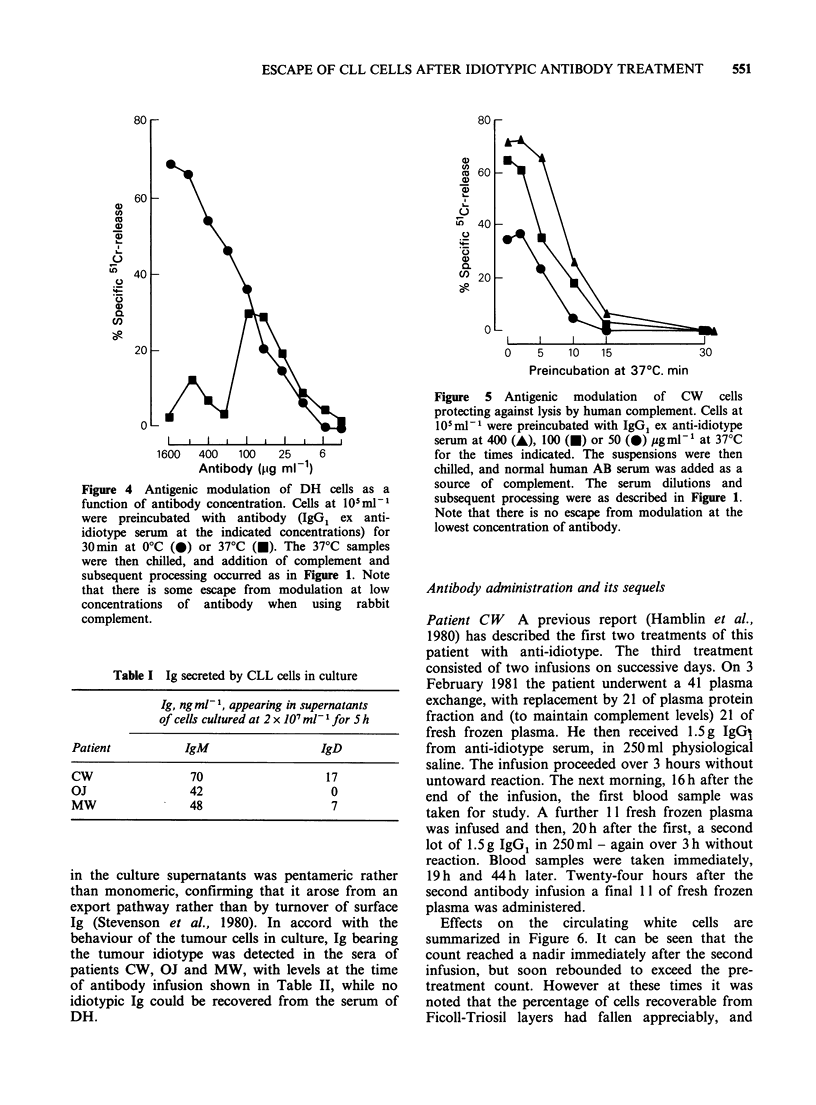
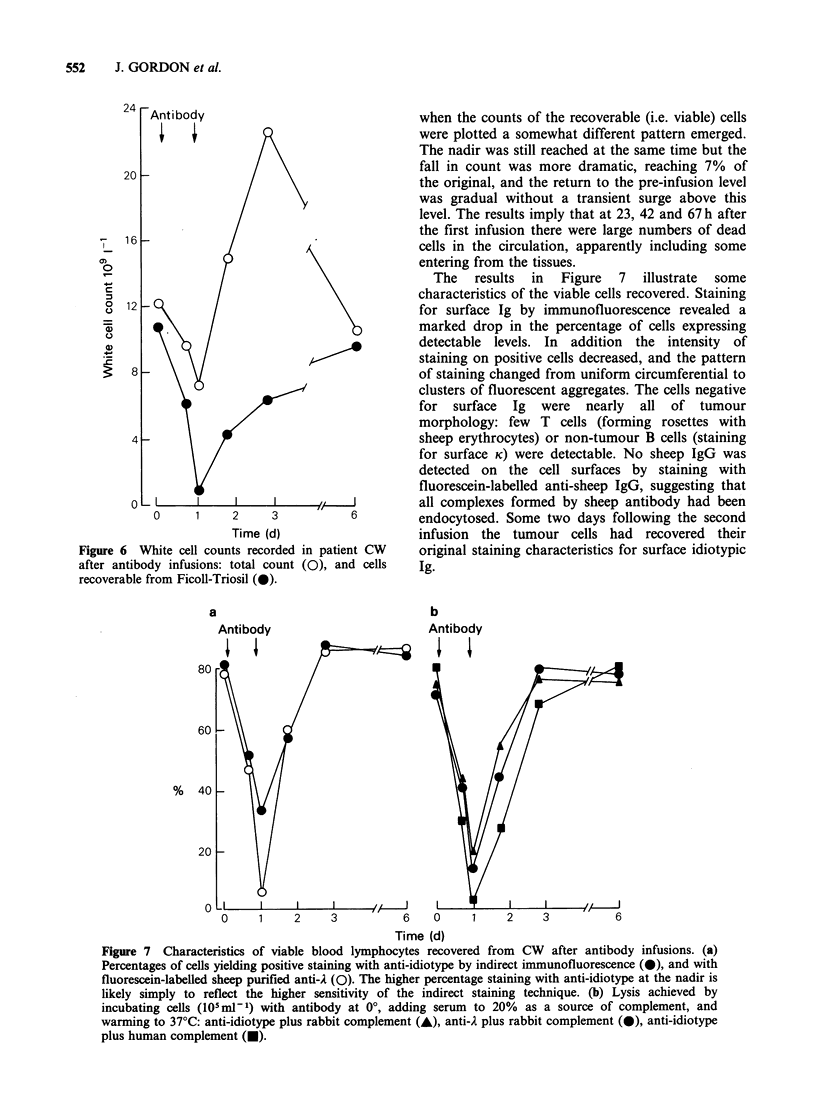
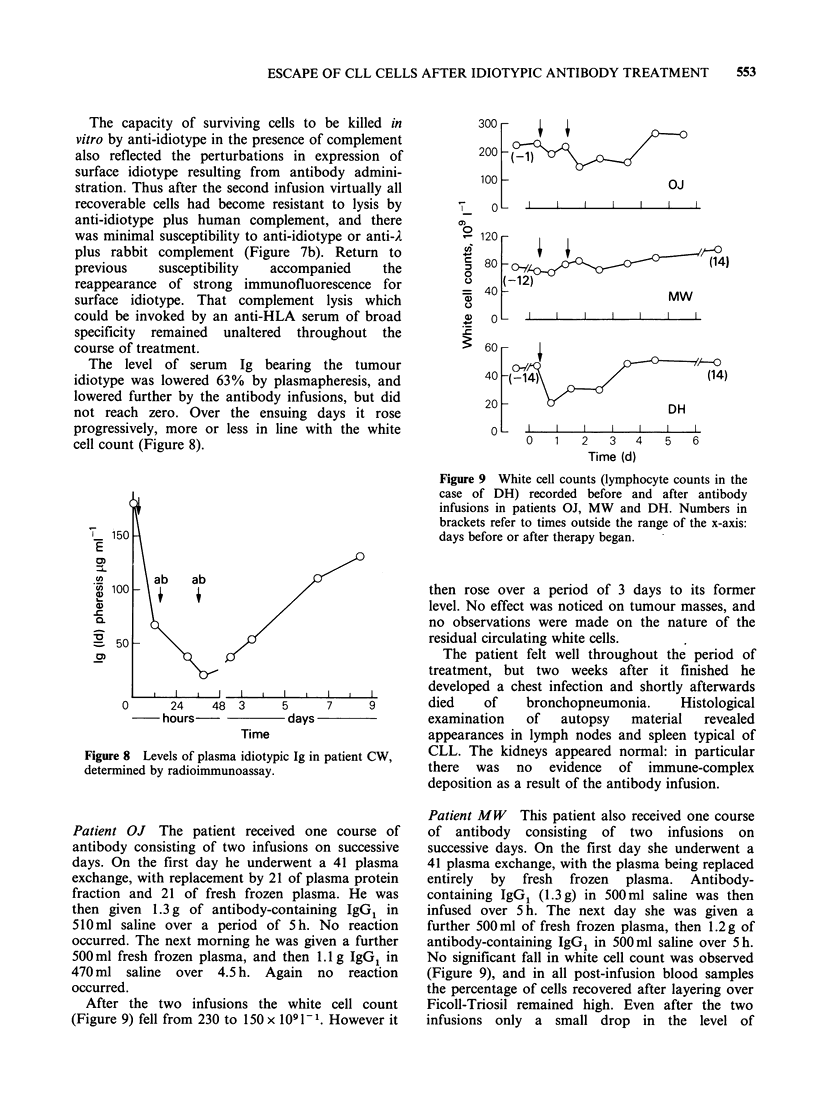
Four patients with chronic lymphocytic leukaemia were treated by one or more infusions of polyclonal antibody specific for the immunoglobulin idiotype expressed on their leukaemic cells. The antibody was in the form of IgG from sheep antiserum. Three of the 4 cases showed a significant fall in blood lymphocyte count. On one occasion most of the residual circulating lymphocytes were apparently dead. However on all occasions the cell counts rebounded to near pre-infusion levels within one week. Viable lymphocytes recovered from the blood after infusion always showed evidence of antigenic modulation: a diminished level of surface idiotype in a patched distribution, with an accompanying refractoriness to lysis by anti-idiotype plus complement. When cultured in vitro blood lymphocytes from three of the four patients revealed an appreciable export of idiotypic Ig. These 3 patients showed plasma levels of idiotypic Ig up to 400 micrograms ml-1, reduced by plasma exchange prior to infusion. The fourth patient had a level of less than 4 micrograms ml-1, and was the only one in whom free antibody could be found in the plasma after infusion. These cases demonstrate two major factors which thwart antibody attack on leukaemic cells--extracellular antigen and antigenic modulation--as well as problems relating to sparseness of surface antigen, recruitment of effectors, and exhaustion of effectors.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Eady R. P., Chapple J. C., Hough D. W., Stevenson G. T. The specificity of a solid phase radioimmunoassay for human immunoglobulins. J Immunol Methods. 1975 Jun;7(2-3):179–186. doi: 10.1016/0022-1759(75)90015-0. [DOI] [PubMed] [Google Scholar]
- Galton D. A., Goldman J. M., Wiltshaw E., Catovsky D., Henry K., Goldenberg G. J. Prolymphocytic leukaemia. Br J Haematol. 1974 May;27(1):7–23. doi: 10.1111/j.1365-2141.1974.tb06769.x. [DOI] [PubMed] [Google Scholar]
- Glennie M. J., Stevenson G. T. Univalent antibodies kill tumour cells in vitro and in vivo. Nature. 1982 Feb 25;295(5851):712–714. doi: 10.1038/295712a0. [DOI] [PubMed] [Google Scholar]
- Gordon J., Anderson V. A., Robinson D. S., Stevenson G. T. The influence of antigen density and a comparison of IgG and IgM antibodies in the anti-complementary modulation of lymphocytic surface immunoglobulin. Scand J Immunol. 1982 Feb;15(2):169–177. doi: 10.1111/j.1365-3083.1982.tb00635.x. [DOI] [PubMed] [Google Scholar]
- Gordon J., Robinson D. S., Stevenson G. T. Antigenic modulation of lymphocytic surface immunoglobulin yielding resistance to complement-mediated lysis. I. Characterization with syngeneic and xenogeneic complements. Immunology. 1981 Jan;42(1):7–12. [PMC free article] [PubMed] [Google Scholar]
- Gordon J., Stevenson G. T. Antigenic modulation of lymphocytic surface immunoglobulin yielding resistance to complement-mediated lysis. II. Relationship to redistribution of the antigen. Immunology. 1981 Jan;42(1):13–17. [PMC free article] [PubMed] [Google Scholar]
- Griffin F. M., Jr, Griffin J. A., Silverstein S. C. Studies on the mechanism of phagocytosis. II. The interaction of macrophages with anti-immunoglobulin IgG-coated bone marrow-derived lymphocytes. J Exp Med. 1976 Sep 1;144(3):788–809. doi: 10.1084/jem.144.3.788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamblin T. J., Abdul-Ahad A. K., Gordon J., Stevenson F. K., Stevenson G. T. Preliminary experience in treating lymphocytic leukaemia with antibody to immunoglobulin idiotypes on the cell surfaces. Br J Cancer. 1980 Oct;42(4):495–502. doi: 10.1038/bjc.1980.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haughton G., Lanier L. L., Babcock G. F., Lynes M. A. Antigen-induced murine B cell lymphomas. II. Exploitation of the surface idiotype as tumor specific antigen. J Immunol. 1978 Dec;121(6):2358–2362. [PubMed] [Google Scholar]
- Hough D. W., Eady R. P., Hamblin T. J., Stevenson F. K., Stevenson G. T. Anti-idiotype sera raised against surface immunoglobulin of human neoplastic lymphocytes. J Exp Med. 1976 Oct 1;144(4):960–969. doi: 10.1084/jem.144.4.960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krolick K. A., Isakson P. C., Uhr J. W., Vitetta E. S. BCL1, a murine model for chronic lymphocytic leukemia: use of the surface immunoglobulin idiotype for the detection and treatment of tumor. Immunol Rev. 1979;48:81–106. doi: 10.1111/j.1600-065x.1979.tb00299.x. [DOI] [PubMed] [Google Scholar]
- Kumagai S., Steinberg A. D., Green I. Antibodies to T cells in patients with systemic lupus erythematosus can induce antibody-dependent cell-mediated cytotoxicity against human T cells. J Clin Invest. 1981 Mar;67(3):605–614. doi: 10.1172/JCI110074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanier L. L., Babcock G. F., Raybourne R. B., Arnold L. W., Warner N. L., Haughton G. Mechanism of B cell lymphoma immunotherapy with passive xenogeneic anti-idiotype serum. J Immunol. 1980 Oct;125(4):1730–1736. [PubMed] [Google Scholar]
- Lawson A. D., Stevenson G. T. Macrophages induce antibody-dependent cytostasis but not lysis in guinea pig leukaemic cells. Br J Cancer. 1983 Aug;48(2):227–237. doi: 10.1038/bjc.1983.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesley J., Hyman R., Dennert G. Effect of antigen density on complement-mediated lysis, T-cell-mediated killing, and antigenic modulation. J Natl Cancer Inst. 1974 Dec;53(6):1759–1765. [PubMed] [Google Scholar]
- Linch D. C., Beverley P. C., Newland A., Turnbull A. Treatment of a low grade T cell proliferation with monoclonal antibody. Clin Exp Immunol. 1983 Jan;51(1):133–140. [PMC free article] [PubMed] [Google Scholar]
- Linscott W. D. An antigen density effect on the hemolytic efficiency of complement. J Immunol. 1970 May;104(5):1307–1309. [PubMed] [Google Scholar]
- Miller R. A., Levy R. Response of cutaneous T cell lymphoma to therapy with hybridoma monoclonal antibody. Lancet. 1981 Aug 1;2(8240):226–230. doi: 10.1016/s0140-6736(81)90475-x. [DOI] [PubMed] [Google Scholar]
- Miller R. A., Maloney D. G., McKillop J., Levy R. In vivo effects of murine hybridoma monoclonal antibody in a patient with T-cell leukemia. Blood. 1981 Jul;58(1):78–86. [PubMed] [Google Scholar]
- Miller R. A., Maloney D. G., Warnke R., Levy R. Treatment of B-cell lymphoma with monoclonal anti-idiotype antibody. N Engl J Med. 1982 Mar 4;306(9):517–522. doi: 10.1056/NEJM198203043060906. [DOI] [PubMed] [Google Scholar]
- Nadler L. M., Stashenko P., Hardy R., Kaplan W. D., Button L. N., Kufe D. W., Antman K. H., Schlossman S. F. Serotherapy of a patient with a monoclonal antibody directed against a human lymphoma-associated antigen. Cancer Res. 1980 Sep;40(9):3147–3154. [PubMed] [Google Scholar]
- Nisonoff A., Bangasser S. A. Immunological suppression of idiotypic specificities. Transplant Rev. 1975;27:100–134. doi: 10.1111/j.1600-065x.1975.tb00186.x. [DOI] [PubMed] [Google Scholar]
- Ojo E., Wigzell H. Natural killer cells may be the only cells in normal mouse lymphoid cell populations endowed with cytolytic ability for antibody-coated tumour target cells. Scand J Immunol. 1978 Apr;7(4):297–306. doi: 10.1111/j.1365-3083.1978.tb00457.x. [DOI] [PubMed] [Google Scholar]
- Ritz J., Pesando J. M., Sallan S. E., Clavell L. A., Notis-McConarty J., Rosenthal P., Schlossman S. F. Serotherapy of acute lymphoblastic leukemia with monoclonal antibody. Blood. 1981 Jul;58(1):141–152. [PubMed] [Google Scholar]
- Ritz J., Schlossman S. F. Utilization of monoclonal antibodies in the treatment of leukemia and lymphoma. Blood. 1982 Jan;59(1):1–11. [PubMed] [Google Scholar]
- Stackpole C. W., Jacobson J. B., Lardis M. P. Antigenic modulation in vitro. I. Fate of thymus-leukemia (TL) antigen-antibody complexes following modulation of TL antigenicity from the surfaces of mouse leukemia cells and thymocytes. J Exp Med. 1974 Oct 1;140(4):939–953. doi: 10.1084/jem.140.4.939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevenson F. K., Elliott E. V., Stevenson G. T. Some effects on leukaemic B lymphocytes of antibodies to defined regions of their surface immunoglobulin. Immunology. 1977 Apr;32(4):549–557. [PMC free article] [PubMed] [Google Scholar]
- Stevenson F. K., Hamblin T. J., Stevenson G. T. The nature of the immunoglobulin G on the surface of B lymphocytes in chronic lymphocytic leukemia. J Exp Med. 1981 Dec 1;154(6):1965–1969. doi: 10.1084/jem.154.6.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevenson F. K., Hamblin T. J., Stevenson G. T., Tutt A. L. Extracellular idiotypic immunoglobulin arising from human leukemic B lymphocytes. J Exp Med. 1980 Dec 1;152(6):1484–1496. doi: 10.1084/jem.152.6.1484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevenson G. T., Elliott E. V., Stevenson F. K. Idiotypic determinants on the surface immunoglobulin of neoplastic lymphocytes: a therapeutic target. Fed Proc. 1977 Aug;36(9):2268–2271. [PubMed] [Google Scholar]
- Stevenson G. T., Stevenson F. K. Antibody to a molecularly-defined antigen confined to a tumour cell surface. Nature. 1975 Apr 24;254(5502):714–716. doi: 10.1038/254714a0. [DOI] [PubMed] [Google Scholar]
- Wernet P., Feizi T., Kunkel H. G. Idiotypic determinants of immunoglobulin M detected on the surface of human lymphocytes by cytotoxicity assays. J Exp Med. 1972 Sep 1;136(3):650–655. doi: 10.1084/jem.136.3.650. [DOI] [PMC free article] [PubMed] [Google Scholar]


