Abstract
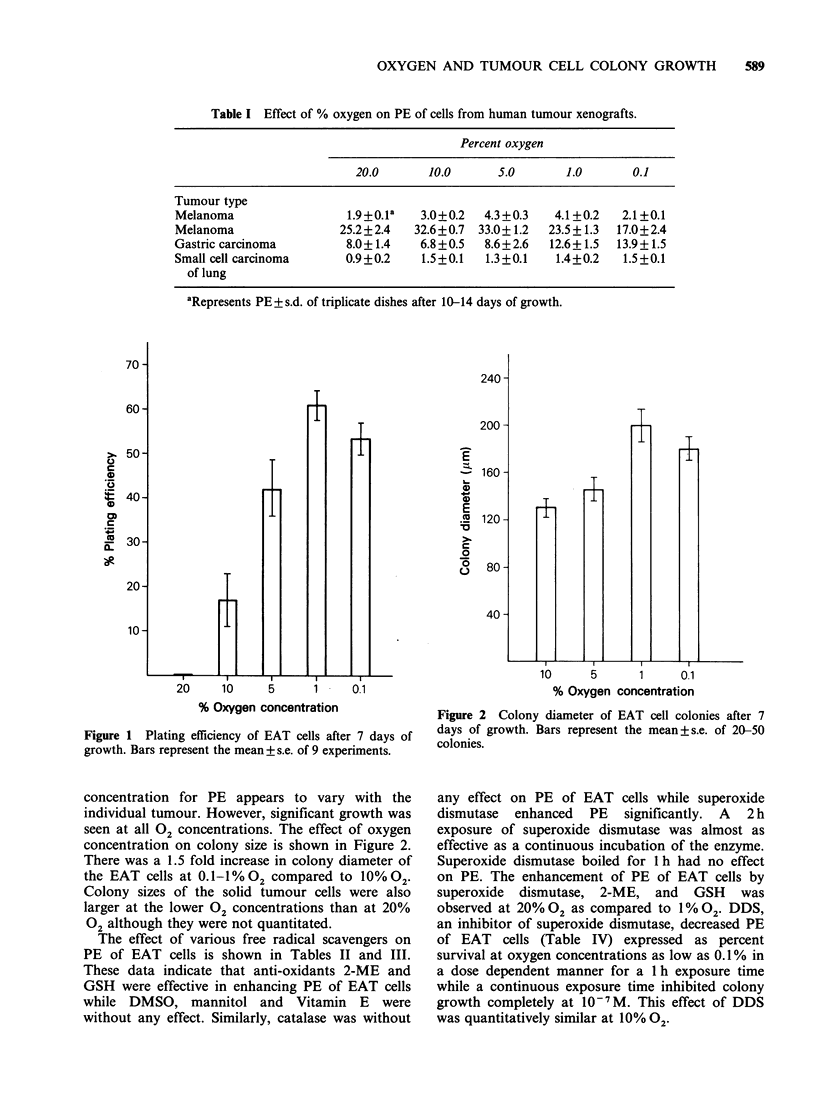
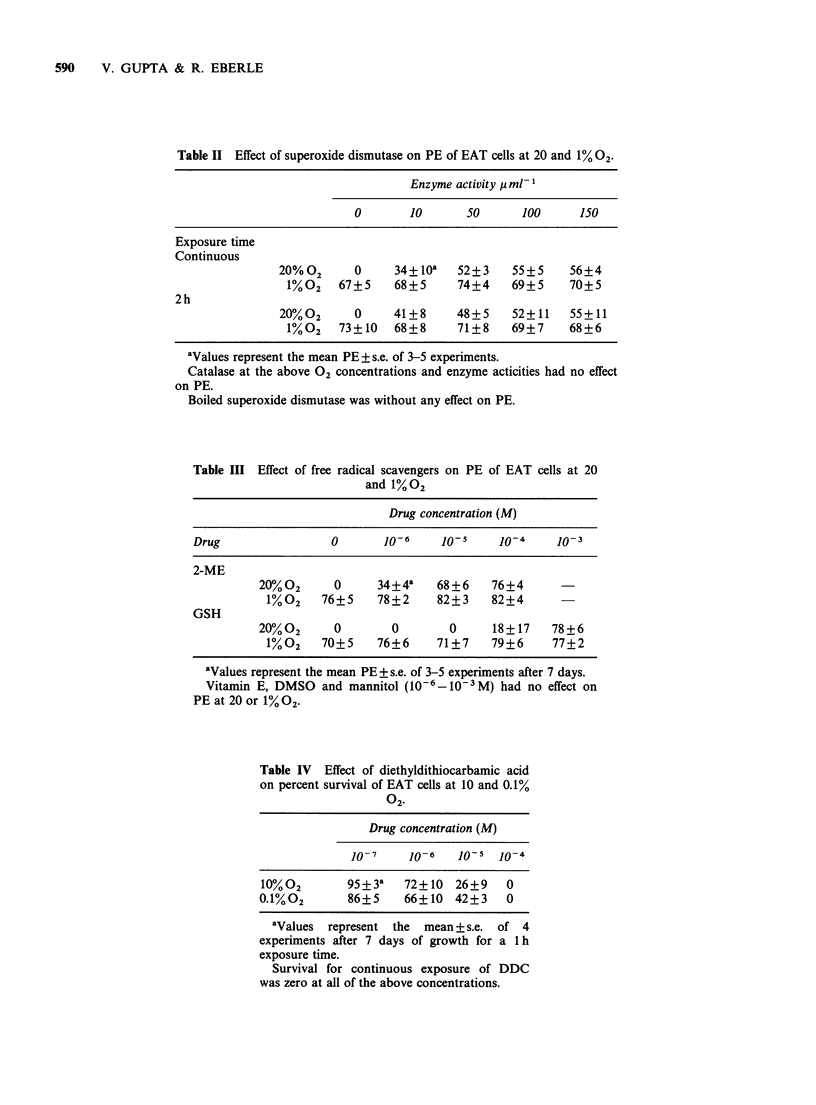
A simple technique for maintaining low oxygen concentrations (0.1-20%) is described. These conditions were then used to study the effect of oxygen on colony growth of neoplastic cells in soft-agar. Physiologically low oxygen concentrations (0.1-10%) compared to 20% O2 were found to enhance plating efficiency and colony size of tumour cells. The optimal oxygen concentration for plating efficiency varied with tumour studied and may be as low as 0.1%. Having established that tumour cell colonies will grow better at 0.1-10% O2 compared to 20% O2, the mechanism by which this enhancement occurs was investigated. Observations on the effect of free radical scavengers and superoxide dismutase on plating efficiency of Ehrlich's ascites tumour cells suggests that this phenomenon occurs through oxygen toxicity mediated by superoxide anion.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boag J. W. Cell respiration as a function of oxygen tension. Int J Radiat Biol Relat Stud Phys Chem Med. 1970;18(5):475–478. doi: 10.1080/09553007014551361. [DOI] [PubMed] [Google Scholar]
- Bradley T. R., Hodgson G. S., Rosendaal M. The effect of oxygen tension on haemopoietic and fibroblast cell proliferation in vitro. J Cell Physiol. 1978 Dec;97(3 Pt 2 Suppl 1):517–522. doi: 10.1002/jcp.1040970327. [DOI] [PubMed] [Google Scholar]
- CATER D. B., SILVER I. A. Quantitative measurements of oxygen tension in normal tissues and in the tumours of patients before and after radiotherapy. Acta radiol. 1960 Mar;53:233–256. doi: 10.3109/00016926009171671. [DOI] [PubMed] [Google Scholar]
- Chapman J. D., Sturrock J., Boag J. W., Crookall J. O. Factors affecting the oxygen tension around cells growing in plastic Petri dishes. Int J Radiat Biol Relat Stud Phys Chem Med. 1970;17(4):305–328. doi: 10.1080/09553007014550381. [DOI] [PubMed] [Google Scholar]
- Courtenay V. D. A soft agar colony assay for Lewis lung tumour and B16 melanoma taken directly from the mouse. Br J Cancer. 1976 Jul;34(1):39–45. doi: 10.1038/bjc.1976.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FROESE G. The respiration of ascites tumour cells at low oxygen concentrations. Biochim Biophys Acta. 1962 Mar 12;57:509–519. doi: 10.1016/0006-3002(62)91158-7. [DOI] [PubMed] [Google Scholar]
- Ganfield R. A., Nair P., Whalen W. J. Mass transfer, storage, and utilization of O2 in cat cerebral cortex. Am J Physiol. 1970 Sep;219(3):814–821. doi: 10.1152/ajplegacy.1970.219.3.814. [DOI] [PubMed] [Google Scholar]
- Gupta V., Krishan A. Effect of oxygen concentration on the growth and drug sensitivity of human melanoma cells in soft-agar clonogenic assay. Cancer Res. 1982 Mar;42(3):1005–1007. [PubMed] [Google Scholar]
- Halliwell B. Biochemical mechanisms accounting for the toxic action of oxygen on living organisms: the key role of superoxide dismutase. Cell Biol Int Rep. 1978 Mar;2(2):113–128. doi: 10.1016/0309-1651(78)90032-2. [DOI] [PubMed] [Google Scholar]
- Hamburger A. W., Salmon S. E. Primary bioassay of human tumor stem cells. Science. 1977 Jul 29;197(4302):461–463. doi: 10.1126/science.560061. [DOI] [PubMed] [Google Scholar]
- Hodgkiss R. J., Middleton R. W. Enhancement of misonidazole radiosensitization by an inhibitor of glutathione biosynthesis. Int J Radiat Biol Relat Stud Phys Chem Med. 1983 Feb;43(2):179–183. doi: 10.1080/09553008314550201. [DOI] [PubMed] [Google Scholar]
- Izaguirre C. A., Curtis J., Messner H., McCulloch E. A. A colony assay for blast cell progenitors in non-B non-T (common) acute lymphoblastic leukemia. Blood. 1981 May;57(5):823–829. [PubMed] [Google Scholar]
- JAMIESON D., VANDENBRENK H. A. EFFECT OF ELECTRODE DIMENSIONS ON TISSUE PO-2 MEASUREMENT IN VIVO. Nature. 1964 Mar 21;201:1227–1228. doi: 10.1038/2011227a0. [DOI] [PubMed] [Google Scholar]
- JAMIESON D., VANDENBRENK H. A. OXYGEN TENSION IN HUMAN MALIGNANT DISEASE UNDER HYPERBARIC CONDITIONS. Br J Cancer. 1965 Mar;19:139–150. doi: 10.1038/bjc.1965.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolstad P. Intercapillary distance, oxygen tension and local recurrence in cervix cancer. Scand J Clin Lab Invest Suppl. 1968;106:145–157. [PubMed] [Google Scholar]
- Lynch R. E., Fridovich I. Effects of superoxide on the erythrocyte membrane. J Biol Chem. 1978 Mar 25;253(6):1838–1845. [PubMed] [Google Scholar]
- Marklund S. L., Westman N. G., Lundgren E., Roos G. Copper- and zinc-containing superoxide dismutase, manganese-containing superoxide dismutase, catalase, and glutathione peroxidase in normal and neoplastic human cell lines and normal human tissues. Cancer Res. 1982 May;42(5):1955–1961. [PubMed] [Google Scholar]
- Meister A. Selective modification of glutathione metabolism. Science. 1983 Apr 29;220(4596):472–477. doi: 10.1126/science.6836290. [DOI] [PubMed] [Google Scholar]
- Mueller-Klieser W. F., Sutherland R. M. Oxygen tensions in multicell spheroids of two cell lines. Br J Cancer. 1982 Feb;45(2):256–264. doi: 10.1038/bjc.1982.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OSGOOD E. E., KRIPPAEHNE M. L. The gradient tissue culture method. Exp Cell Res. 1955 Aug;9(1):116–127. doi: 10.1016/0014-4827(55)90165-8. [DOI] [PubMed] [Google Scholar]
- Oberley L. W., Buettner G. R. Role of superoxide dismutase in cancer: a review. Cancer Res. 1979 Apr;39(4):1141–1149. [PubMed] [Google Scholar]
- Rich I. N., Kubanek B. The effect of reduced oxygen tension on colony formation of erythropoietic cells in vitro. Br J Haematol. 1982 Dec;52(4):579–588. doi: 10.1111/j.1365-2141.1982.tb03934.x. [DOI] [PubMed] [Google Scholar]
- Richter A., Sanford K. K., Evans V. J. Influence of oxygen and culture media on plating efficiency of some mammalian tissue cells. J Natl Cancer Inst. 1972 Dec;49(6):1705–1712. doi: 10.1093/jnci/49.6.1705. [DOI] [PubMed] [Google Scholar]
- Sahu S. K., Oberley L. W., Stevens R. H., Riley E. F. Superoxide dismutase activity of Ehrlich ascites tumor cells. J Natl Cancer Inst. 1977 Apr;58(4):1125–1128. doi: 10.1093/jnci/58.4.1125. [DOI] [PubMed] [Google Scholar]
- Smith S. D., Wood G. W., Fried P., Lowman J. T. In vitro growth of lymphoma colonies from children with non-Hodgkin's lymphoma. Cancer. 1981 Dec 15;48(12):2612–2623. doi: 10.1002/1097-0142(19811215)48:12<2612::aid-cncr2820481213>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- Vaupel P. W., Frinak S., Bicher H. I. Heterogeneous oxygen partial pressure and pH distribution in C3H mouse mammary adenocarcinoma. Cancer Res. 1981 May;41(5):2008–2013. [PubMed] [Google Scholar]
- Whalen W. J., Nair P. Intracellular PO2 and its regulation in resting skeletal muscle of the guinea pig. Circ Res. 1967 Sep;21(3):251–261. doi: 10.1161/01.res.21.3.251. [DOI] [PubMed] [Google Scholar]
- Whillans D. W., Rauth A. M. An experimental and analytical study of oxygen depletion in stirred cell suspensions. Radiat Res. 1980 Oct;84(1):97–114. [PubMed] [Google Scholar]
- Wilson D. F., Erecińska M., Drown C., Silver I. A. The oxygen dependence of cellular energy metabolism. Arch Biochem Biophys. 1979 Jul;195(2):485–493. doi: 10.1016/0003-9861(79)90375-8. [DOI] [PubMed] [Google Scholar]


