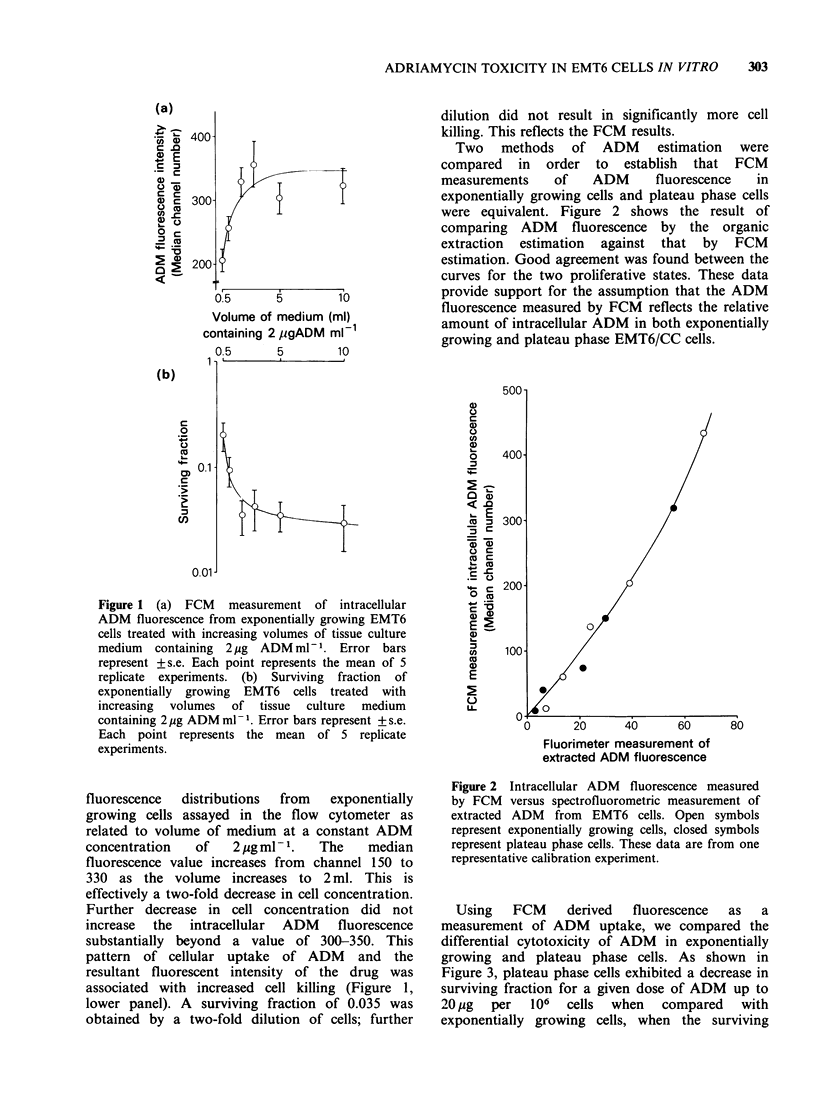
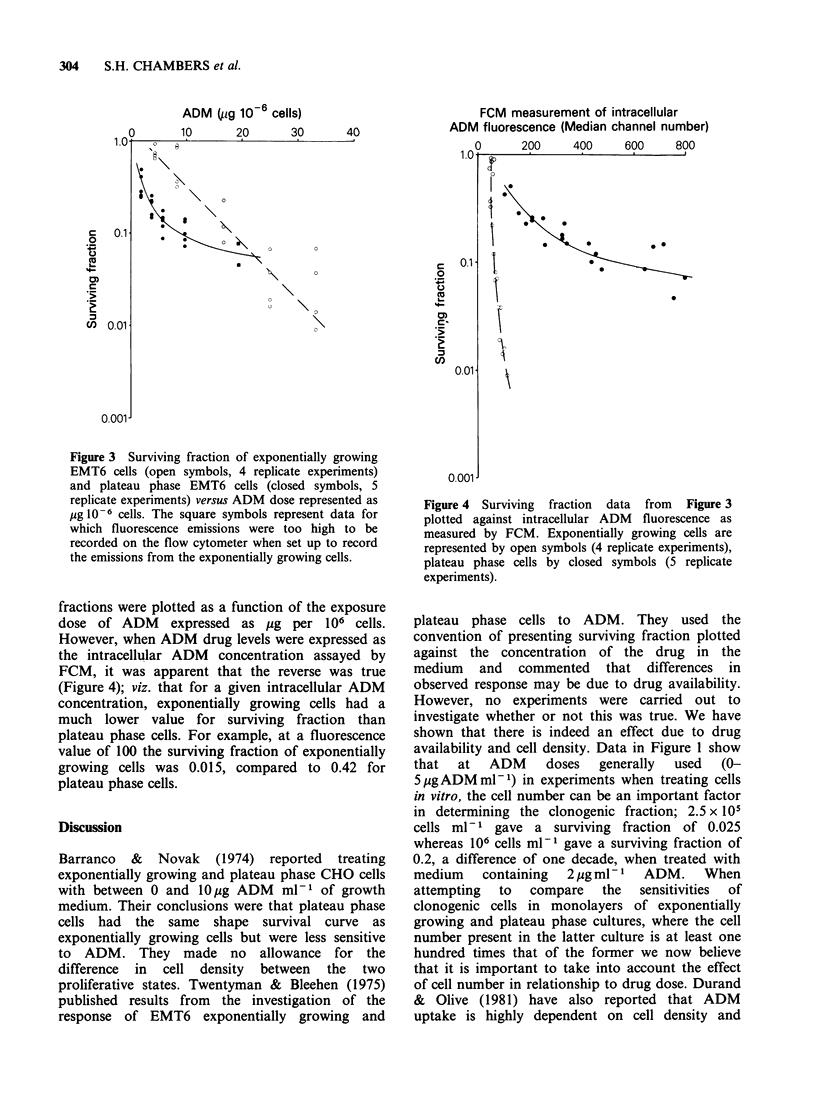
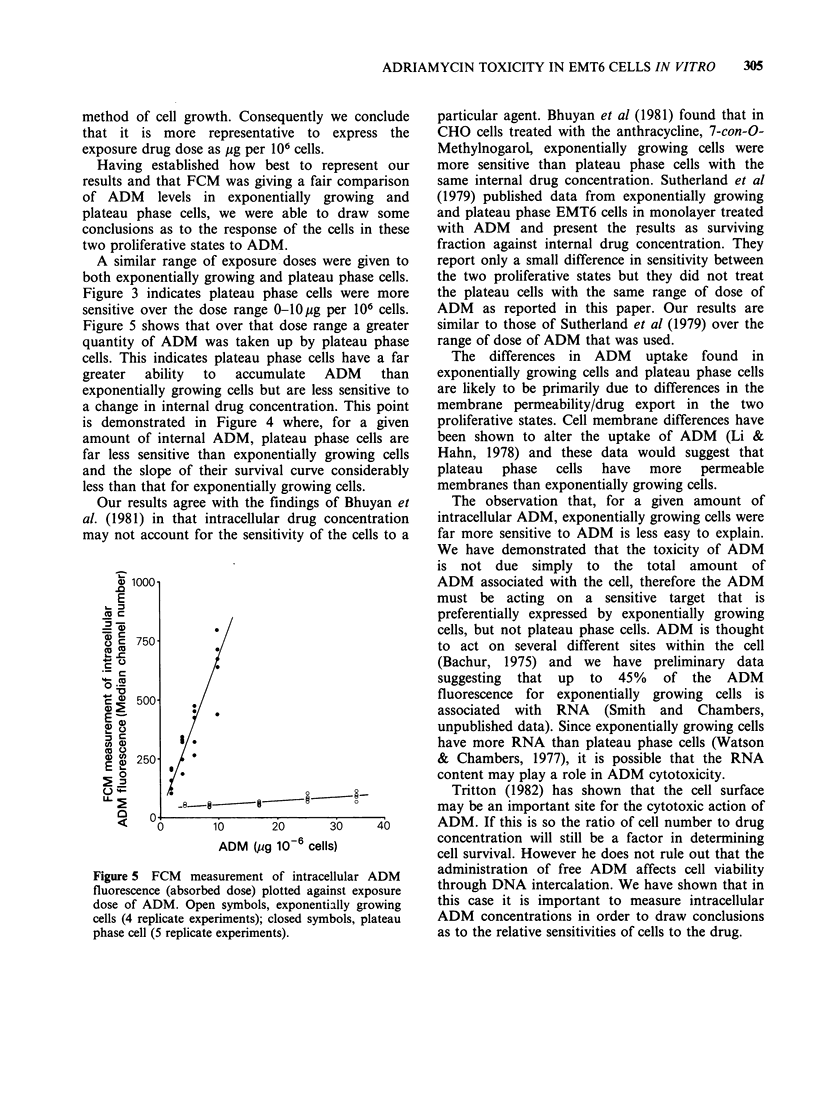
Abstract
The relationship between cell number and available Adriamycin (ADM) has been investigated in EMT6 cells. Results have shown that the ratio between cell number and total available ADM is important in determining in vitro ADM uptake and surviving fraction. Having established this effect, the sensitivity of exponentially growing and plateau phase EMT6 cells to ADM was investigated. ADM was assayed by extraction followed by spectrofluorimetry and also by flow cytometry (FCM); both methods were found to give the same ratio of intracellular ADM between exponentially growing and plateau phase cells. We found that for a given exposure dose plateau phase cells were more sensitive than exponentially growing cells. For the same dose per cell, plateau cells take up more ADM than exponentially growing cells. But for a given intracellular ADM concentration exponentially growing cells have a lower surviving fraction than plateau phase cells. We conclude that the surviving fraction is dependent on the proliferative state of the cells and in order to draw that conclusion it is important to relate the ADM effect on cells in vitro to the total ADM available to each cell.
Full text
PDF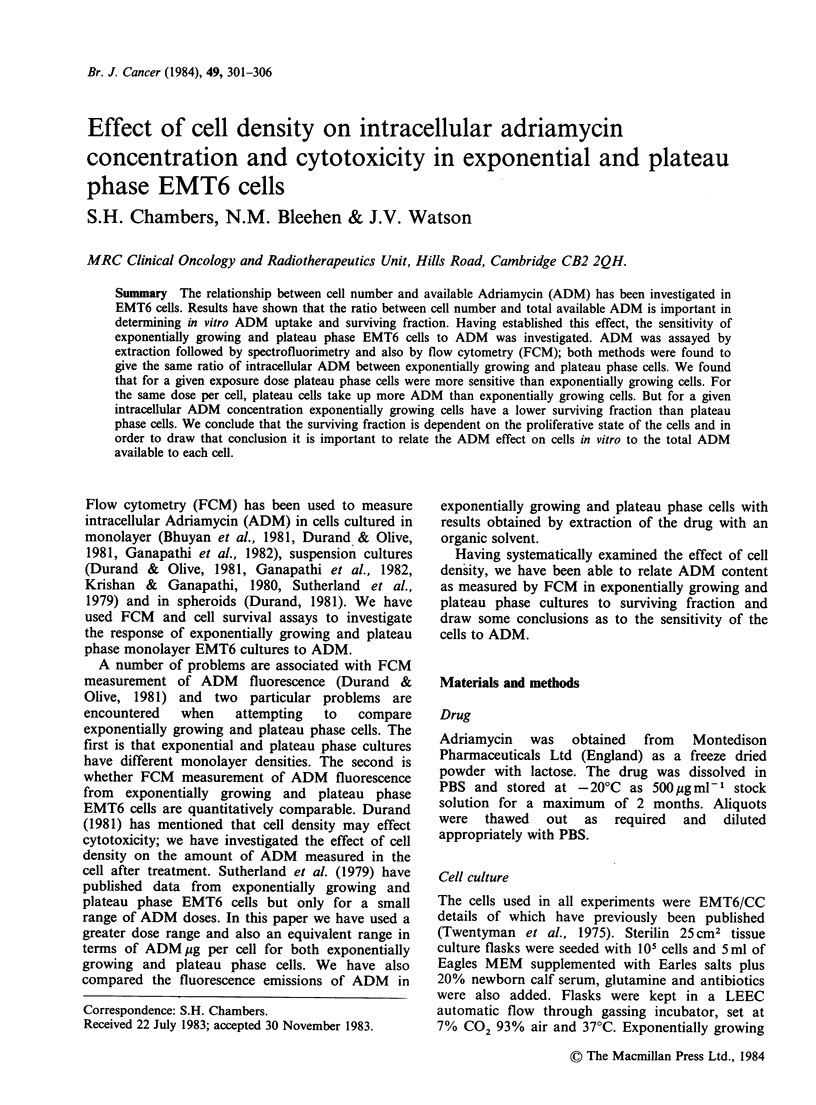


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barranco S. C., Novak J. K. Survival responses of dividing and nondividing mammalian cells after treatment with hydroxyurea, arabinosylcytosine, or adriamycin. Cancer Res. 1974 Jul;34(7):1616–1618. [PubMed] [Google Scholar]
- Bhuyan B. K., McGovren J. P., Crampton S. L. Intracellular uptake of 7-con-o-methylnogarol and adriamycin by cells in culture and its relationship to cell survival. Cancer Res. 1981 Mar;41(3):882–887. [PubMed] [Google Scholar]
- Durand R. E. Flow cytometry studies of intracellular adriamycin in multicell spheroids in vitro. Cancer Res. 1981 Sep;41(9 Pt 1):3495–3498. [PubMed] [Google Scholar]
- Durand R. E., Olive P. L. Flow cytometry studies of intracellular adriamycin in single cells in vitro. Cancer Res. 1981 Sep;41(9 Pt 1):3489–3494. [PubMed] [Google Scholar]
- Ganapathi R., Reiter W., Krishan A. Intracellular adriamycin levels and cytotoxicity in adriamycin-sensitive and adriamycin-resistant P388 mouse leukemia cells. J Natl Cancer Inst. 1982 Jun;68(6):1027–1032. [PubMed] [Google Scholar]
- Krishan A., Ganapathi R. Laser flow cytometric studies on the intracellular fluorescence of anthracyclines. Cancer Res. 1980 Nov;40(11):3895–3900. [PubMed] [Google Scholar]
- Li G. C., Hahn G. M. Ethanol-induced tolerance to heat and to adriamycin. Nature. 1978 Aug 17;274(5672):699–701. doi: 10.1038/274699a0. [DOI] [PubMed] [Google Scholar]
- Schwartz H. S. A fluorometric assay for daunomycin and adriamycin in animal tissues. Biochem Med. 1973 Jun;7(3):396–404. doi: 10.1016/0006-2944(73)90060-4. [DOI] [PubMed] [Google Scholar]
- Sutherland R. M., Eddy H. A., Bareham B., Reich K., Vanantwerp D. Resistance to adriamycin in multicellular spheroids. Int J Radiat Oncol Biol Phys. 1979 Aug;5(8):1225–1230. doi: 10.1016/0360-3016(79)90643-6. [DOI] [PubMed] [Google Scholar]
- Triton T. R., Yee G. The anticancer agent adriamycin can be actively cytotoxic without entering cells. Science. 1982 Jul 16;217(4556):248–250. doi: 10.1126/science.7089561. [DOI] [PubMed] [Google Scholar]
- Twentyman P. R., Bleehen N. M. Changes in sensitivity to cytotoxic agents occurring during the life history of monolayer cultures of a mouse tumour cell line. Br J Cancer. 1975 Apr;31(4):417–423. doi: 10.1038/bjc.1975.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Twentyman P. R., Watson J. V., Bleehen N. M., Rowles P. M. Changes in cell proliferation kinetics occurring during the life history of monolayer cultures of a mouse tumour cell line. Cell Tissue Kinet. 1975 Jan;8(1):41–50. doi: 10.1111/j.1365-2184.1975.tb01205.x. [DOI] [PubMed] [Google Scholar]
- Watson J. V., Chambers S. H. Fluorescence discrimination between diploid cells on their RNA content: a possible distinction between clonogenic and non-clonogenic cells. Br J Cancer. 1977 Nov;36(5):592–600. doi: 10.1038/bjc.1977.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson J. V. Enzyme kinetic studies in cell populations using fluorogenic substrates and flow cytometric techniques. Cytometry. 1980 Sep;1(2):143–151. doi: 10.1002/cyto.990010209. [DOI] [PubMed] [Google Scholar]


