Abstract
There is now abundant evidence to substantiate an important role of hepatitis C virus (HCV) core protein in cellular gene expression as well as in the viral cycle. Thus the subcellular localization of this protein has important implications. However, several studies have shown controversial results: the HCV core has been, indeed, described as cytoplasmic or nuclear depending on the size of the protein or on the genotype analyzed. We have studied the localization of the HCV core protein in two different cell lines, one nonhepatic (CHO) and the other hepatic (HepG2). Double immunofluorescence staining using a nuclear membrane marker and confocal analysis showed the core protein pattern to be cytoplasmic and globular. This pattern is not cell cycle-regulated. Electron microscopy analysis revealed the nature of the globular staining observed in immunofluorescence. The HCV core protein accumulated at the surface of lipid droplets that were also the unique morphological feature of nonhepatic core transfected cells. The lipid droplets were isolated by sequential ultracentrifugation on the basis of their density; biochemical analysis revealed a prevalence of triglycerides. In addition the core protein colocalized with apolipoprotein AII at the surface of the lipid droplets as revealed by confocal microscopy. Moreover analysis of liver biopsies from chronically HCV-infected chimpanzees revealed that HCV core is cytoplasmic and localized on the endoplasmic reticulum and on lipid droplets. These results clearly define the subcellular localization of the HCV core protein and suggest a relationship between the expression of the HCV core protein and cellular lipid metabolism.
The hepatitis C virus (HCV), the major causative agent of non-A/non-B hepatitis (1), is a positive-stranded RNA virus of about 10 kb evolutionary related to pestivirus and flavivirus (2, 3). The HCV ORF is flanked by a 341-bp long 5′ untranslated region and a 3′ untranslated region with a poly(U) or a poly(A) tail (3, 4) and encodes for a precursor polyprotein of about 3000 aa that is then cleaved into structural and nonstructural proteins (5, 6).
A major characteristic of HCV infection is the extremely high (up to 80%) risk of chronicity; in addition, chronic infection can lead to liver cirrhosis and liver cancer (7, 8). An important issue regarding the pathogenesis of HCV-associated liver lesions is to determine whether or not HCV proteins might have a direct effect on cellular phenotype as suggested by some recent works (9, 10). In this view, a regulative effect by HCV core protein, one of the structural proteins of the virus, has been shown by transfection both on hepatitis B viral genome expression and replication (11) and on expression of different cellular genes such as c-myc oncogene (12) or genes encoding for interferon β in a human cell line (13).
The observations reported above would suggest that the HCV core protein could have not only a packaging function in the cytoplasm, but also a regulatory role on cell functions. Precise information on the subcellular localization of HCV core is therefore necessary to interpret these observations. So far only a few studies have analyzed, in the absence of an efficient cell culture system for HCV, expression of recombinant cDNAs. Discrepancies have been observed among these analysis, and the core protein has been indeed described as cytoplasmic, although some authors have reported a nuclear localization under particular conditions such as the truncation of the hydrophobic C-terminal region (13). Whether this form exists in vivo during viral infection remains to be demonstrated. These discrepancies could reflect a change in subcellular localization dependent on the phase of the cell cycle as has been already reported for the HBV core protein (14, 15).
To address further this important question, we have undertaken a detailed analysis of the HCV core localization by using a combination of cell cycle synchronization and confocal and electron microscopy. We have analyzed two cell lines (CHO and HepG2) stably expressing this protein. Our results clearly exclude an intranuclear localization. They also demonstrate that, upon expression of HCV core, cells show cytoplasmic accumulation of lipid (triglyceride-rich) droplets on which the core accumulates. Finally, a colocalization with apolipoprotein (apo) AII has been detected by confocal microscopy. In addition, liver biopsies from HCV chronically infected chimpanzees show presence of steatosis in comparison to normal control liver biopsies, and electron microscopic localization of HCV core protein in these samples shows an accumulation of the protein on the surface of lipid droplets. Our data therefore indicate an interaction between synthesis and intracellular transport of HCV core protein and lipid metabolism.
MATERIALS AND METHODS
Cells.
CHO cells were transfected with the vector pChmBp1 316 carrying under the control of SRα promoter the HCV cDNA covering core and E1 regions inserted as one transcription units or, as negative control, with the empty vector. HepG2 cells were transfected with the vector pEF352neo carrying under the control of elongation factor (EF)-1α promoter the HCV cDNA covering from core to NS3 region inserted as one transcript unit or, as negative control, with the empty vector. Two independent clones of each cell line have been analyzed.
Immunofluorescence.
CHO cells or HepG2 cells were plated at a density of 5 × 104 on glass coverslips. After 2 days cells were fixed in acetone at −20°C and incubated with the primary antibodies. The following antibodies were used: (i) anticore mAb 2E5 recognizing the aa 105–112 (16); (ii) antinuclear lamin B polyclonal antibody developed in rabbit (gift of J. C. Courvalin, Institut Jacques Monod, Paris); (iii) anti-apoAI and -apoAII polyclonal antibodies developed in rabbit (gift of N. Vu-Dac, Institut Pasteur, Lille, France).
Cells were then labeled with fluorescein isothiocyanate-conjugated sheep anti-mouse Ig (Amersham) or Texas-red-conjugated donkey anti-rabbit Ig (Amersham). Nuclei were stained with propidium iodide at 1 μg/ml (Sigma).
Cell Synchronization.
Briefly, CHO cells were synchronized in G1/S and G2/M with hydroxyurea (Sigma) at 2 mM for 16 h, and nocodazole (Sigma) at 100 ng/ml for 16 h, respectively, with standard protocol.
Electron Microscopy.
For standard electron microscopic techniques, cells monolayers were fixed at 4°C for 1 h with 1.6% glutaraldehyde (TAAB Laboratory Equipment, Reading, U.K.). The monolayer was then scraped from the Petri dish, washed once in phosphate buffer, and postfixed either in 2% osmium tetroxide or directly dehydrated in increasing concentration of ethanol and embedded in Epon.
For immunolabeling, monolayers were fixed in 4% paraformaldehyde (Merck) for 1 h at 4°C. Pellets were dehydrated in increasing concentrations of methanol and embedded at low temperature in Lowicryl K 4 M (Chemische Werke Lowi, Waldkraiburg, Germany). The grids were incubated for 30 min at room temperature with anticore mAb 2E5, washing in PBS, and incubated for 30 min with goat anti-mouse IgG conjugated to gold particles, 10 nm in diameter (Biocell Research Laboratories, Cardiff, U.K.). The grids were then washed in PBS, rinsed in distilled water, air-dried, and stained for 10 min with 5% acqueous uranyl acetate. For controls, normal mouse serum instead of anticore antibody were used.
Growth Curve.
Cells were seeded at a density of 0.3 × 106 per 100-mm Petri dish and counted each day for 10 days. The medium was changed daily and the experiment was done in triplicate. The growth curve was obtained by plotting the log of cell number against the days of growth.
Viability of the cells was also tested by measuring the activity of mitochondrial dehydrogenases in the 3-(4,5-dimethylthiaziol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) test (Boehringer Mannheim).
Isolation of Lipid Droplets.
Isolation of lipid droplets from cellular extract was performed by a sequential ultracentrifugal procedure (17). About 30 or 80 × 106 cells for the CHO and 50 or 120 × 106 cells for the HepG2 were collected in 12 ml of PBS and homogenized by several passages through needles of decreasing size (18G–21G–26G). The homogenate was ultracentrifuged at 35,000 rpm for 2 h at 15°C in a SW41 rotor using a L8-S5 Beckmann centrifuge. About 1 ml from the meniscus downwards was collected and taken as fraction I (top d < 1.006 g/ml). The infranatant was resuspended and adjusted to a density of 1.063 g/ml by adding solid KBr, then centrifuged at 35,000 rpm in SW41 rotor at 15°C overnight. One milliliter was then collect from the meniscus downwards and taken as fraction II (top d 1.006–1.063 g/ml), the infranatant was taken as fraction III (infranatant d < 1.063), and the pellet was resuspended in SDS buffer (1% SDS/10 mM Tris, pH 7.4) and taken as fraction IV (pellet d > 1.063).
All fractions were exhaustively dialyzed at 4°C against a solution containing 0.15 M NaCl and 1 mM EDTA (pH 7.4).
Chemical Analysis.
The protein concentration was determined by the Lowry procedure (18); fractions I and II were delipidated by adding sodiumdesoxycholate (0.5 M). Triglyceride, total cholesterol, and phospholipid content was determined as described (17). The core protein was detected by Western blot and by a recently established ELISA assay (19). Briefly 2–50 μl of sample were treated with 50 μl of 10 M urea and 50 μl of 0.5 M NaOH and incubated for 10 min at room temperature. For neutralization 50 μl of 0.5 M NaH2PO4 was added to a total volume of 152–200 μl. The samples were added to tubes precoated with an anticore mAb 5F11 and blocked with 2% casein. After 10 min at 37°C, mAb 5E3 conjugated to β-d-galactosidase was added for 9 min at 37°C. 4-Methylumbelliferyl β-d-galactopyranosid was used as substrate. After 9 min incubation at 37°C the reaction was blocked by adding 0.1 M glycine-NaOH (pH 10.3) and the relative fluorescence intensity was determined as described (20).
Liver Biopsies from HCV Chronic-Infected Chimpanzees.
Two chimpanzees (E4 and E5) were inoculated i.v. in 1985 with an HCV-infected plasma pool. Nine years later both animals were challenged with an inoculum of factor VIII concentrate produced from HCV-infected plasma pool. Both animals developed chronic hepatitis C.†† Liver needle biopsy samples were taken from chimpanzees E4 and E5 and from one uninfected chimpanzee (F4). The biopsy samples were fixed in 1% paraformaldehyde mixed with 0.1% glutaraldehyde at 4°C for 20 min and washed in PBS. Thin slices (20–30 μm) of liver tissue were incubated with the anticore mAb 2E5 for 1 h at room temperature. After washing in PBS, the sections were incubated with goat anti-mouse IgG conjugated to gold particles, 10 nm in diameter (GAMAG 10; Amersham) for 1 h at room temperature. As control the primary antibody was substituted by normal mouse Igs. After washing in PBS, the sections were postfixed in 2% osmium tetroxide for 5 min at room temperature, washed in PBS, and embedded into poly(B) (Polysciences). Ultrathin sections were cut, not counterstained, and examined in a Philips CM10 electron microscope.
RESULTS
Confocal Analysis of Asynchronous Cells.
To investigate the subcellular localization of the HCV core protein we used CHO and HepG2 cells stably expressing this protein.
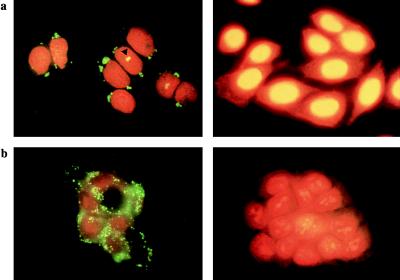
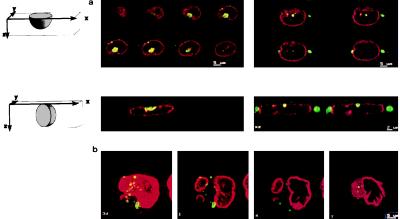
Indirect immunofluorescence analysis on CHO asynchronous cells revealed the core protein in the cytoplasm of the cells with a characteristic globular staining and a perinuclear localization, whereas the negative control did not show any staining (Fig. 1a). In addition a minority of the cells showed a superposition of the fluorescein stained core protein and propidium iodide staining in the nucleus (Fig. 1a). This pattern was further analyzed by confocal microscopy to verify if it could represent a nuclear localization of the core protein. The cells were analyzed along the horizontal axis in slices of 0.3 μm each and were analyzed also along the vertical axis. By a double staining for the core and nuclear membrane lamin proteins together with the use of confocal analysis of the vertical sections, we were able to clearly exclude an intranuclear localization of the core protein (Fig. 2a). The images suggested localization on the cytoplasmic side of the nuclear membrane, although, with such analysis, we cannot definitely exclude a localization on the inner membrane of the nuclear envelope. Fig. 2b shows the origin of the pseudo-nuclear staining observed in some cells, even by confocal microscopy: in the reconstruction of the different slices of the cells, the core seems to be nuclear, but from the analysis of the single slices, the border of the nucleus is clearly visible and shows that the staining is cytoplasmic and the core protein lies in a nuclear invagination.
Figure 1.
Indirect immunofluorescence analysis to detect the HCV core protein. (a) CHO cells expressing the HCV core protein (Left) and negative control (Right). The triangle shows the superposition between the core protein and the nucleus. (b) HepG2 cells expressing the HCV core protein (Left) and negative control (Right).
Figure 2.
Confocal analysis of double immunofluorescence in CHO cells stained for the HCV core and the nuclear membrane marker lamin B. (a Upper) Horizontal sections of 0.5 μm each of two cells with the core protein in the nucleus. (Lower) Vertical sections of 0.3 μm of the same cells. The core protein is visible on the cytoplasmic site of the nuclear membrane and has been never found in the nucleus. (b) Explanation of the pseudo-nuclear localization of the core protein. Shown from left to right are superposition of all the slices: the core protein seems to be in the nucleus; successive slices from the bottom of the cell: the core protein is in the cytoplasm and lies in a nuclear invagination.
In the HepG2 cells the core protein also showed a globular pattern but with a different distribution. No nuclear staining was detected and the protein was localized in the cytoplasm without a predominant perinuclear localization and showing a preferential submembranous accumulation. The negative control showed no staining (Fig. 1b).
Immunofluorescence of Synchronized Cells.
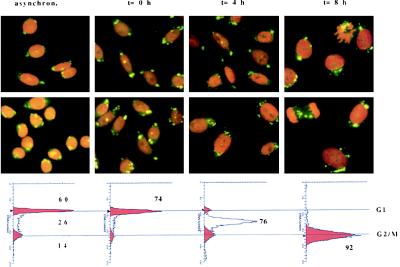
Fig. 3 shows the results of an experiment where synchronization was achieved by hydroxyurea treatment. Immunofluorescence analysis of the different phases of the cell cycle did not reveal any obvious change in the globular perinuclear pattern of the core protein. Therefore we concluded that the cell cycle does not influence the distribution of the core protein in the cell.
Figure 3.
Indirect immunofluorescence analysis of synchronized CHO cells. (Lower) Schematic representation of the synchronization obtained by plotting the DNA content against the number of cells. The number indicates the percent of cells in a specific phase of the cell cycle. The immunofluorescence analysis shows that the core protein does not change its globular perinuclear distribution during the different phases of the cell cycle.
Localization by Electron Microscopy.
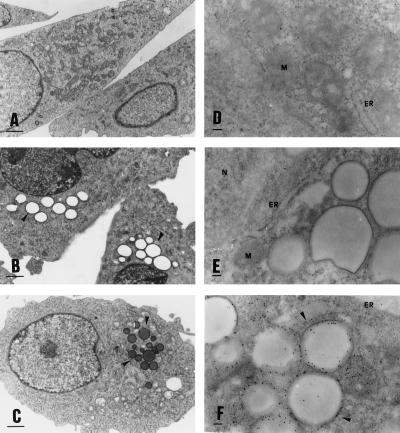
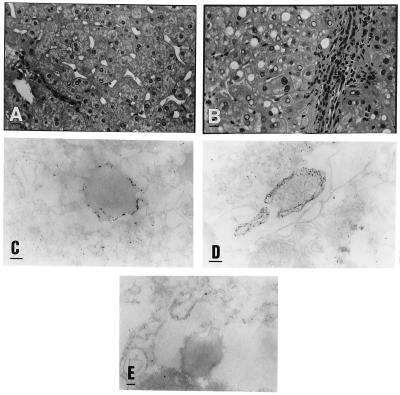
The pattern of the core protein was further analyzed by electron microscopy to identify the globular structures of the staining. The analysis of the slices fixed by glutaraldehyde revealed the presence of lipid droplets in core-expressing CHO cells that are absent in the negative control (Fig. 4 B, C, and A, respectively). The lipid content of the droplets is demonstrated by the osmium tetroxide staining (Fig. 4C). Lipids are in fact preserved if fixed with osmic acid and show a gray staining (Fig. 4C). Lipids are usually dissolved leaving an empty space or vacuole if not properly fixed as the case when using only glutaraldehyde (Fig. 4B). These droplets are delimited by an electron dense region similar to a membrane and show a perinuclear localization. The presence of droplets represent the unique morphologic difference between the core-expressing cells and the negative control. The size of the droplets ranged between 1.4 μm and 0.2 μm (average 0.75 μm).
Figure 4.
Electron microscopy analysis of core-expressing CHO cells. (Left) (A) CHO-negative control cells. (B) Core-expressing CHO cells fixed only with glutaraldehyde. (C) Core-expressing CHO cells fixed with glutaraldehyde and postfixed with osmic acid. Only the core-expressing cells show an accumulation in the cytoplasm of droplets that show a lipid content only by a fixation which preserve lipids as the osmic staining. (×8000; bar = 1 μm.) (Right) (D) CHO-negative control cells fixed with paraformaldehyde and immunostained by an anticore mAb. (E) Core-expressing CHO cells immunostained by normal mouse serum. (F) Core-expressing CHO cells immunostained by an anticore mAb. The two negative controls show absence of unspecific staining. The core protein is concentrated on the surface of the lipid droplets, and only minor staining is present on the endoplasmic reticulum (ER). (×45,000; bar = 0.1 μm.)
Immunostaining with a gold-conjugated anticore antibody revealed the protein on the surface of these droplets and only minor staining was found on the ER (Fig. 4F). Negative controls show the specificity of the staining (Fig. 4 D and E). The accumulation of the core protein on the surface of the lipid droplets provides an explanation of the globular staining detected in the immunofluorescence analysis.
To assess if the lipid droplets are due to toxicity and/or reduced cell viability due to the expression of the core protein we constructed a growth curve of the core-expressing cells in comparison to the negative control cells. The presence of the core did not influence the growth kinetic of the cells; the same conclusion was obtained by the MTT test, an assay for the quantification of the metabolically active cells by measuring mitochondrial dehydrogenases activity (data not shown).
In the HepG2 cells, the core protein also accumulated on the surface of lipid droplets which were not exclusively present in the HepG2 core-expressing cells, but were also present in the control cells. The immunostaining with an anticore antibody revealed the core protein on the surface of the lipid droplets of the core-expressing cells whereas no staining was detected on the lipid droplets of the negative cells (data not shown). The size of the droplets was smaller and more homogeneous than that in CHO cells, ranging between 0.6 to 0.2 μm (average 0.47 μm). Mitochondrial staining was also observed in the HepG2 cells.
Liver Biopsies from HCV-Infected Chimpanzees.
The liver biopsy samples taken from the HCV-infected chimpanzees have shown the ultrastructural alterations typical for HCV infection (21, 22). Namely, undulating membranes of the ER, reticular aggregates of the intracytoplasmic membranes, were seen. Large lipid droplets were also characteristic for both biopsy materials obtained from the HCV-infected chimpanzees, while were absent in the control chimpanzee (Fig. 5 B and A, respectively). Accumulation of the gold particles was shown in a few hepatocytes at different localization, around some of the lipid droplets and along the ER (Fig. 5 C and D). No labeling was seen inside the lipid droplets or in association with the mitochondria, nuclear membrane, or nucleoplasm. No accumulation of the gold particles was seen in the section incubated with normal mouse immunoglobulins instead of the primary antibody or by omitting the first antibody (Fig. 5E). The average size of the droplets was 0.8 μm.
Figure 5.
(A) Normal liver tissue taken from an uninfected chimpanzee (F4). (B) Liver tissue taken from a chimpanzee (E5) chronically infected with HCV. Inflammatory infiltration in the portal area, fatty degeneration of the hepatocytes. (×320; bar = 20 μm.) (C) Immunostaining with an anticore mAb. Shown are 10 nm gold particles surrounding a lipid droplet located in the cytoplasm of an hepatocyte taken from an HCV-infected chimpanzee. (D) Accumulation of gold particles in an hepatocyte along the membrane of the ER taken from a chimpanzee infected with HCV. (×78,000; bar = 0.1 μm.) (E) Negative control. Part of the cytoplasm of an hepatocyte taken from an HCV-infected chimpanzee (E5). No primary antibody to core was applied. No accumulation of gold particles around the lipid droplets (not contrasted). (×58,900; bar = 0.1 μm.)
Composition of the Lipid Droplets.
Data obtained from two independent experiments using chemical and Western blot assays for analysis of fractions I, III, and IV of cell lysates after density fractionation are reported in Table 1. Only traces of material were recovered from fraction II (<100 μg), and it was not possible to perform biochemical analyses on this fraction. Fraction I (top d < 1.006 g/ml) contained the lipid droplets observed in the whole cells as revealed by negative staining electron microscopy (data not shown). Fractions I but CHO-negative cells are enriched in triglycerides (>40% by weight) and the content of such lipid was increased more than 2.2-fold in the CHO core-expressing cells in comparison to the CHO control cells (Table 1). In the HepG2 cells, the lipid droplets were present both in the control cells as well as in the core-expressing cells, in which case both cell lines showed triglyceride as a major lipid component. The variation between the two experiments performed likely reflects the isolation of distinct populations of lipid droplets in the two different clones analyzed for each cell line; cellular lipid droplets, indeed, show marked heterogeneity in size and triglyceride/phospholipid ratio (23)—i.e., large droplets have a high content of triglyceride and are poor in phospholipid whereas small droplets have less triglycerides and are richer in phospholipid. However, the ratio of triglyceride content between CHO-negative and CHO-positive cells remained invariate. Cellular cholesterol and phospholipid content did not vary significantly between control cells and core-expressing cells (data not shown). The core protein was detected by Western blot in fractions I and IV of core-expressing cells and the ELISA test (limit of sensitivity, 10 pg/ml) confirmed that the core protein co-isolated with the triglyceride-rich fraction I in both CHO and HepG2 core-expressing cells (Table 1). No significant amount of core protein was detected in fractions II and III for both cell lines.
Table 1.
Triglyceride and HCV core protein content of cellular subfractions isolated by ultracentrifugation from CHO and HepG2 cell lysate
| Top fraction (fraction I, d < 1.006)
|
Infranatant fraction (fraction III, d < 1.063)
|
Pellet fraction in SDS (fraction IV, d > 1.063)
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Triglyceride, | ELISA core, ng/ml | W.B. core | Triglyceride, | ELISA core ng/ml | W.B. core | Triglyceride, | ELISA core ng/ml | W.B. core | |||
| % weight | % weight | % weight | |||||||||
| CHO− | 19 | − | − | CHO−* | 7.5 | − | − | CHO− | 1.5 | − | − |
| (9–29) | (6–9) | (0–1.5) | |||||||||
| CHO+ | 43 | 99.5 | + | CHO+* | 8 | 3.8 | − | CHO+ | 1.2 | 1943 | + |
| (18–68) | (53–146) | (2–14) | (0.–3.8) | (0–1.2) | (686–3200) | ||||||
| Hep− | 41 | − | − | Hep− | 10 | − | − | Hep− | 3.3 | − | − |
| (27–55) | (10–10) | (0–3.3) | |||||||||
| Hep+ | 42 | 37.25 | + | Hep+ | 8.5 | 4.6 | − | Hep+ | 3 | 171.5 | + |
| (22–62) | (36–38.5) | (6–11) | (0–4.6) | (0–3) | (101–242) | ||||||
The results are taken from two separate experiments using two independent clones for each cell line. All the analyses were performed in duplicate. W.B., Western blot from 5 μg of total extract; anticore mAb 2E5 and anti-mouse peroxidase conjugated antibody were used at 1:2000 dilution. Bold numbers indicate the change in triglyceride content in CHO cells.
*Fraction III from CHO negative and positive cells contained traces of material (<100 μg).
Colocalization of the HCV Core Protein and ApoAII by Confocal Microscopy.
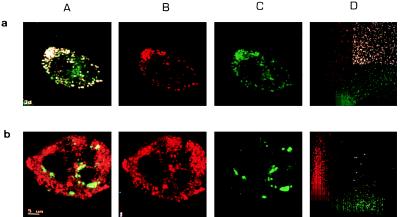
Double immunofluorescence staining for the HCV core protein and apoAII was performed on HepG2 cells. The subcellular distribution of both proteins was analyzed by confocal microscopy. Fig. 6a shows a colocalization of the HCV core and the endogenous apoAII in cytoplasmic globular structures as schematized in the diagram obtained by plotting the intensity of fluorescein- and Texas-red fluorescence for each point of the cell. In contrast, no colocalization was detected between the core and the endogenous apoAI (Fig. 6b).
Figure 6.
(a) Confocal analysis of double immunofluorescence staining for the HCV core and apoAII in HepG2 cells. Lanes: A, superposition of the staining for HCV core and apoAII; B, HCV core protein stained by Texas-red; C, apoAII stained by fluorescein; D, cytofluorogram. The cytofluorogram was obtained by plotting for each point of the cell the fluorescence green on the x axis and the fluorescence red on the y axis. Colocalization is indicated from the white points (strongest fluorescence green and red), and from the general shape of the graphic, the points are indeed concentrated on the diagonal of the graphic meaning that the most points are fluorescent for both fluorochromes. (b) Confocal analysis of double immunofluorescence staining for the HCV core and apoAI in HepG2 cells. Lanes: A, superposition of the staining for HCV core and apoAI; B, HCV core protein stained by Texas-red; C, apoAI stained by fluorescein; D, cytofluorogram. Only few points show a superposition of the red and green fluorescence. The cytofluorogram shows two different peaks for the red and the green fluorescence, indicating no colocalization.
DISCUSSION
The combination of immunofluorescence and confocal and electron microscopy in our study has provided an accurate picture of the subcellular organization of the HCV core protein expressed in two different stable transfected cell lines, including a differentiated hepatoma derived cell line (HepG2) as well as liver biopsy samples. Immunofluorescence staining showed that the protein was concentrated on globular structures independently of the cell type (CHO or HepG2) and of the level of core protein expressed (high or low level in CHO and HepG2, respectively). The combination of confocal microscopic and electron microscopic analyses allowed us to exclude an intranuclear localization. Colocalization with lamin B suggested that a part of the core might associate to the nuclear envelope. In addition, electron microscopic analysis revealed that the cytoplasmic globular structures were lipid droplets. Confocal microscopy also revealed colocalization of the core protein and lipid-associated protein apoAII. Finally, immunoelectron microscopic staining revealed the core protein on the surface of lipid droplets present in the liver of HCV chronic-infected chimpanzees.
The subcellular localization of the HCV core protein remains a controversial subject. In tissue sections, HCV antigens have been exclusively detected in the cytoplasm of the infected hepatocytes, whereas the nuclei were never stained (24, 25). However, most studies have involved HCV-positive sera as the source of antibody and have thus failed to specifically analyze core localization. In cell culture, several authors have demonstrated a nuclear localization for truncated forms of the core protein (11, 13, 16). In this study, in agreement with the in vivo observations, we demonstrate an exclusive cytoplasmic localization of the core protein. We would like to stress the necessity of using vertical sections in confocal microscopy in combination with nuclear membrane staining as the only method that can eliminate the artefacts due to localization of the protein in nuclear invaginations. The cell cycle did not influence the subcellular distribution of the core protein, which exhibited the same cytoplasmic globular pattern at all phases of the cycle.
Three different core protein products have been described: p21, p19, and p16 (26). Both p21 and p19, which correspond to the 191- and 173-aa protein fragments, respectively, display a cytoplasmic pattern associated with the ER membrane; by contrast, the p16 fragment, which consists of about 151 aa, shows a nuclear localization with enrichment in nucleoli (27). This truncated form shows an amino acid substitution at codon 9 and has been so far only found as a product of genotype 1a. In this regard, the HCV isolate used in our system was 1b and our observation is in fact consistent with the absence of nuclear localization reported by the same group for this HCV genotype (27). In addition, Western blot analysis of our cell extracts showed a unique band at 21 kDa (data not shown).
A cytoplasmic globular pattern has been described in other cell lines transiently transfected with different plasmids encoding the core protein (11, 28, 29). This cytoplasmic localization of the core protein has been described as ER-associated on the basis of immunofluorescent images. Our images in immunofluorescence are comparable to that reported by other authors (27). Electron microscopic analysis, however, could detect only a minor staining on the ER, whereas most of the core protein was present on the surface of lipid droplets.
Double staining of core protein and an ER marker does not apparently reveal a complete overlap, as both perinuclear and peripheral small round structures distinct from cellular structures have indeed been detected and described as undefined subcellular compartment in which the core protein can accumulated (29). These undefined subcellular structures could be lipid droplets. Moreover “granules” that do not correspond to cellular structures have been described to react specifically with an HCV patient’s serum in HCV-infected liver specimen (25).
Several authors have detected strong positive cytoplasmic staining for structural and nonstructural HCV antigens in infected human and chimpanzees livers (30, 31). We have detected by electron microscopy in liver biopsies from HCV chronic-infected chimpanzees the core protein exclusively in the cytoplasm. The protein was localized on the ER and on the surface of lipid droplets present in abundance in the liver in comparison to the normal liver of an uninfected chimpanzee. These data obtained in vivo by infection with the complete virus are comparable to our results obtained in the cell system in which only a part of the HCV genome is expressed. They therefore show the in vivo relevance of the association of HCV core to lipid storage vesicle.
Fractionation of cellular lysates by sequential ultracentrifugation allowed us to isolate the lipid droplets in a fraction of d < 1.006 g/ml. Analysis of lipid composition revealed a prevalence of triglycerides that confer a character of intracellular lipid storage droplets. These droplets are, however, not the typical hepatic triglyceride-rich storage droplets isolated at d < 1.006 g/ml, as such droplets usually contain >80–90% triglycerides. It is interesting to note that in fraction I of the nonhepatic cell line CHO, the protein/triglyceride ratio is 2.14 and that in the same cell line the expression of the core protein induces a change of this ratio to 0.74, which is the range found in the HepG2 cell line. Therefore, it seems that core protein expression stimulates a change in the cellular metabolism of triglycerides. The core protein in the cell can be divided into two different classes: one associated with triglycerides (fraction I) and the other associated to membranes (fraction IV). Comparison between the lipid droplets in the whole cell and in fraction I shows in this latter a reduction of size (data not shown), indicating a disruption and fragmentation of the droplets during the manipulation of cell lysis. Thus we cannot exclude that a part of the core protein detected in fraction IV could result from coalescence and destruction of part of the lipid droplets. The fact that the lipid droplets in the CHO core-expressing cells display a unique morphologic appearance when compared with the control cells would suggest an interference of the HCV core protein in the lipid metabolism of the cell. The core protein has been described to regulate, as an intact protein, several cellular promoters (12, 13); in view of its cytoplasmic localization, one might speculate that it could regulate the expression of cellular genes involved in lipid metabolism by binding the specific mRNAs. The core protein has indeed been described to be an RNA binding protein (29).
Whether the association to the triglycerides is direct or mediated by other structures remains to be defined. A bridge structure between the core protein and triglyceride-rich lipid droplets could be hypothesized from the electron microscopy images: the core protein is indeed localized on an electron dense region that could delineate polar interfaces between lipid and protein layers. The core protein could interact directly with cellular proteins as lipid-associated proteins, and this interaction could be involved in maturation of HCV particles. Apo, which are proteins that bind lipids and render them water soluble in the form of lipoproteins, could be a good candidate for an interaction with the core protein. Our study by double immunofluorescence and confocal analysis for the core and apo has, indeed, revealed colocalization of apoAII and the HCV core protein in the globular structures.
On the other hand, HCV has been described as a lipid-containing virus and in plasma of patients shows a heterogeneous density distribution partially due to the binding to low density lipoprotein, very low density lipoprotein, IgG, and to a minor degree to IgM and high density lipoprotein (32). It is important, therefore, to emphasize that several in vivo observations regarding HCV infection might be related to our findings. Firstly, a characteristic of HCV infection is the presence of liver steatosis; it is plausible that this steatosis could arise, at least in part, from direct effects of HCV proteins on lipid metabolism. The importance of the association of HCV to lipids is further reinforced by recent evaluation of blood lipid composition in patients with chronic hepatitis C treated by interferon α. These studies showed that the levels of apoAI and high density lipoprotein are independent predictive factors for response to treatment (33).
Considered together, therefore, our study as well as these reports point to the potential important role of the association of HCV to lipids in the viral cycle and pathogenesis.
Acknowledgments
We thank F. Le Deist for the fluorescence-activated cell sorter analysis and R. Hellio for the confocal microscopic analysis. We also thank M. Chaudhary and J. C. Courvalin for providing us the anti-human lamin B antibody. G.B. was supported by a fellowship of the French Foundation for Medical Research.
Footnotes
Abbreviations: HCV, hepatitis C virus; apo, apolipoprotein; MTT, 3-(4,5-dimethylthiaziol-2-yl)-2,5-diphenyl tetrazolium bromide; ER, endoplasmic reticulum.
Eder, G. & Schaff, Zs., Abstracts of the Fifth International Symposium on Hepatitis C Virus and Related Viruses, Aug. 28–Sept. 3, 1995, Gold Coast, Australia, abstr. C 54.
References
- 1.Choo Q-L, Kuo G, Weiner A J, Overby L R, Bradley D W, Houghton M. Science. 1989;244:359–362. doi: 10.1126/science.2523562. [DOI] [PubMed] [Google Scholar]
- 2.Miller R H, Purcell R H. Proc Natl Acad Sci USA. 1990;87:2057–2061. doi: 10.1073/pnas.87.6.2057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Han J H, Shyamala V, Richman K H, Brauer M J, Irvine B, Urdea M S, Tekamp-Olson P, Kuo G, Choo Q-L, Houghton M. Proc Natl Acad Sci USA. 1991;88:1711–1715. doi: 10.1073/pnas.88.5.1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tanaka T, Kato N, Cho M-J, Sugiyama K, Shimotohno K. J Virol. 1996;70:3307–3312. doi: 10.1128/jvi.70.5.3307-3312.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Houghton M, Weiner A, Han J, Kuo G, Choo Q-L. Hepatology. 1991;14:381–388. [PubMed] [Google Scholar]
- 6.Matsuura Y, Miyamura T. Virology. 1993;4:297–304. [Google Scholar]
- 7.Saito I, Miyamura T, Ohbayashi A, Harada H, Katayama S, Kikuchi S, Watanabe Y, Koi S, Onji M, Choo Q-L, Houghton M, Kuo G. Proc Natl Acad Sci USA. 1990;87:6547–6549. doi: 10.1073/pnas.87.17.6547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Colombo M, Romeo R. In: Primary Liver Cancer: Etiological and Progression Factors. Bréchot C, editor. Boca Raton, FL: CRC; 1994. pp. 49–56. [Google Scholar]
- 9.Sakamuro D, Furukawa T, Takegami T. J Virol. 1995;69:3893–3896. doi: 10.1128/jvi.69.6.3893-3896.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ray R B, Lagging L M, Meyer K, Ray R. J Virol. 1996;70:4438–4443. doi: 10.1128/jvi.70.7.4438-4443.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shih C-M, Lo J, Miyamura T, Chen S-Y, Lee Y-H. J Virol. 1993;67:5823–5832. doi: 10.1128/jvi.67.10.5823-5832.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ray R B, Lagging L M, Meyer K, Steele R, Ray R. Virus Res. 1995;37:209–220. doi: 10.1016/0168-1702(95)00034-n. [DOI] [PubMed] [Google Scholar]
- 13.Kim D W, Suzuki R, Harada T, Saito I, Miyamura T. Jpn J Med Sci Biol. 1994;47:211–220. doi: 10.7883/yoken1952.47.211. [DOI] [PubMed] [Google Scholar]
- 14.Ou J-H, Yeh C-T, Benedict Yen T S. J Virol. 1989;63:5238–5243. doi: 10.1128/jvi.63.12.5238-5243.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yeh C-T, Wong S W, Fung Y-K, Ou J-H. Proc Natl Acad Sci USA. 1993;90:6459–6463. doi: 10.1073/pnas.90.14.6459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Suzuki R, Matsuura Y, Suzuki T, Ando A, Chiba J, Harada S, Saito I, Miyamura T. J Gen Virol. 1995;76:53–61. doi: 10.1099/0022-1317-76-1-53. [DOI] [PubMed] [Google Scholar]
- 17.Chapman M J, Goldstein S, Lagrange D, Laplaud P M. J Lipid Res. 1981;22:339–358. [PubMed] [Google Scholar]
- 18.Lowry O H, Rosebrough N J, Farr A L, Randall R J. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 19.Tanaka T, Lau J Y L, Mizokami M, Orito E, Tanaka E, Kiyosawa K, Yasui K, Ohta Y, Hasegawa A, Tanaka S, Kohara M. J Hepatol. 1995;22:742–745. doi: 10.1016/0168-8278(95)80043-3. [DOI] [PubMed] [Google Scholar]
- 20.Yoshitake S, Hamaguchi Y, Ishikawa E. Scand J Immunol. 1979;10:81–86. doi: 10.1111/j.1365-3083.1979.tb01338.x. [DOI] [PubMed] [Google Scholar]
- 21.Schimizu Y K, Feinstone S M, Purcell R H, London W T. Science. 1979;205:197–200. doi: 10.1126/science.451589. [DOI] [PubMed] [Google Scholar]
- 22.Schaff Z, Tabor E, Jackson D R, Gerety R J. Virchows Arch Cell Pathol. 1984;45:301–312. doi: 10.1007/BF02889872. [DOI] [PubMed] [Google Scholar]
- 23.Ontko J A, Perrin L W, Horne L S. J Lipid Res. 1986;27:1097–1103. [PubMed] [Google Scholar]
- 24.Hiramatsu N, Hayashi N, Haruna Y, Kasahara A, Fusamoto H, Mori C, Fuke I, Okayama H, Kamada T. Hepatology. 1992;16:306–311. doi: 10.1002/hep.1840160205. [DOI] [PubMed] [Google Scholar]
- 25.Sansonno D, Dammacco F. Hepatology. 1993;18:240–245. [PubMed] [Google Scholar]
- 26.Lo S-Y, Selby M, Tong M, Ou J-H. Virology. 1994;199:124–131. doi: 10.1006/viro.1994.1104. [DOI] [PubMed] [Google Scholar]
- 27.Lo S-Y, Masiarz F, Hwang S B, Lai M M C, Ou J-H. Virology. 1995;213:455–461. doi: 10.1006/viro.1995.0018. [DOI] [PubMed] [Google Scholar]
- 28.Harada S, Watanabe Y, Takeuchi K, Suzuki T, Katayama T, Takebe Y, Saito I, Miyamura T. J Virol. 1991;65:3015–3021. doi: 10.1128/jvi.65.6.3015-3021.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Santolini E, Migliaccio G, La Monica N. J Virol. 1994;67:3631–3641. doi: 10.1128/jvi.68.6.3631-3641.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Haruna Y, Hayashi N, Kamada T, Hytiroglon P, Thung S N, Gerber M A. Cancer. 1994;73:2253–2258. doi: 10.1002/1097-0142(19940501)73:9<2253::aid-cncr2820730904>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 31.Krawczynski K, Beach M J, Bradley D W, Kuo G, Di Bisceglie A M, Houghton M, Reyes G R, Kim J P, Choo Q-L, Alter M J. Gastroenterology. 1992;103:622–629. doi: 10.1016/0016-5085(92)90856-t. [DOI] [PubMed] [Google Scholar]
- 32.Thomssen R, Bonk S, Thiele A. Med Microbiol Immunol. 1993;182:329–334. doi: 10.1007/BF00191948. [DOI] [PubMed] [Google Scholar]
- 33.Soardo G, Pirisi M, Fonda M, Fabris C, Falleti E, Toniutto P, Vitulli D, Cattin L, Gonano F, Bartoli E. J Interferon Cytokine Res. 1995;15:705–712. doi: 10.1089/jir.1995.15.705. [DOI] [PubMed] [Google Scholar]