Abstract
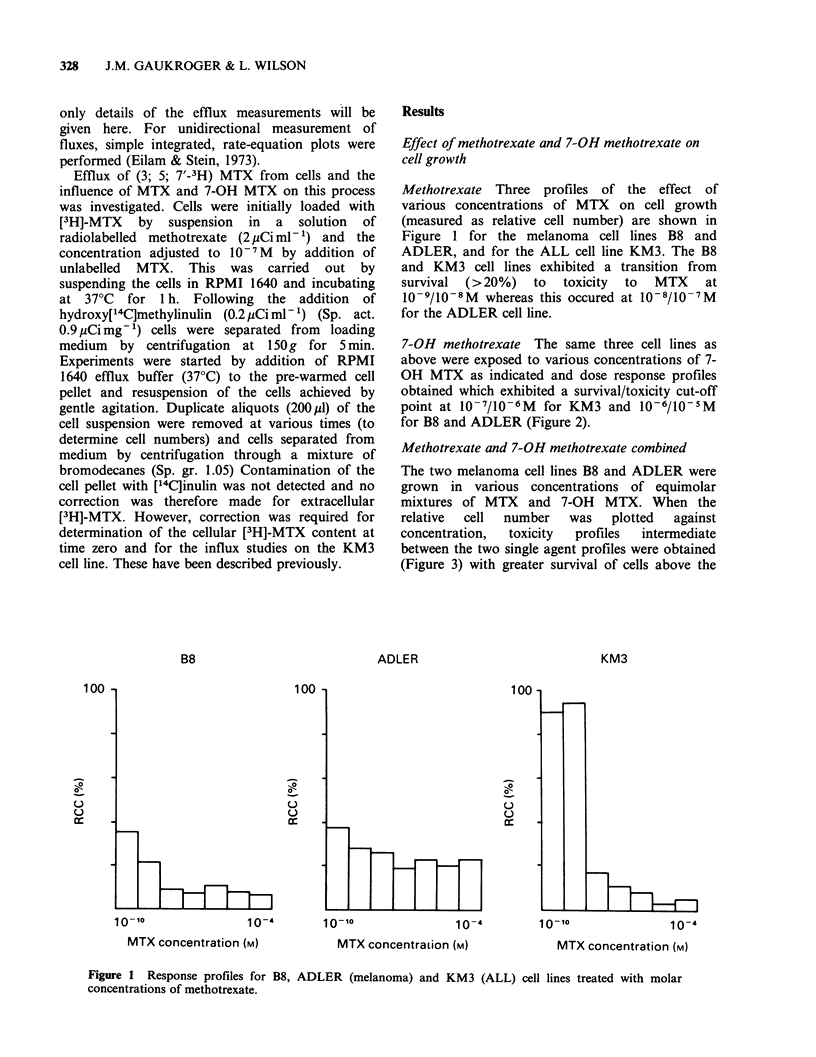
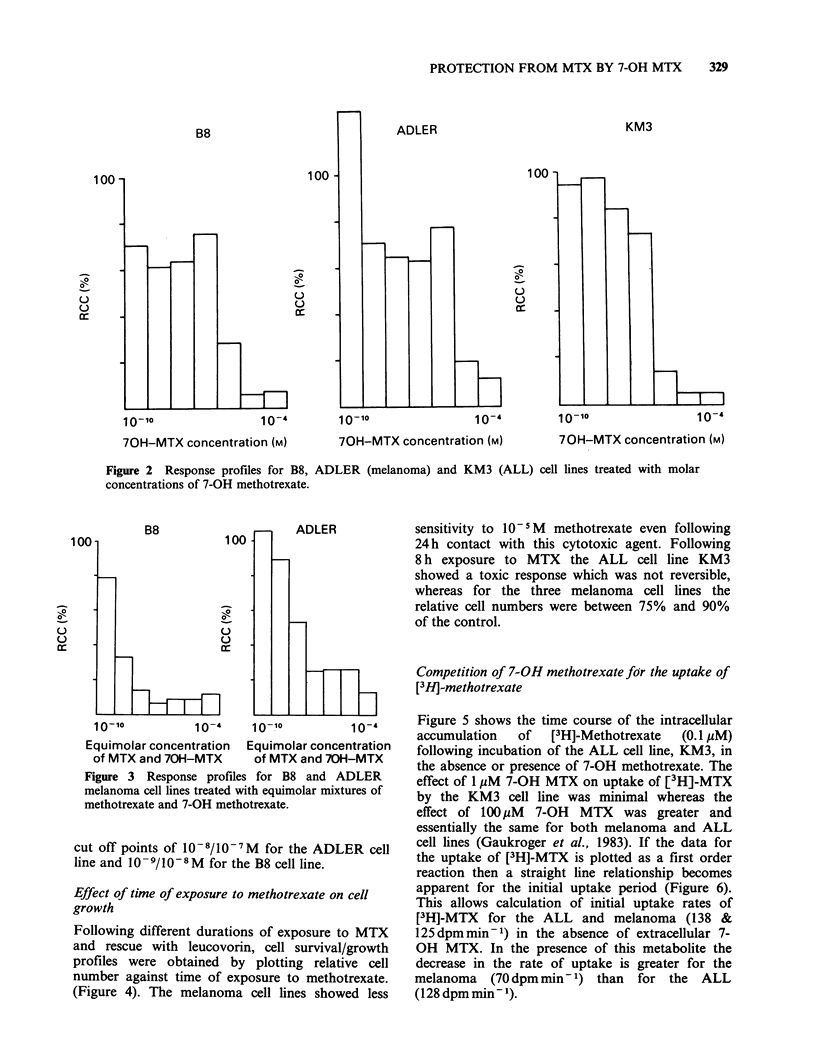
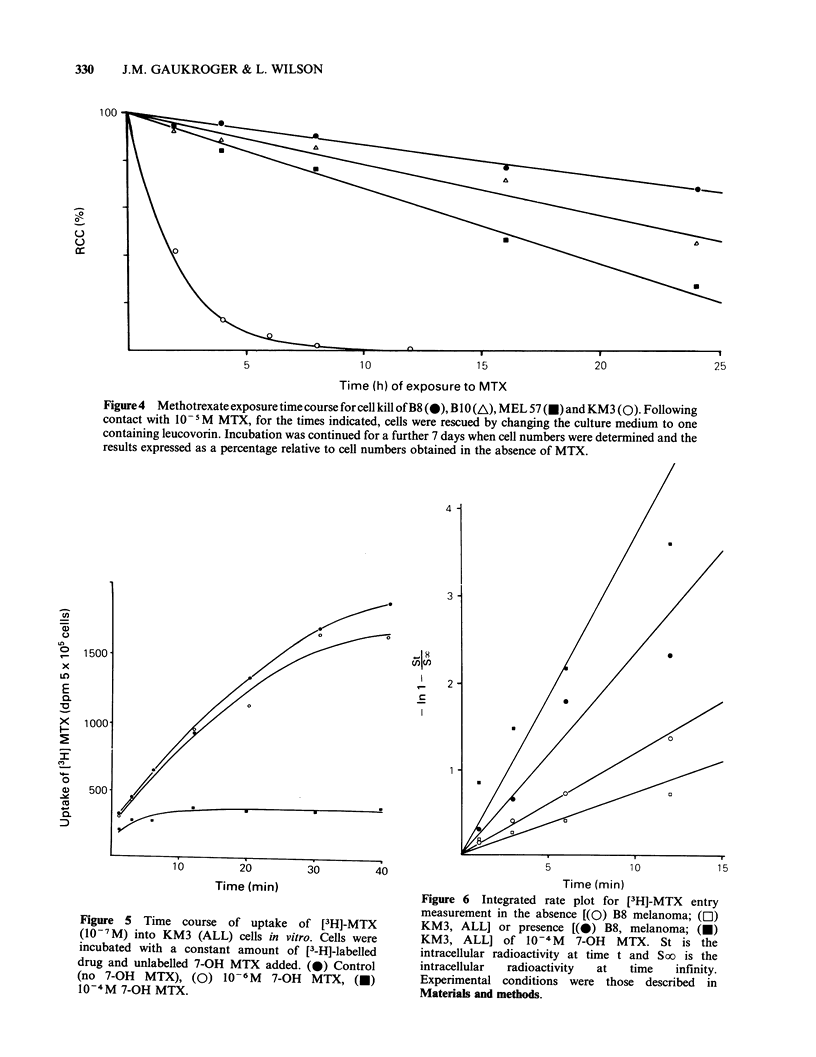
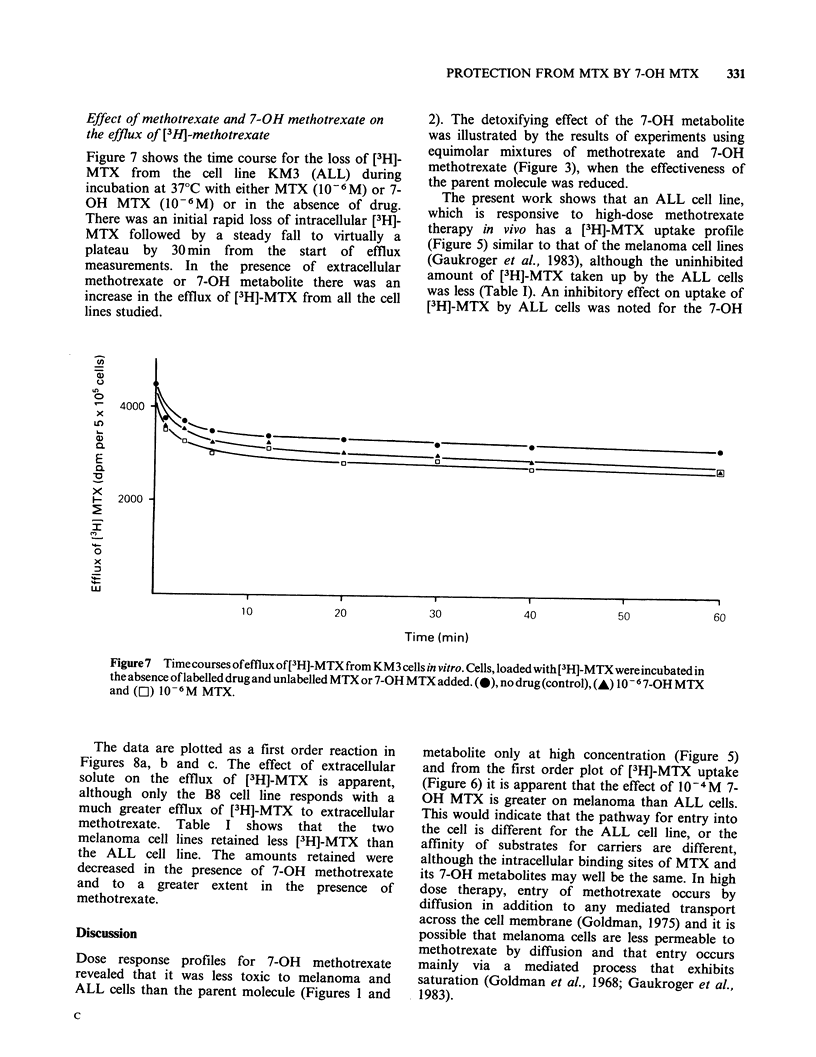
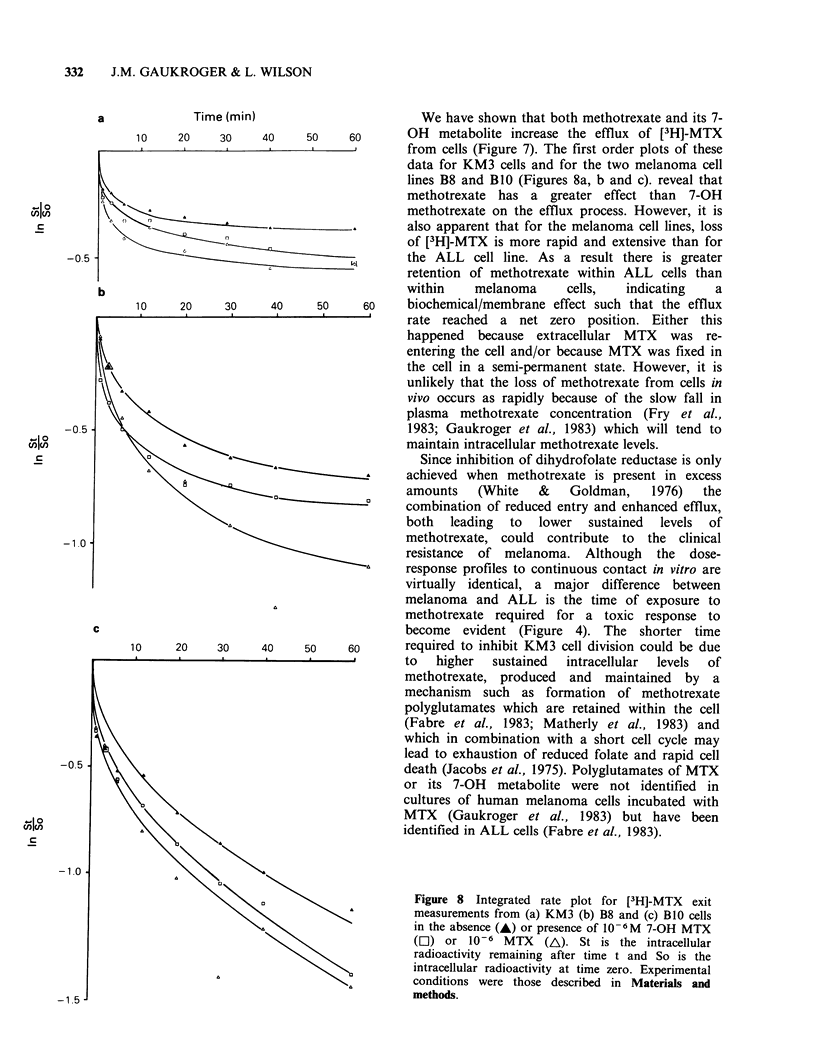
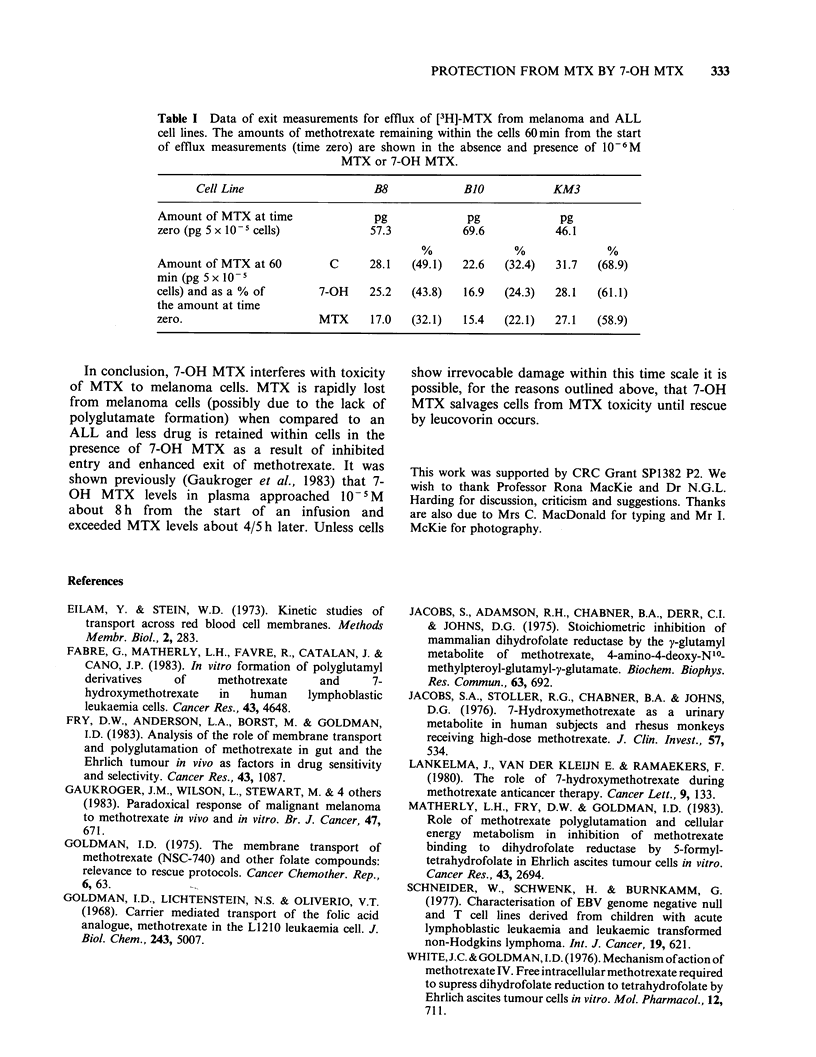
Cell growth survival studies have revealed that 7-OH methotrexate is two orders of magnitude less cytotoxic to human melanoma and human acute lymphoblastic leukaemia (ALL) cells in vitro than methotrexate. The influence of 7-OH methotrexate on methotrexate toxicity was investigated by studying cell growth in the presence of methotrexate and its 7-OH metabolite and by studying [3H]-methotrexate movement across the plasma membrane of isolated human cells. Transport was followed for net entry of the drug into drug-free cells, net exit of drug into drug-free medium and for unidirectional exit fluxes with drug and/or metabolite in the extracellular medium (exchange exit). Results indicate that 7-OH methotrexate (10(-6) M) interacts with melanoma cells to reduce the initial cellular uptake rate of [3H]-methotrexate but that no such interaction occurs with ALL cells. Efflux measurements revealed that a stimulatory effect of extracellular methotrexate on [3H]-methotrexate exit was apparent and that extracellular 7-OH methotrexate had a less stimulatory effect. Overall, loss of intracellular drug was greater from melanoma cells than from ALL cells. The results suggest that the drug resistance encountered following high dose therapy may be due to reduced cellular uptake and/or increased efflux of methotrexate from cells, both events being enhanced by 7-OH methotrexate. In addition, there is an apparently endogenous resistance of the melanomas to methotrexate as regards time of exposure to this agent which could also contribute to the lack of clinical response when compared to ALL.
Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Fabre G., Matherly L. H., Favre R., Catalin J., Cano J. P. In vitro formation of polyglutamyl derivatives of methotrexate and 7-hydroxymethotrexate in human lymphoblastic leukemia cells. Cancer Res. 1983 Oct;43(10):4648–4652. [PubMed] [Google Scholar]
- Fry D. W., Anderson L. A., Borst M., Goldman I. D. Analysis of the role of membrane transport and polyglutamation of methotrexate in gut and the Ehrlich tumor in vivo as factors in drug sensitivity and selectivity. Cancer Res. 1983 Mar;43(3):1087–1092. [PubMed] [Google Scholar]
- Gaukroger J., Wilson L., Stewart M., Farid Y., Habeshaw T., Harding N., Mackie R. Paradoxical response of malignant melanoma to methotrexate in vivo and in vitro. Br J Cancer. 1983 May;47(5):671–679. doi: 10.1038/bjc.1983.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman I. D., Lichtenstein N. S., Oliverio V. T. Carrier-mediated transport of the folic acid analogue, methotrexate, in the L1210 leukemia cell. J Biol Chem. 1968 Oct 10;243(19):5007–5017. [PubMed] [Google Scholar]
- Jacobs S. A., Adamson R. H., Chabner B. A., Derr C. J., Johns D. C. Stoichiometric inhibition of mammalian dihydrofolate reductase by the gamma-glutamyl metabolite of methotrexiate, 4-amino-4-deoxy-N-10-methylpteroylglutamyl-gamma-glutamate. Biochem Biophys Res Commun. 1975 Apr 7;63(3):692–698. doi: 10.1016/s0006-291x(75)80439-6. [DOI] [PubMed] [Google Scholar]
- Jacobs S. A., Stoller R. G., Chabner B. A., Johns D. G. 7-Hydroxymethotrexate as a urinary metabolite in human subjects and rhesus monkeys receiving high dose methotrexate. J Clin Invest. 1976 Feb;57(2):534–538. doi: 10.1172/JCI108308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lankelma J., van der Klein E., Ramaekers F. The role of 7-hydroxymethotrexate during methotrexate anti-cancer therapy. Cancer Lett. 1980 Apr;9(2):133–142. doi: 10.1016/0304-3835(80)90117-2. [DOI] [PubMed] [Google Scholar]
- Matherly L. H., Fry D. W., Goldman I. D. Role of methotrexate polyglutamylation and cellular energy metabolism in inhibition of methotrexate binding to dihydrofolate reductase by 5-formyltetrahydrofolate in Ehrlich ascites tumor cells in vitro. Cancer Res. 1983 Jun;43(6):2694–2699. [PubMed] [Google Scholar]
- Schneider U., Schwenk H. U., Bornkamm G. Characterization of EBV-genome negative "null" and "T" cell lines derived from children with acute lymphoblastic leukemia and leukemic transformed non-Hodgkin lymphoma. Int J Cancer. 1977 May 15;19(5):621–626. doi: 10.1002/ijc.2910190505. [DOI] [PubMed] [Google Scholar]
- White J. C., Goldman I. D. Mechanism of action of methotrexate. IV. Free intracellular methotrexate required to suppress dihydrofolate reduction to tetrahydrofolate by Ehrlich ascites tumor cells in vitro. Mol Pharmacol. 1976 Sep;12(5):711–719. [PubMed] [Google Scholar]


