Abstract
We have recently shown that the adenovirus type 5 E4orf6 protein interacts with the cellular tumor suppressor protein p53 and blocks p53 transcriptional functions. Here we report that the E4orf6 protein can promote focus formation of primary rodent epithelial cells in cooperation with adenovirus E1A and E1A plus E1B proteins. The E4orf6 protein can also inhibit p53-mediated suppression of E1A plus E1B-19kDa-induced focus formation. Mutant analysis of the E4orf6 protein demonstrates that these activities correlate with the ability of the adenovirus protein to relieve transcriptional repression mediated by the carboxyl-terminal region of p53 in transient transfection assays. We further demonstrate that expression of wild-type E4orf6 correlates with a dramatic reduction of p53 steady-state levels in transformed rat cells. Our data demonstrate that adenovirus type 5 encodes two different proteins, E1B-55kDa and E4orf6, that bind to p53 and contribute to transformation by modulating p53 transcriptional functions.
Keywords: transcription, activation, repression
The cellular phoshoprotein p53 is a sequence-specific DNA-binding protein and a transcription factor, capable of both transactivating and repressing transcription (1, 2). p53 can suppress oncogenic transformation (3, 4), negatively regulate mammalian cell cycle progression, and induce apoptosis. It has been well established that these activities are necessary for p53 to function as a tumor suppressor (5). Inhibition of its ability to regulate transcription by mutations or by association with viral oncoproteins correlates with oncogenesis (reviewed in refs. 6 and 7).
Human adenovirus type 5 (Ad5) encodes two different proteins, E1B-55kDa and E1B-19kDa, that can independently cooperate with adenovirus E1A protein in the transformation of primary cells in culture (8). This function is most likely due to their ability to inhibit at least in part E1A-induced p53-dependent apoptosis (reviewed in ref. 9). The E1B-55kDa protein binds to the amino-terminal transactivation domain of p53 (10) and blocks the ability of p53 to activate transcription (11). It appears that E1B-55kDa functions as a direct transcriptional repressor that is targeted to p53-responsive promoters by binding to p53 (11). Mutant analysis of the 55-kDa product indicates that inhibition of p53-mediated transactivation correlates with the transformation potential of E1B-55kDa (12). The E1B-19kDa protein shares functional and structural homologies with the cellular Bcl-2 protein (13). Both the E1B-19kDa and Bcl-2 proteins block apoptosis induced by p53 (14), tumor necrosis factor α (TNF-α), and Fas antigen (15, 16). It has been suggested that E1B-19kDa may overcome p53-induced apoptosis by alleviating p53-mediated transcriptional repression (15, 17) and by binding to the p53-inducible death-promoting protein Bax (18).
Recently we have shown that a second adenoviral protein encoded by open reading frame 6 (orf6) of the early region 4 (E4) transcription unit forms a stable complex with p53 in virus-infected cells and in vitro (19). In contrast to E1B-55kDa, the E4orf6 protein binds to the carboxyl-terminal region of p53. Transient transfection experiments demonstrate that the E4orf6 protein, like E1B-55kDa, can block p53-mediated transcriptional activation (19). The E4orf6 protein blocks p53-stimulated transcription by interfering with the ability of the amino-terminal domain of p53 to contact TAFII31, a component of TFIID. The mechanism by which E4orf6 binding abrogates the p53–TAFII31 interaction is not clear yet. The carboxyl-terminal domain of p53 has several biological functions that regulate DNA binding, nuclear localization, oligomerization, and both nonspecific DNA binding and recognition of DNA damage (reviewed in refs. 7 and 20). Considerable evidence suggests that the carboxyl-terminal region of p53 plays an important role in p53-mediated induction of apoptosis that may involve the interaction with two subunits (XPB and XPD) of the transcription-repair complex TFIIH (21). In addition, recent evidence indicates that the transcriptional repression function of the carboxyl-terminal region of p53 may be important in mediating apoptosis (15, 17). These observations raise the possibility that binding of E4orf6 to the carboxyl-terminal domain of p53 might antagonize transcriptional and other functional properties of the tumor suppressor protein that may be most critical for tumor suppressor function of p53.
To examine this model, wild-type E4orf6 and two mutants of the E4orf6 protein were tested for their ability to inhibit p53 transcriptional functions and their ability to transform primary baby rat kidney (BRK) cells in cooperation with E1A and E1A plus E1B proteins.
MATERIALS AND METHODS
Plasmids and Transient Transfections.
The construction of plasmids using the cytomegalovirus (CMV) major immediate early promoter to express wild-type E4orf6 and E4orf6/7 proteins and deletion mutants of the E4orf6 protein (E4ΔC and E4ΔN) has been described previously (22). pCMV-E4ΔC and pCMV-E4ΔN express E4orf6 amino acids 1–151 and 109–294, respectively. pC53-SN3 encodes human wild-type p53 from the pCMV/neo vector. pGal4-p53 and pGal4-p53C express human wild-type p53 (amino acids 1–393) or mutant p53 (amino acids 80–393) fused to the Gal4 DNA-binding domain (23) from the pSG424 vector (24). The luciferase reporter plasmid pC3G5-LUC was made by cloning the XbaI/PvuII fragment from pFC53G5 (25) containing five Gal4-binding sites and the human c-fos promoter (−53 to +42) between the NheI/SmaI sites of pGL2basic (Promega). pGalTK-LUC contains five Gal4-binding sites upstream of the herpes virus thymidine kinase promoter that controls expression of luciferase (19). The p53-negative cell line H1299 (26) was grown in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal calf serum (FCS). For luciferase assays, subconfluent H1299 cells in 35-mm-diameter tissue culture dishes were transfected by calcium phosphate coprecipitation using the indicated amounts of reporter and effector plasmids and 0.5 μg of pSVβ-Gal (Promega). Total DNA was brought to 10 μg per plate by adding salmon sperm DNA. Total cell extracts were prepared 36 h after transfection in 100 μl of lysis buffer (Promega) and luciferase activity was assayed with 20 μl of extract as described (19). All samples were normalized for transfection efficiency by measuring β-galactosidase activity.
Transformation Assays.
Kidneys were obtained from 6-day-old Sprague Dawley rats. Organs were incubated with 1 mg/ml dispase-collagenase (Boehringer Mannheim) at 37°C for 4 h, and single cells were seeded in DMEM supplemented with 10% FCS on 90-mm-diameter tissue culture dishes. Then 3 × 106 primary baby rat kidney cells were transfected with the indicated amount of each plasmid by calcium phosphate coprecipitation. To avoid effects of promoter competition, all DNA combinations were adjusted to the same amount of cytomegalovirus promoter with pCMV/neo vector, and all combinations were brought to 20 μg of DNA per dish by the addition of salmon sperm DNA. Three days after transfection cells were trypsinized and seeded on three 90-mm-diameter dishes in DMEM supplemented with 10% FCS. They were fed with fresh medium every 4 days. Three weeks after transfection foci were stained with crystal violet and scored. To establish permanent cell lines single foci or pools of foci were isolated and expanded. The following plasmids were additionally used in these experiments: pAd5 XhoI-C contains the left end (1–15.5 map units) of the adenovirus type 5 genome and expresses the E1A and E1B proteins (27). pAd5 dl338XhoI-C is a derivative of pAd5 XhoI-C and contains a 524-bp deletion in the E1B-55kDa coding region located between nucleotides 2805 and 3329 (28). pCMV-E1A, an E1A expression plasmid, has been described (29), and pEJ6.6 encodes mutant Ha-ras (30).
Protein and DNA Analysis.
Total cell extracts were prepared as described (22) and analyzed by immunoblotting using anti-E1B-55kDa (2A6, ref. 31), anti-E4orf6 (RSA3, ref. 32), anti-p53 (PAb421, ref. 33), and anti-HA (12CA5, Boehringer Mannheim) monoclonal antibodies. For PCRs genomic DNA was isolated using the QIAamp tissue kit (Qiagen, Chatsworth, CA). The E4orf6 cDNAs were PCR amplified from 100 ng of genomic DNA using primers orf6fw, 861rev, 461rev, 331fw, and flufix exactly as described previously (22).
RESULTS
The Carboxyl-Terminal Region of E4orf6 Is Required to Block Both Transcriptional Functions of p53.
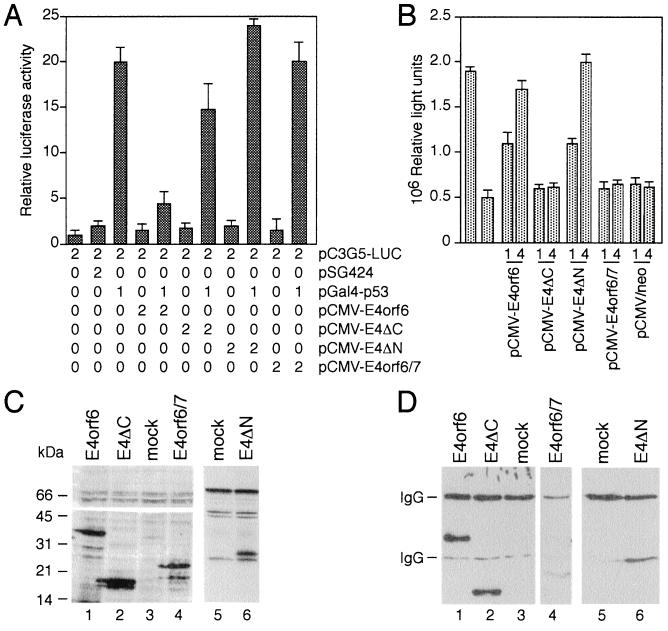
The p53 tumor suppressor protein activates transcription of genes with promoters containing p53-binding sites (reviewed in ref. 7). p53 also represses expression of a number of cellular and viral genes with promoters lacking p53-responsive elements. Although the mechanism of the repression is not yet known, it appears that only those promoters containing TATA boxes are inhibited by p53, whereas those containing an initiator element are not repressed by p53. Wild-type E4orf6 protein blocks the ability of p53 to enhance expression from reporter constructs that contain p53-binding sites (19). This inhibition is also evident in transient transfections using a Gal4-p53 fusion protein where DNA binding is mediated by Gal4 sequences (19). Furthermore, E4orf6 can relieve repression of a Gal4 fusion protein that contains the carboxyl-terminal region of p53 (19). To reveal the effect of mutations in the E4orf6 protein on both transcriptional properties of p53 we performed transient transfection assays in p53-negative H1299 cells. Additionally, we assayed the effect of the E4orf6/7 protein, which shares 58 amino acids at the amino terminus with the E4orf6 protein. This protein has also been found to bind to p53 in adenovirus-infected cells (19). First, we tested the ability of wild-type E4orf6 and E4orf6 mutants to inhibit transcriptional activation of a Gal4-p53 fusion protein. As expected, E4orf6 but not E4orf6/7 blocked the induction of a reporter gene containing five Gal4-binding sites (pC3G5-LUC) by Gal4-p53 (Fig. 1A). Activation by Gal4-VP16 was not affected by the E4orf6 protein, demonstrating that its inhibition was specific (data not shown). Coexpression of E4ΔC reproducibly resulted in a slight inhibition of p53-stimulated transcription. These inhibitory effects were not evident in transient transfections using the Gal4-VP16 fusion protein (data not shown). In contrast, E4ΔN did not block transactivation by the Gal4-p53 fusion protein; rather, coexpression of E4ΔN reproducibly enhanced p53-mediated transactivation (Fig. 1A).
Figure 1.
Effects of E4orf6, E4orf6/7, and E4orf6 deletion mutants on p53 transcriptional functions. (A) Inhibition of Gal4-p53 transactivation. Subconfluent H1299 cells were transfected with the indicated amounts of reporter and effector plasmids (μg of DNA) tabulated below each bar in the graphs, and luciferase activity was determined. The mean and standard deviation are presented for four experiments, each performed in duplicate. (B) Relief of Gal4-p53C-mediated repression. Three micrograms of reporter plasmid pGalTK-LUC and the indicated amount of pCMV-E4 effector plasmids were cotransfected with 1 μg of pGal4-p53C into H1299 cells and luciferase activity was determined. Bar 1 (counting from the left) represents luciferase activity produced by transfecting 3 μg of the reporter plasmid in the absence of any effector plasmids. Bar 2 represents luciferase activity produced by cotransfecting 3 μg of pGalTK-LUC and 1 μg of pGal4-p53C. The mean and standard deviation are presented for three experiments, each performed in duplicate. (C) Expression of wild-type E4orf6 and E4orf6 mutant proteins in transfected H1299 cells. Luciferase assay samples were immunoblotted by normalizing the amount of extract used according to β-galactosidase activity and probing the immunoblot with anti-E4orf6 monoclonal antibody RSA3 (lanes 1–4) and anti-HA monoclonal antibody 12CA5 (lanes 5 and 6). The position of molecular mass markers is indicated at left. (D) In vivo interaction of p53 with E4orf6, E4orf6/7, and E4orf6 deletion mutants in H1299 cells. The same extracts were subjected to immunoprecipitation using anti-p53 monoclonal antibody DO-1 (34). The precipitates were resolved on the same SDS/15% polyacrylamide gel, and coprecipitated E4 proteins were visualized by immunoblotting using RSA3 (lanes 1–4) or 12CA5 (lanes 5 and 6) monoclonal antibodies on two separate filters. The coimmunoprecipitation reaction with E4orf6/7 (lane 4) was resolved on a separate SDS/15% polyacrylamide gel. The bands representing the IgG proteins are indicated at left.
The ability of p53 to repress transcription maps to the carboxyl-terminal domain of this protein and requires the interaction with the TATA-box-binding protein, TBP (23). To examine the effect of mutations in the E4orf6 protein on p53-mediated transcriptional repression we used a Gal4 fusion protein that contains p53 amino acids 80–393 (Gal4-p53C, ref. 23). Cotransfection of plasmids pCMV-E4orf6 or pCMV-E4ΔN with pGal4-p53C alleviated the repression of the reporter plasmid pGalTK-LUC in a dose-dependent manner, whereas coexpression of E4orf6/7, E4ΔC, and the expression cassette without an inserted E4orf6 cDNA (pCMV/neo) had no effect (Fig. 1B). To determine whether these results are due to differences in expression of wild-type and mutant proteins or might be explained by a failure of both truncated polypeptides to bind to p53, we analyzed extracts from transfected H1299 cells by Western blotting and coimmunoprecipitation assays (Fig. 1 C and D). The results from these experiments demonstrate that all transfected plasmids express stable proteins at roughly the same level. Wild-type E4orf6 and E4ΔC proteins efficiently coprecipitated with the p53 polypeptide, while deletion of amino acids 1–108 in mutant E4ΔN decreased the interaction but did not abolish it. The E4orf6/7 protein interacted weakly with p53 in this assay, which is consistent with previous observations that E4orf6/7 inefficiently binds to p53 in vitro. Our data suggest that an 186-amino acid segment from the carboxyl-terminal region of E4orf6 is required but not sufficient to inhibit Gal4-p53-stimulated transcription, while expression of this segment is sufficient to relieve Gal4-p53C-mediated transcriptional repression. Additionally, our data indicate that the amino-terminal region of E4orf6 is involved in the p53 interaction.
E4orf6 Interferes with p53-Mediated Suppression of E1A/E1B-19kDa-Induced Focus Formation.
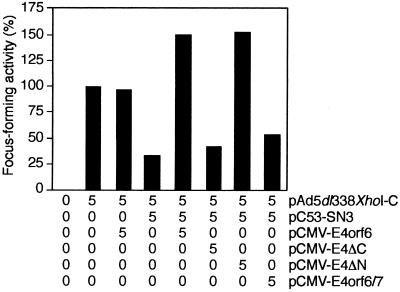
Plasmids encoding wild-type p53 efficiently interfere with the ability of adenovirus E1A and E1B proteins to elicit neoplastically transformed foci upon transfection of primary cells in tissue culture (4). In light of the fact that E4orf6 modulates the transcriptional properties of p53 we asked whether the E4orf6 protein overcomes the inhibitory effect of wild-type p53 on E1A- and E1B-mediated focus induction. To assay directly the effect of E4orf6 and E4orf6 mutants on p53 “suppressor” activity it was necessary to exclude expression of the E1B-55kDa protein because this protein binds to both p53 and E4orf6 (19, 35). We therefore studied the effect of p53 and E4orf6 on the transformation of primary rodent epithelial cells by pAd5 dl338XhoI-C, which directs the synthesis of E1A and E1B-19kDa proteins. Primary BRK cells could be transformed efficiently with plasmid pAd5 dl338XhoI-C (Fig. 2). Cotransfection of pCMV-E4orf6 resulted in a slight decrease in the number of foci, whereas inclusion of plasmid pC53-SN3, which expresses human wild-type p53 under the control of the cytomegalovirus promoter, reduced the number of foci by almost 70%. No such reduction was seen when wild-type E4orf6 or mutant E4ΔN proteins were coexpressed in combination with p53 and E1A plus E1B-19kDa proteins. Instead, on average there was a reproducible increase of over 50% in the number of foci. In contrast, cotransfection of plasmids pCMV-E4ΔC or pCMV-E4orf6/7 with pC53-SN3 and pAd5 dl338XhoI-C had no significant effect on p53-mediated suppression on focus formation. Hence, wild-type E4orf6 and mutant E4ΔN proteins antagonize p53 functions which are necessary for p53 to prevent focus formation. Apparently, this activity correlates with the ability of E4orf6 and mutant E4ΔN to modulate the transcriptional properties of the carboxyl-terminal domain of p53 in transient transfection assays.
Figure 2.
Wild-type E4orf6 and E4ΔN efficiently interfere with p53-mediated suppression of pAd5 dl338XhoI-C-induced transformation. Primary BRK cells were transfected with the indicated amounts of plasmids (μg of DNA per 3 × 106 cells). Focus-forming activity is represented as a percentage of E1A plus E1B-19kDa activity. The average number of foci for pAd5 dl338XhoI-C was 21 in four independent experiments.
E4orf6 Promotes Focus Formation in Cooperation with E1A and E1B.
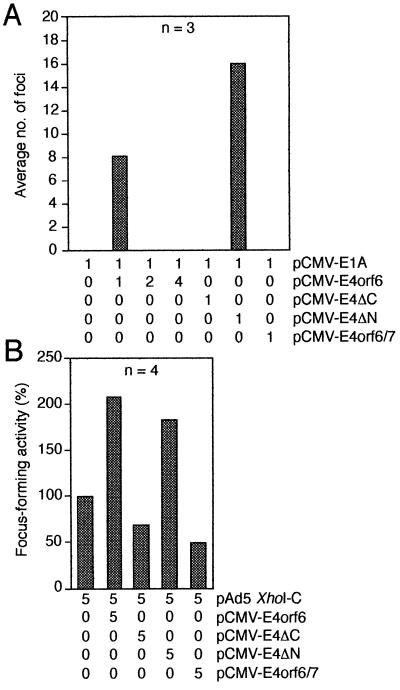
Blocking p53 transcriptional functions is an integral part of the molecular mechanisms by which adenovirus E1B proteins contribute to transformation of primary cells in cooperation with adenovirus E1A proteins (12, 15). Because the E4orf6 protein binds to p53, modulates transcriptional properties of p53, and overcomes p53-mediated inhibition of transformation, we analyzed the ability of wild-type and mutant E4orf6 proteins to transform primary BRK cells in conjunction with E1A and E1A plus E1B proteins. Primary BRK cells were transfected with plasmids expressing the E1A gene in combination with wild-type E4orf6, E4ΔC, E4ΔN, or E4orf6/7 (Fig. 3A). To monitor the efficiency of the transformation experiment E1A was cotransfected with activated Ha-ras, which is known to strongly cooperate with E1A in the transformation of rat cells (4). Coexpression of E1A with Ha-ras resulted in an average of 108 foci in three independent experiments (data not shown). Transfection with plasmids expressing E1A and E4orf6 or E4ΔN produced on average 8 and 16 morphologically transformed cells, respectively. No foci were observed in transfections with E1A alone, E1A plus E4ΔC, and E1A plus E4orf6/7, or when increasing amounts of pCMV-E4orf6 plasmid were included in the transformation mixture (Fig. 3A). E1A/E4orf6 and E1A/E4ΔN foci developed more slowly and differed in their morphology from the E1A plus ras ones, but they could be easily propagated into permanent cell lines. Curiously, all cell lines established from foci generated in the presence of pCMV-E1A plus pCMV-E4orf6 or pCMV-E4ΔN failed to express any detectable E1A and E4orf6 proteins and did not contain the viral DNAs, whereas expression of E1A was detected in cell lines established from foci transformed by E1A and Ha-ras (data not shown).
Figure 3.
Transformation experiments. (A) E4orf6 cooperates with E1A to promote focus formation. Primary BRK cells were transfected with the indicated amounts of plasmids, and morphologically transformed colonies were scored 3 weeks after transfection. The mean number of dense foci from three independent experiments is presented. (B) E4orf6 cooperates with E1A and E1B proteins to enhance the number of cell transformants. Focus-forming activity is represented as a percentage of pAd5 XhoI-C activity. The average number of foci for pAd5 XhoI-C was 33 in four independent experiments.
To assay the effect of E4orf6 and E4orf6 mutant derivatives on E1A- plus E1B-induced transformation, primary BRK cells were transfected with a plasmid expressing the E1A and E1B genes (pAd5 XhoI-C) in combination with the E4orf6- and E4orf6/7-expressing plasmids (Fig. 3B). Cotransfection of wild-type pCMV-E4orf6 with pAd5 XhoI-C resulted in a more than 2-fold increase of foci compared with pAd5 XhoI-C alone. Slightly lower numbers of foci were obtained when pAd5 XhoI-C and pCMV-E4ΔN were cotransfected. No such increase was seen when wild-type E4orf6/7 or mutant E4ΔC proteins were coexpressed with E1A plus E1B proteins. Remarkably, on average there was a reproducible reduction of 35% and 50% in the number of foci with pCMV-E4ΔC and pCMV-E4orf6/7, respectively. Together, these experiments demonstrate that E4orf6 can promote focus formation in cooperation with E1A and can also cooperate with E1A and E1B proteins to increase the number of transformed cells. Apparently, these activities depend also on the function of the carboxyl-terminal region of E4orf6.
E4orf6 Antagonizes the E1A-Induced Metabolic Stabilization of p53 in Transformed Rat Cells.
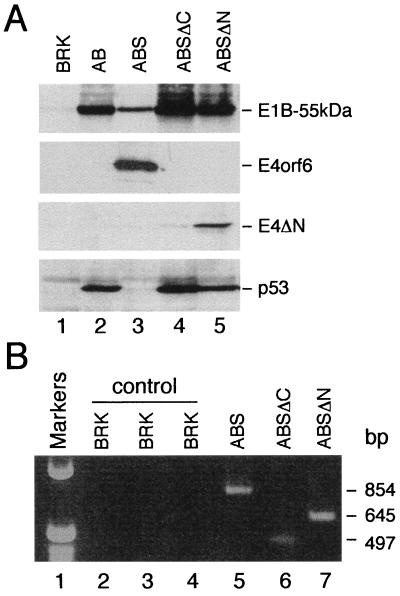
Individual foci or pools of foci were selected for G418 resistance and expanded into cell lines. Transformants derived from cotransfections of pAd5 XhoI-C with pCMV-E4orf6/7 grew very slowly and could not be cloned into cell lines. In contrast, all other foci were readily cloned into permanent cell lines, which were subsequently subjected to protein and DNA analyses. High levels of wild-type E4orf6 and mutant E4ΔN proteins could be detected in five monoclonal cell lines originating from cultures cotransfected with pAd5 XhoI-C. Identical results were obtained when two polyclonal cell lines derived from pools of foci were analyzed for expression of E4orf6 (ABS cells) and E4ΔN proteins (ABSΔN cells; Fig. 4A). At this time we cannot say with certainty whether mutant protein E4ΔC is expressed in cell lines established from individual or pooled foci (ABSΔC cells), although PCRs confirmed the presence of the E4ΔC-cDNA in all cell lines tested (Fig. 4B, lane 6). The p53 protein accumulated to high levels in cells expressing E1A and E1B proteins (AB cells; Fig. 4A, lane 2), most likely due to the E1A-induced metabolic stabilization of p53 (36). In contrast, the levels of p53 were greatly reduced in ABS cells expressing wild-type E4orf6 and E1A plus E1B proteins (Fig. 4A, lane 3). However, ABSΔC and ABSΔN cells contained p53 protein in amounts comparable to those in AB cells. Since p53 accumulates in ABSΔN cells which express the mutant E4ΔN protein, these results indicate that the observed reduction of p53 steady-state levels in transformed ABS cells correlates with the expression of wild-type E4orf6 protein and that an amino-terminal segment of E4orf6 (residues 1–108) is required for this activity.
Figure 4.
Protein and DNA analysis. (A) Immunoblot showing the steady-state levels of E1B-55kDa, E4orf6, E4ΔN, and p53 proteins in cell pools originating from cotransfections with pAd5 XhoI-C. The same amount of total protein form each pool was resolved on SDS/12% polyacrylamide gels and subjected to immunoblot analysis with monoclonal antibodies 2A6 (E1B-55kDa), RSA3 (E4orf6), 12CA5 (E4ΔN), and PAb421 (p53). The bands representing the viral proteins and p53 are indicated at right. (B) Analysis of viral cDNAs in ABS, ABSΔC, and ABSΔN pool cells. The viral cDNAs were PCR amplified as described in the text. The following primers were used: orf6fw and 861rev (lanes 2 and 5); orf6fw and 461rev (lanes 3 and 6); and 331fw and flufix (lanes 4 and 7). Genomic DNA isolated from BRK cells was used in the control reactions. The size of the PCR products (bp) is indicated at right.
DISCUSSION
In this report we have demonstrated that the adenovirus E4orf6 protein can cooperate with E1A and E1A plus E1B proteins to promote focus formation of primary rat cells. Similar effects of the E4orf6 protein in transformation experiments have been recently reported by Shenk and coworkers (37). We found a positive correlation between the transforming potential of E4orf6 and its ability to alleviate transcriptional repression of the carboxyl-terminal region of p53. We further demonstrate that E4orf6 antagonizes the E1A-induced metabolic stabilization of p53 in transformed rat cells.
The ability of E4orf6 to promote focus formation is dependent on the function of its carboxyl-terminal region. Deletion of a 142-amino-acid segment from the carboxyl terminus of E4orf6 did not inhibit p53 binding (Fig. 1D) but abolished its ability to inhibit p53-stimulated transcription and its ability to relieve p53-mediated repression (Fig. 1 A and B). Thus, the carboxyl-terminal region of E4orf6 interferes with both transcriptional functions of p53. The transforming properties of E4orf6 correlated with its ability to alleviate p53-mediated transcriptional repression (Fig. 1B). Considerable evidence indicates that transcriptional repression mediated by the carboxyl-terminal region of p53 may be important in mediating apoptosis (reviewed in ref. 7). This function requires the interaction with TBP and presumably TAFs (38). Since the E4orf6 protein blocks the p53–TAFII31 interaction and interferes with p53-mediated transcriptional repression it seems most likely that the adenovirus protein promotes focus formation by blocking p53-induced apoptosis. In fact, Moore et al. (37) have recently shown that the E4orf6 protein can indeed block the induction of p53-mediated apoptosis, but not apoptosis induced by tumor necrosis factor α or cycloheximide. According to our results this activity is dependent on the ability of the carboxyl-terminal region of E4orf6 to relieve transcriptional repression mediated by the carboxyl-terminal region of p53.
The function of the amino-terminal region of E4orf6 in the modulation of p53 function is unclear. Although this region seems to be required to inhibit p53-stimulated transcription (Fig. 1A), it is clearly dispensable for the transforming properties of E4orf6, since deletion of this segment (residues 1–108) even increases the focus-forming activity of the E4orf6 protein in cooperation with E1A (Fig. 3A). However, it appears that the amino-terminal region of the E4orf6 protein is required to antagonize the E1A-induced metabolic stabilization of p53. Transformants expressing the mutant E4ΔN protein (residues 109–294) contain high levels of p53 in comparison to transformants expressing wild-type E4orf6 (Fig. 4A). In fact, this activity might be responsible for the observed effect that more foci were obtained with wild-type E4orf6 than with E4ΔN in cotransfections with E1A and E1B (Fig. 3B). The work from Moore et al. (37) demonstrates that the E4orf6 protein reduces the half-life of p53 in transformed cells. A similar effect has been described previously for productively adenovirus-infected cells (39). However, the work by Grand and colleagues (39) indicates that this activity additionally requires the expression of the E1B-55kDa protein, which forms a physical complex with E4orf6 in lytically infected cells (40). Moreover, we have recently shown that E1B-55kDa binds to the amino-terminal 55 amino acids of the E4orf6 protein in vitro and in 293 cells (22). Thus, is seems very likely that the reduction of p53 steady-state levels in virus-infected and transformed cells is mediated by the E1B-55kDa–E4orf6 protein interaction.
It is noteworthy that immunoprecipitation experiments from adenovirus-infected cells indicate that the E4orf6/7 protein also binds to p53 (19). Weak binding of E4orf6/7 to p53 was also evident in coimmunoprecipitation assays from transfected H1299 cells (Fig. 1D) and in glutathione S-transferase binding experiments (ref. 19 and M.N., unpublished results). However, our data demonstrate that E4orf6/7 has no effect on both p53 transcriptional functions (Fig. 1) and does not cooperate with E1A and E1A plus E1B proteins in the transformation of primary rat cells (Fig. 3). Rather, coexpression of E4orf6/7 significantly decreased the focus-forming activity of pAd5 XhoI-C (Fig. 3B). Similar effects were observed with mutant E4ΔC (Fig. 3B). These data indicate, but do not prove, that overexpression of E4orf6/7 and E4ΔC may cause cytotoxic effects in rat cells, which are obviously reduced in the presence of the carboxyl-terminal region of E4orf6. Because the two proteins share 58 amino acids at their amino termini it is tempting to speculate that this region might contribute to these effects. This might also explain the observation that increasing amounts of pCMV-E4orf6 reduced the number of foci in the E1A/E4orf6 transformation assay. The recent observation by Moore et al. (37), which shows that E1A/E4orf6 transformants contain very low to nondetectable levels of E4orf6 protein, provides additional support for this view.
Our data fit well in a model in which binding of E4orf6 to the carboxyl-terminal region of the p53 protein inactivates p53 function and promotes focus formation in cooperation with E1A and E1A plus E1B proteins. However, the lack of cooperation with E4orf6 and E1A plus E1B-19kDa (pAd5 dl338XhoI-C) in the transformation assay is a notable exception to this correlation (Fig. 2). One possible explanation for this result is that E4orf6 and E1B-19kDa have redundant activities that modulate p53 function. According to our data, E4orf6 and E1B-19kDa may inhibit, at least in part, p53-induced apoptosis by modulating the same transcriptional repression function of p53. Remarkably, we observed a significant increase in focus-forming activity when cells were transfected with pAd5 dl338XhoI-C and plasmids encoding human wild-type p53, wild-type E4orf6, or E4ΔN (when compared with pAd5 dl338XhoI-C alone). This enhancement is similar to previously described results showing that mutant, but not wild-type, p53 stimulates focus formation in combination with E1A (3, 4). Possibly, the E4orf6 interaction near the carboxyl terminus induces a “mutant” conformation (reviewed in ref. 20) in the overexpressed p53 protein that additionally enhances focus formation.
Finally, it should be noted that all established cell lines originating from cotransfections with E1A and E4orf6 or E4ΔN did not contain the viral cDNAs and consequently the viral proteins. Curiously, these transformants could be easily cloned and propagated and were morphologically different from all other transformants. Hence, it appears that transient expression of E1A with E4orf6 or E4ΔN, but not with E4orf6/7 or E4ΔC, allows the accumulation of mutations in cellular genes that result at least in the morphological transformation of these primary epithelial rat cells.
There is accumulating evidence that other viral proteins from different DNA tumor viruses—i.e., human papilloma virus E6, hepatitis B virus Hbx, and Epstein–Barr virus BZLF1—bind to the carboxyl-terminal region of p53. Given the importance of this domain in the regulation of the tumor-suppressor function of p53, these protein interactions may represent a general mechanism by which these viruses modulate p53 function and trigger oncogenic transformation.
Acknowledgments
We thank J. Wiesenmeyer for animal work and H. Schütt for helpful discussions. S.R. received a scholarship from the Universität Regensburg. T.D. was supported by a grant from the Infektionsforschung AIDS-Stipendienprogramm of the Deutsches Krebsforschungszentrum, Heidelberg.
References
- 1.Farmer G, Bargonetti H, Zhu H, Friedman P, Prywes R, Prives C. Nature (London) 1992;358:83–86. doi: 10.1038/358083a0. [DOI] [PubMed] [Google Scholar]
- 2.Ginsberg D, Mechta F, Yaniv M, Oren M. Proc Natl Acad Sci USA. 1991;88:9979–9983. doi: 10.1073/pnas.88.22.9979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Finlay C A, Hinds P W, Levine A J. Cell. 1989;57:1083–1093. doi: 10.1016/0092-8674(89)90045-7. [DOI] [PubMed] [Google Scholar]
- 4.Eliyahu D, Michalovitz D, Eliyahu S, Pinhasi-Kimhi O, Oren M. Proc Natl Acad Sci USA. 1989;86:8763–8767. doi: 10.1073/pnas.86.22.8763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vogelstein B, Kinzler K W. Cell. 1992;70:523–526. doi: 10.1016/0092-8674(92)90421-8. [DOI] [PubMed] [Google Scholar]
- 6.Levine A J. Annu Rev Biochem. 1993;62:623–651. doi: 10.1146/annurev.bi.62.070193.003203. [DOI] [PubMed] [Google Scholar]
- 7.Ko L J, Prives C. Genes Dev. 1996;10:1054–1072. doi: 10.1101/gad.10.9.1054. [DOI] [PubMed] [Google Scholar]
- 8.Barker D D, Berk A J. Virology. 1987;156:107–121. doi: 10.1016/0042-6822(87)90441-7. [DOI] [PubMed] [Google Scholar]
- 9.Shenk T. In: Fields Virology. Fields B N, Knipe D M, Howley P M, editors. New York: Lippincott–Raven; 1996. pp. 2111–2148. [Google Scholar]
- 10.Kao C C, Yew P R, Berk A J. Virology. 1990;179:806–814. doi: 10.1016/0042-6822(90)90148-k. [DOI] [PubMed] [Google Scholar]
- 11.Yew P R, Liu X, Berk A J. Genes Dev. 1994;8:190–202. doi: 10.1101/gad.8.2.190. [DOI] [PubMed] [Google Scholar]
- 12.Yew P R, Berk A J. Nature (London) 1992;357:82–85. doi: 10.1038/357082a0. [DOI] [PubMed] [Google Scholar]
- 13.Rao L, Debbas M, Sabbatini P, Hockenbery D, Korsmeyer S, White E. Proc Natl Acad Sci USA. 1992;89:7742–7746. doi: 10.1073/pnas.89.16.7742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Debbas M, White E. Genes Dev. 1993;7:546–554. doi: 10.1101/gad.7.4.546. [DOI] [PubMed] [Google Scholar]
- 15.Sabbatini P, Chiou S K, Rao L, White E. Mol Cell Biol. 1995;15:1060–1070. doi: 10.1128/mcb.15.2.1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.White E, Sabbatini P, Debbas M, Wold W S, Kusher D I, Gooding L R. Mol Cell Biol. 1992;12:2570–2580. doi: 10.1128/mcb.12.6.2570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shen Y, Shenk T. Proc Natl Acad Sci USA. 1994;91:8940–8944. doi: 10.1073/pnas.91.19.8940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Han J, Sabbatini P, Perez L, Modha D, White E. Genes Dev. 1996;10:461–477. doi: 10.1101/gad.10.4.461. [DOI] [PubMed] [Google Scholar]
- 19.Dobner T, Horikoshi N, Rubenwolf S, Shenk T. Science. 1996;272:1470–1473. doi: 10.1126/science.272.5267.1470. [DOI] [PubMed] [Google Scholar]
- 20.Soussi T, May P. J Mol Biol. 1996;260:623–637. doi: 10.1006/jmbi.1996.0425. [DOI] [PubMed] [Google Scholar]
- 21.Wang X W, Vermeulen W, Coursen J D, Gibson M, Lupold S E, Forrester K, Xu G X, Elmore L, Yeh H Y, Hoeijmakers J H J, Harris C C. Genes Dev. 1996;10:1219–1232. doi: 10.1101/gad.10.10.1219. [DOI] [PubMed] [Google Scholar]
- 22.Rubenwolf, S., Schütt, H., Nevels, M., Wolf, H. & Dobner, T. (1997) J. Virol. 71, in press. [DOI] [PMC free article] [PubMed]
- 23.Horikoshi N, Usheva A, Chen J, Levine A J, Weinmann R, Shenk T. Mol Cell Biol. 1995;15:227–234. doi: 10.1128/mcb.15.1.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sadowski I, Ptashne M. Nucleic Acids Res. 1989;17:7539. doi: 10.1093/nar/17.18.7539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Prywes R, Zhu H. Nucleic Acids Res. 1990;20:513–520. doi: 10.1093/nar/20.3.513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mitsudomi T, Steinberg S M, Nau M M, Carbone D, D’Amico D, Bodner H K, Oie H K, Linnoila R I, Mulshine J L, Minna J D, Gazdar A F. Oncogene. 1992;7:171–180. [PubMed] [Google Scholar]
- 27.Logan J, Pilder S, Shenk T. Cancer Cells. 1984;2:527–532. [Google Scholar]
- 28.Pilder S, Moore M, Logan J, Shenk T. Mol Cell Biol. 1986;6:470–476. doi: 10.1128/mcb.6.2.470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Neill S D, Hemstrom C, Virtanen A, Nevins J R. Proc Natl Acad Sci USA. 1990;87:2008–2012. doi: 10.1073/pnas.87.5.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shih C, Weinberg R A. Cell. 1982;29:161–169. doi: 10.1016/0092-8674(82)90100-3. [DOI] [PubMed] [Google Scholar]
- 31.Sarnow P, Sullivan C A, Levine A J. Virology. 1982;120:510–517. doi: 10.1016/0042-6822(82)90054-x. [DOI] [PubMed] [Google Scholar]
- 32.Marton M J, Baim S B, Ornelles D A, Shenk T. J Virol. 1990;64:2345–2359. doi: 10.1128/jvi.64.5.2345-2359.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Harlow E, Pim D C, Crawford L V. J Virol. 1981;37:564–573. doi: 10.1128/jvi.37.2.564-573.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vojtesek B, Bartek J, Midgley C A, Lane D P. J Immunol Methods. 1992;151:237–244. doi: 10.1016/0022-1759(92)90122-a. [DOI] [PubMed] [Google Scholar]
- 35.Sarnow P, Ho Y S, Williams J, Levine A J. Cell. 1982;28:387–394. doi: 10.1016/0092-8674(82)90356-7. [DOI] [PubMed] [Google Scholar]
- 36.Lowe S W, Ruley H E. Genes Dev. 1993;7:535–545. doi: 10.1101/gad.7.4.535. [DOI] [PubMed] [Google Scholar]
- 37.Moore M, Horikoshi N, Shenk T. Proc Natl Acad Sci USA. 1996;93:11295–11301. doi: 10.1073/pnas.93.21.11295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sabbatini P, Lin J, Levine A J, White E. Genes Dev. 1995;9:2184–2192. doi: 10.1101/gad.9.17.2184. [DOI] [PubMed] [Google Scholar]
- 39.Grand R J, Grant M L, Gallimore P H. Virology. 1994;203:229–240. doi: 10.1006/viro.1994.1480. [DOI] [PubMed] [Google Scholar]
- 40.Sarnow P, Hearing P, Anderson C W, Halbert D N, Shenk T, Levine A J. J Virol. 1984;49:692–700. doi: 10.1128/jvi.49.3.692-700.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]