Abstract
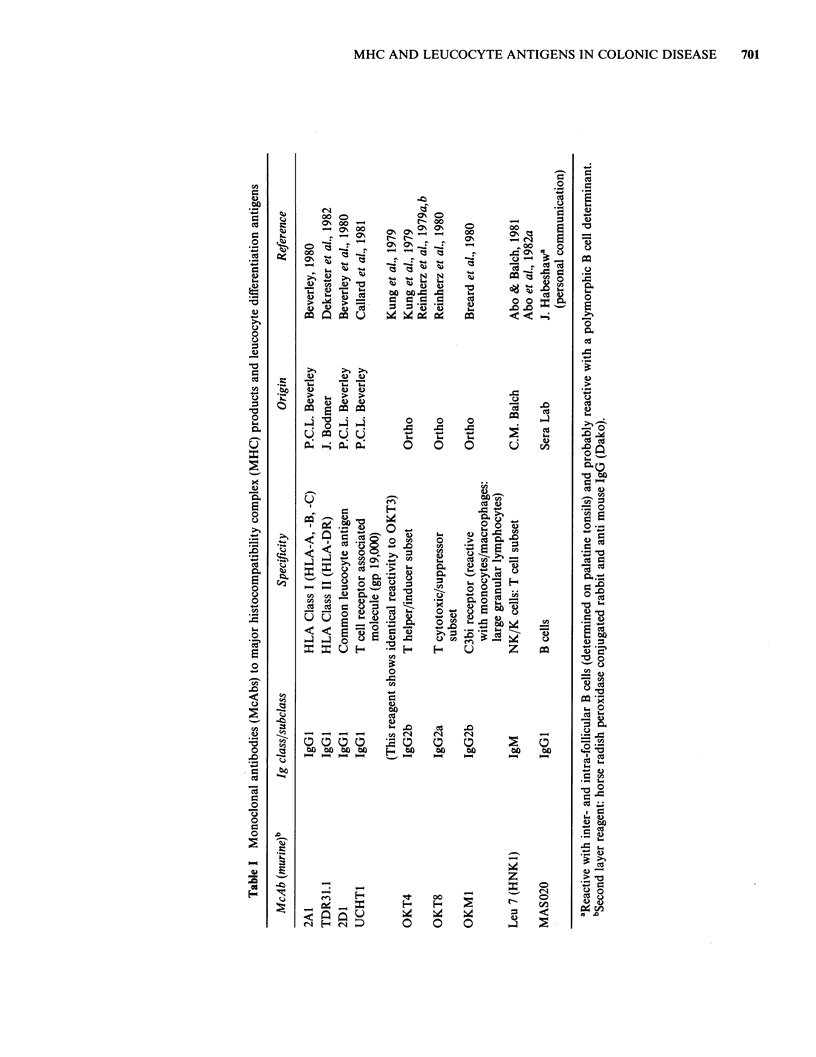
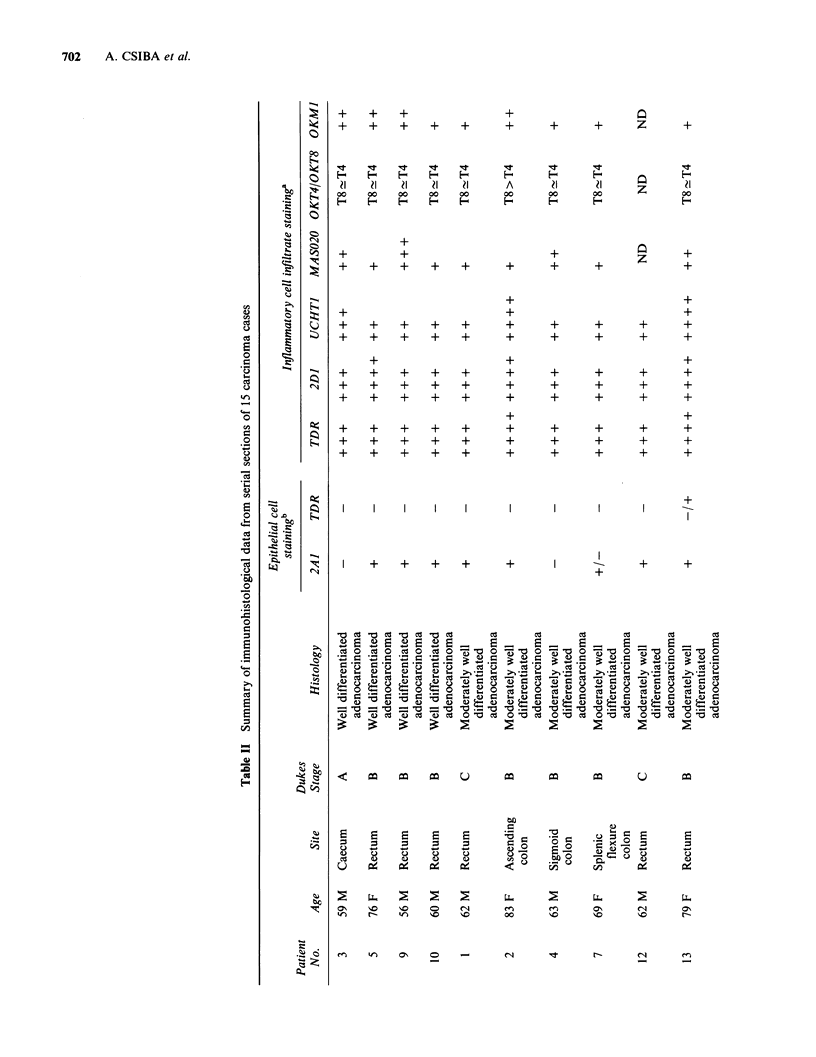
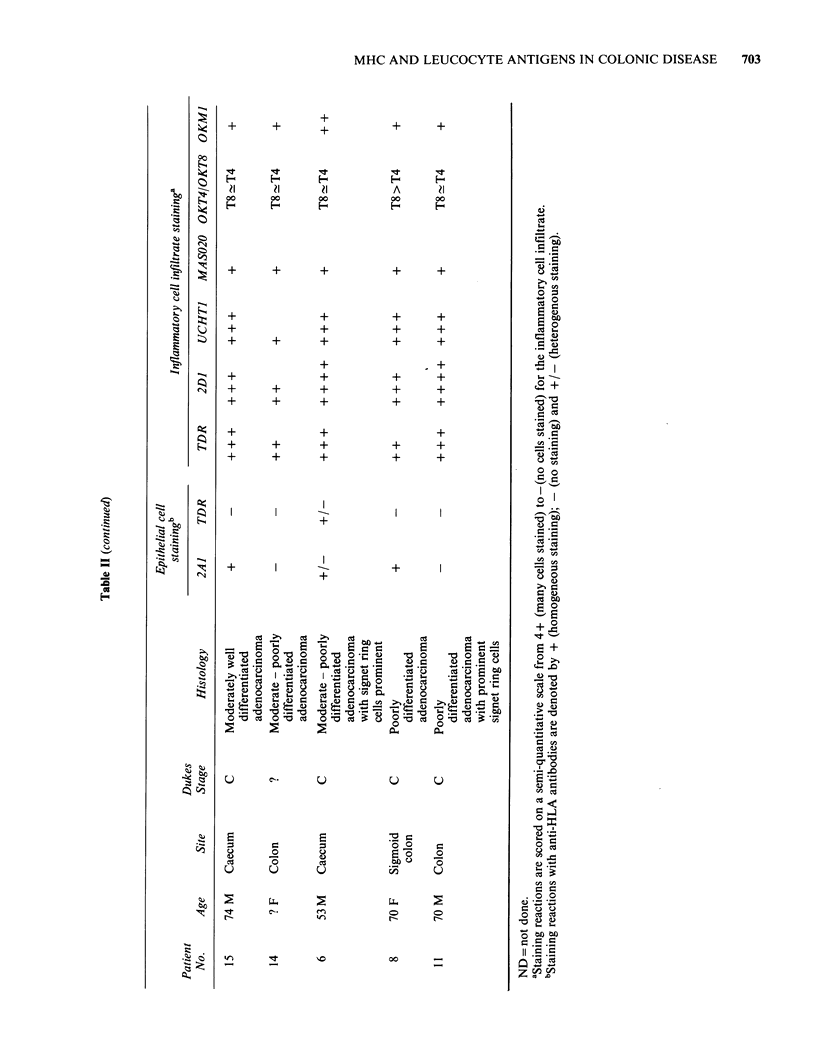
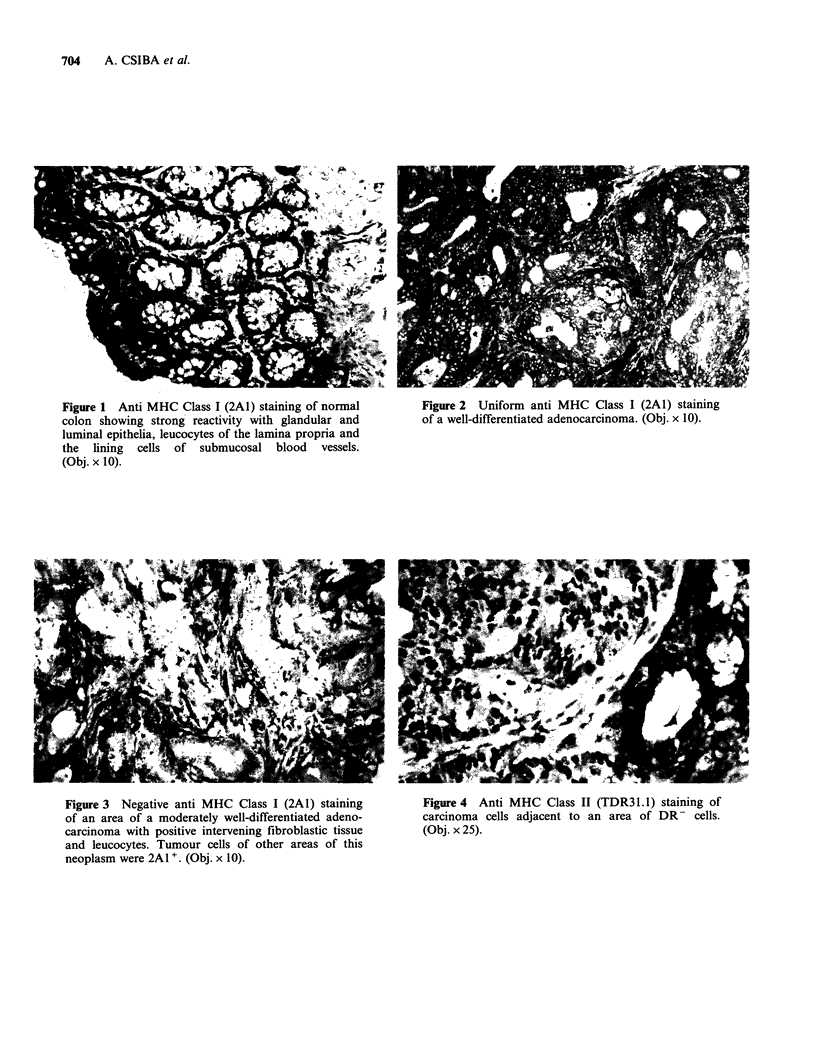
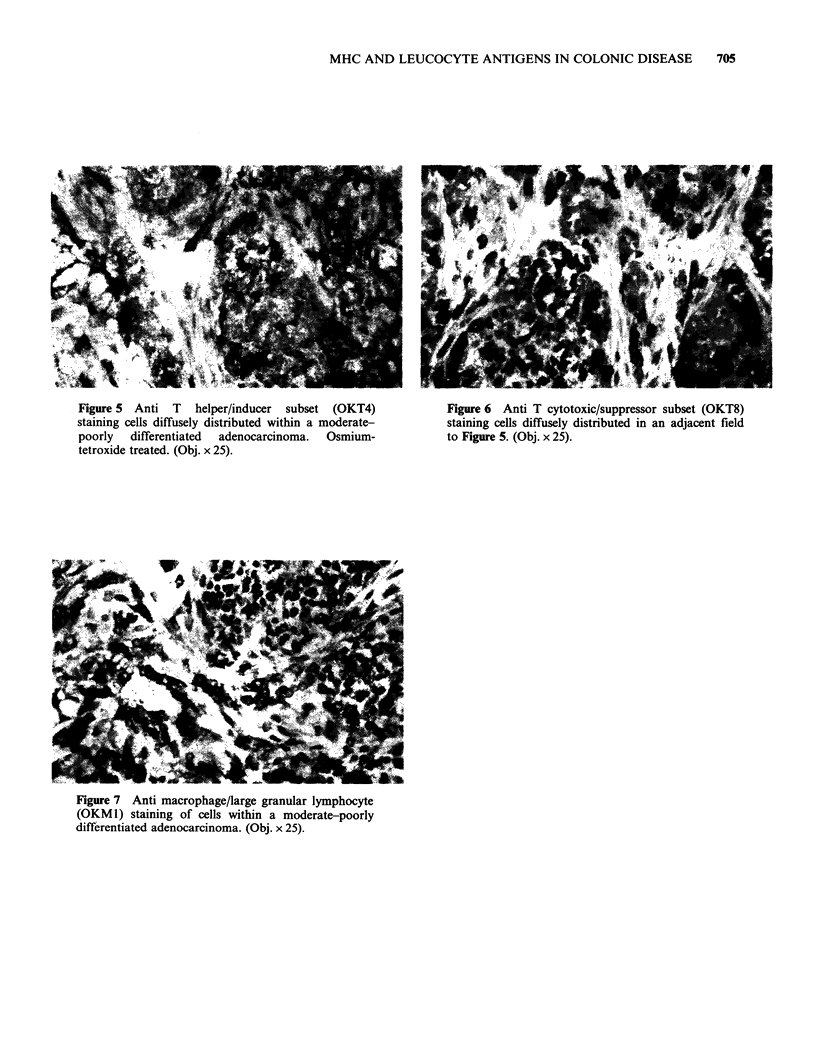
Monoclonal antibodies (McAbs) directed against the framework determinants of Class I and Class II products of the major histocompatibility complex (MHC) and against leucocyte differentiation antigens were used in an indirect immunoperoxidase technique to study their expression in normal, benign (adenomatous polyps) and malignant disease of the colon. Class I products (detected by the McAb 2A1) were strongly expressed on all cell types in normal and benign tissues but some carcinomas exhibited a heterogenous pattern of epithelial cell staining and 4/15 were completely negative. Class II products (detected by TDR31.1) were strongly expressed on cells (mainly B lymphocytes) within the lamina propria. In carcinomas TDR31.1 staining was mainly interstitial, but in 2/15, DR + epithelial cells were also detected. In normal and benign tissues, leucocytes (reactive with 2D1) found predominantly in the lamina propria, comprised T cells mainly of the helper/inducer (OKT4) subset, DR + cells in approx. equivalent proportion and a few OKM1+ cells mostly of macrophage morphology. Occasional intraepithelial lymphocytes were of cytotoxic/suppressor (OKT8) phenotype. In malignant neoplasms, there was wide inter and intra-tumour variation in the proportion of leucocytes which were heterogeneous with respect to cell type and confined mainly to the stroma. T cells were consistently predominant, but B cells and macrophages were also present. Two neoplasms showed unequivocal evidence of a shift (relative to peripheral blood) in favour of the OKT8+ subset, but in the majority of tumours OKT4+; and OKT8+ cells were present in roughly similar proportions. Natural killer cells (monitored with Leu7, HNK1) were virtually undetectable in both normal and malignant tissues. There were no apparent correlations between the extent and type of leucocyte infiltration, tumour differentiation or expression of MHC products. Some implications for the extrapolation of in vitro data on leucocyte function to the in vivo situation are discussed.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abo T., Balch C. M. A differentiation antigen of human NK and K cells identified by a monoclonal antibody (HNK-1). J Immunol. 1981 Sep;127(3):1024–1029. [PubMed] [Google Scholar]
- Abo T., Cooper M. D., Balch C. M. Characterization of HNK-1+ (Leu-7) human lymphocytes. I. Two distinct phenotypes of human NK cells with different cytotoxic capability. J Immunol. 1982 Oct;129(4):1752–1757. [PubMed] [Google Scholar]
- Abo T., Cooper M. D., Balch C. M. Postnatal expansion of the natural killer and keller cell population in humans identified by the monoclonal HNK-1 antibody. J Exp Med. 1982 Jan 1;155(1):321–326. doi: 10.1084/jem.155.1.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beverley P. C., Linch D., Delia D. Isolation of human haematopoietic progenitor cells using monoclonal antibodies. Nature. 1980 Sep 25;287(5780):332–333. doi: 10.1038/287332a0. [DOI] [PubMed] [Google Scholar]
- Bhan A. K., DesMarais C. L. Immunohistologic characterization of major histocompatibility antigens and inflammatory cellular infiltrate in human breast cancer. J Natl Cancer Inst. 1983 Sep;71(3):507–516. [PubMed] [Google Scholar]
- Breard J., Reinherz E. L., Kung P. C., Goldstein G., Schlossman S. F. A monoclonal antibody reactive with human peripheral blood monocytes. J Immunol. 1980 Apr;124(4):1943–1948. [PubMed] [Google Scholar]
- Callard R. E., Smith C. M., Worman C., Linch D., Cawley J. C., Beverley P. C. Unusual phenotype and function of an expanded subpopulation of T cells in patients with haemopoietic disorders. Clin Exp Immunol. 1981 Mar;43(3):497–505. [PMC free article] [PubMed] [Google Scholar]
- DUKES C. E., BUSSEY H. J. The spread of rectal cancer and its effect on prognosis. Br J Cancer. 1958 Sep;12(3):309–320. doi: 10.1038/bjc.1958.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daar A. S., Fabre J. W. The membrane antigens of human colorectal cancer cells: demonstration with monoclonal antibodies of heterogeneity within and between tumours and of anomalous expression of HLA-DR. Eur J Cancer Clin Oncol. 1983 Feb;19(2):209–220. doi: 10.1016/0277-5379(83)90419-4. [DOI] [PubMed] [Google Scholar]
- Daar A. S., Fuggle S. V., Ting A., Fabre J. W. Anomolous expression of HLA-DR antigens on human colorectal cancer cells. J Immunol. 1982 Aug;129(2):447–449. [PubMed] [Google Scholar]
- De Kretser T. A., Crumpton M. J., Bodmer J. G., Bodmer W. F. Two-dimensional gel analysis of the polypeptides precipitated by a polymorphic HLA-DR1,2,w6 monoclonal antibody: evidence for a third locus. Eur J Immunol. 1982 Jul;12(7):600–606. doi: 10.1002/eji.1830120713. [DOI] [PubMed] [Google Scholar]
- Eremin O., Coombs R. R., Ashby J. Lymphocytes infiltrating human breast cancers lack K-cell activity and show low levels of NK-cell activity. Br J Cancer. 1981 Aug;44(2):166–176. doi: 10.1038/bjc.1981.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleming K. A., McMichael A., Morton J. A., Woods J., McGee J. O. Distribution of HLA class 1 antigens in normal human tissue and in mammary cancer. J Clin Pathol. 1981 Jul;34(7):779–784. doi: 10.1136/jcp.34.7.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerry D., 4th, Alexander M. A., Herlyn M. F., Zehngebot L. M., Mitchell K. F., Zmijewski C. M., Lusk E. J. HLA-DR histocompatibility leukocyte antigens permit cultured human melanoma cells from early but not advanced disease to stimulate autologous lymphocytes. J Clin Invest. 1984 Jan;73(1):267–271. doi: 10.1172/JCI111201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ioachim H. L. The stromal reaction of tumors: an expression of immune surveillance. J Natl Cancer Inst. 1976 Sep;57(3):465–475. doi: 10.1093/jnci/57.3.465. [DOI] [PubMed] [Google Scholar]
- Klareskog L., Forsum U., Peterson P. A. Hormonal regulation of the expression of Ia antigens on mammary gland epithelium. Eur J Immunol. 1980 Dec;10(12):958–963. doi: 10.1002/eji.1830101212. [DOI] [PubMed] [Google Scholar]
- Kung P., Goldstein G., Reinherz E. L., Schlossman S. F. Monoclonal antibodies defining distinctive human T cell surface antigens. Science. 1979 Oct 19;206(4416):347–349. doi: 10.1126/science.314668. [DOI] [PubMed] [Google Scholar]
- Lampert I. A., Suitters A. J., Chisholm P. M. Expression of Ia antigen on epidermal keratinocytes in graft-versus-host disease. Nature. 1981 Sep 10;293(5828):149–150. doi: 10.1038/293149a0. [DOI] [PubMed] [Google Scholar]
- Mason D. W., Dallman M., Barclay A. N. Graft-versus-host disease induces expression of Ia antigen in rat epidermal cells and gut epithelium. Nature. 1981 Sep 10;293(5828):150–151. doi: 10.1038/293150a0. [DOI] [PubMed] [Google Scholar]
- McMichael A. HLA restriction of human cytotoxic T lymphocytes specific for influenza virus. Poor recognition of virus associated with HLA A2. J Exp Med. 1978 Dec 1;148(6):1458–1467. doi: 10.1084/jem.148.6.1458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore M., Vose B. M. Extravascular natural cytotoxicity in man: Anti-K562 activity of lymph-node and tumour-infiltrating lymphocytes. Int J Cancer. 1981 Mar 15;27(3):265–272. doi: 10.1002/ijc.2910270303. [DOI] [PubMed] [Google Scholar]
- Murray D., Hreno A., Dutton J., Hampson L. G. Prognosis in colon cancer: a pathologic reassessment. Arch Surg. 1975 Aug;110(8):908–913. doi: 10.1001/archsurg.1975.01360140052011. [DOI] [PubMed] [Google Scholar]
- Ortaldo J. R., Sharrow S. O., Timonen T., Herberman R. B. Determination of surface antigens on highly purified human NK cells by flow cytometry with monoclonal antibodies. J Immunol. 1981 Dec;127(6):2401–2409. [PubMed] [Google Scholar]
- Perussia B., Acuto O., Terhorst C., Faust J., Lazarus R., Fanning V., Trinchieri G. Human natural killer cells analyzed by B73.1, a monoclonal antibody blocking Fc receptor functions. II. Studies of B73.1 antibody-antigen interaction on the lymphocyte membrane. J Immunol. 1983 May;130(5):2142–2148. [PubMed] [Google Scholar]
- Perussia B., Starr S., Abraham S., Fanning V., Trinchieri G. Human natural killer cells analyzed by B73.1, a monoclonal antibody blocking Fc receptor functions. I. Characterization of the lymphocyte subset reactive with B73.1. J Immunol. 1983 May;130(5):2133–2141. [PubMed] [Google Scholar]
- Pizzolo G., Semenzato G., Chilosi M., Morittu L., Ambrosetti A., Warner N., Bofill M., Janossy G. Distribution and heterogeneity of cells detected by HNK-1 monoclonal antibody in blood and tissues in normal, reactive and neoplastic conditions. Clin Exp Immunol. 1984 Jul;57(1):195–206. [PMC free article] [PubMed] [Google Scholar]
- Reinherz E. L., Kung P. C., Goldstein G., Schlossman S. F. A monoclonal antibody reactive with the human cytotoxic/suppressor T cell subset previously defined by a heteroantiserum termed TH2. J Immunol. 1980 Mar;124(3):1301–1307. [PubMed] [Google Scholar]
- Reinherz E. L., Kung P. C., Goldstein G., Schlossman S. F. A monoclonal antibody with selective reactivity with functionally mature human thymocytes and all peripheral human T cells. J Immunol. 1979 Sep;123(3):1312–1317. [PubMed] [Google Scholar]
- Reinherz E. L., Kung P. C., Goldstein G., Schlossman S. F. Further characterization of the human inducer T cell subset defined by monoclonal antibody. J Immunol. 1979 Dec;123(6):2894–2896. [PubMed] [Google Scholar]
- Rognum T. O., Brandtzaeg P., Thorud E. Is heterogeneous expression of HLA-dr antigens and CEA along with DNA-profile variations evidence of phenotypic instability and clonal proliferation in human large bowel carcinomas? Br J Cancer. 1983 Oct;48(4):543–551. doi: 10.1038/bjc.1983.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe D. J., Beverley P. C. Characterisation of breast cancer infiltrates using monoclonal antibodies to human leucocyte antigens. Br J Cancer. 1984 Feb;49(2):149–159. doi: 10.1038/bjc.1984.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiter D. J., Bhan A. K., Harrist T. J., Sober A. J., Mihm M. C., Jr Major histocompatibility antigens and mononuclear inflammatory infiltrate in benign nevomelanocytic proliferations and malignant melanoma. J Immunol. 1982 Dec;129(6):2808–2815. [PubMed] [Google Scholar]
- Selby W. S., Janossy G., Goldstein G., Jewell D. P. T lymphocyte subsets in human intestinal mucosa: the distribution and relationship to MHC-derived antigens. Clin Exp Immunol. 1981 Jun;44(3):453–458. [PMC free article] [PubMed] [Google Scholar]
- Spratt J. S., Jr, Spjut H. J. Prevalence and prognosis of individual clinical and pathologic variables associated with colorectal carcinoma. Cancer. 1967 Nov;20(11):1976–1985. doi: 10.1002/1097-0142(196711)20:11<1976::aid-cncr2820201125>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- Svennevig J. L., Lunde O. C., Holter J., Bjørgsvik D. Lymphoid infiltration and prognosis in colorectal carcinoma. Br J Cancer. 1984 Mar;49(3):375–377. doi: 10.1038/bjc.1984.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson J. J., Herlyn M. F., Elder D. E., Clark W. H., Steplewski Z., Koprowski H. Expression of DR antigens in freshly frozen human tumors. Hybridoma. 1982;1(2):161–168. doi: 10.1089/hyb.1.1982.1.161. [DOI] [PubMed] [Google Scholar]
- Tötterman T. H., Häyry P., Saksela E., Timonen T., Eklund B. Cytological and functional analysis of inflammatory infiltrates in human malignant tumors. II. Functional investigations of the infiltrating inflammatory cells. Eur J Immunol. 1978 Dec;8(12):872–875. doi: 10.1002/eji.1830081209. [DOI] [PubMed] [Google Scholar]
- Uchiyama T., Nelson D. L., Fleisher T. A., Waldmann T. A. A monoclonal antibody (anti-Tac) reactive with activated and functionally mature human T cells. II. Expression of Tac antigen on activated cytotoxic killer T cells, suppressor cells, and on one of two types of helper T cells. J Immunol. 1981 Apr;126(4):1398–1403. [PubMed] [Google Scholar]
- Underwood J. C. Lymphoreticular infiltration in human tumours: prognostic and biological implications: a review. Br J Cancer. 1974 Dec;30(6):538–548. doi: 10.1038/bjc.1974.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vose B. M., Gallagher P., Moore M., Schofield P. F. Specific and non-specific lymphocyte cytotoxicity in colon carcinoma. Br J Cancer. 1981 Dec;44(6):846–855. doi: 10.1038/bjc.1981.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vose B. M., Moore M. Suppressor cell activity of lymphocytes infiltrating human lung and breast tumours. Int J Cancer. 1979 Nov 15;24(5):579–585. doi: 10.1002/ijc.2910240510. [DOI] [PubMed] [Google Scholar]
- Vánky F., Péterffy A., Bök K., Willems J., Klein E., Klein G. Correlation between lymphocyte-mediated auto-tumor reactivities and the clinical course. II. Evaluation of 69 patients with lung carcinoma. Cancer Immunol Immunother. 1983;16(1):17–22. doi: 10.1007/BF00199900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe S., Sato Y., Kodama T., Shimosato Y. Immunohistochemical study with monoclonal antibodies on immune response in human lung cancers. Cancer Res. 1983 Dec;43(12 Pt 1):5883–5889. [PubMed] [Google Scholar]
- Watt A. G., House A. K. Colonic carcinoma: a quantitative assessment of lymphocyte infiltration at the periphery of colonic tumors related to prognosis. Cancer. 1978 Jan;41(1):279–282. doi: 10.1002/1097-0142(197801)41:1<279::aid-cncr2820410139>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- Werkmeister J. A., Pihl E., Nind A. P., Flannery G. R., Nairn R. C. Immunoreactivity by intrinsic lymphoid cells in colorectal carcinoma. Br J Cancer. 1979 Dec;40(6):839–847. doi: 10.1038/bjc.1979.274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitwell H. L., Hughes H. P., Moore M., Ahmed A. Expression of major histocompatibility antigens and leucocyte infiltration in benign and malignant human breast disease. Br J Cancer. 1984 Feb;49(2):161–172. doi: 10.1038/bjc.1984.28. [DOI] [PMC free article] [PubMed] [Google Scholar]









