Abstract
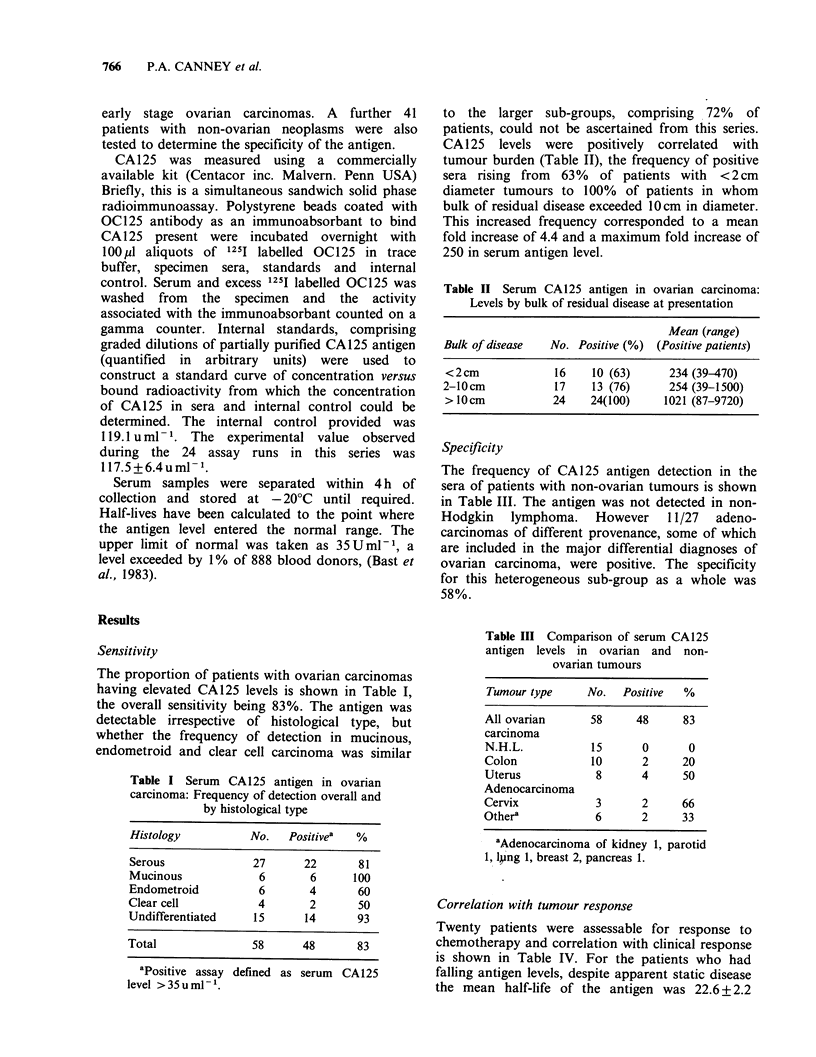
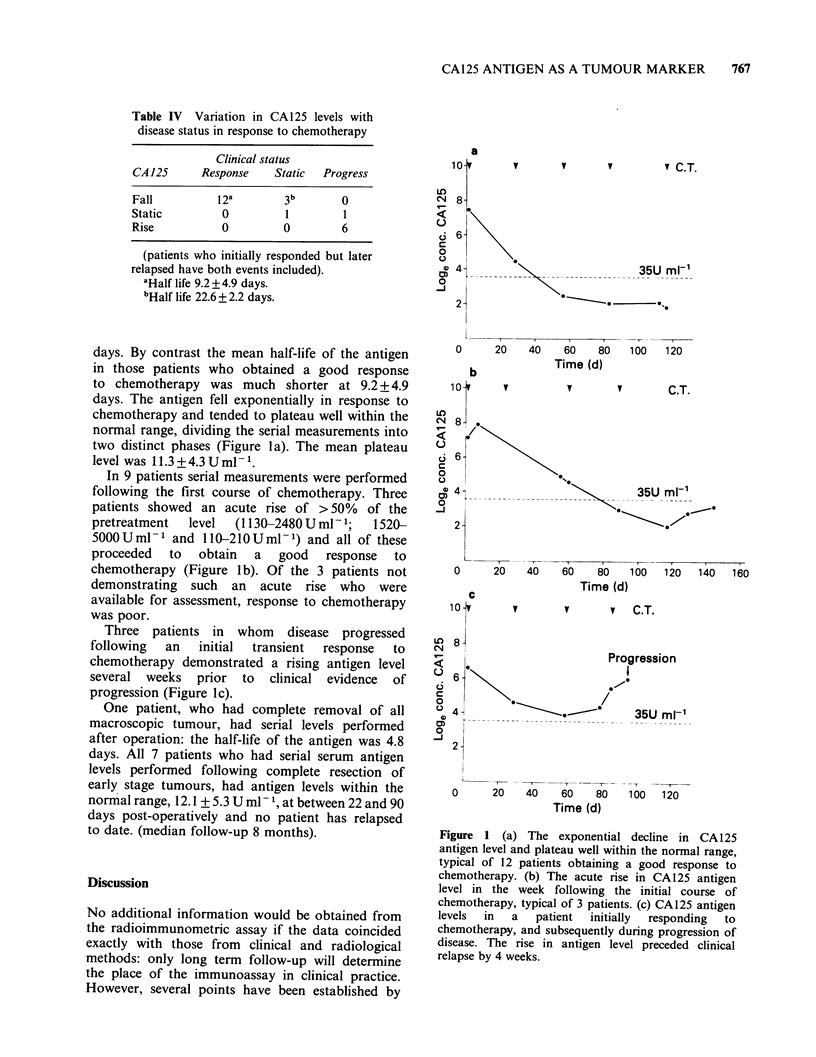
Serum CA 125, quantified by an immunoradiometric assay employing the monoclonal antibody 0C125 was found to be elevated in 48/58 (83%) of patients with established ovarian cancer. All histological types of carcinoma were antigen positive and there was a positive correlation between the frequency and level of serum CA125 and body burden of tumour. Twenty patients undergoing chemotherapy had serial CA125 estimations following a prospective protocol. Variation in CA125 level reflected disease progression or regression in 21/23 instances. Three of 9 patients tested showed an acute elevation of CA125 in the first week following chemotherapy and this effect predicted a good response to treatment. The natural half-life of CA125 in serum was estimated at approximately 4.8 days, sufficiently short to allow changes in tumour volume to be rapidly reflected by a change in circulating antigen level. Although none of 15 patients with non-Hodgkin lymphoma demonstrated antigen levels outside the normal range, 11/27 patients with non-ovarian adenocarcinoma showed elevated CA125 levels, a specificity of 58% for this latter group. The value of CA125 in the management of ovarian malignancy is discussed.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bast R. C., Jr, Feeney M., Lazarus H., Nadler L. M., Colvin R. B., Knapp R. C. Reactivity of a monoclonal antibody with human ovarian carcinoma. J Clin Invest. 1981 Nov;68(5):1331–1337. doi: 10.1172/JCI110380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bast R. C., Jr, Klug T. L., St John E., Jenison E., Niloff J. M., Lazarus H., Berkowitz R. S., Leavitt T., Griffiths C. T., Parker L. A radioimmunoassay using a monoclonal antibody to monitor the course of epithelial ovarian cancer. N Engl J Med. 1983 Oct 13;309(15):883–887. doi: 10.1056/NEJM198310133091503. [DOI] [PubMed] [Google Scholar]
- Bhattacharya M., Chatterjee S. K., Barlow J. J., Fuji H. Monoclonal antibodies recognizing tumor-associated antigen of human ovarian mucinous cystadenocarcinomas. Cancer Res. 1982 May;42(5):1650–1654. [PubMed] [Google Scholar]
- Cohen C. J., Goldberg J. D., Holland J. F., Bruckner H. W., Deppe G., Gusberg S. B., Wallach R. C., Kabakow B., Rodin J. Improved therapy with cisplatin regimens for patients with ovarian carcinoma (FIGO Stages III and IV) as measured by surgical end-staging (second-look operation). Am J Obstet Gynecol. 1983 Apr 15;145(8):955–967. doi: 10.1016/0002-9378(83)90849-9. [DOI] [PubMed] [Google Scholar]
- Donaldson E. S., van Nagell J. R., Jr, Pursell S., Gay E. C., Meeker W. R., Kashmiri R., van deVoorde J. Multiple biochemical markers in patients with gynecologic malignancies. Cancer. 1980 Mar 1;45(5):948–953. doi: 10.1002/1097-0142(19800301)45:5<948::aid-cncr2820450519>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- Johnson R. J., Blackledge G., Eddleston B., Crowther D. Abdomino-pelvic computed tomography in the management of ovarian carcinoma. Radiology. 1983 Feb;146(2):447–452. doi: 10.1148/radiology.146.2.6849092. [DOI] [PubMed] [Google Scholar]
- Kabawat S. E., Bast R. C., Welch W. R., Knapp R. C., Colvin R. B. Immunopathologic characterization of a monoclonal antibody that recognizes common surface antigens of human ovarian tumors of serous, endometrioid, and clear cell types. Am J Clin Pathol. 1983 Jan;79(1):98–104. doi: 10.1093/ajcp/79.1.98. [DOI] [PubMed] [Google Scholar]
- Newlands E. S., Begent R. H., Kaye S. B., Rustin G. J., Bagshawe K. D. Chemotherapy of advanced malignant teratomas. Br J Cancer. 1980 Sep;42(3):378–391. doi: 10.1038/bjc.1980.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith J. P., Delgado G., Rutledge F. Second-look operation in ovarian carcinoma: postchemotherapy. Cancer. 1976 Sep;38(3):1438–1442. doi: 10.1002/1097-0142(197609)38:3<1438::aid-cncr2820380351>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]


