Abstract
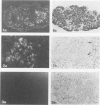
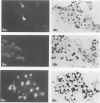
Foetal acinar components associated with the development of the hamster pancreas have been previously defined with the aid of an antifoetal pancreas serum. In immunohistology this antiserum also stained malignant ductal cells in N-nitrobis (2-oxopropyl) amine (BOP)-induced pancreatic adenocarcinoma. It did not stain adult pancreas structures including acini, ducts and islets of Langerhans. In this study, re-expression of foetal acinar antigens was disclosed before formation of tumours. Adenocarcinomas were not detected by conventional histology before the 24th week following initiation of the chemical treatment. However, staining with the antiserum was observed from the 7th week appearing in the apex of some acini cells having an almost normal histological appearance. Later, foetal acinar expression was frequently associated with evident morphological alterations in acini like dyskaryosis, enlarged cytoplasm or lumina. Staining of ducts with marked atypical epithelium and (as already reported) of neoplastic ducts was also observed. It was not detected in other pancreatic lesions viz. cystadenomas, mucoid glands and regular hyperplastic ducts. Acinar dedifferentiation as assessed by expression of foetal components preceded formation of tumours in all instances.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Banwo O., Versey J., Hobbs J. R. New oncofetal antigen for human pancreas. Lancet. 1974 Apr;1(7859):643–645. doi: 10.1016/s0140-6736(74)93197-3. [DOI] [PubMed] [Google Scholar]
- Benedi V. J., Escribano M. J., Zuinghedau J., Burtin P. Fetal pancreatic antigens in the Syrian golden hamster and their relationship to development and carcinogenesis. Cancer Res. 1984 Mar;44(3):1135–1141. [PubMed] [Google Scholar]
- Bockman D. E. Cells of origin of pancreatic cancer: experimental animal tumors related to human pancreas. Cancer. 1981 Mar 15;47(6 Suppl):1528–1534. doi: 10.1002/1097-0142(19810315)47:6+<1528::aid-cncr2820471415>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- Cubilla A. L., Fitzgerald P. J. Morphological lesions associated with human primary invasive nonendocrine pancreas cancer. Cancer Res. 1976 Jul;36(7 Pt 2):2690–2698. [PubMed] [Google Scholar]
- Cubilla A. L., Fitzgerald P. J. Morphological patterns of primary nonendocrine human pancreas carcinoma. Cancer Res. 1975 Aug;35(8):2234–2248. [PubMed] [Google Scholar]
- Flaks B., Moore M. A., Flaks A. Ultrastructural analysis of pancreatic carcinogenesis. V. Changes in differentiation of acinar cells during chronic treatment with N-nitrosobis(2-hydroxypropyl)amine. Carcinogenesis. 1982;3(5):485–498. doi: 10.1093/carcin/3.5.485. [DOI] [PubMed] [Google Scholar]
- Gelder F. B., Reese C. J., Moossa A. R., Hall T., Hunter R. Purification, partial characterization, and clinical evaluation of a pancreatic oncofetal antigen. Cancer Res. 1978 Feb;38(2):313–324. [PubMed] [Google Scholar]
- Herlyn M., Sears H. F., Steplewski Z., Koprowski H. Monoclonal antibody detection of a circulating tumor-associated antigen. I. Presence of antigen in sera of patients with colorectal, gastric, and pancreatic carcinoma. J Clin Immunol. 1982 Apr;2(2):135–140. doi: 10.1007/BF00916897. [DOI] [PubMed] [Google Scholar]
- Levin D. L., Connelly R. R., Devesa S. S. Demographic characteristics of cancer of the pancreas: mortality, incidence, and survival. Cancer. 1981 Mar 15;47(6 Suppl):1456–1468. doi: 10.1002/1097-0142(19810315)47:6+<1456::aid-cncr2820471404>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- Longnecker D. S., Curphey T. J., Kuhlmann E. T., Schaeffer B. K. Experimental induction of pancreatic carcinomas in the hamster with N delta-(N-methyl-N-nitrosocarbamoyl)-L-ornithine. J Natl Cancer Inst. 1983 Dec;71(6):1327–1336. [PubMed] [Google Scholar]
- Longnecker D. S., Roebuck B. D., Yager J. D., Jr, Lilja H. S., Siegmund B. Pancreatic carcinoma in azaserine-treated rats: induction, classification and dietary modulation of incidence. Cancer. 1981 Mar 15;47(6 Suppl):1562–1572. doi: 10.1002/1097-0142(19810315)47:6+<1562::aid-cncr2820471419>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Magnani J. L., Steplewski Z., Koprowski H., Ginsburg V. Identification of the gastrointestinal and pancreatic cancer-associated antigen detected by monoclonal antibody 19-9 in the sera of patients as a mucin. Cancer Res. 1983 Nov;43(11):5489–5492. [PubMed] [Google Scholar]
- Moore M. A., Takahashi M., Ito N., Bannasch P. Early lesions during pancreatic carcinogenesis induced in Syrian hamster by DHPN or DOPN. I. Histologic, histochemical and radioautographic findings. Carcinogenesis. 1983;4(4):431–437. doi: 10.1093/carcin/4.4.431. [DOI] [PubMed] [Google Scholar]
- Pour P. M., Runge R. G., Birt D., Gingell R., Lawson T., Nagel D., Wallcave L., Salmasi S. Z. Current knowledge of pancreatic carcinogenesis in the hamster and its relevance to the human disease. Cancer. 1981 Mar 15;47(6 Suppl):1573–1589. doi: 10.1002/1097-0142(19810315)47:6+<1573::aid-cncr2820471420>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- Pour P. M., Salmasi S. Z., Runge R. G. Selective induction of pancreatic ductular tumors by single doses of N-nitrosobis(2-oxopropyl)amine in Syrian golden hamsters. Cancer Lett. 1978 Jun;4(6):317–323. doi: 10.1016/s0304-3835(78)95502-7. [DOI] [PubMed] [Google Scholar]
- Pour P., Althoff J., Takahashi M. Early lesions of pancreatic ductal carcinoma in the hamster model. Am J Pathol. 1977 Aug;88(2):291–308. [PMC free article] [PubMed] [Google Scholar]
- Rao M. S., Reddy J. K. Histogenesis of pseudo-ductular changes induced in the pancreas of guinea pigs treated with N-methyl-N-nitrosourea. Carcinogenesis. 1980;1(12):1027–1037. doi: 10.1093/carcin/1.12.1027. [DOI] [PubMed] [Google Scholar]
- Reddy J. K., Rao M. S. Pancreatic adenocarcinoma in inbred guinea pigs induced by n-methyl-N-nitrosourea. Cancer Res. 1975 Aug;35(8):2269–2277. [PubMed] [Google Scholar]
- Scarpelli D. G., Kokkinakis D. M., Rao M. S., Subbarao V., Luetteke N., Hollenberg P. F. Metabolism of the pancreatic carcinogen N-nitroso-2,6-dimethylmorpholine by hamster liver and component cells of pancreas. Cancer Res. 1982 Dec;42(12):5089–5095. [PubMed] [Google Scholar]
- Scarpelli D. G., Rao M. S. Early changes in regenerating hamster pancreas following a single dose of N-nitrosobis (2-oxopropyl)amine (NBOP) administered at the peak of DNA synthesis. Cancer. 1981 Mar 15;47(6 Suppl):1552–1561. doi: 10.1002/1097-0142(19810315)47:6+<1552::aid-cncr2820471418>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- Shimano T., Loor R. M., Papsidero L. D., Kuriyama M., Vincent R. G., Nemoto T., Holyoke E. D., Berjian R., Douglass H. O., Chu T. M. Isolation, characterization and clinical evaluation of a pancreas cancer-associated antigen. Cancer. 1981 Mar 15;47(6 Suppl):1602–1613. doi: 10.1002/1097-0142(19810315)47:6+<1602::aid-cncr2820471424>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- Watabe H. Early appearance of embryonic -globulin in rat serum during carcinogenesis with 4-dimethylaminoazobenzene. Cancer Res. 1971 Sep;31(9):1192–1194. [PubMed] [Google Scholar]