Abstract
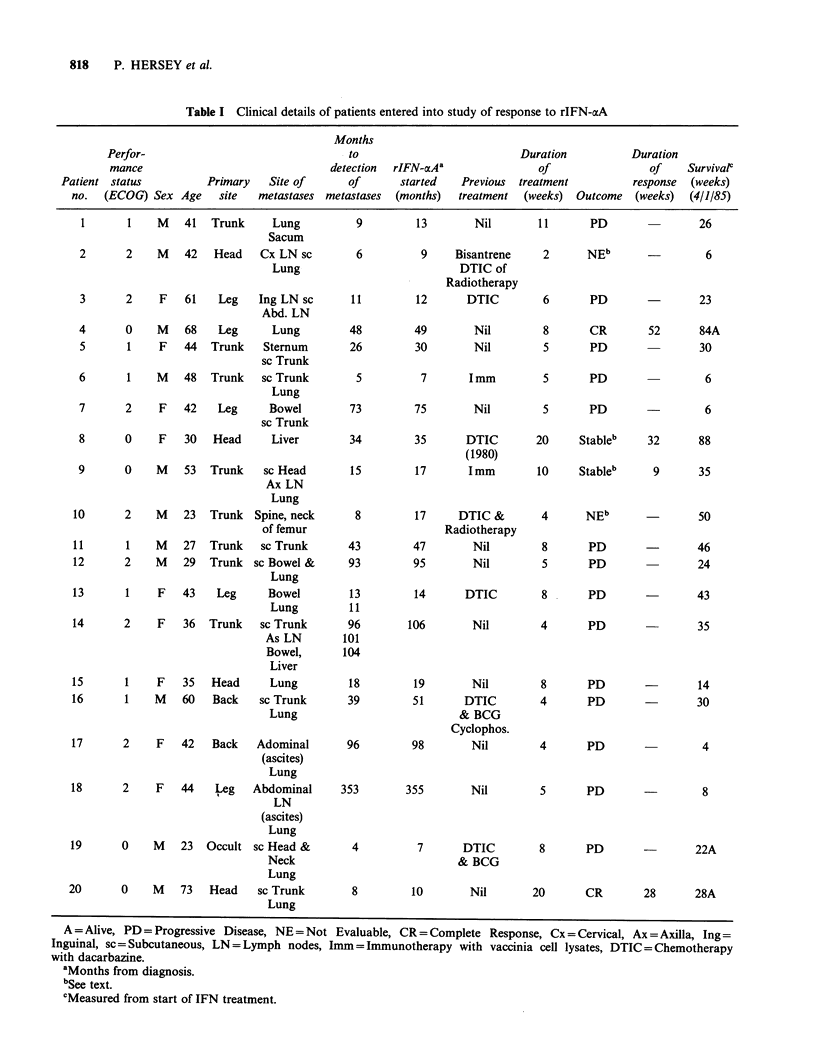
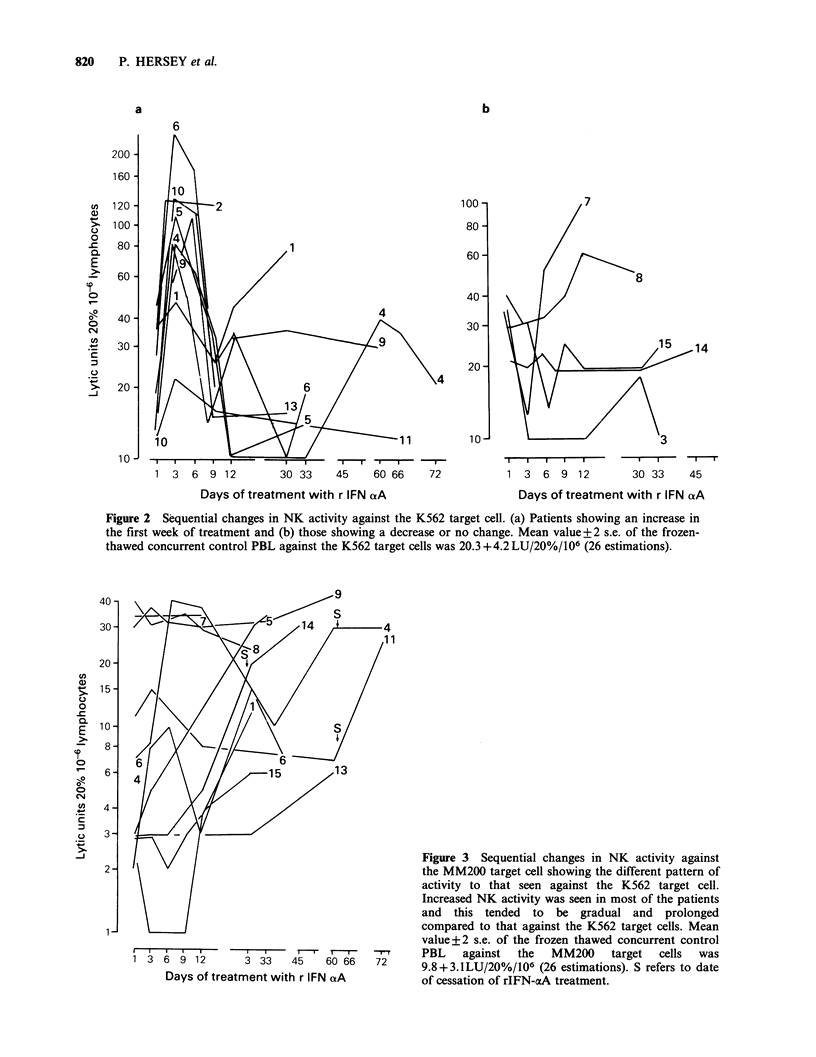
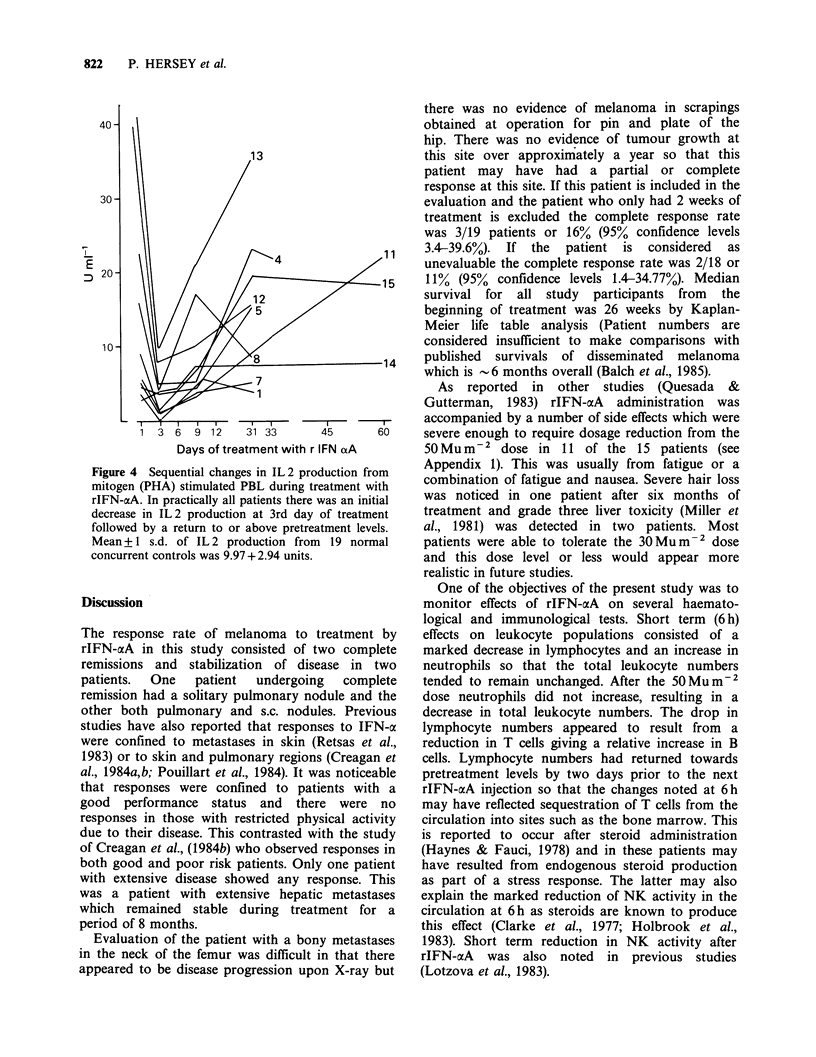
Studies were initiated to assess the response of patients with disseminated melanoma to recombinant alpha interferon (rIFN-alpha A) and to monitor effects of rIFN-alpha A on several tests of immune function. Twenty patients were treated with rIFN-alpha A given by i.m. injection in escalating doses from 15 to 50 X 10(6) um-2. The responses of two patients were considered unevaluable. Of the remainder there was complete remission of tumour in two and stable disease in two. Subsequent progression of tumour in one of the latter patients coincided with development of antibodies to IFN. Side effects (usually fatigue) were dose rate limiting in 11 patients. Laboratory tests on samples taken 6 hours after rIFN-alpha A indicated a marked lymphopenia and a reduction in natural killer (NK) cell activity particularly against K562 target cells. Longer term changes measured in samples taken 2 days after the previous rIFN-alpha A injections consisted of neutropenia and an increase in the T4/T8 ratio due mainly to a relative increase in OKT4 positive T cells compared to OKT8 positive T cells. NK activity against the K562 target cell increased in most patients during the first week of treatment and then returned to below or near pretreatment levels thereafter against the K562 target cell. This contrasted with NK activity against the melanoma target cell which showed a more gradual increase over the duration of the treatment in 6 patients. The latter correlated with an increase in mitogen stimulated IL 2 production from their blood lymphocytes and may indicate that the cytotoxic activity resulted from lymphokine-activated killer (LAK) cells. These results confirm the activity of rIFN-alpha A against melanoma in certain patients. They suggest that further studies are needed to select patients who may respond to rIFN-alpha A and to optimize treatment regimens. Tests of IL 2 production and LAK activity may assisted in achieving these objectives.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Belardelli F., Gresser I., Maury C., Duvillard P., Prade M., Maunoury M. T. Antitumor effects of interferon in mice injected with interferon-sensitive and interferon-resistant Friend leukemia cells. III. Inhibition of growth and necrosis of tumors implanted subcutaneously. Int J Cancer. 1983 May 15;31(5):649–653. doi: 10.1002/ijc.2910310518. [DOI] [PubMed] [Google Scholar]
- Blomgren H., Einhorn S. Lymphokine production by PHA-stimulated human lymphocytes is enhanced by interferon. Int Arch Allergy Appl Immunol. 1981;66(2):173–178. doi: 10.1159/000232816. [DOI] [PubMed] [Google Scholar]
- Broxmeyer H. E., Lu L., Platzer E., Feit C., Juliano L., Rubin B. Y. Comparative analysis of the influences of human gamma, alpha and beta interferons on human multipotential (CFU-GEMM), erythroid (BFU-E) and granulocyte-macrophage (CFU-GM) progenitor cells. J Immunol. 1983 Sep;131(3):1300–1305. [PubMed] [Google Scholar]
- Clarke J. R., Gagnon R. F., Gotch F. M., Heyworth M. R., Maclennan I. C., Truelove S. C., Waller C. A. The effects of prednisolone in leucocyte function in man. A double blind controlled study. Clin Exp Immunol. 1977 May;28(2):292–301. [PMC free article] [PubMed] [Google Scholar]
- Creasey A. A., Bartholomew J. C., Merigan T. C. Role of G0-G1 arrest in the inhibition of tumor cell growth by interferon. Proc Natl Acad Sci U S A. 1980 Mar;77(3):1471–1475. doi: 10.1073/pnas.77.3.1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feun L. G., Gutterman J., Burgess M. A., Hersh E. M., Mavligit G., McBride C. M., Benjamin R. S., Richman S. P., Murphy W. K., Bodey G. P. The natural history of resectable metastatic melanoma (Stage IVA melanoma). Cancer. 1982 Oct 15;50(8):1656–1663. doi: 10.1002/1097-0142(19821015)50:8<1656::aid-cncr2820500833>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- Golub S. H., Dorey F., Hara D., Morton D. L., Burk M. W. Systemic administration of human leukocyte interferon to melanoma patients. I. Effects on natural killer function and cell population. J Natl Cancer Inst. 1982 May;68(5):703–710. [PubMed] [Google Scholar]
- Grimm E. A., Mazumder A., Zhang H. Z., Rosenberg S. A. Lymphokine-activated killer cell phenomenon. Lysis of natural killer-resistant fresh solid tumor cells by interleukin 2-activated autologous human peripheral blood lymphocytes. J Exp Med. 1982 Jun 1;155(6):1823–1841. doi: 10.1084/jem.155.6.1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutterman J. U., Fine S., Quesada J., Horning S. J., Levine J. F., Alexanian R., Bernhardt L., Kramer M., Spiegel H., Colburn W. Recombinant leukocyte A interferon: pharmacokinetics, single-dose tolerance, and biologic effects in cancer patients. Ann Intern Med. 1982 May;96(5):549–556. doi: 10.7326/0003-4819-96-5-549. [DOI] [PubMed] [Google Scholar]
- Haynes B. F., Fauci A. S. The differential effect of in vivo hydrocortisone on the kinetics of subpopulations of human peripheral blood thymus-derived lymphocytes. J Clin Invest. 1978 Mar;61(3):703–707. doi: 10.1172/JCI108982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hersey P., Bindon C., Edwards A., Murray E., Phillips G., McCarthy W. H. Induction of cytotoxic activity in human lymphocytes against autologous and allogeneic melanoma cells in vitro by culture with interleukin 2. Int J Cancer. 1981 Dec;28(6):695–703. doi: 10.1002/ijc.2910280607. [DOI] [PubMed] [Google Scholar]
- Hersey P., Edwards A., McCarthy W. H. Tumour-related changes in natural killer cell activity in melanoma patients. Influence of stage of disease, tumour thickness and age of patients. Int J Cancer. 1980 Feb 15;25(2):187–194. doi: 10.1002/ijc.2910250204. [DOI] [PubMed] [Google Scholar]
- Hersey P., Grace J., Murray E., Palmer A., McCarthy W. H. Expression of Thy-1 antigen on human melanoma cells. Int J Cancer. 1983 Jul 15;32(1):21–25. doi: 10.1002/ijc.2910320105. [DOI] [PubMed] [Google Scholar]
- Holbrook N. J., Cox W. I., Horner H. C. Direct suppression of natural killer activity in human peripheral blood leukocyte cultures by glucocorticoids and its modulation by interferon. Cancer Res. 1983 Sep;43(9):4019–4025. [PubMed] [Google Scholar]
- Krown S. E., Burk M. W., Kirkwood J. M., Kerr D., Morton D. L., Oettgen H. F. Human leukocyte (alpha) interferon in metastatic malignant melanoma: the American Cancer Society phase II trial. Cancer Treat Rep. 1984 May;68(5):723–726. [PubMed] [Google Scholar]
- Lotzová E., Savary C. A., Quesada J. R., Gutterman J. U., Hersh E. M. Analysis of natural killer cell cytotoxicity of cancer patients treated with recombinant interferon. J Natl Cancer Inst. 1983 Nov;71(5):903–910. [PubMed] [Google Scholar]
- Masucci M. G., Klein E., Argov S. Disappearance of the NK effect after explantation of lymphocytes and generation of similar nonspecific cytotoxicity correlated to the level of blastogenesis in activated cultures. J Immunol. 1980 May;124(5):2458–2463. [PubMed] [Google Scholar]
- Miller A. B., Hoogstraten B., Staquet M., Winkler A. Reporting results of cancer treatment. Cancer. 1981 Jan 1;47(1):207–214. doi: 10.1002/1097-0142(19810101)47:1<207::aid-cncr2820470134>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- Retsas S., Priestman T. J., Newton K. A., Westbury G. Evaluation of human lymphoblastoid interferon in advanced malignant melanoma. Cancer. 1983 Jan 15;51(2):273–276. doi: 10.1002/1097-0142(19830115)51:2<273::aid-cncr2820510218>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- Silver H. K., Connors J. M., Karim K. A., Kong S., Spinelli J. J., de Jong G., McLean D. M., Salinas F. A. Effect of lymphoblastoid interferon on lymphocyte subsets in cancer patients. J Biol Response Mod. 1983;2(5):428–440. [PubMed] [Google Scholar]
- Suzuki R., Handa K., Itoh K., Kumagai K. Natural killer (NK) cells as a responder to interleukin 2 (IL 2). I. Proliferative response and establishment of cloned cells. J Immunol. 1983 Feb;130(2):981–987. [PubMed] [Google Scholar]


