Abstract
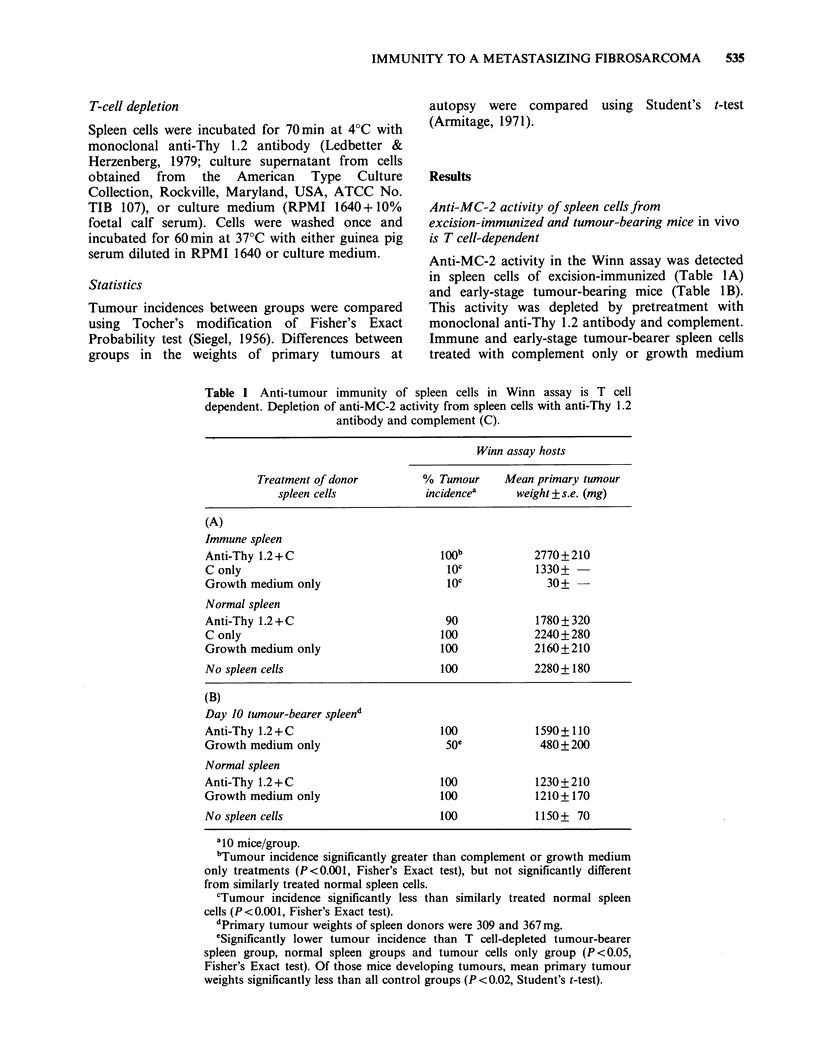
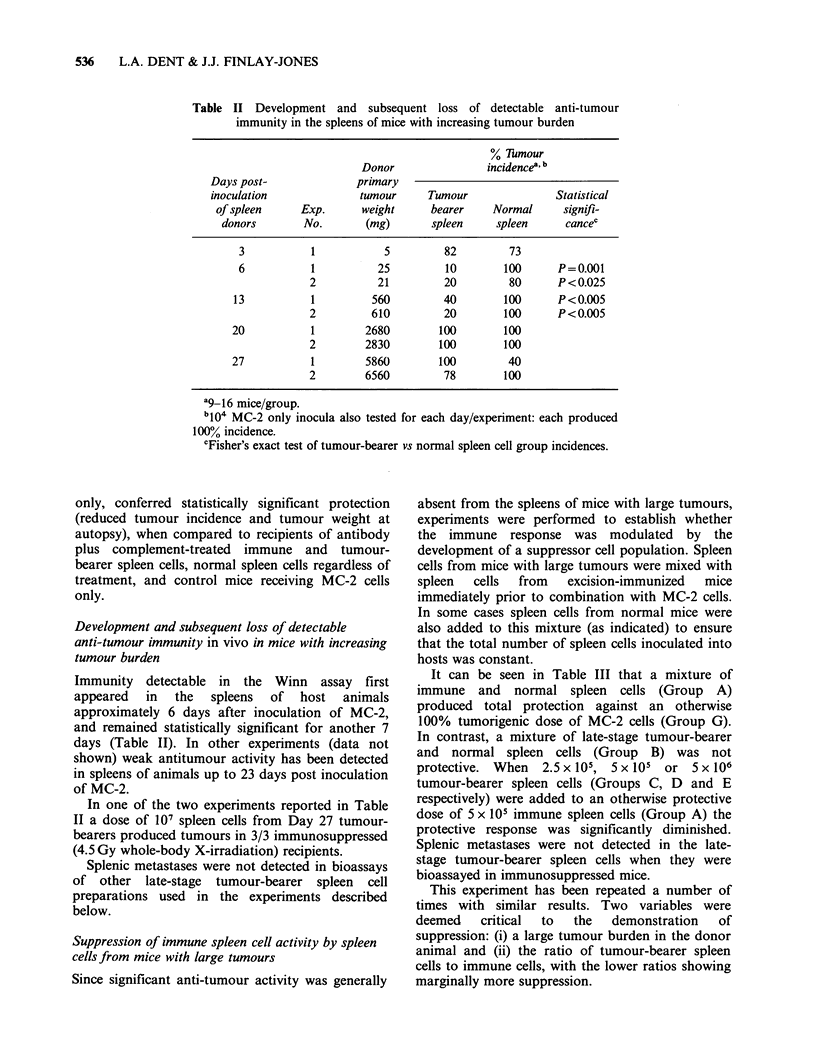
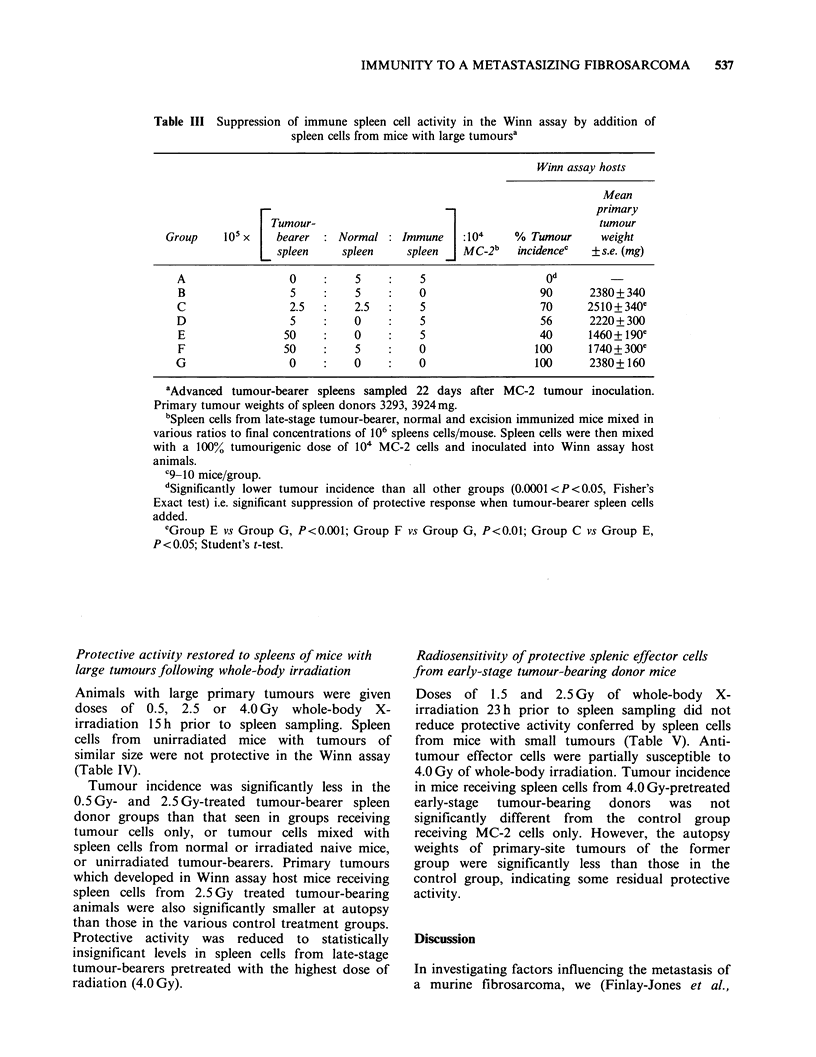
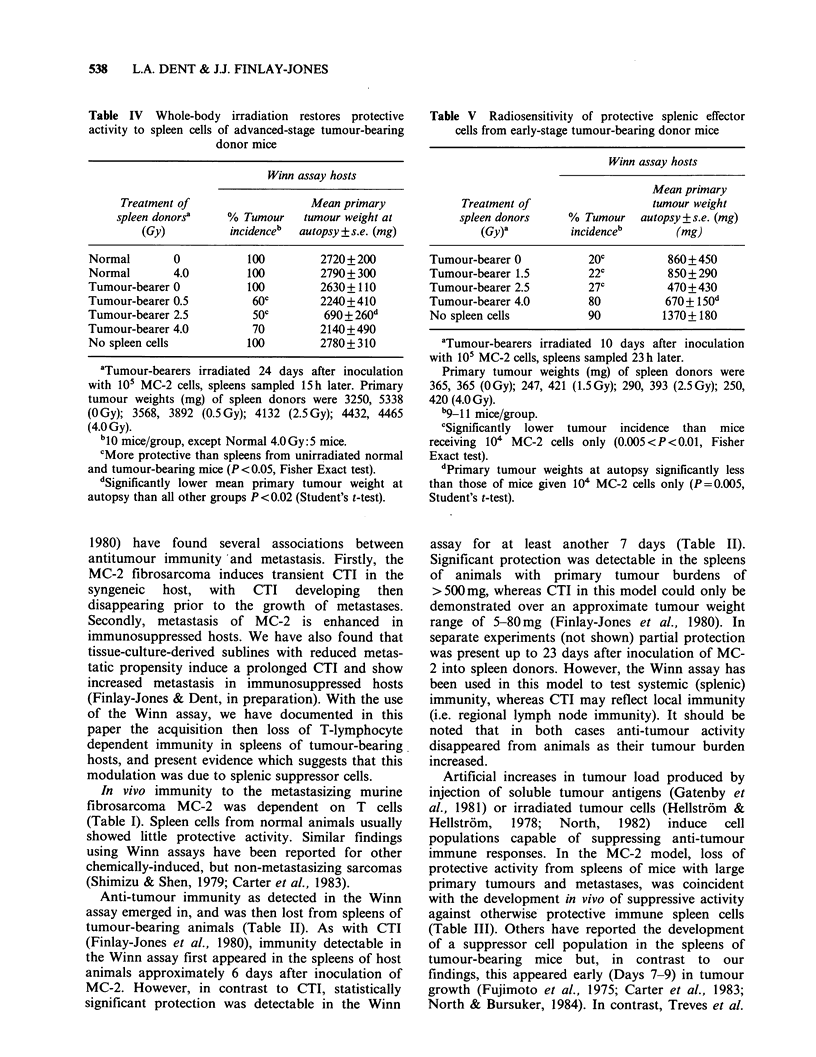
The MC-2 fibrosarcoma, which is a transplantable tumour syngeneic for BALB/c mice, metastasizes to lymph nodes draining subcutaneous inoculation sites, and also to the lungs. T cell-mediated immunity was detected in Winn assays using spleens from excision immunized mice. T cell-mediated anti-tumour immunity was also detected in spleens from mice with small tumours but disappeared as the tumour burden increased. Protective immune spleen cell activity in the Winn assay was inhibited by prior addition of spleen cells from mice with large tumours, causing increased tumour incidence. Splenic metastases occasionally occurred in the MC-2 model, but were not demonstrable by bioassay in any of the experiments detecting splenic suppressor cell activity. In vivo protective activity was restored to advanced-stage tumour-bearer spleens by whole-body ionizing irradiation (0.5 and 2.5 Gy) of donor mice 15 h before sampling. Spleen cells from mice with small tumours remained protective after 1.5, 2.5 and 4.0 Gy of irradiation in vivo. These results are consistent with the properties of radiosensitive suppressor T cells. In contrast to reports in other tumour models, suppressor cells were not detected until late in the course of MC-2 development. This is surprising in view of the aggressively metastatic nature of MC-2. It is postulated that modulation of the anti-tumour immune response by the suppressor cells is associated with metastasis in this tumour model. The late appearance of both suppressor cells and metastatic cells in the spleen may reflect similar processes occurring earlier in regional lymph nodes.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson R. E., Lefkovits I., Troup G. M. Radiation-induced augmentation of the immune response. Contemp Top Immunobiol. 1980;11:245–274. doi: 10.1007/978-1-4684-3701-0_8. [DOI] [PubMed] [Google Scholar]
- Anderson R. E., Warner N. L. Ionizing radiation and the immune response. Adv Immunol. 1976;24:215–335. doi: 10.1016/s0065-2776(08)60331-4. [DOI] [PubMed] [Google Scholar]
- Ando K., Hunter N., Peters L. J. Inhibition of artificial lung metastases in mice by pre-irradiation of abdomen. Br J Cancer. 1980 Feb;41(2):250–258. doi: 10.1038/bjc.1980.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartholomaeus W. N., Bray A. E., Papadimitriou J. M., Keast D. Immune response to a transplantable malignant melanoma in mice. J Natl Cancer Inst. 1974 Oct;53(4):1065–1072. doi: 10.1093/jnci/53.4.1065. [DOI] [PubMed] [Google Scholar]
- Basten A., Miller J. F., Johnson P. T cell-dependent suppression of an anti-hapten antibody response. Transplant Rev. 1975;26:130–169. doi: 10.1111/j.1600-065x.1975.tb00178.x. [DOI] [PubMed] [Google Scholar]
- Carter R. H., Drebin J. A., Schatten S., Perry L. L., Greene M. I. Regulation of the immune response to tumor antigens. IX. In vitro Lyt-1+2- cell proliferative responses to cellbound or subcellular tumor antigen. J Immunol. 1983 Feb;130(2):997–1002. [PubMed] [Google Scholar]
- Currie G. A., Alexander P. Spontaneous shedding of TSTA by viable sarcoma cells: its possible role in facilitating metastatic spread. Br J Cancer. 1974 Jan;29(1):72–75. doi: 10.1038/bjc.1974.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeVita V. T., Jr, Young R. C., Canellos G. P. Combination versus single agent chemotherapy: a review of the basis for selection of drug treatment of cancer. Cancer. 1975 Jan;35(1):98–110. doi: 10.1002/1097-0142(197501)35:1<98::aid-cncr2820350115>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- Doria G., Agarossi G., Adorini L. Selective effects of ionizing radiations on immunoregulatory cells. Immunol Rev. 1982;65:23–54. doi: 10.1111/j.1600-065x.1982.tb00426.x. [DOI] [PubMed] [Google Scholar]
- Eccles S. A., Alexander P. Macrophage content of tumours in relation to metastatic spread and host immune reaction. Nature. 1974 Aug 23;250(5468):667–669. doi: 10.1038/250667a0. [DOI] [PubMed] [Google Scholar]
- Enker W. E., Jacobitz J. L. In vivo splenic irradiation eradicates suppressor T-cells causing the regression and inhibition of established tumor. Int J Cancer. 1980 Jun 15;25(6):819–825. doi: 10.1002/ijc.2910250619. [DOI] [PubMed] [Google Scholar]
- Finlay-Jones J. J., Bartholomaeus W. N., Fimmel P. J., Keast D., Stanley N. F. Biologic and immunologic studies on a murine model of regional lymph node metastasis. J Natl Cancer Inst. 1980 Jun;64(6):1363–1372. [PubMed] [Google Scholar]
- Flannery G. R., Chalmers P. J., Rolland J. M., Nairn R. C. Immune response to a syngeneic rat tumour: evolution of serum cytotoxicity and blockade. Br J Cancer. 1973 Oct;28(4):293–298. doi: 10.1038/bjc.1973.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujimoto S., Greene M., Sehon A. H. Immunosuppressor T cells in tumor bearing host. Immunol Commun. 1975;4(3):201–217. doi: 10.3109/08820137409055774. [DOI] [PubMed] [Google Scholar]
- Gatenby P. A., Basten A., Creswick P. "Sneaking through": a T-cell-dependent phenomenon. Br J Cancer. 1981 Nov;44(5):753–756. doi: 10.1038/bjc.1981.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glaser M. Regulation of specific cell-mediated cytotoxic response against SV40-induced tumor associated antigens by depletion of suppressor T cells with cyclophosphamide in mice. J Exp Med. 1979 Mar 1;149(3):774–779. doi: 10.1084/jem.149.3.774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellström K. E., Hellström I. Evidence that tumor antigens enhance tumor growth in vivo by interacting with a radiosensitive (suppressor?) cell population. Proc Natl Acad Sci U S A. 1978 Jan;75(1):436–440. doi: 10.1073/pnas.75.1.436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellström K. E., Hellström I., Kant J. A., Tamerius J. D. Regression and inhibition of sarcoma growth by interference with a radiosensitive T-cell population. J Exp Med. 1978 Sep 1;148(3):799–804. doi: 10.1084/jem.148.3.799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kearney R., Nelson D. S. Concomitant immunity to syngeneic methylcholanthrene-induced tumours in mice. Occurrence and specificity of concomitant immunity. Aust J Exp Biol Med Sci. 1973 Dec;51(6):723–735. doi: 10.1038/icb.1973.70. [DOI] [PubMed] [Google Scholar]
- Ledbetter J. A., Herzenberg L. A. Xenogeneic monoclonal antibodies to mouse lymphoid differentiation antigens. Immunol Rev. 1979;47:63–90. doi: 10.1111/j.1600-065x.1979.tb00289.x. [DOI] [PubMed] [Google Scholar]
- Mills C. D., North R. J. Expression of passively transferred immunity against an established tumor depends on generation of cytolytic T cells in recipient. Inhibition by suppressor T cells. J Exp Med. 1983 May 1;157(5):1448–1460. doi: 10.1084/jem.157.5.1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minami A., Mizushima Y., Takeichi N., Hosokawa M., Kobayashi H. Dissociation of anti-tumor immune responses in rats immunized with solubilized tumor-associated antigens from a methylcholanthrene-induced fibrosarcoma. Int J Cancer. 1979 Mar 15;23(3):358–365. doi: 10.1002/ijc.2910230314. [DOI] [PubMed] [Google Scholar]
- North R. J., Bursuker I. Generation and decay of the immune response to a progressive fibrosarcoma. I. Ly-1+2- suppressor T cells down-regulate the generation of Ly-1-2+ effector T cells. J Exp Med. 1984 May 1;159(5):1295–1311. doi: 10.1084/jem.159.5.1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- North R. J. Cyclophosphamide-facilitated adoptive immunotherapy of an established tumor depends on elimination of tumor-induced suppressor T cells. J Exp Med. 1982 Apr 1;155(4):1063–1074. doi: 10.1084/jem.155.4.1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pike M. C., Snyderman R. Depression of macrophage function by a factor produced by neoplasms: a merchanism for abrogation of immune surveillance. J Immunol. 1976 Oct;117(4):1243–1249. [PubMed] [Google Scholar]
- Roos E., Dingemans K. P. Mechanisms of metastasis. Biochim Biophys Acta. 1979 Feb 4;560(1):135–166. doi: 10.1016/0304-419x(79)90005-2. [DOI] [PubMed] [Google Scholar]
- Röllinghoff M., Starzinski-Powitz A., Pfizenmaier K., Wagner H. Cyclophosphamide-sensitive T lymphocytes suppress the in vivo generation of antigen-specific cytotoxic T lymphocytes. J Exp Med. 1977 Feb 1;145(2):455–459. doi: 10.1084/jem.145.2.455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheridan J. W., Finlay-Jones J. J. Studies on a fractionated murine fibrosarcoma: a reproducible method for the cautious and a caution for the unwary. J Cell Physiol. 1977 Mar;90(3):535–552. doi: 10.1002/jcp.1040900316. [DOI] [PubMed] [Google Scholar]
- Shimizu K., Shen F. W. Role of different T cells sets in the rejection of syngeneic chemically induced tumors. J Immunol. 1979 Mar;122(3):1162–1165. [PubMed] [Google Scholar]
- Talmadge J. E., Key M., Fidler I. J. Macrophage content of metastatic and nonmetastatic rodent neoplasms. J Immunol. 1981 Jun;126(6):2245–2248. [PubMed] [Google Scholar]
- WINN H. J. Immune mechanisms in homotransplantation. II. Quantitative assay of the immunologic activity of lymphoid cells stimulated by tumor homografts. J Immunol. 1961 Feb;86:228–239. [PubMed] [Google Scholar]


