Abstract
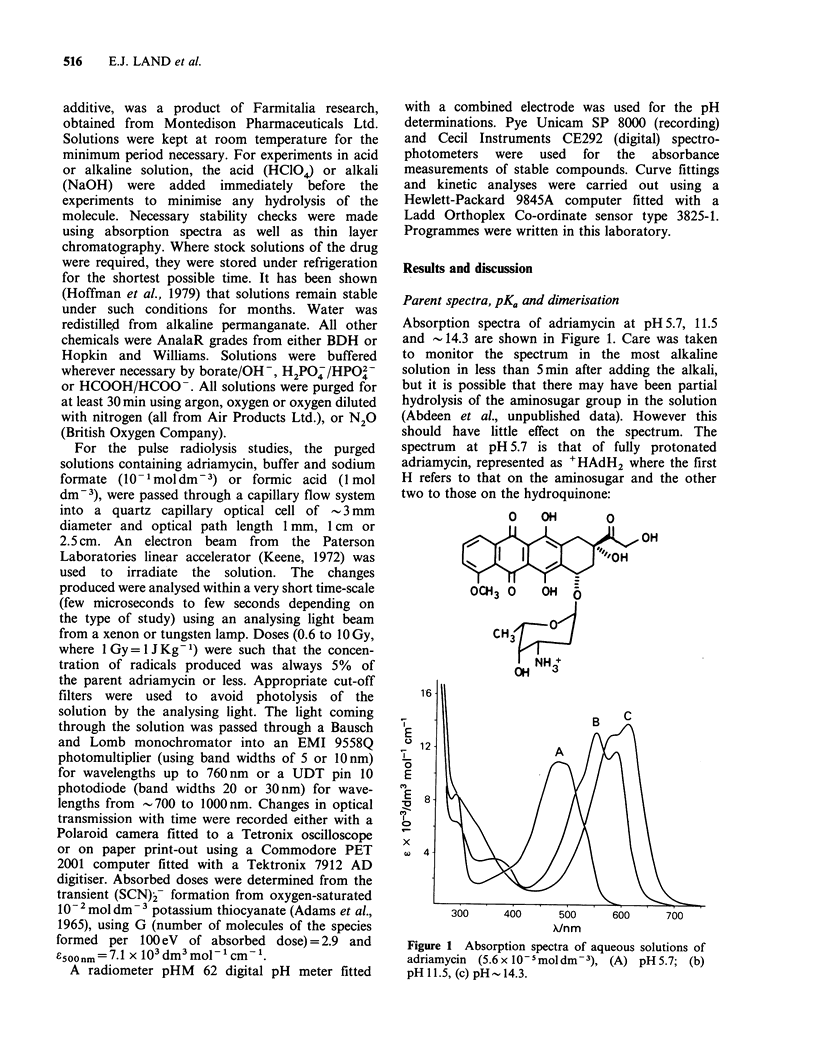
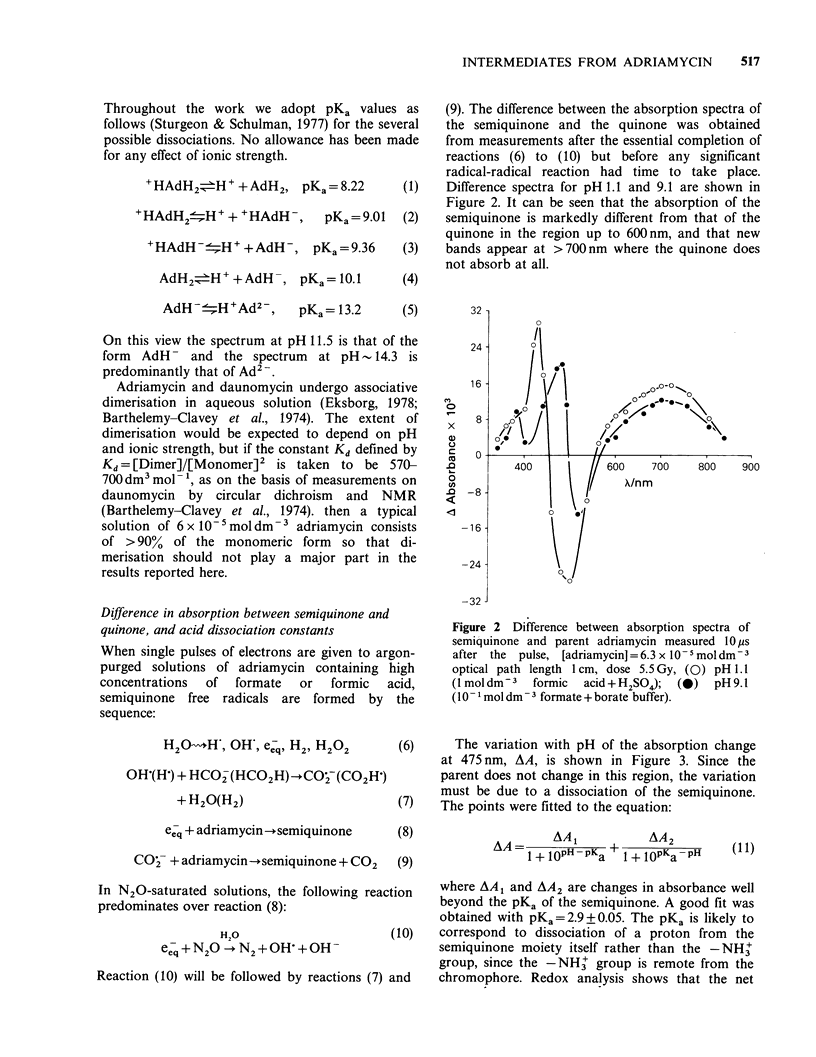
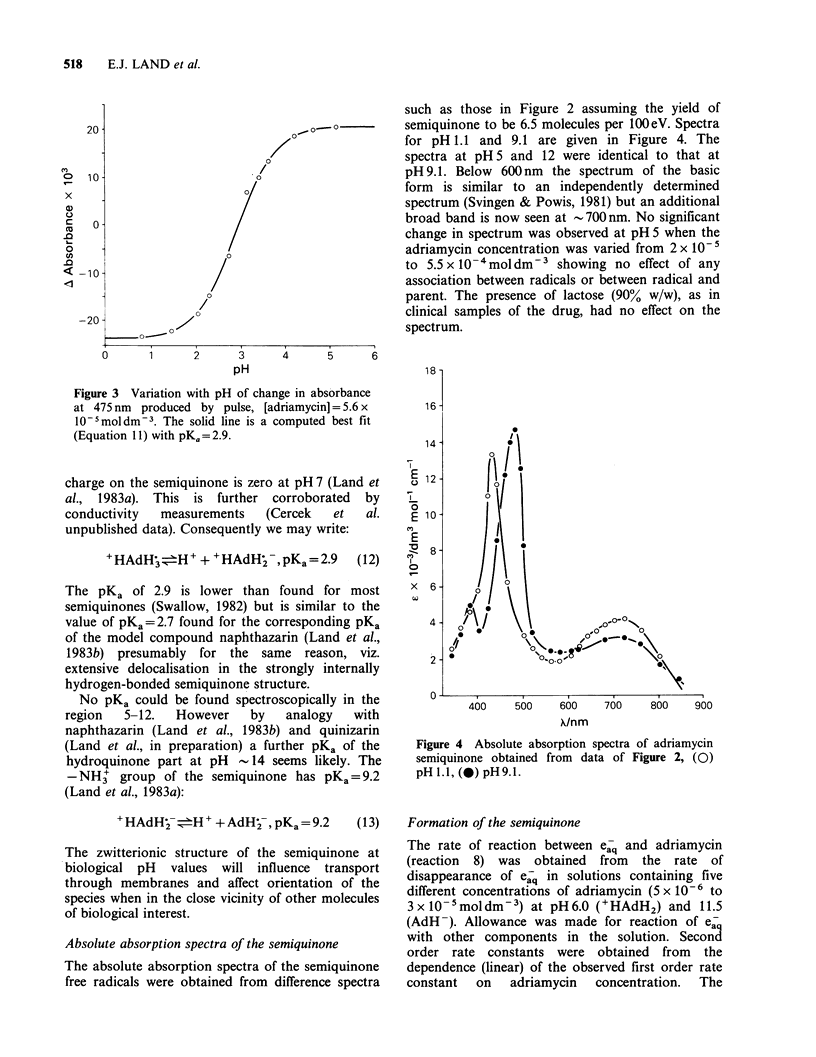
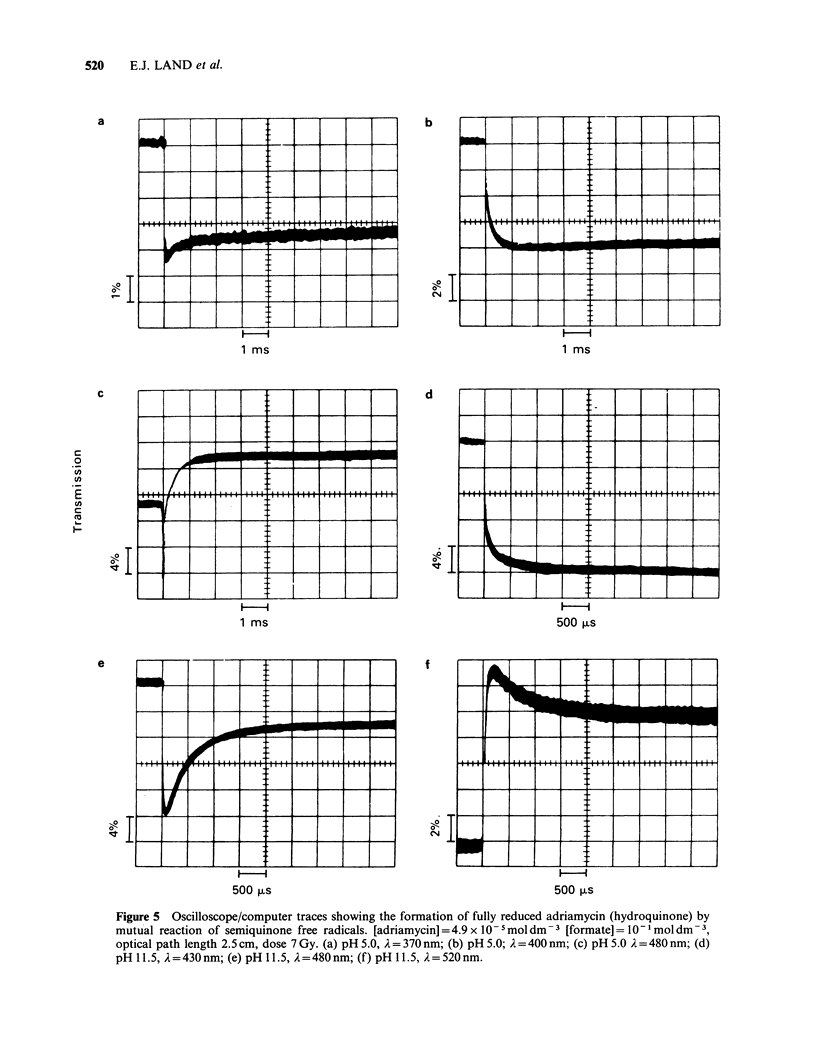
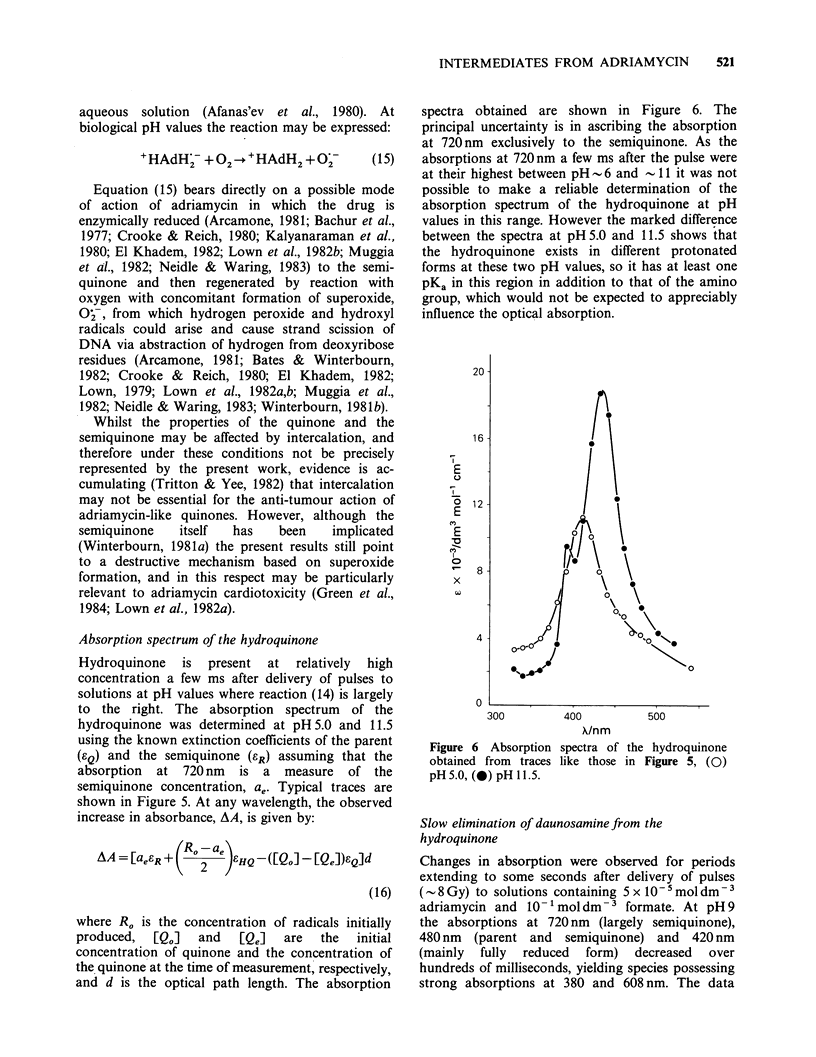
Over a wide range of pH, the semiquinone free radicals formed by reduction of adriamycin exist as a form which is strongly stabilised by internal hydrogen bonding and resonance. They protonate with pKa = 2.9. Below this pH they exhibit absorption maxima at 430 nm (Emax = 13,200 dm3 mol-1 cm-1) and approximately 720 nm (Emax = 4,200 dm3 mol-1 cm-1). Above pH 2.9 they have maxima at 480 nm (Emax = 14,600 dm3 mol-1 cm-1) and approximately 700 nm (Emax = 3,400 dm3 mol-1 cm-1). In acid and alkaline solution the radicals rapidly disappear by disproportionation, but within the approximate pH range 6 to 11 they appear to be relatively stable for at least 10-20 ms, existing in transient equilibrium with parent adriamycin and the full reduced form. Some rate constants for the formation and reactions of the semiquinone are given, including the reaction with oxygen to give O2.-. Fully reduced adriamycin has absorption maxima at 410 nm (Emax = 11,000 dm3 mol-1 cm-1) at pH 5 and 430 nm (Emax = 19,000 dm3 mol-1 cm-1) at pH 11. It undergoes decomposition within a few hundred ms. The intermediates from daunomycin would be expected to have properties similar to those from adriamycin.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bachur N. R., Gordon S. L., Gee M. V. A general mechanism for microsomal activation of quinone anticancer agents to free radicals. Cancer Res. 1978 Jun;38(6):1745–1750. [PubMed] [Google Scholar]
- Bachur N. R., Gordon S. L., Gee M. V. Anthracycline antibiotic augmentation of microsomal electron transport and free radical formation. Mol Pharmacol. 1977 Sep;13(5):901–910. [PubMed] [Google Scholar]
- Bates D. A., Winterbourn C. C. Deoxyribose breakdown by the adriamycin semiquinone and H2O2: evidence for hydroxyl radical participation. FEBS Lett. 1982 Aug 16;145(1):137–142. doi: 10.1016/0014-5793(82)81222-2. [DOI] [PubMed] [Google Scholar]
- Eksborg S. Extraction of daunorubicin and doxorubicin and their hydroxyl metabolites: self-association in aqueous solution. J Pharm Sci. 1978 Jun;67(6):782–785. doi: 10.1002/jps.2600670613. [DOI] [PubMed] [Google Scholar]
- Green M. D., Speyer J. L., Muggia F. M. Cardiotoxicity of anthracyclines. Eur J Cancer Clin Oncol. 1984 Feb;20(2):293–296. doi: 10.1016/0277-5379(84)90200-1. [DOI] [PubMed] [Google Scholar]
- Hoffman D. M., Grossano D. D., Damin L., Woodcock T. M. Stability of refrigerated and frozen solutions of doxorubicin hydrochloride. Am J Hosp Pharm. 1979 Nov;36(11):1536–1538. [PubMed] [Google Scholar]
- Johnston J. B., Zwelling L. A., Kerrigan D., Lloyd L. S., Glazer R. I. Comparison of DNA scission and cytotoxicity produced by Adriamycin and 5-iminodaunorubicin in human colon carcinoma cells. Biochem Pharmacol. 1983 Jul 15;32(14):2255–2258. doi: 10.1016/0006-2952(83)90235-6. [DOI] [PubMed] [Google Scholar]
- Kalyanaraman B., Perez-Reyes E., Mason R. P. Spin-trapping and direct electron spin resonance investigations of the redox metabolism of quinone anticancer drugs. Biochim Biophys Acta. 1980 Jun 5;630(1):119–130. doi: 10.1016/0304-4165(80)90142-7. [DOI] [PubMed] [Google Scholar]
- Land E. J., Mukherjee T., Swallow A. J., Bruce J. M. One-electron reduction of adriamycin: properties of the semiquinone. Arch Biochem Biophys. 1983 Aug;225(1):116–121. doi: 10.1016/0003-9861(83)90013-9. [DOI] [PubMed] [Google Scholar]
- Lown J. W., Chen H. H., Plambeck J. A., Acton E. M. Further studies on the generation of reactive oxygen species from activated anthracyclines and the relationship to cytotoxic action and cardiotoxic effects. Biochem Pharmacol. 1982 Feb 15;31(4):575–581. doi: 10.1016/0006-2952(82)90162-9. [DOI] [PubMed] [Google Scholar]
- Lown J. W., Joshua A. V., Lee J. S. Molecular mechanisms of binding and single-strand scission of deoxyribonucleic acid by the antitumor antibiotics saframycins A and C. Biochemistry. 1982 Feb 2;21(3):419–428. doi: 10.1021/bi00532a001. [DOI] [PubMed] [Google Scholar]
- Rosen G., Wollner N., Tan C., Wu S. J., Hajdu S. I., Cham W., D'Angio G. J., Murphy M. L. Proceedings: Disease-free survival in children with Ewing's sarcoma treated with radiation therapy and adjuvant four-drug sequential chemotherapy. Cancer. 1974 Feb;33(2):384–393. doi: 10.1002/1097-0142(197402)33:2<384::aid-cncr2820330213>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- Sato S., Iwaizumi M., Handa K., Tamura Y. Electron spin resonance study on the mode of generation of free radicals of daunomycin, adriamycin, and carboquone in NAD(P)H-microsome system. Gan. 1977 Oct;68(5):603–608. [PubMed] [Google Scholar]
- Sinha B. K., Gregory J. L. Role of one-electron and two-electron reduction products of adriamycin and daunomycin in deoxyribonucleic acid binding. Biochem Pharmacol. 1981 Sep 15;30(18):2626–2629. doi: 10.1016/0006-2952(81)90594-3. [DOI] [PubMed] [Google Scholar]
- Sturgeon R. J., Schulman S. G. Electronic absorption spectra and protolytic equilibria of doxorubicin: direct spectrophotometric determination of microconstants. J Pharm Sci. 1977 Jul;66(7):958–961. doi: 10.1002/jps.2600660714. [DOI] [PubMed] [Google Scholar]
- Svingen B. A., Powis G. Pulse radiolysis studies of antitumor quinones: radical lifetimes, reactivity with oxygen, and one-electron reduction potentials. Arch Biochem Biophys. 1981 Jun;209(1):119–126. doi: 10.1016/0003-9861(81)90263-0. [DOI] [PubMed] [Google Scholar]
- Triton T. R., Yee G. The anticancer agent adriamycin can be actively cytotoxic without entering cells. Science. 1982 Jul 16;217(4556):248–250. doi: 10.1126/science.7089561. [DOI] [PubMed] [Google Scholar]
- Winterbourn C. C. Cytochrome c reduction by semiquinone radicals can be indirectly inhibited by superoxide dismutase. Arch Biochem Biophys. 1981 Jun;209(1):159–167. doi: 10.1016/0003-9861(81)90268-x. [DOI] [PubMed] [Google Scholar]



