Abstract
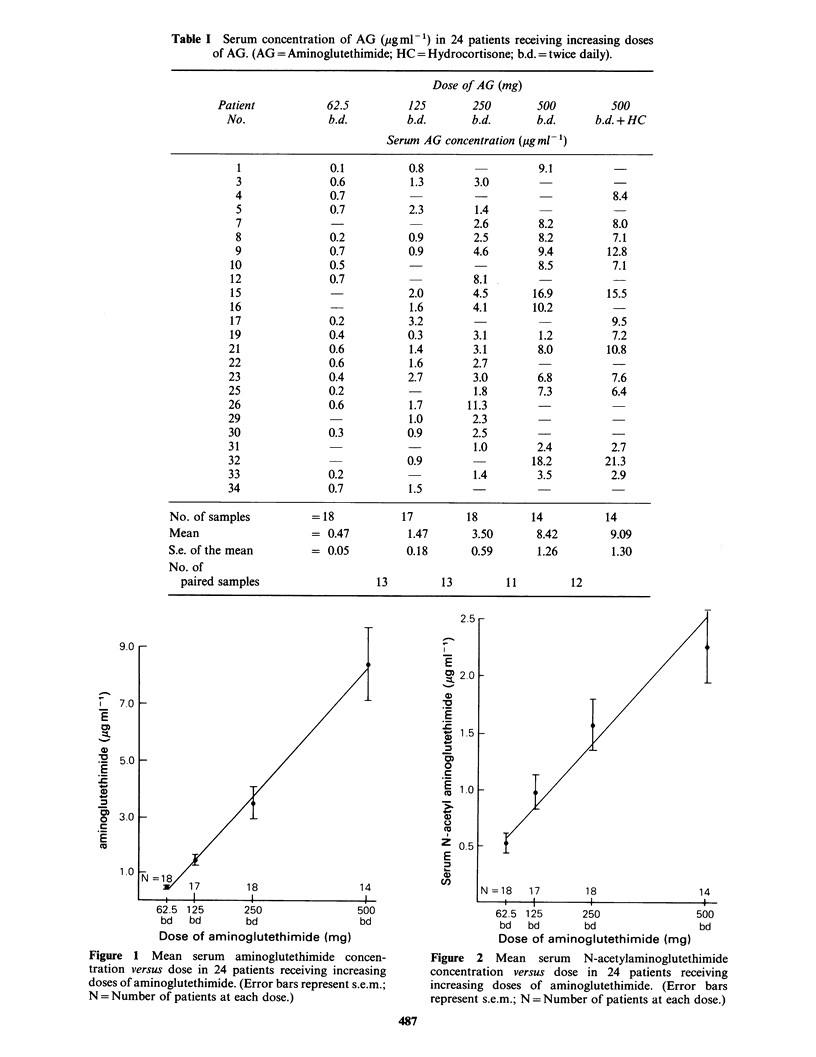
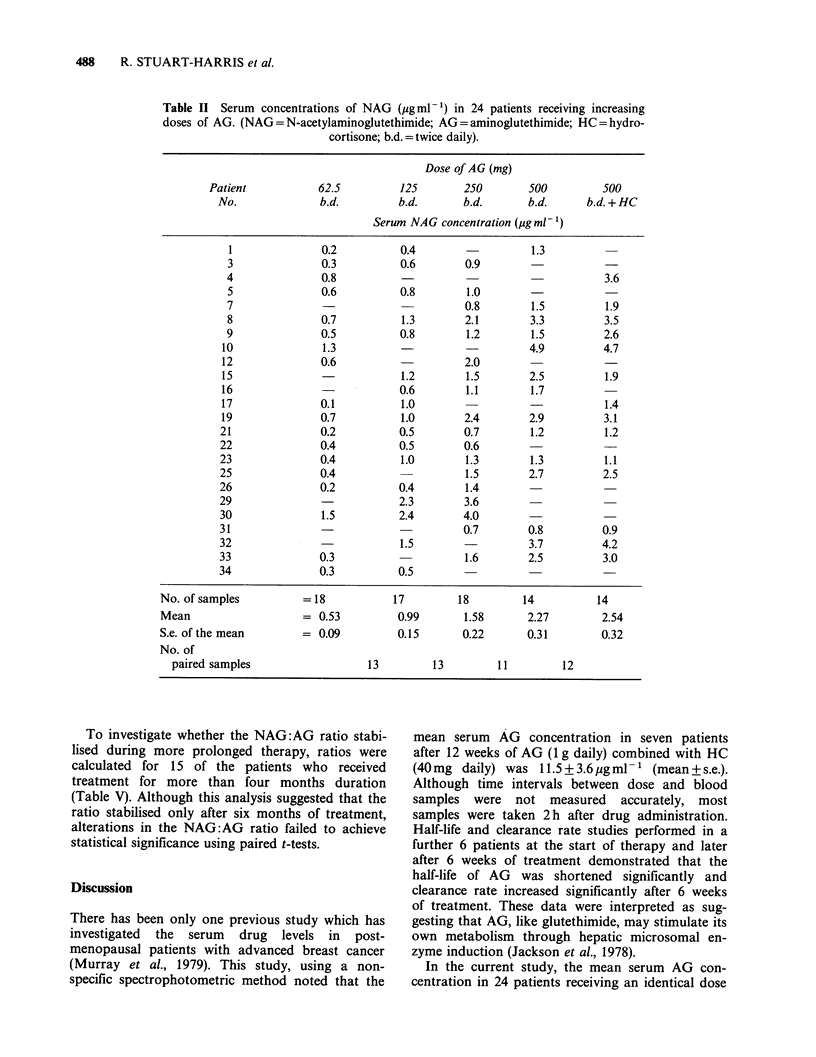
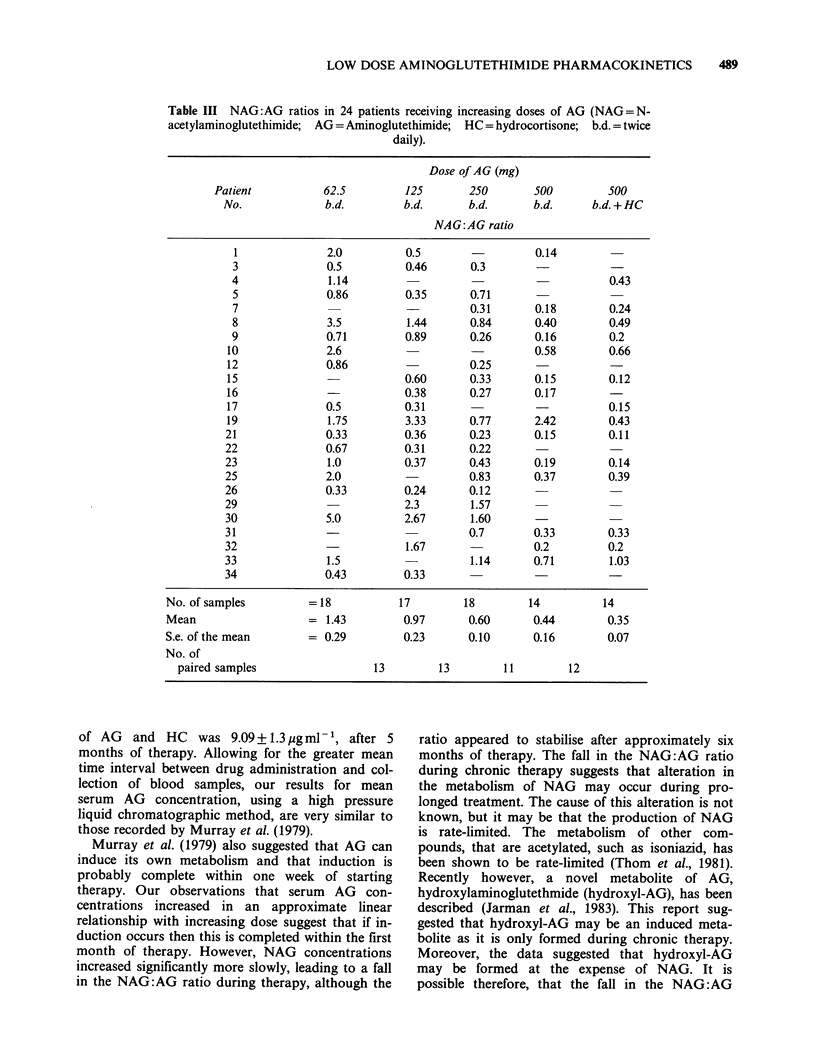
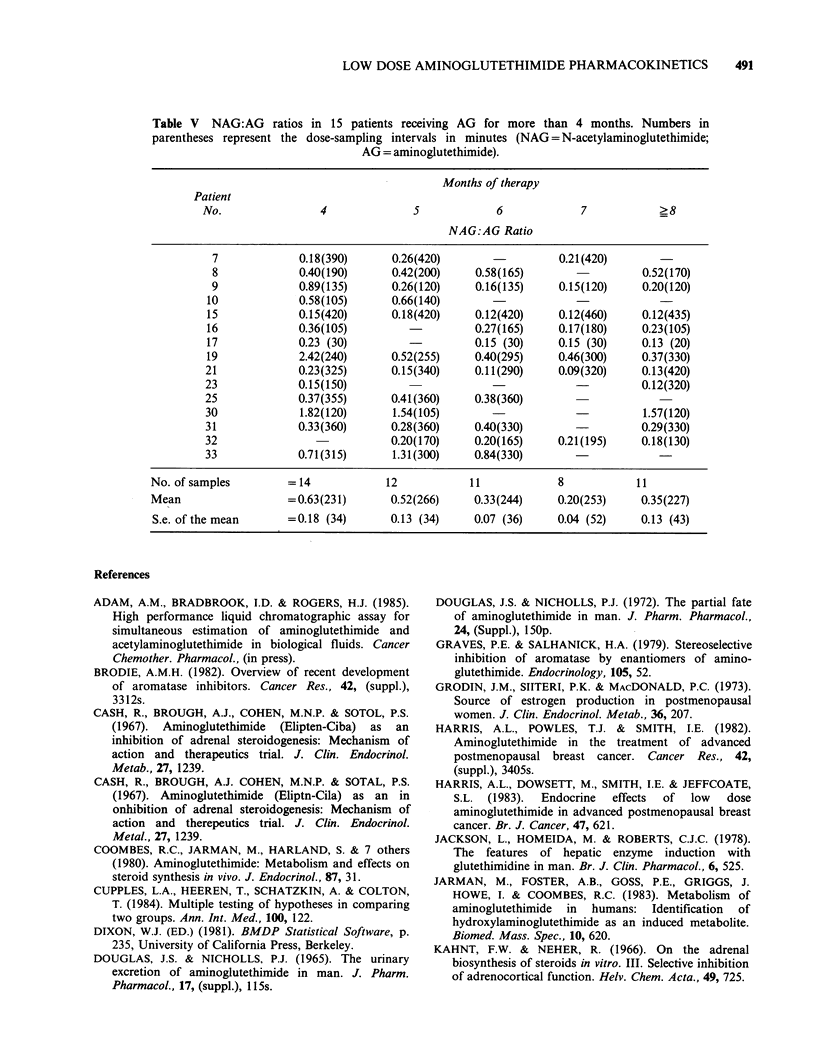
Serum aminoglutethimide (AG) and N-acetylaminoglutethimide (NAG) concentrations were measured by high pressure liquid chromatography (HPLC) in 24 postmenopausal women with advanced breast cancer receiving increasing doses of oral AG. Patients received 62.5 mg b.d., 125 mg b.d., 250 mg b.d., and 500 mg b.d. of AG alone, and 500 mg b.d. of AG combined with hydrocortisone (HC) 20 mg b.d. Dose was increased at monthly intervals. Each dose increment was accompanied by a significant rise in serum AG and NAG levels (P less than 0.05). The addition of HC to the dose of 500 mg b.d. of AG did not alter serum AG or NAG concentrations significantly. Although serum AG and NAG levels appeared to increase linearly with dose, serum NAG increased significantly more slowly, leading to a fall in the NAG:AG ratio during therapy. The NAG:AG ratio appeared to stabilise only after about 6 months of treatment.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brodie A. M. Overview of recent development of aromatase inhibitors. Cancer Res. 1982 Aug;42(8 Suppl):3312s–3314s. [PubMed] [Google Scholar]
- Cash R., Brough A. J., Cohen M. N., Satoh P. S. Aminoglutethimide (Elipten-Ciba) as an inhibitor of adrenal steroidogenesis: mechanism of action and therapeutic trial. J Clin Endocrinol Metab. 1967 Sep;27(9):1239–1248. doi: 10.1210/jcem-27-9-1239. [DOI] [PubMed] [Google Scholar]
- Cupples L. A., Heeren T., Schatzkin A., Colton T. Multiple testing of hypotheses in comparing two groups. Ann Intern Med. 1984 Jan;100(1):122–129. doi: 10.7326/0003-4819-100-1-122. [DOI] [PubMed] [Google Scholar]
- Douglas J. S., Nicholls P. J. The partial fate of aminoglutethimide in man. J Pharm Pharmacol. 1972 Dec;24(Suppl):150P–150P. [PubMed] [Google Scholar]
- Graves P. E., Salhanick H. A. Stereoselective inhibition of aromatase by enantiomers of aminoglutethimide. Endocrinology. 1979 Jul;105(1):52–57. doi: 10.1210/endo-105-1-52. [DOI] [PubMed] [Google Scholar]
- Grodin J. M., Siiteri P. K., MacDonald P. C. Source of estrogen production in postmenopausal women. J Clin Endocrinol Metab. 1973 Feb;36(2):207–214. doi: 10.1210/jcem-36-2-207. [DOI] [PubMed] [Google Scholar]
- Harris A. L., Dowsett M., Smith I. E., Jeffcoate S. L. Endocrine effects of low dose aminoglutethimide alone in advanced postmenopausal breast cancer. Br J Cancer. 1983 May;47(5):621–627. doi: 10.1038/bjc.1983.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris A. L., Powles T. J., Smith I. E. Aminoglutethimide in the treatment of advanced postmenopausal breast cancer. Cancer Res. 1982 Aug;42(8 Suppl):3405s–3408s. [PubMed] [Google Scholar]
- Jackson L., Homeida M., Roberts C. J. The features of hepatic enzyme induction with glutethimide in man. Br J Clin Pharmacol. 1978 Dec;6(6):525–528. doi: 10.1111/j.1365-2125.1978.tb00877.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarman M., Foster A. B., Goss P. E., Griggs L. J., Howe I., Coombes R. C. Metabolism of aminoglutethimide in humans: identification of hydroxylaminoglutethimide as an induced metabolite. Biomed Mass Spectrom. 1983 Nov;10(11):620–625. doi: 10.1002/bms.1200101108. [DOI] [PubMed] [Google Scholar]
- MacDonald P. C., Rombaut R. P., Siiteri P. K. Plasma precursors of estrogen. I. Extent of conversion of plasma delta-4-androstenedione to estrone in normal males and nonpregnant normal, castrate and adrenalectomized females. J Clin Endocrinol Metab. 1967 Aug;27(8):1103–1111. doi: 10.1210/jcem-27-8-1103. [DOI] [PubMed] [Google Scholar]
- Murray F. T., Santner S., Samojlik E., Santen R. J. Serum aminoglutethimide levels: studies of serum half-life, clearance, and patient compliance. J Clin Pharmacol. 1979 Nov-Dec;19(11-12):704–711. doi: 10.1002/j.1552-4604.1979.tb01640.x. [DOI] [PubMed] [Google Scholar]
- Santen R. J., Misbin R. I. Aminoglutethimide: review of pharmacology and clinical use. Pharmacotherapy. 1981 Sep-Oct;1(2):95–120. doi: 10.1002/j.1875-9114.1981.tb03557.x. [DOI] [PubMed] [Google Scholar]
- Santen R. J., Santner S., Davis B., Veldhuis J., Samojlik E., Ruby E. Aminoglutethimide inhibits extraglandular estrogen production in postmenopausal women with breast carcinoma. J Clin Endocrinol Metab. 1978 Dec;47(6):1257–1265. doi: 10.1210/jcem-47-6-1257. [DOI] [PubMed] [Google Scholar]
- Smith I. E., Fitzharris B. M., McKinna J. A., Fahmy D. R., Nash A. G., Neville A. M., Gazet J. C., Ford H. T., Powles T. J. Aminoglutethimide in treatment of metastatic breast carcinoma. Lancet. 1978 Sep 23;2(8091):646–649. doi: 10.1016/s0140-6736(78)92759-9. [DOI] [PubMed] [Google Scholar]
- Stuart-Harris R., Dowsett M., Bozek T., McKinna J. A., Gazet J. C., Jeffcoate S. L., Kurkure A., Carr L., Smith I. E. Low-dose aminoglutethimide in treatment of advanced breast cancer. Lancet. 1984 Sep 15;2(8403):604–607. doi: 10.1016/s0140-6736(84)90596-8. [DOI] [PubMed] [Google Scholar]
- Thompson T. A., Vermeulen J. D., Wagner W. E., Jr, Le Sher A. R. Aminoglutethimide bioavailability, pharmacokinetics, and binding to blood constituents. J Pharm Sci. 1981 Sep;70(9):1040–1043. doi: 10.1002/jps.2600700919. [DOI] [PubMed] [Google Scholar]
- Vermeulen A., Paridaens R., Heuson J. C. Effects of aminoglutethimide on adrenal steroid secretion. Clin Endocrinol (Oxf) 1983 Dec;19(6):673–682. doi: 10.1111/j.1365-2265.1983.tb00044.x. [DOI] [PubMed] [Google Scholar]


