Abstract
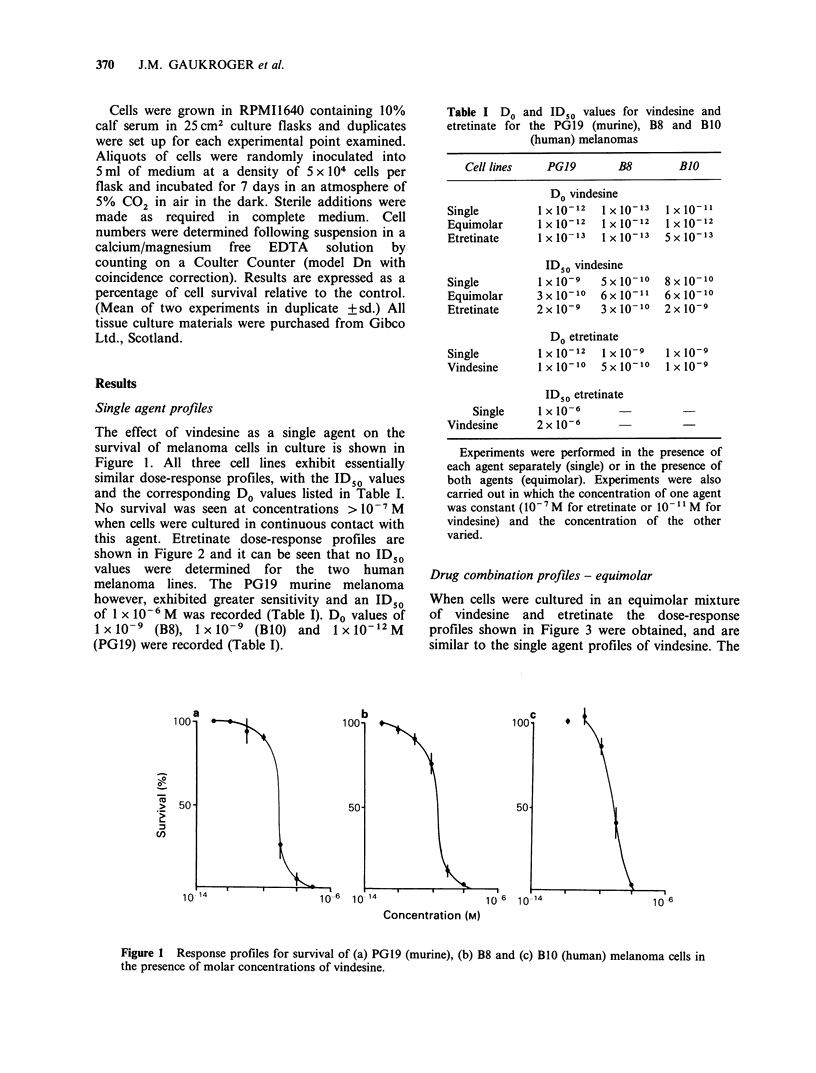
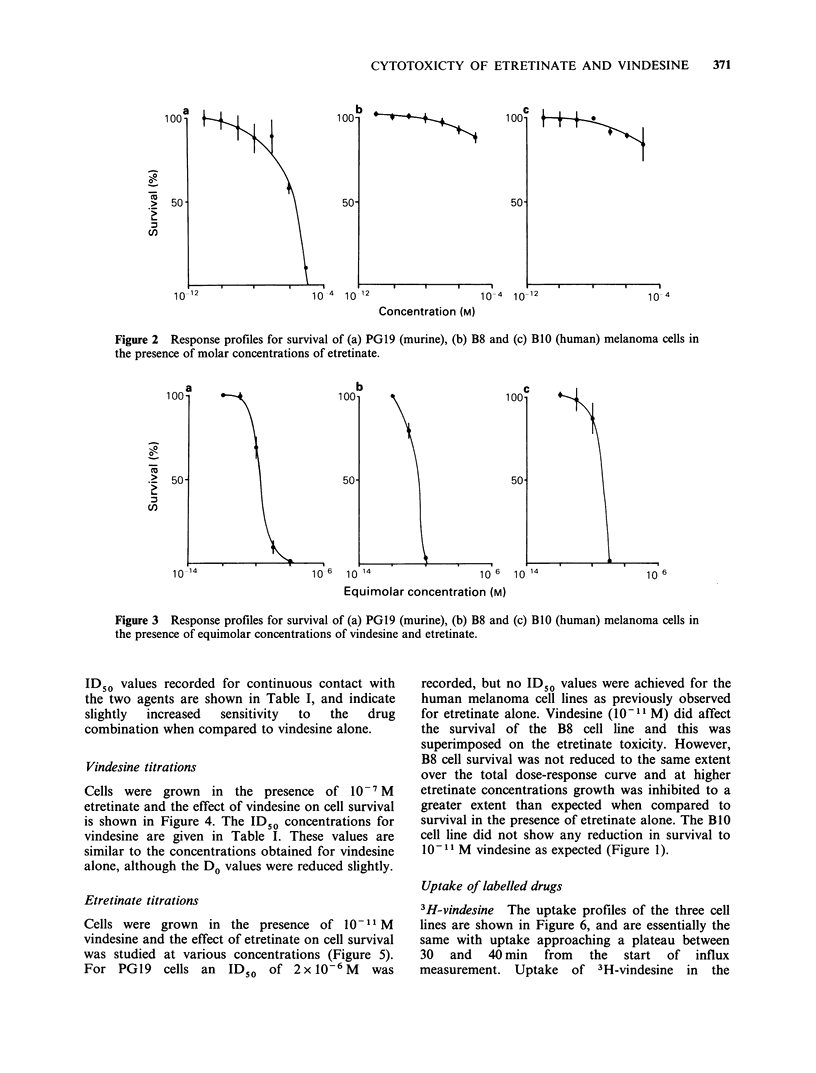
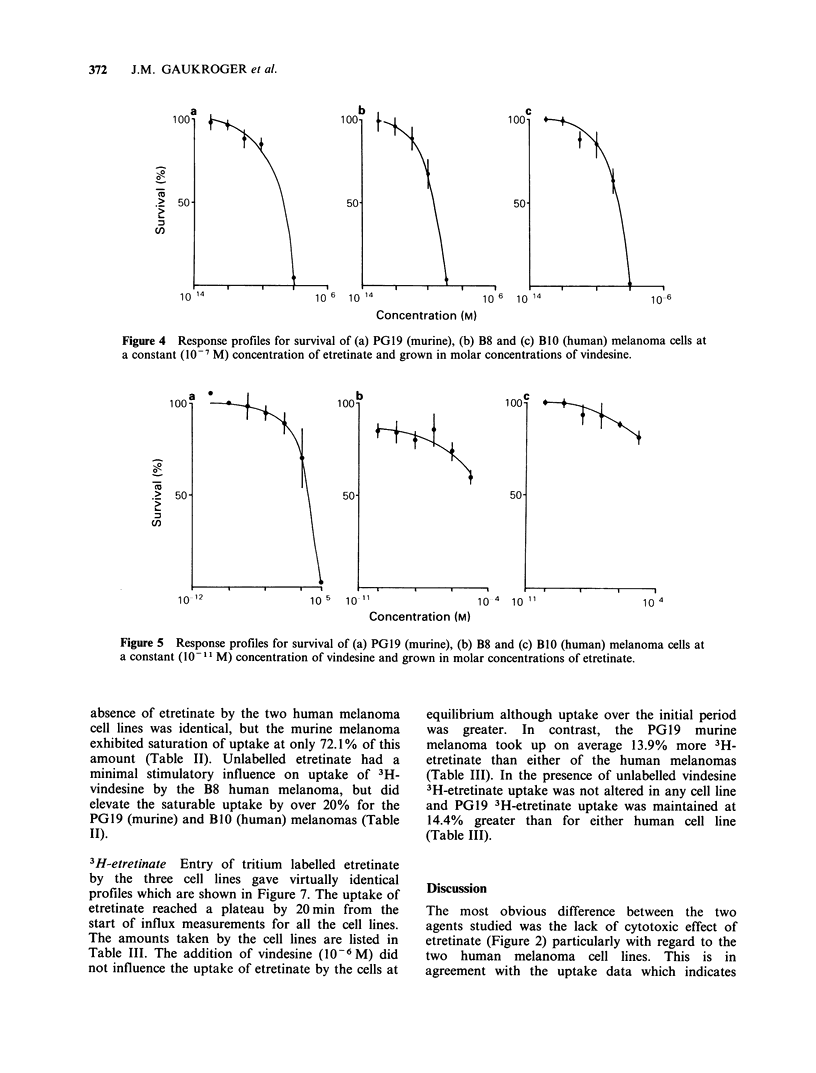
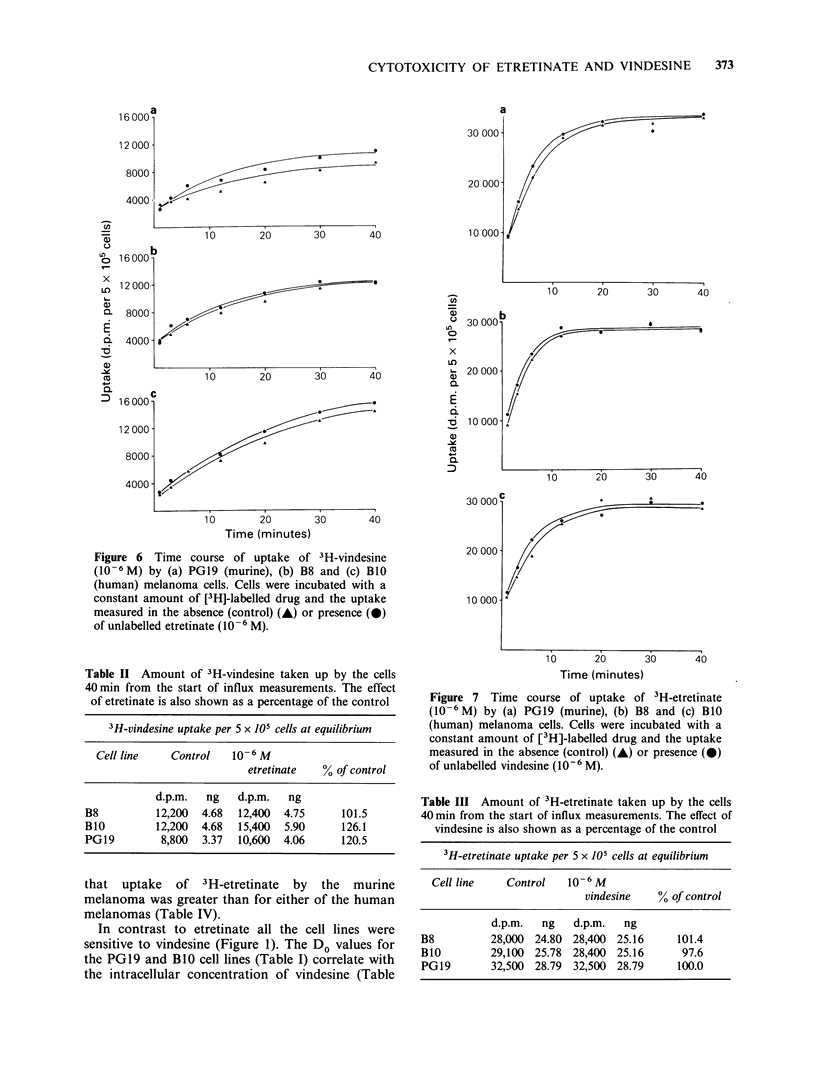
The effects of an aromatic retinoid, etretinate and a vinca alkaloid, vindesine were investigated by culture of malignant melanoma cells in vitro with these two agents; either separately or in combination. Etretinate inhibited growth of a murine melanoma but only minimal effects were recorded with two human melanomas. Vindesine however, was inhibitory for all of the cell lines and this effect was enhanced in the presence of the retinoid. Entry of 3H labelled vindesine or etretinate into drug free cells was followed in the absence or presence of unlabelled drug. It was found that etretinate enhanced cellular uptake of vindesine in two of the cell lines and this may be responsible for the enhanced toxicity of vindesine in the presence of etretinate. The human melanoma which did not exhibit retinoid stimulated vindesine uptake, appeared to be intrinsically sensitive to the vinca alkaloid. No effect on cellular retinoid uptake by vindesine was recorded in any of the melanomas. The results indicate that the intracellular concentrations combined with the intrinsic sensitivities of each cell line to etretinate and vindesine determines the toxic response.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Astrup E. G., Paulsen J. E. Effect of retinoic acid pretreatment on 12-O-tetradecanoylphorbol-13-acetate-induced cell population kinetics and polyamine biosynthesis in hairless mouse epidermis. Carcinogenesis. 1982;3(3):313–320. doi: 10.1093/carcin/3.3.313. [DOI] [PubMed] [Google Scholar]
- Carmichael J., Atkinson R. J., Calman K. C., Mackie R. M., Naysmith A. M., Smyth J. F. A multicentre phase II trial of vindesine in malignant melanoma. Eur J Cancer Clin Oncol. 1982 Dec;18(12):1293–1295. doi: 10.1016/0277-5379(82)90131-6. [DOI] [PubMed] [Google Scholar]
- Cohen M. H., Carbone P. P. Enhancement of the antitumor effects of 1,3-bis(2-chloroethyl)-1-nitrosourea and cyclophosphamide by vitamin A. J Natl Cancer Inst. 1972 Apr;48(4):921–926. [PubMed] [Google Scholar]
- Dyke R. W., Nelson R. L. Phase I anti-cancer agents: vindesine (desacetyl vinblastine amide sulfate). Cancer Treat Rev. 1977 Jun;4(2):135–142. doi: 10.1016/s0305-7372(77)80010-8. [DOI] [PubMed] [Google Scholar]
- Felix E. L., Loyd B., Cohen M. H. Inhibition of the growth and development of a transplantable murine melanoma by vitamin A. Science. 1975 Sep 12;189(4206):886–888. doi: 10.1126/science.1154026. [DOI] [PubMed] [Google Scholar]
- Gainer J. L., Wallis D. A., Jones J. R. The effect of crocetin on skin papillomas and Rous sarcoma. Oncology. 1976;33(5-6):222–224. doi: 10.1159/000225150. [DOI] [PubMed] [Google Scholar]
- Gaukroger J. M., Wilson L. Protection of cells from methotrexate toxicity by 7-hydroxymethotrexate. Br J Cancer. 1984 Sep;50(3):327–333. doi: 10.1038/bjc.1984.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaukroger J., Wilson L., Stewart M., Farid Y., Habeshaw T., Harding N., Mackie R. Paradoxical response of malignant melanoma to methotrexate in vivo and in vitro. Br J Cancer. 1983 May;47(5):671–679. doi: 10.1038/bjc.1983.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill B. T., Whelan R. D., Bellamy A. S. Identification of differential drug responses and mechanism(s) of resistance in vincristine-resistant cell lines developed either by exposure to the drug or to fractionated radiation. Cancer Treat Rev. 1984 Mar;11 (Suppl A):73–79. doi: 10.1016/0305-7372(84)90045-8. [DOI] [PubMed] [Google Scholar]
- Hill B. T., Whelan R. D. Comparative effects of vincristine and vindesine on cell cycle kinetics in vitro. Cancer Treat Rev. 1980 Sep;7 (Suppl 1):5–15. doi: 10.1016/s0305-7372(80)80002-8. [DOI] [PubMed] [Google Scholar]
- Hoal E., Wilson E. L., Dowdle E. B. Variable effects of retinoids on two pigmenting human melanoma cell lines. Cancer Res. 1982 Dec;42(12):5191–5195. [PubMed] [Google Scholar]
- Lotan R. Effects of vitamin A and its analogs (retinoids) on normal and neoplastic cells. Biochim Biophys Acta. 1980 Mar 12;605(1):33–91. doi: 10.1016/0304-419x(80)90021-9. [DOI] [PubMed] [Google Scholar]
- Lotan R., Fischer I., Meromsky L., Moldave K. Effects of retinoic acid on protein synthesis in cultured melanoma cells. J Cell Physiol. 1982 Oct;113(1):47–55. doi: 10.1002/jcp.1041130110. [DOI] [PubMed] [Google Scholar]
- Meyskens F. L., Jr, Salmon S. E. Inhibition of human melanoma colony formation by retinoids. Cancer Res. 1979 Oct;39(10):4055–4057. [PubMed] [Google Scholar]
- Sherman M. I., Matthaei K. I., Schindler J. Studies on the mechanism of induction of embryonal carcinoma cell differentiation by retinoic acid. Ann N Y Acad Sci. 1981 Feb 27;359:192–199. doi: 10.1111/j.1749-6632.1981.tb12747.x. [DOI] [PubMed] [Google Scholar]
- Sporn M. B., Roberts A. B. Role of retinoids in differentiation and carcinogenesis. Cancer Res. 1983 Jul;43(7):3034–3040. [PubMed] [Google Scholar]
- Tucker R. W., Owellen R. J., Harris S. B. Correlation of cytotoxicity and mitotic spindle dissolution by vinblastine in mammalian cells. Cancer Res. 1977 Dec;37(12):4346–4351. [PubMed] [Google Scholar]



