Abstract
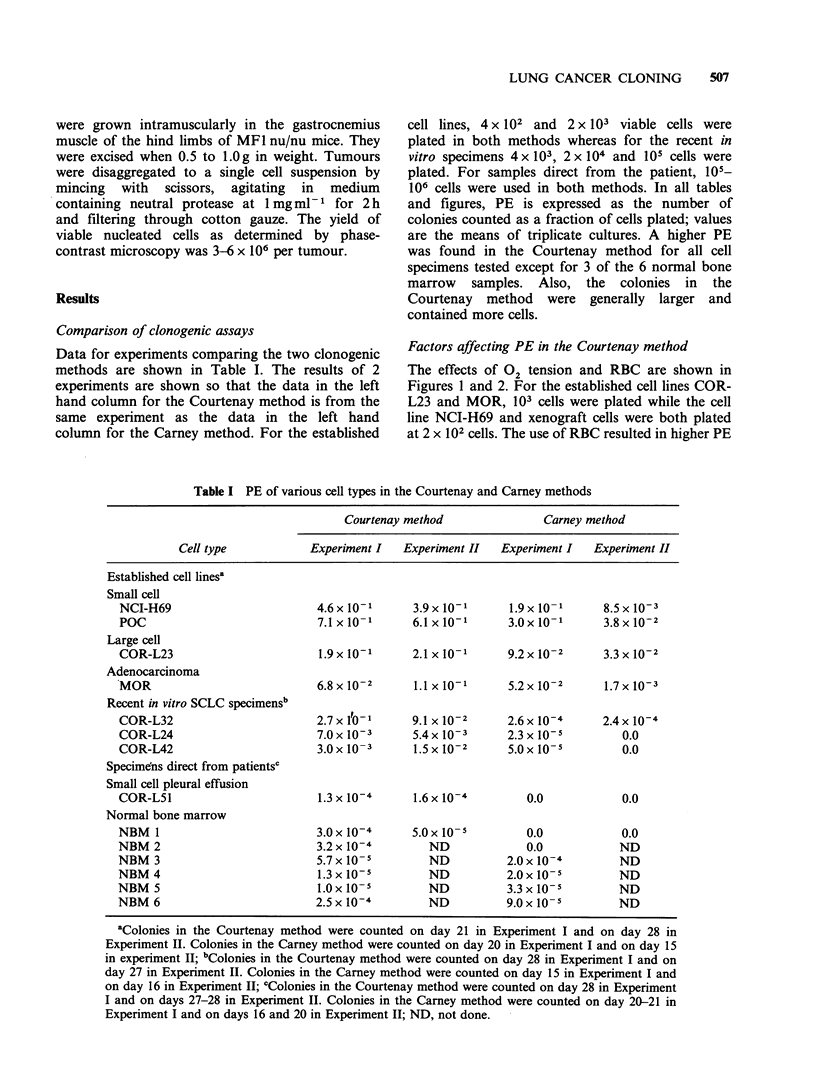
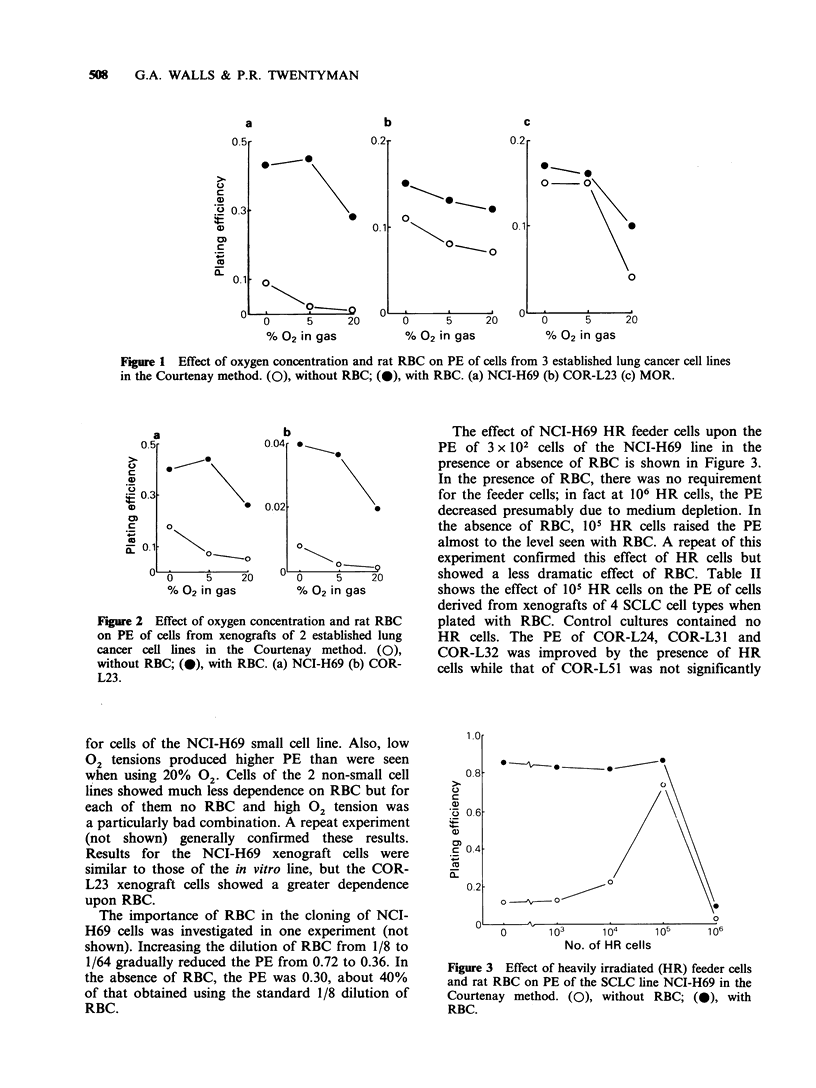
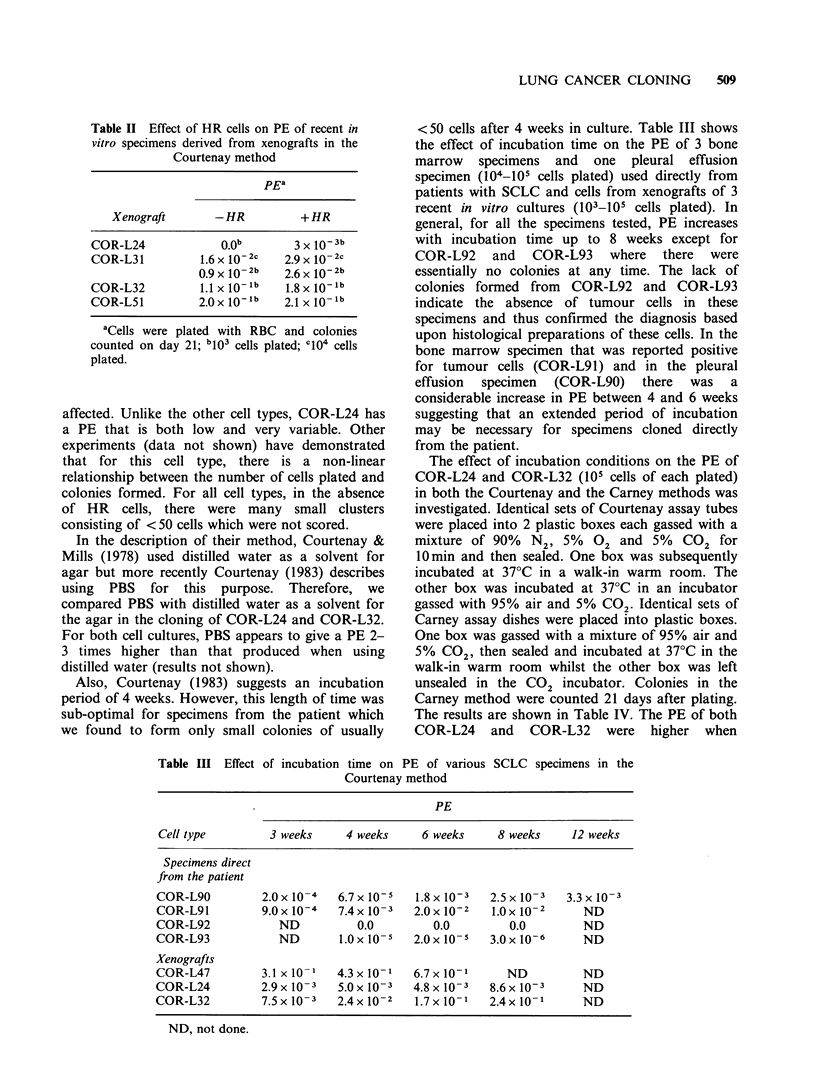
We have carried out a comparison of two different methods for cloning human lung cancer cells. The method of Courtenay & Mills (1978) generally gave higher plating efficiencies (PE) than the method of Carney et al. (1980). The number of colonies increased with incubation time in both methods and the weekly medium replenishment in the Courtenay method was advantageous for longer incubation times of several weeks. In the Courtenay method, the use of August rat red blood cells (RBC) and low oxygen tension were both found to be necessary factors for maximum plating efficiency. The usefulness of heavily irradiated feeder cells in improving PE is less certain; each cell type may have its own requirement.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bradley T. R., Metcalf D. The growth of mouse bone marrow cells in vitro. Aust J Exp Biol Med Sci. 1966 Jun;44(3):287–299. doi: 10.1038/icb.1966.28. [DOI] [PubMed] [Google Scholar]
- Bradley T. R., Telfer P. A., Fry P. The effect of erythrocytes on mouse bone marrow colony development in vitro. Blood. 1971 Sep;38(3):353–359. [PubMed] [Google Scholar]
- Carney D. N., Gazdar A. F., Minna J. D. Positive correlation between histological tumor involvement and generation of tumor cell colonies in agarose in specimens taken directly from patients with small-cell carcinoma of the lung. Cancer Res. 1980 Jun;40(6):1820–1823. [PubMed] [Google Scholar]
- Courtenay V. D. A soft agar colony assay for Lewis lung tumour and B16 melanoma taken directly from the mouse. Br J Cancer. 1976 Jul;34(1):39–45. doi: 10.1038/bjc.1976.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courtenay V. D., Mills J. An in vitro colony assay for human tumours grown in immune-suppressed mice and treated in vivo with cytotoxic agents. Br J Cancer. 1978 Feb;37(2):261–268. doi: 10.1038/bjc.1978.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gazdar A. F., Carney D. N., Russell E. K., Sims H. L., Baylin S. B., Bunn P. A., Jr, Guccion J. G., Minna J. D. Establishment of continuous, clonable cultures of small-cell carcinoma of lung which have amine precursor uptake and decarboxylation cell properties. Cancer Res. 1980 Oct;40(10):3502–3507. [PubMed] [Google Scholar]
- Gupta V., Eberle R. Modulation of tumour cell colony growth in soft agar by oxygen and its mechanism. Br J Cancer. 1984 May;49(5):587–593. doi: 10.1038/bjc.1984.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta V., Krishan A. Effect of oxygen concentration on the growth and drug sensitivity of human melanoma cells in soft-agar clonogenic assay. Cancer Res. 1982 Mar;42(3):1005–1007. [PubMed] [Google Scholar]
- Hamburger A. W., Salmon S. E. Primary bioassay of human tumor stem cells. Science. 1977 Jul 29;197(4302):461–463. doi: 10.1126/science.560061. [DOI] [PubMed] [Google Scholar]
- Richter A., Sanford K. K., Evans V. J. Influence of oxygen and culture media on plating efficiency of some mammalian tissue cells. J Natl Cancer Inst. 1972 Dec;49(6):1705–1712. doi: 10.1093/jnci/49.6.1705. [DOI] [PubMed] [Google Scholar]
- Tveit K. M., Endresen L., Rugstad H. E., Fodstad O., Pihl A. Comparison of two soft-agar methods for assaying chemosensitivity of human tumours in vitro: malignant melanomas. Br J Cancer. 1981 Oct;44(4):539–544. doi: 10.1038/bjc.1981.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tveit K. M., Fodstad O., Pihl A. Cultivation of human melanomas in soft agar. Factors influencing plating efficiency and chemosensitivity. Int J Cancer. 1981 Sep 15;28(3):329–334. doi: 10.1002/ijc.2910280312. [DOI] [PubMed] [Google Scholar]


